Abstract
This study was designed to assess whether heat shock protein Hsp72 is an early and sensitive biomarker of acute kidney injury (AKI) as well as to monitor a renoprotective strategy. Seventy-two Wistar rats were divided into six groups: sham-operated and rats subjected to 10, 20, 30, 45 and 60 min of bilateral ischemia (I) and 24 h of reperfusion (R). Different times of reperfusion (3, 6, 9, 12, 18, 24, 48, 72, 96 and 120 h) were also evaluated in 30 other rats subjected to 30 min of ischemia. Hsp72 messenger RNA (mRNA) and protein levels were determined in both kidney and urine. Hsp72-specificity as a biomarker to assess the success of a renoprotective intervention was evaluated in rats treated with different doses of spironolactone before I/R. Renal Hsp72 mRNA and protein, as well as urinary Hsp72 levels, gradually increased relative to the extent of renal injury induced by different periods of ischemia quantified by histomorphometry as a benchmark of kidney damage. Urinary Hsp72 increased significantly after 3 h and continued rising until 18 h, followed by restoration after 120 h of reperfusion in accord with histopathological findings. Spironolactone renoprotection was associated with normalization of urinary Hsp72 levels. Accordingly, urinary Hsp72 was significantly increased in patients with clinical AKI before serum creatinine elevation. Our results show that urinary Hsp72 is a useful biomarker for early detection and stratification of AKI. In addition, urinary Hsp72 levels are sensitive enough to monitor therapeutic interventions and the degree of tubular recovery following an I/R insult.
Keywords: early biomarker, stratifying biomarker, spironolactone renoprotection, patients with AKI
INTRODUCTION
Ischemia/reperfusion (I/R) and nephrotoxic injuries are the major causes of acute kidney injury (AKI) in native and transplanted kidneys (Friedewald & Rabb, 2004). AKI occurs in about 5% of hospitalized patients and up to 40–60% of intensive care unit (ICU) patients (Kelly, 2006). Despite technical improvements in clinical care and the development of preventive strategies, the prevalence of AKI has risen significantly in the last 15 years due to population aging and the rising pandemics of obesity, diabetes and hypertension (Liano & Pascual, 1996; Waikar et al, 2006). Despite efforts and advances in the development of new therapeutics, the mortality rate of AKI remains between 40 and 80% and has not been reduced in the last four decades, mainly because current tools used for the early detection of AKI are not adequately sensitive or specific (Wu & Parikh, 2008). In current clinical practice, AKI is typically diagnosed by a rise in serum creatinine (SCr). Indeed, acute kidney injury network (AKIN) and risk injury failure lost end stage renal disease (RIFLE) classifications for the detection of AKI are based on elevation of SCr (Bagshaw, 2010; Lopes et al, 2008; Wu & Parikh, 2008). It is generally accepted, however, that SCr is an unreliable and delayed marker of kidney injury. Substantial injury to the kidney may occur without affecting glomerular filtration; for example, extensive kidney mass reduction may occur without changes in SCr levels, and urinary obstruction caused by post-renal factors may be associated with an elevation in SCr without renal tubular injury (Coca & Parikh, 2008). Moreover, it has been demonstrated that SCr rises too late to facilitate early diagnosis of AKI; creatinine elevation is detected after 48 h of ischemic insult (Parikh et al, 2006).
It is known that early initiation of treatment substantially improves the prognosis for patients with AKI. Therefore, the development of novel, sensitive renal biomarkers is crucial for the identification of new therapeutic strategies for AKI; such biomarkers will facilitate early treatment and monitoring of the disease course (Yamamoto et al, 2007). Ideally, a sensitive biomarker for AKI should comprise the following characteristics: easy detection, non-invasive and capable of detecting AKI early in the clinical course. Because acute tubular necrosis (ATN), which is characterized by loss of brush border and polarity in the tubular epithelium with necrosis and apoptosis as well as cell tubular detachment (Devarajan, 2006; Liu & Brakeman, 2008; Price et al, 2009), is a common feature of most AKI, a good biomarker should also possess the ability to predict the extent of tubular injury. The detection of the extent of insult would identify those patients with severe tubular damage and who could be at risk for developing end stage chronic kidney disease. Consequently, a good biomarker could also be used to identify patients that will require subsequent follow-up to decrease or slow the progression of chronic renal failure. In addition to early diagnosis and prognosis, it would also be desirable to identify biomarkers capable of discerning AKI subtypes, identifying etiologies, predicting clinical outcomes, allowing for risk stratification and monitoring the response to interventions (Coca & Parikh, 2008).
It is well known that, during AKI, several mechanisms are activated to compensate for the resultant cell injury; one of these compensatory mechanisms is the up-regulation of heat shock proteins (Hsp) (Csermely et al, 2007; Ritossa, 1962), which help to restore cell homeostasis. These proteins, which have molecular weights ranging from 10 to 150 kDa (Lindquist & Craig, 1988), are encoded by different genes. The Hsp70 subfamily is composed of four isoforms: Hsc70, the inducible isoform Hsp72, mHsp75 and Grp78. Hsp72 is expressed in response to cell stress, and its induction can be as great as 15% of the total cell protein (Fan et al, 2003). Several studies have shown that Hsp72 is up-regulated in damaged tubules after ischemic and toxic kidney injury (Hernadez-Pando et al, 1995; Kelly, 2002; Kelly et al, 2001; Molinas et al, 2010; Mueller et al, 2003; Turman & Rosenfeld, 1999; Zhipeng et al, 2006). Given that Hsp72 is induced in renal tubules during AKI and that proximal tubular detachment is projected to the urinary space, we reasoned that the urinary Hsp72 level could serve as an early biomarker to detect, monitor and/or stratify AKI. The performance of Hsp72 as a sensitive biomarker to detect different degrees of renal injury and recovery was corroborated by using histopathological analysis as a benchmark of kidney damage. Here, we provide evidence that Hsp72 is an early and sensitive biomarker of AKI in both rats subjected to I/R and in humans with clinical AKI.
RESULTS
Different degrees of renal injury were induced in five groups of rats that underwent various periods of bilateral renal ischemia (10 to 60 min); all rats were studied after 24 h of reperfusion. Figure 1 shows the renal function parameters in the six groups studied together with the quantification of two classic tubular injury markers. Although increases in SCr were only significant after 30 min of bilateral ischemia (Fig 1A), all rats that underwent I/R exhibited renal dysfunction characterized by gradual reduction in creatinine clearance (Fig 1B) and renal plasma flow (Fig 1C). These alterations were not associated with changes in mean arterial pressure (MAP) (Fig 1D). As expected, the worst renal dysfunction was observed in the animals subjected to 60 min of bilateral ischemia. Tubular injury induced by different periods of ischemia was assessed by the elevation of urinary N-acetyl-β-d-glucosaminidase (NAG) and proteinuria. Statistical differences in NAG were seen only after 30 min of ischemia, suggesting that NAG is not adequately sensitive to detect slight or mild renal injury.
Figure 1. Renal functional parameters in rats underwent which different periods of bilateral renal ischemia (10, 20, 30, 45, and 60 min) and 24 h of reperfusion compared to sham-operated rats (white bars).
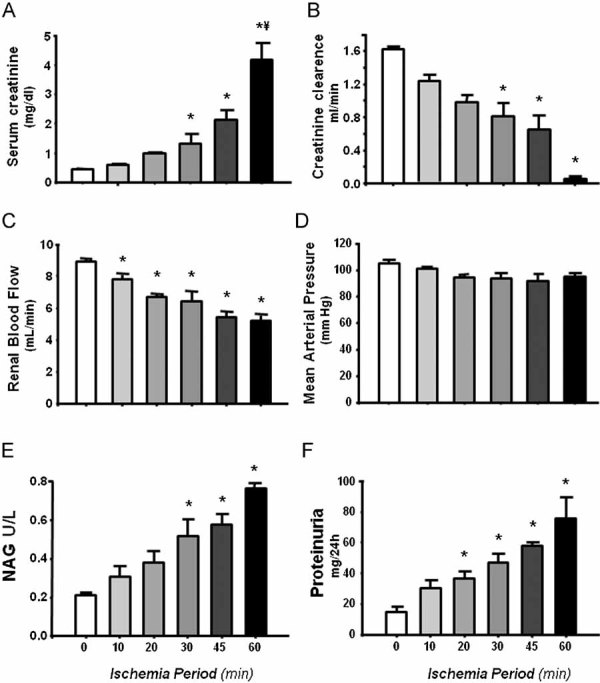
- Serum creatinine levels.
- Creatinine clearance.
- Renal blood flow.
- Mean arterial pressure.
- Urinary NAG excretion.
- Protein excretion.
n = 6, *p < 0.05 versus sham-operated rats and ¥p < 0.05 versus 45 min I/R group.
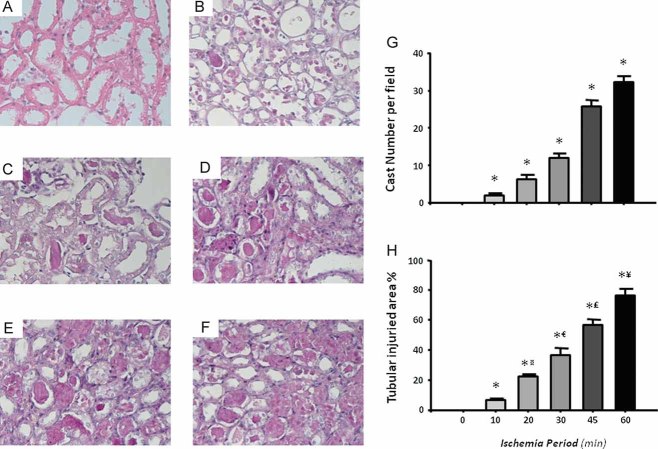
Tubular injury was also detected by light microscopy, the gold standard in evaluating acute renal injury. The left panels of Fig 2 show representative images of kidney sections from rats subjected to different periods of ischemia, and the right panels show quantified cast numbers and the percentage of injured tubular area quantified by morphometry. Tubular histopathology induced by I/R was characterized by brush border loss, lumen dilatation or collapse and cellular detachment from tubular basement membranes (Fig 2A–F). Thus, a proportional increase in tubular injury correlates to a longer period of induced renal ischemia. After 24 h of reperfusion, the smallest degree of tubular injury was observed in rats that underwent 10 min of bilateral ischemia, and the worst injury was observed in rats subjected to 60 min of ischemia (Fig 2 G–H). Therefore, progressive increases in tubular damage are proportional to the ischemia period provoked.
Figure 2. Representative images and morphometry of subcortical histopathological lesions induced by different periods of ischemia and 24 h of reperfusion.
- Sham-operated rats.
- 10 min.
- 20 min.
- 30 min.
- 45 min.
- 60 min.
- Mean cast number per field.
- Percentage of tubular affected area.
*p < 0.05 versus sham-operated rats, ¤p < 0.05 versus 10 min, €p < 0.05 versus 20 min, £p < 0.05 versus 30 min and ¥p < 0.05 versus 45 min of ischemia group.
I/R induced renal Hsp72 up-regulation
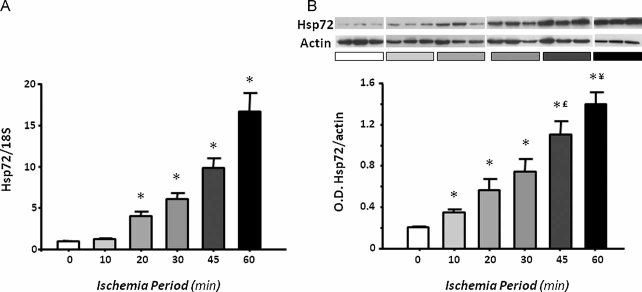
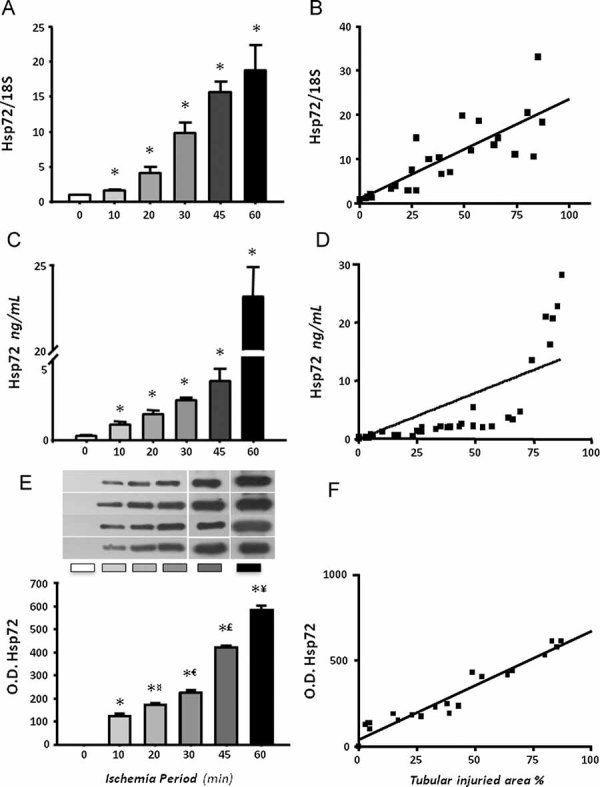
To evaluate if Hsp72 is proportionally induced with different degrees of ischemic injury, messenger RNA (mRNA) and protein levels were detected in the renal cortex from rats subjected to different periods of ischemia. As shown in Fig 3A, after 24 h of reperfusion, Hsp72 mRNA levels were significantly and progressively increased following 10 min of bilateral ischemia. These findings were reflected at protein level, as is shown in Fig 3B. Renal injury induced by different periods of renal ischemia was associated with a gradual and significant increase in Hsp72 expression, compared with almost undetectable levels in sham-operated rats. The greatest expression of Hsp72 was observed in the group with severe tubular injury, around 30-fold greater compared to control levels. These results show that Hsp72 is gradually increased relative to the intensity of induced renal injury.
Figure 3. Renal Hsp72 expression in rats subjected to different periods of ischemia.
- Total RNA was individually extracted from the renal cortex of all studied groups (n = 5) and Hsp72 mRNA levels were determined by real time RT-PCR.
- Renal cortex proteins were individually extracted from three rats of each group, and Hsp72 protein levels were assessed by western blot. Upper inset shows a representative image of the autoradiography of the membrane and the lower graph depicts densitometric analyses of the ratio of Hsp72 to β-actin. *p < 0.05 versus sham-operated rats, £p < 0.05 versus 30 min and ¥p < 0.05 versus 45 min of ischemia group.
Urinary Hsp72 levels as a biomarker of AKI
Consequently, we addressed whether Hsp72 could be detected in the urine of animals that suffered from AKI. First, Hsp72 mRNA levels were analyzed in collected urine samples of the different groups studied. As shown in Fig 4A, Hsp72 mRNA levels were increased following 10 min of ischemia compared to the control group, and the incremental increase in Hsp72 was progressively enhanced relative to the duration of ischemia. When these values were compared to the number of casts, a significant correlation was found (r2 = 0.72 and p < 0.0001, data not shown). Similar results were observed when Hsp72 mRNA levels and tubular affected area were correlated, as is shown in Fig 4B (r2 = 0.82 and p < 0.0001).
Figure 4. Urinary Hsp72 mRNA and protein levels from rats subjected to different periods of ischemia.
- Total RNA was individually extracted from the urine of six rats per group and Hsp72 mRNA levels were determined by real time RT-PCR.
- Relationship between mRNA levels and tubular affected area.
- Urinary Hsp72 levels assessed by ELISA.
- Relationship between urinary Hsp72 levels and the % of tubular affected area.
- Urinary Hsp72 levels assessed by WB analysis from four rats of each group.
- Relationship between urinary Hsp72 levels detected by WB and tubular injured area. *p < 0.05 versus sham-operated rats, ¤p < 0.05 versus 10 min, €p < 0.05 versus 20 min, £p < 0.05 versus 30 min and ¥p < 0.05 versus 45 min of ischemia group.
Urinary protein levels of Hsp72 were assessed by two immunoassays: enzyme-linked immunosorbent assay (ELISA) and Western blot analysis. The urinary determination of Hsp72 by ELISA is shown in Fig 4C and revealed that this protein can be detected in urine samples and that it is an excellent marker of acute renal injury as it is capable of differentiating scant (since 10 min), moderate (20 and 30 min) and severe renal injury (45 and 60 min of ischemia). Thus, Hsp72 correlates with the extent of renal injury induced by different periods of ischemia. The amount of Hsp72 detected was the greatest in the group that underwent 60 min of ischemia; it increased by 23-fold compared to the control group. As demonstrated in Fig 4D, the progressive urinary elevation of Hsp72 correlated with the intensity of renal injury, as measured by tubular injured area, r2 = 0.62 (p < 0.0001). Similar results, but with a greater sensitivity, were observed when urinary Hsp72 protein levels were detected by Western blot. The upper panel of Fig 4E shows Western blots of the urine from four different rats from each group studied. Hsp72 was undetectable in the urine of sham-operated rats; in contrast, Hsp72 was progressively increased in the urine from rats that suffered increasing periods of ischemia. As shown in Fig 4E, Hsp72 protein levels, assessed by Western blot, were significantly enhanced after 10 min of ischemia and increased in animals subjected to longer ischemia periods. However, the sensitivity to detect different degrees of renal damage was greater with Western blot analysis than with ELISA. Urinary Hsp72 was 40-fold increased in the group subjected to 10 min of ischemia and 535-fold in the group with severe renal damage (60 min of ischemia) compared to sham-operated rats. As shown in Fig 4F, stronger correlations were found between the amount of Hsp72 in the urine and the % affected tubular area, r2 = 0.89 (p < 0.0001). These findings show that Hsp72 is found in the urine of ischemic rats and its urinary detection is adequately sensitive to assess the extent of tubular injury.
Urinary Hsp72 as an early biomarker of AKI
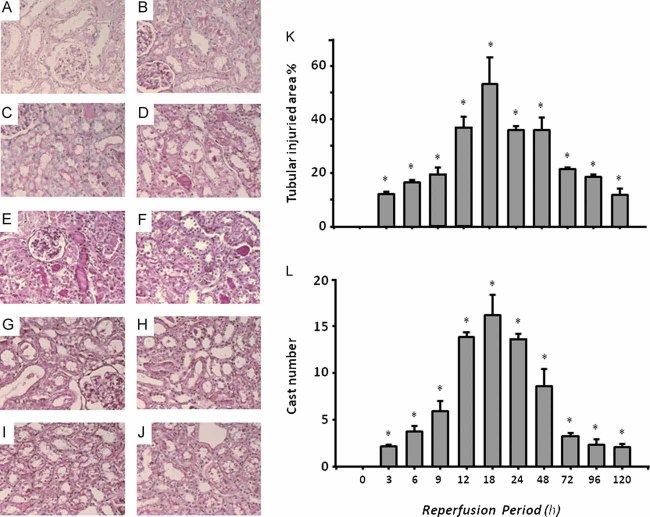
To evaluate if Hsp72 could be utilized as an early biomarker of AKI and if it is adequately sensitive to detect tubular recovery after an ischemic insult, urinary Hsp72 concentrations were assessed in animals subjected to 30 min of bilateral ischemia followed by 3–120 h of reperfusion. Figure 5 shows representative microphotographs from kidneys after reperfusion periods of 3, 6, 9, 12, 18, 24, 48, 72 and 120 h (Fig 5A–J). In the right panels, quantification of injured areas and casts number per field are shown. As the morphometric analysis shows in Fig 5K, there was a progressive increment in tubular injury, reaching the maximal degree after 18 h of reperfusion, at which point more than 50% of the tubular area was damaged. After this point, there was progressive tubular recovery until 120 h of reperfusion, at which point only 12% of tubular area was injured. The same pattern was observed when casts number were quantified, as depicted in Fig 5L.
Figure 5. Representative images and morphometry of subcortical histopathological lesions induced by 30 min of ischemia and different periods of reperfusion.
- 3 h
- 6 h
- 9 h
- 12 h
- 18 h
- 24 h
- 48 h
- 72 h
- 96 h and
- 120 h of reperfusion.
- Morphometric quantification of affected tubular area.
- Mean cast number per field *p < 0.05 versus sham-operated rats.
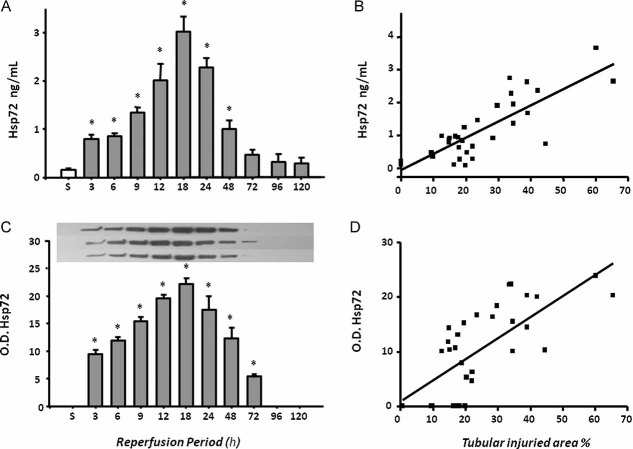
In Fig 6A (urinary detection of Hsp72 by ELISA), a significant elevation was detected from 3 h after reperfusion. Similarly to histological injury, the greatest urinary Hsp72 was found after 18 h and return to basal values after 96 h of reperfusión. Again a significant correlation between urinary Hsp72 and % of tubular injured area was observed, r2 = 0.65 (p < 0.0001). Insets in the top of Fig 6C show Western blots from three rats of each group; densitometric analysis is shown below. Urinary Hsp72 was almost undetectable in sham-operated rats; in contrast, there was a significant increase in Hsp72 in groups subjected to renal ischemia followed by 3 h of reperfusion and a progressive increase in the amount of Hsp72 detected in the urine was observed relative to the reperfusion period, peaking at 18 h of reperfusion with a subsequent decrease in excretion of this protein. Intriguingly, there was a significant correlation between urinary Hsp72 and the % area of tubular injury, r2 = 0.55 (p < 0.0001) as depicted in Fig 6D. A better correlation was found between Hsp72 and cast formation, r2 = 0.73 (p < 0.0001, data not shown). These results demonstrate that Hsp72 is an early biomarker to detect AKI and suggest that the amount of Hsp72 detected in the urine might reveal the state of tubular injury and recovery.
Figure 6. Urinary Hsp72 levels in rats which underwent to 30 min of ischemia with different periods of reperfusion.
- Urinary Hsp72 levels assessed by ELISA.
- Relationship between urinary Hsp72 and % of tubular damaged area.
- Urinary Hp72 protein levels determined by Western blot.
- Relationship between Hsp72 and % of tubular injured area.
n = 3 per period of reperfusion, *p < 0.05 versus sham operated rats.
Kim-1, NGAL, IL-18 as biomarkers for stratifying and early detecting AKI
The performance of the kidney injury molecule 1 (Kim-1), neutrophil gelatinase-associated lipocalin (NGAL) and Interleukin 18 (IL-18) for stratification and early detection of AKI was also investigated to compare it with performance of Hsp72. As shown in figure Fig 7A, urinary Kim-1 levels significantly increased after 20 min of ischemia, and these values reached the maximum increment at 30 min; thus Kim-1 was unable to differentiate greater renal injury induced by 45 and 60 min of ischemia. As a result, a lower correlation between percent of tubular injured area and Kim-1 levels was found (r2 = 0.46). NGAL levels progressively increased with the extent of renal injury induced by different periods of ischemia, r2 = 0.61 (p < 0.001) as shown in Fig 7B. However, statistical differences between different periods of ischemia were not found. Thus, NGAL was not as good a biomarker as Hsp72 to predict different degrees of renal damage. Similarly to Hsp72 and NGAL, IL-18 increased proportionally to the renal injury induced from 20 min of ischemia (r2 = 0.59, p < 0.001), as depicted in Fig 7C.
Figure 7. Urinary levels of Kim-1, NGAL and IL-18 in rats which underwent different periods of ischemia and reperfusion (n = 6).
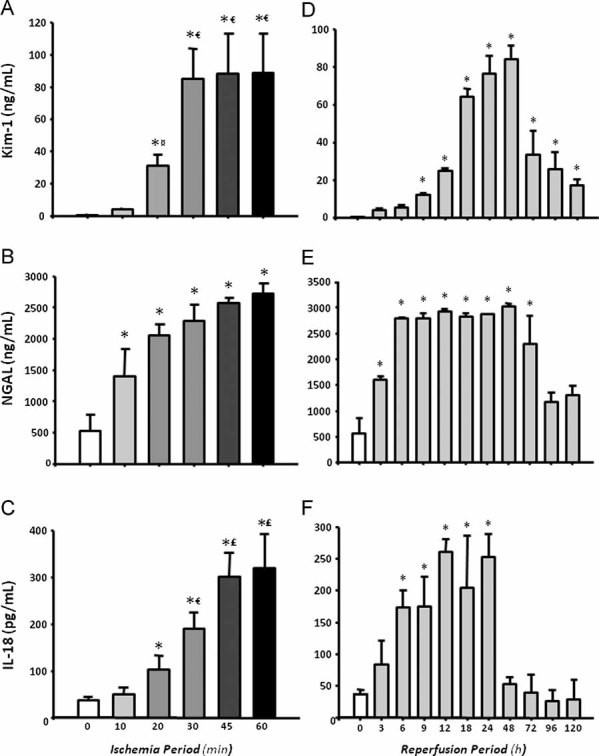
- A, B, C. Urinary levels of Kim-1, NGAL, and IL-18 in different degrees of renal injury induced by increasing periods of ischemia (10, 20, 30, 45 and 60 min), respectively.
- D, E, F. Urinary concentrations of Kim-1, NGAL and IL-18 after several times of reperfusion (3, 6, 9, 12, 18, 24, 48, 72, 96 and 120 h), respectively.
¤p < 0.05 versus 10 min, €p < 0.05 versus 20 min, £p < 0.05 versus 30 min and ¥p < 0.05 versus 45 min of ischemia group.
The usefulness of these biomarkers for early diagnosis of AKI was also assessed. Figure 7D shows the urinary Kim-1 values from rats exposed to different periods of reperfusion. A significant elevation of Kim-1 was observed after 9 h of reperfusion, reaching the maximal value after 48 h. As a result, a low correlation between tubular affected area and urinary Kim-1 levels (r2 = 0.27) was found. In the case of urinary NGAL, a significant elevation was found after 3 h of reperfusion; however, NGAL levels reached the maximum peak at 6 h and did not reflect the greater renal injury that is observed after 18 h. As a result, a lower correlation between urinary NGAL levels and tubular affected area was observed, r2 = 0.48 versus r2 = 0.65 observed with Hsp72. When IL-18 was assessed, a significant increase was observed after 6 h and levels remained elevated after 24 h of reperfusion. As a result, the correlation between tubular affected area and urinary IL-18 was r2 = 0.51, p < 0.001 (Fig 7F), which was similar to NGAL (r2 = 0.48), but lower than Hsp72 (r2 = 0.65).
Urinary Hsp72 levels as a monitor of a renoprotective intervention in AKI
Because we previously reported that aldosterone blockade is a helpful treatment to prevent renal injury induced by I/R (Mejia-Vilet et al, 2007; Ramirez et al, 2009), we assessed if this renoprotective effect could be reflected by the prevention of Hsp72 elevation. Figure 8A and B shows the SCr and creatinine clearance in two groups of rats subjected to ischemia and 24 h of reperfusion without treatment (I/R) and pre-treated with spironolactone 3 days before I/R (I/R + Sp). Spironolactone administration prevented renal dysfunction. This renoprotective effect was associated with a significant reduction in urinary Hsp72 levels detected by ELISA and Western blot, as shown in Fig 8C and D, respectively. In order to evaluate if Hsp72 might allow to monitor different degrees of renoprotection in the absence of creatinine elevation, different groups with I/R were pre-treated with lower doses of spironolactone. As is shown in Fig 8E, rats pre-treated with spironolactone at 10 and 5 mg/kg exhibited normal values of SCr that contrast with the 2.5 mg/kg dose, in which the values were similar to untreated I/R group. Interestingly, urinary Hsp72 levels were significantly increased from 10 mg/kg of spironolactone and progressively enhanced when the lower doses of were administrated, as is depicted in Fig 8F. These findings suggest that the lower the dose of spironolactone, the lower the renoprotection. This can be efficiently detected by urinary Hsp72.
Figure 8. Urinary Hsp72 as a biomarker of renoprotection conferred by spironolactone in I/R rats (n = 5).
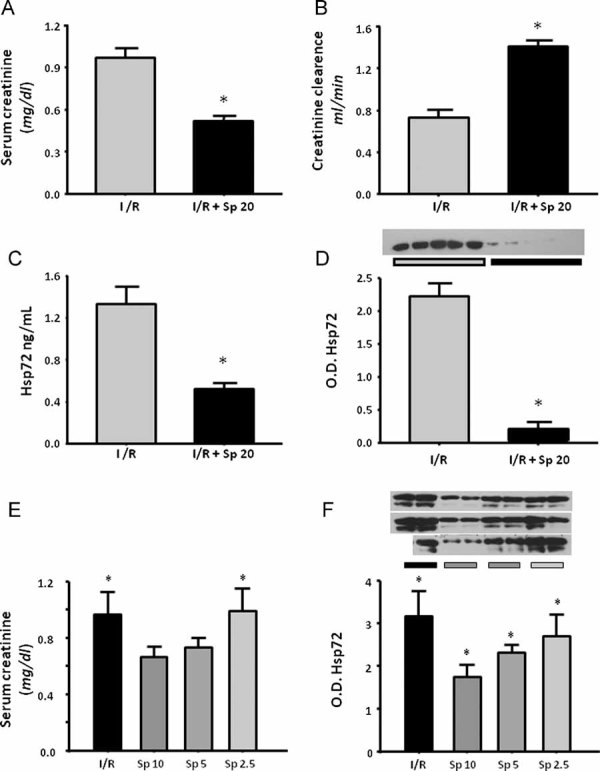
- Serum creatinine in I/R group without treatment (gray bar) and rats pre-treated with spironolactone (black bar),
- Creatinine clearance,
- Urinary Hsp72 assessed by ELISA and
- By WB analysis, the inset shows the individual analysis from five individual urines.
- Serum creatinine in I/R group and in rats pre-treated with lower doses of spironolactone (10, 5 and 2.5 mg/kg) and
- WB analysis of Hsp72 in rats pre-treated with lower doses of spironolactone, the superior inset shows the individual Hsp72 level from five different animals. *p < 0.05 versus I/R group.
Urinary Hsp72 levels in healthy kidney donors and patients with AKI
To determine whether Hsp72 is a sensitive biomarker to detect AKI in humans, the levels of this protein were assessed by Western blot in the urine of normal subjects and compared to those patients who developed AKI in the ICU of our institution. AKI was defined as an increase in SCr by at least 0.3 mg/dL, or urine output ≤0.5 ml/kg/h over 6 h. The Supplementary Table 1 shows general characteristics and renal function of five healthy kidney donors and nine patients with septic AKI. AKI patients included five females and four males aged between 24 and 84 years. At admission to the ICU, all patients exhibited normal creatinine levels; however, increases in SCr from 0.55 ± 0.05 to 2.30 ± 0.52 mg/dl were subsequently observed (p = 0.004). Urinary Hsp72 levels are depicted in Fig 9A. In the urine of healthy kidney donors, Hsp72 was almost undetectable (3.4 ± 0.7 arbitrary units), whereas urinary Hsp72 levels significantly increased by 17.3-fold in patients with clinical AKI (58.3 ± 8.5 arbitrary units). These results were confirmed by ELISA. The basal levels of urinary Hsp72 from healthy living donors were 0.22 ± 0.07, contrasting with the values in patients diagnosed with AKI of 4.90 ± 1.5 ng/ml (22-fold increase). Of note, two patients diagnosed with AKI died during hospitalization; these patients exhibited greater urinary values of Hsp72 as assessed by either Western blot or ELISA.
Figure 9. Urinary Hsp72 levels as a biomarker of AKI in humans.
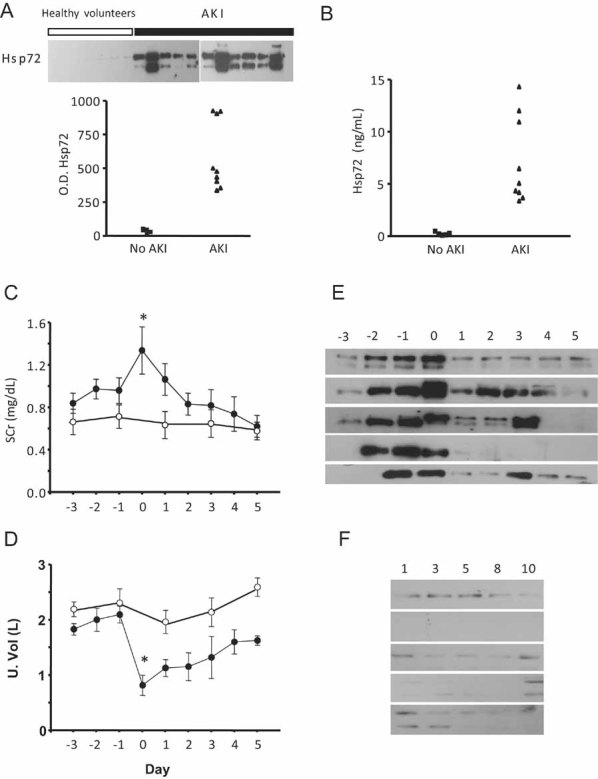
- A, B. Hsp72 levels assessed by Western blot and ELISA, respectively, in five healthy kidney donors (▪) and nine patients that were diagnosed with AKI (▴).
- C. Serum creatinine from five patients with no evidence of AKI (○) or in five patients with AKI diagnosed with AKIN criteria (•).
- D. Urine output from five patients with no evidence of AKI (○) or in five patients with AKI diagnosed with AKIN criteria (•).
- E. Daily urinary Hsp72 levels from patients with diagnosed AKI.
- F. Daily urinary Hsp72 levels in patients without AKI.
The samples were collected during three days before and 5 days after AKI was diagnosed. *p < 0.05 versus 3 days before AKI.
To identify if Hsp72 is an early biomarker to detect AKI in humans, daily urine samples were collected from patients who were admitted to the ICU exhibited and respiratory failure (mechanical ventilation) complicated with other organ failure. These patients had normal renal function at the admission. Five patients that developed AKI and five patients with no evidence of clinical AKI were included. Urine samples taken every day were analyzed to investigate if an elevation of Hsp72 could be detected before AKI criteria was fulfilled. Figure 9C and 9D show the mean daily serum creatinine and urine output from patients with or without AKI. Day 0 means the day at which AKI was diagnosed based on AKI criteria. Figure 9E depicts Western blots showing the daily urinary Hsp72 levels from the patients diagnosed with AKI, compared with the Western blots from patients without AKI (Fig 9F). The basal levels of Hsp72 were minimal in patients without AKI and three days before AKI was diagnosed in AKI patients. In agreement with our experimental studies, one or two days before AKI diagnosis, there was a significant increase in urinary Hsp72 levels, suggesting that, indeed, Hsp72 can be an early marker of AKI in humans.
DISCUSSION
In this study, we found that urinary Hsp72 is a reliable biomarker for the early detection of AKI. This novel biomarker was adequately sensitive for stratifying different degrees of tubular injury and recovery, as well as for monitoring a renoprotective intervention in an experimental rat model of AKI. In addition, these experimental findings were corroborated in humans who suffered from AKI. Thus, a significant increase in urinary Hsp72 was observed in patients diagnosed with AKI even 48 h before SCr elevation occurred, revealing that this Hsp is a promising biomarker for both early detection and stratification of AKI.
Because Hsp72 expression could increase during stress conditions to as much as 15% of total cellular proteins (Fan et al, 2003) and tubular detachment is proportional to the extent of renal injury, we reasoned that urinary Hsp72 levels could be used to stratify renal injury induced by ischemic insult. First, we observed that, after 24 h of reperfusion, kidney Hsp72 expression was proportionally increased in response to the ischemic insult provoked by different periods of ischemia. Thus, Hsp72 increased significantly following slight injury induced by 10 min of ischemia and increased progressively more with mild to severe ischemia, suggesting that the induction of this protein in the kidney is proportional to the degree of the resulting damage. Furthermore, the same pattern was found in the urine of these animals when Hsp72 was detected both at mRNA and at protein levels. Significant increase in urinary Hsp72 was observed in rats that underwent 10 min of ischemia and greater amounts were found in rats following 60 min of ischemia. In contrast, increase in SCr, NAG and urinary protein excretion reached significance after 30 min of ischemia.
In this study, we performed a blindly morphometric quantification of the tubular affected area and the casts number per field. Thus, when the amount of Hsp72 was interrelated with the tubular injured area affected by different periods of ischemia, a significant correlation was found at mRNA and protein levels. These data show that Hsp72 seems to be a sensitive biomarker to stratify the extent of the renal insult.
To investigate if urinary Hsp72 levels could detect early stages of AKI, different groups of rats were subjected to renal ischemia of 30 min and were studied at different periods of reperfusion. Histopathologic analysis showed that the greatest tubular injury was found after 18 h of reperfusion, and the extent was progressively reduced with longer periods of reperfusion due to the concomitant tubular recovery observed after 72 h. Interestingly, the amount of urinary Hsp72 detected also reflected the extent of tubular epithelium injury during different periods of reperfusion and the regeneration of tubular epithelium. Urinary Hsp72 increased significantly after 3 h and continually rose until 18 h of reperfusion. After this time, a progressive reduction was detected until 120 h of reperfusion, at which point Hsp72 returned to basal levels. The pattern of urinary Hsp72 expression observed with different periods of reperfusion significantly correlated with the tubular injury quantified by morphometry. This finding suggests that this protein was not only able to detect early AKI but that it could also reflect the tubular recovery processes that occur after the epithelium is exposed following ischemic/reperfusion insult.
Recently, a sequence of studies which evaluated seven different biomarkers of glomerular or tubular injury induced by different nephrotoxic drugs in the rat was reported. These biomarkers included urinary total protein, cystatin C, α2-microglobulin, Kim-1, urinary trefoil factor 3 (TFF3), clusterin (CLU) and albumin urinary excretion. Renal histopathology was used as the benchmark or gold standard to define renal injury (Dieterle et al, 2010a; Vaidya et al, 2010; Yu et al, 2010). As was discussed by the FDA-EMEA and Predictive Safety Testing Consortium, in these rat toxicology studies, Kim-1, CLU, urinary albumin excretion and TFF3 were helpful to diagnose drug-induced acute kidney tubular injury in the rat and were superior to the traditional markers such as SCr and blood urea nitrogen (BUN), whereas urinary total protein, cystatin C, and α2-microglobulin were useful to identify acute drug-induced glomerular damage or impairment of kidney tubular reabsorption (Dieterle et al, 2010b). This consortium emphasized, however, that these findings must wait validation in order to be employed for clinical practice. The renal injury induced by I/R, as the most common cause of AKI in ICUs and during cardiovascular surgery, was not addressed in most of these studies (Dieterle et al, 2010; Vaidya et al, 2010; Yu et al, 2010).
Regarding AKI induced by renal ischemia in animals and humans, several urinary biomarkers have been identified over the past few years. These biomarkers include the following: NAG (Tsutsumi & Neckers, 2007), neutrophil gelatinase associated lipocalin (NGAL) (McIlroy et al, 2010; Mishra et al, 2003, 2005), Kim-1 (Han et al, 2009; Vaidya et al, 2006, 2009), cystatin C (Nejat et al, 2010; Uchida & Gotoh, 2002), interleukin-18 (IL-18) (Melnikov et al, 2001; Parikh et al, 2005, 2006), hepatocyte growth factor (HGF) (Taman et al, 1997) and liver fatty acid binding protein (L-FABP) (Ferguson et al, 2010; Negishi et al, 2009; Yamamoto et al, 2007). In humans with AKI induced by an ischemic insult, NGAL, NAG and IL-18 have shown to be early biomarkers (Han et al, 2009; Parikh et al, 2006; Wagener et al, 2006), whereas Kim-1 elevation is seen several hours after AKI has occurred and remains elevated along the time (Han et al, 2009). In addition, it is still unknown if these biomarkers individually possess the features that an ideal biomarker should have, such as the ability to detect AKI early in the disease course, to stratify different degrees of renal injury, to identify tubular recovery, to predict patients at risk of developing end stage renal disease (ESRD) and those at increased risk of death. In fact, it has been proposed that a panel of biomarkers might be a powerful tool to diagnose AKI before SCr elevation occurs; however, this strategy represents greater effort and cost (Han et al, 2009; Liangos et al, 2007; Vaidya et al, 2008). In this study, we compared the performance of Kim-1, NGAL and IL-18 for stratifying and early detecting AKI compared to Hsp72. As was shown before (Han et al, 2009), although Kim-1 differentiated low and moderate renal injury, it was unable to stratify severe renal injury degrees (Fig 7A). This is supported by the low correlation between tubular affected area and urinary Kim-1 levels (r2 = 0.27). In addition, Kim-1 did not perform well as an early biomarker, since a significant elevation of Kim-1 was observed only after 9 h of reperfusion. With respect to NGAL, this biomarker increased progressively with the extent of renal injury induced by different periods of ischemia, but statistical differences between different periods of ischemia were not found. Although NGAL was an early biomarker as Hsp72, it reached the maximum peak at 6 h and thus did not reflect the greater renal injury that is observed after 18 h of reperfusion. In the case of IL-18, it showed a similar pattern to Hsp72 for stratifying renal injury induced by different periods of ischemia; however, it was unable to detect slight renal injury induced by 10 min of ischemia. In addition, IL-18 was not useful for early AKI detection.
It has been reported that a sensitive biomarker must sense if a therapeutic strategy could reduce AKI (Yamamoto et al, 2007). In this regard, we have previously shown that mineralocorticoid receptor blockade is an effective intervention to prevent renal injury induced by I/R (Mejia-Vilet et al, 2007; Ramirez et al, 2009). Therefore, in this study, we pre-treated rats with spironolactone 3 days before induction of ischemia. Then, renal function and urinary Hsp72 were detected. In untreated rats, renal function was significantly reduced, and a significant rise in urinary Hsp72 was observed. The renoprotection conferred by spironolactone was associated with the prevention of this Hsp72 increase, and this effect was consistent with the preservation of tubular architecture, as we previously reported (Mejia-Vilet et al, 2007; Ramirez et al, 2009). In this study, we also evaluated urinary Hsp72 performance for monitoring different degrees of renoprotection. For this purpose, rats subjected to I/R were pre-treated with different doses of spironolactone. The lower dose employed (2.5 mg/kg) was not able to protect the kidneys from ischemic injury, as demonstrated by a similar increase in SCr in rats treated with this dose as compared to those untreated (Fig 8E). In contrast, some degree of protection was observed with administration of 5 or 10 mg/kg. Consistent with urinary Hsp72 being a good biomarker, the lower the renoprotection, the higher the Hsp72 amount in urine. These data suggest that urinary Hsp72 is a helpful tool for monitoring the effectiveness of a pharmacological intervention to prevent ischemic insult.
Acute kidney injury remains a common syndrome in hospitalized patients and has consistently been associated with increased morbidity and mortality. However, advances in reducing this complication have long been hindered by the lack of early and sensitive biomarkers. In spite of creatinine limitations, current AKIN and RIFLE classifications for diagnosing AKI are based on the elevation of SCr or urine output reduction. In this study, we included patients that developed AKI and were diagnosed by the AKIN classification. Once the diagnosis was made, a urine sample was taken to evaluate urinary Hsp72 levels to compare with healthy living donors. To determine Hsp72 concentration, the urine sample was analyzed by Western blot analysis and ELISA. Hsp72 levels were almost undetectable in healthy subjects; in contrast, patients with clinical AKI exhibited an abrupt increase in this protein. The variability in the AKI patients observed might result from different degrees and extension of the renal injury. In this regard, two of three patients that exhibited high urinary Hsp72 died during their hospitalization, suggesting that this protein could also serve as a biomarker of AKI-associated death. However, more studies are necessary to evaluate this issue.
To evaluate the potential of urinary Hsp72 as an early biomarker in humans, daily urine samples from ten patients with normal renal function at the admission to ICU in severe conditions (mechanical ventilation and other organ failure) were included. In accord with our experimental data, an increase in urinary Hsp72 was found even two days before AKI criteria were fulfilled (SCr elevation or urine output reduction), suggesting that urinary Hsp72 could be a promising early biomarker of AKI in humans. Most of the biomarkers studies include patients that are expected to develop AKI, such as after cardiovascular surgery or organ transplantation (Han et al, 2009; McIlroy et al, 2010; Mishra et al, 2005; Parikh et al, 2006; Yamamoto et al, 2007), however, in the daily clinical setting, we cannot predict which patients will develop AKI. In this study, urinary Hsp72 had the potential to predict AKI 48 h before AKIN criteria were fulfilled. Further clinical studies will be required to validate its use in multiple cohort studies. In this regard, it has been reported that there are five critical phases in a biomarker development that include: (1) experimental studies that identify proteins that are up-regulated in AKI mice and rat models, (2) development of clinical assays to detect the biomarker in samples from patients with clinical AKI that should be obtained non-invasively, (3) evaluation of the biomarker's ability to detect preclinical disease prior to clinical diagnosis, and (4) phase IV and V studies that involve large-scale biomarker validation (Coca & Parikh, 2008).
In summary, in an experimental model of AKI, our results revealed that Hsp72 possesses most of the specific characteristics of an ideal sensitive biomarker. Hsp72 is an early, non-invasive and easily detected biomarker that can be used to stratify renal injury that correlates with tubular injury and recovery. It also seems to be a tool to monitor the effectiveness of a pharmacological intervention. In addition, the preliminary data that we found in humans support the notion that Hsp72 could be an early predictor of AKI in the clinical setting. Taken together, these results suggest that urinary Hsp72 detection is a promising and sensitive biomarker in a clinical translational context.
MATERIALS AND METHODS
All experiments involving animals were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington, DC, 1996) and were approved by the Animal Care and Use Committee of our Institution. One hundred and five male Wistar rats, weighing 270–300 g, were included in this study and divided into two different experimental protocols. In the first protocol, seventy-two rats were randomly divided into six groups: sham-operated (sham) or subjected to bilateral ischemia of 10, 20, 30, 45 or 60 min. All of these groups were studied after 24 h of reperfusion. One half of each group was used only for the determination of urinary Hsp72 mRNA levels, as is described below. In the second protocol, thirty rats were subjected to 30 min of bilateral ischemia and divided in ten different groups, which differed by reperfusion time: 3, 6, 9, 12, 18, 24, 48, 72, 96 and 120 h. A sham-operated group was also included in this protocol.
Kidney I/R injury animal model
Rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (30 mg/kg), placed on a heating pad to maintain core body temperature at 37°C and monitored with a rectal thermometer. A midline abdominal incision was made, and both kidneys were exposed. Renal pedicles were isolated, and bilateral ischemia was induced by non-traumatic clamps over the pedicles for different times, as we previously described (Mejia-Vilet et al, 2007; Ramirez et al, 2009). Ischemia was verified visually by change in kidney colour. Reperfusion was achieved by release of the clips and confirmed by return of oxygenated blood to the kidney. After reperfusion, the incision was closed in two layers with 3–0 sutures. Sham-operated rats underwent anesthesia, laparotomy and renal pedicle dissection only.
Functional studies
Rats were placed in metabolic cages at 22°C with a 12:12 h light–dark cycle and allowed free access to water. Individual urine samples were collected from all studied rats. For urine ribonucleic acid (RNA) isolation, metabolic cages were previously cleaned with RNAse ZAP (Ambion) and 300 µL of RNAlater (Ambion) added to the urine recipient. In both cases, urine samples were aliquoted and quickly frozen at −80°C until biomarker analysis.
After the reperfusion period, rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (30 mg/kg) and placed on a homoeothermic table. The trachea and femoral arteries were catheterized with polyethylene tubing (PE-240 and PE-50). The rats were maintained under euvolemic conditions by infusing 10 ml of rat plasma per kg of body weight during surgery. MAP was monitored with a pressure transducer (model p23 db, Gould) and recorded on a polygraph (Grass Instruments, Quincy, MA). An ultrasound transit-time flow probe was placed around the left artery and filled with ultrasonic coupling gel (HR Lubricating Jelly, Carter-Wallace, New York, NY) to record renal blood flow. Blood samples were taken at the end of the study. Serum and creatinine concentrations were measured with an autoanalyzer (Technicon RA-1000, Bayer, Tarrytown, NY), and renal creatinine clearance was calculated by the standard formula C = (U × V)/P, where U is the concentration in urine, V is the urine flow rate and P is the serum concentration. Urinary protein excretion was measured by a trichloroacetic acid (TCA) turbidimetric method, and NAG was measured spectrophotometrically.
The paper explained
PROBLEM
Acute kidney injury is a common and serious complication in critically ill patients, especially in the ICU in both native and transplanted kidneys and may also be associated with long-term chronic kidney disease development. AKI may occur in about 5% of hospitalized patients and up to 40–60% patients in ICU. In addition, over the last decade the incidence of AKI has increased mainly due to population aging and the rising pandemics of obesity, diabetes and hypertension. Despite efforts and advances in new therapeutics, the mortality rate of AKI remains between 40 and 80% and has not been reduced in the last four decades, mainly because current tools used for early AKI detection are not adequately sensitive or specific. Therefore, novel renal early biomarkers indicating tubular injury extent are needed.
RESULTS
In an experimental model of AKI, we found that Hsp72 possess most of the specific characteristics of an ideal biomarker should have such as: non-invasive, easily detected, to be able to early detect AKI, to stratify renal injury that correlates with tubular injury and recovery, and to be a tool to monitor the effectiveness of a pharmacological intervention. In addition, urinary Hsp72 levels performance to detect and stratify renal injury induced by different periods of ischemia was better than urinary NGAL, Kim-1 and IL-18. Our preliminary data in humans support the notion that Hsp72 could be an early predictor of AKI in the clinical setting.
IMPACT
Hsp72 may be a useful tool to early AKI diagnose in patients admitted to ICU or with high risk of developing renal injury such as those patients undergoing cardiac surgery, renal transplant or under nephrotoxic treatment. The opportune AKI diagnosis would permit the clinician to make an adequate pharmacologic intervention to improve patient survival and to avoid end stage renal disease development.
Histopathologic studies
At the end of the experiment, the right kidney was removed and quickly frozen for molecular studies, and the left kidney was perfused through the femoral catheter with phosphate buffered saline (PBS). Following blanching of the kidney, the perfusate was replaced by a freshly prepared 4% formalin buffer, and perfusion was continued until fixation was completed. After appropriate dehydration, kidney slices were embedded in paraffin, sectioned at 4 µm and stained via a periodic acid-Schiff (PAS) technique. Ten subcortical and juxtamedullary fields were randomly recorded from each kidney slide using a digital camera incorporated in a Nikon Light microscope. In each microphotograph, tubular cast per field were counted, and the results were expressed as the average of fields observed. The affected tubular area was analyzed blindly. Tubular damage was characterized by a loss of brush border, lumen dilatation or collapse and detachment from basement membrane. Digital microphotographs were recorded for each rat to assess, by morphometric analysis, the total tubular area (excluding luminal, interstitial and glomerular areas) and damaged tubular area, delimited by using eclipse net software (magnification 400×). The damaged tubular area was expressed as a proportion of the affected tubular area and total tubular area.
Hsp72 mRNA levels
Each renal cortex was isolated and snap frozen in liquid nitrogen. Total RNA was isolated with the TRIzol method (Invitrogen) and checked for integrity by 1% agarose gel electrophoresis. To avoid DNA contamination, all total RNA samples were treated with DNAse (DNAase I, Invitrogen). For urine RNA isolation, 24 h urine-collection from each rat was centrifuged at 3000 rpm for 30 min at 4°C. The sediment was resuspended in 1 ml of PBS; afterward, it was centrifuged at 13,000 rpm for 3 min and processed by the TrIzol method for RNA isolation. Reverse transcription (RT) was carried out with 1 µg of total RNA and 200 U of Moloney murine leukemia virus reverse transcriptase (Invitrogen). The mRNA levels of Hsp72 were quantified by real-time PCR on an ABI Prism 7300 Sequence Detection System (TaqMan, ABI, Foster City, CA). Primer and probe for Hsp72 were ordered as a kit: Rn00583013_s1 (Assay-on-Demand, ABI). As an endogenous control, eukaryotic 18S rRNA (predesigned assay reagent Applied by ABI, external run) was used. Relative quantification of Hsp72 gene expression was performed with the comparative threshold cycle (Ct) method (Livak & Schmittgen, 2001).
Kidney and urinary Hsp72 protein levels
Hsp72 detected by Western blot
Total renal proteins were isolated from six cortexes from each group and homogenized separately in lysis buffer (50 mM HEPES pH 7.4, 250 mM NaCl, 5 mM EDTA, 0.1% NP-40 and complete protease inhibitor (Roche)). Protein samples containing 20 µg of total protein were resolved by 8.5% sodium polyacrylamide gel electrophoresis (SDS–PAGE) electrophoresis and electroblotted onto polyvinylidinedifluoride membranes (Amersham). For urinary Hsp72 detection by western blot, urine was diluted 1:100 in 0.9% saline solution, and 10 µL of each dilution was loaded and resolved by 8.5% SDS–PAGE electrophoresis and electroblotted, as previously described. Membranes were then blocked with 5% blotting-grade non-fat dry milk. Membranes were then incubated in 0.1% blotting-grade non-fat dry milk with their respective antibodies. For detection of Hsp72 in the renal cortex, the lower parts of the membranes were incubated with goat anti-actin antibody (1:5000 dilution) overnight at 4°C. (Santa Cruz Biotechnology, Santa Barbara CA). Upper membranes were incubated with monoclonal anti-Hsp72 antibody. Afterward, membranes for Hsp72 were incubated with a secondary antibody, HRP-conjugated goat anti-mouse IgG (1:500, Santa Cruz Biotechnology). Actin detection was performed using donkey anti-goat IgG-HRP (1:5000, Santa Cruz Biotechnology). Proteins were detected with an enhanced chemiluminescence kit (Amersham) and autoradiography, following the manufacturer's recommendations. The bands were scanned for densitometric analysis.
Hsp72 detected by ELISA
Urine Hsp72 was analyzed using a commercially available high-sensitivity ELISA (Assay Designs EKS-715, MI, USA). Briefly, samples and standards were added to wells coated with a mouse monoclonal antibody. Hsp72 was captured by the antibody and then detected by adding a rabbit polyclonal detection antibody. Both antibodies are specific for inducible Hsp72 and do not react with other members of the HSP70 family. A horseradish peroxidase conjugate bound to the detection antibody and colour development was accomplished by the addition of tetramethylbenzidine substrate and stopped with an acid stop solution. The optical density of samples was read at 450 nm by a plate reader and was overlapped with the standard curve generated from known concentrations of recombinant Hsp72 that ranged from 0.1 to 12.5 ng/ml.
Urinary Hsp72 levels as a monitor of a renoprotective intervention in AKI
Because we previously found that aldosterone antagonism with spironolactone at 20 mg/kg prevented renal injury induced by I/R, urinary Hsp72 levels as a monitor of a renoprotective intervention in AKI was assessed. Twenty-five Wistar rats, weighing 270–300 g, were divided in five groups: rats subjected to 30 min of ischemia and 24 h of reperfusion and rats that received spironolactone at different doses (20, 10, 5 or 2.5 mg/kg, by gastric gavage) 3 days before I/R was induced. After ischemia, urine was collected in metabolic cages, and blood samples were taken.
NGAL, Kim-1 and IL-18 detected by ELISA
Urine NGAL, Kim-1 and IL-18 levels were analyzed using commercially available ELISA kits: rat NGAL ELISA kit (Innovative Research IRNGALKT), rat Kim-1 ELISA kit (CosmoBIO Co CSB-E08808r) and rat IL-18 ELISA Kit (Invitrogen KRC2341). All procedures were performed accordingly manufacturer's instructions.
Evaluation of urinary Hsp72 in healthy kidney donors and patients with AKI
Urine samples from five healthy living-kidney donors (controls) and nine patients with septic AKI from the ICU were tested. AKI was defined as a 30% or greater increase in SCr from baseline according to the AKIN criteria (Mehta et al, 2007). In healthy donors, urine samples were collected from the first morning voids one day previous to nephrectomy (previously signed consent). All patients with sepsis in the ICU were followed every day, and, when the AKI criteria were fulfiled, a fresh urine sample was taken after draining the urine collection bag. Moreover, daily fresh urine samples were collected from patients in the ICU that exhibited respiratory failure with mechanical ventilation and together with other organ failure. These patients had normal renal function at the admission. From these patients, we analyzed, five patients that developed AKI and five that did not. In the urine samples Hsp72 levels were assessed in order to test if this biomarker could predict AKI criteria was fulfil (SCr elevation or urine output reduction). All urine samples were frozen and stored at −80°C until Hsp72 levels were evaluated. The human subjects included in these study form part of two ongoing clinical studies, which are in accordance with national and international guidelines and regulations and were approved by our institutional ethical review board. All subjects or designed surrogate (when ICU patients could not sign) signed the informed consent form.
Statistical analysis
Results are presented as means ± SE. Significance of the differences between groups was tested by ANOVA using Bonferroni's correction for multiple comparisons. All comparisons passed the normality test. Statistical significance was defined as p-value <0.05.
Acknowledgments
We are grateful to the members of the Molecular Physiology Unit for their suggestions and to Dr. Octavio Villanueva for his help with animal care, as well as to Martha Carrrasco for her technical assistance. The results presented in this paper have not been published previously neither in whole nor in abstract. This project was supported by grants from the Mexican Council of Science and Technology (CONACyT) 101030 and 112780 to NAB, by a grant from the National University of Mexico IN200909-3 to NAB and by a grant from Fundación Miguel Aleman AC to NAB. JBC is a PhD student supported by fellowship grant from CONACyT.
Supporting information is available at EMBO Molecular Medicine online.
The authors declare that they have no conflict of interest.
Author Contributions
JBC, LEM and NAB: Conceived and designed the experiments. JBC, RPV, CCG and MOC: performed the experiments. JBC, GG, LEMB and NAB: analyzed the data. GG and NAB: contributed reagents or analysis tools. JBC, GG and NAB: wrote the paper.
For more information
Heat Shock 70-kd protein:
http://www.ncbi.nlm.nih.gov/omim/140550
National Kidney Foundation
Supplementary material
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
References
- Bagshaw SM. Acute kidney injury: diagnosis and classification of AKI: AKIN or RIFLE. Nat Rev Nephrol. 2010;6:71–73. doi: 10.1038/nrneph.2009.225. [DOI] [PubMed] [Google Scholar]
- Coca SG, Parikh CR. Urinary biomarkers for acute kidney injury: perspectives on translation. Clin J Am Soc Nephrol. 2008;3:481–490. doi: 10.2215/CJN.03520807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csermely P, Soti C, Blatch GL. Chaperones as parts of cellular networks. Adv Exp Med Biol. 2007;594:55–63. doi: 10.1007/978-0-387-39975-1_6. [DOI] [PubMed] [Google Scholar]
- Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17:1503–1520. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- Dieterle F, Perentes E, Cordier A, Roth DR, Verdes P, Grenet O, Pantano S, Moulin P, Wahl D, Mahl, et al. Urinary clusterin, cystatin C, beta2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat Biotechnol. 2010;28:463–469. doi: 10.1038/nbt.1622. [DOI] [PubMed] [Google Scholar]
- Dieterle F, Sistare F, Goodsaid F, Papaluca M, Ozer JS, Webb CP, Baer W, Senagore A, Schipper MJ, Vonderscher J, et al. Renal biomarker qualification submission: a dialog between the FDA-EMEA and Predictive Safety Testing Consortium. Nat Biotechnol. 2010;28:455–462. doi: 10.1038/nbt.1625. [DOI] [PubMed] [Google Scholar]
- Fan CY, Lee S, Cyr DM. Mechanisms for regulation of Hsp70 function by Hsp40 cell stress. Chaperones. 2003;8:309–316. doi: 10.1379/1466-1268(2003)008<0309:mfrohf>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson MA, Vaidya VS, Waikar SS, Collings FB, Sunderland KE, Gioules CJ, Bonventre JV. Urinary liver-type fatty acid-binding protein predicts adverse outcomes in acute kidney injury. Kidney Int. 2010;77:708–714. doi: 10.1038/ki.2009.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004;66:486–491. doi: 10.1111/j.1523-1755.2004.761_3.x. [DOI] [PubMed] [Google Scholar]
- Han WK, Wagener G, Zhu Y, Wang S, Lee HT. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2009;4:873–882. doi: 10.2215/CJN.04810908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernadez-Pando R, Pedraza-Chaverri J, Orozco-Estevez H, Silva-Serna P, Moreno I, Rondan-Zarate A, Elinos M, Correa-Rotter R, Larriva-Sahd J. Histological and subcellular distribution of 65 and 70 kD heat shock proteins in experimental nephrotoxic injury. Exp Toxicol Pathol. 1995;47:501–508. doi: 10.1016/s0940-2993(11)80337-4. [DOI] [PubMed] [Google Scholar]
- Kelly KJ. Stress response proteins and renal ischemia. Minerva Urol Nefrol. 2002;54:81–91. [PubMed] [Google Scholar]
- Kelly KJ. Acute renal failure: much more than a kidney disease. Semin Nephrol. 2006;26:105–113. doi: 10.1016/j.semnephrol.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Kelly KJ, Baird NR, Greene AL. Induction of stress response proteins and experimental renal ischemia/reperfusion. Kidney Int. 2001;59:1798–1802. doi: 10.1046/j.1523-1755.2001.0590051798.x. [DOI] [PubMed] [Google Scholar]
- Liangos O, Perianayagam MC, Vaidya VS, Han WK, Wald R, Tighiouart H, MacKinnon RW, Li L, Balakrishnan VS, Pereira BJ, et al. Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J AmSoc Nephrol. 2007;18:904–912. doi: 10.1681/ASN.2006030221. [DOI] [PubMed] [Google Scholar]
- Liano F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int. 1996;50:811–818. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Liu KD, Brakeman PR. Renal repair and recovery. Crit Care Med. 2008;36:S187–S192. doi: 10.1097/CCM.0b013e318168ca4a. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lopes JA, Fernandes P, Jorge S, Goncalves S, Alvarez A, Costa e S, Franca C, Prata MM. Acute kidney injury in intensive care unit patients: a comparison between the RIFLE and the acute kidney injury network classifications. Crit Care. 2008;12:R110. doi: 10.1186/cc6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlroy DR, Wagener G, Lee HT. Neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery: the effect of baseline renal function on diagnostic performance. Clin J Am Soc Nephrol. 2010;5:211–219. doi: 10.2215/CJN.04240609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia-Vilet JM, Ramirez V, Cruz C, Uribe N, Gamba G, Bobadilla NA. Renal ischemia-reperfusion injury is prevented by the mineralocorticoid receptor blocker spironolactone. Am J Physiol Renal Physiol. 2007;293:F78–F86. doi: 10.1152/ajprenal.00077.2007. [DOI] [PubMed] [Google Scholar]
- Melnikov VY, Ecder T, Fantuzzi G, Siegmund B, Lucia MS, Dinarello CA, Schrier RW, Edelstein CL. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest. 2001;107:1145–1152. doi: 10.1172/JCI12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- Molinas SM, Rosso M, Wayllace NZ, Pagotto MA, Pisani GB, Monasterolo LA, Trumper L. Heat shock protein 70 induction and its urinary excretion in a model of acetaminophen nephrotoxicity. Pediatr Nephrol. 2010;25:1245–1253. doi: 10.1007/s00467-010-1493-2. [DOI] [PubMed] [Google Scholar]
- Mueller T, Bidmon B, Pichler P, Arbeiter K, Ruffingshofer D, VanWhy SK, Aufricht C. Urinary heat shock protein-72 excretion in clinical and experimental renal ischemia. Pediatr Nephrol. 2003;18:97–99. doi: 10.1007/s00467-002-1037-5. [DOI] [PubMed] [Google Scholar]
- Negishi K, Noiri E, Doi K, Maeda-Mamiya R, Sugaya T, Portilla D, Fujita T. Monitoring of urinary L-type fatty acid-binding protein predicts histological severity of acute kidney injury. Am J Pathol. 2009;174:1154–1159. doi: 10.2353/ajpath.2009.080644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejat M, Pickering JW, Walker RJ, Endre ZH. Rapid detection of acute kidney injury by plasma cystatin C in the intensive care unit. Nephrol Dial Transplant. 2010;25:3283–3289. doi: 10.1093/ndt/gfq176. [DOI] [PubMed] [Google Scholar]
- Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol. 2005;16:3046–3052. doi: 10.1681/ASN.2005030236. [DOI] [PubMed] [Google Scholar]
- Parikh CR, Mishra J, Thiessen-Philbrook H, Dursun B, Ma Q, Kelly C, Dent C, Devarajan P, Edelstein CL. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70:199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- Price PM, Safirstein RL, Megyesi J. The cell cycle and acute kidney injury. Kidney Int. 2009;76:604–613. doi: 10.1038/ki.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez V, Trujillo J, Valdes R, Uribe N, Cruz C, Gamba G, Bobadilla NA. Adrenalectomy prevents renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2009;297:F932–F942. doi: 10.1152/ajprenal.00252.2009. [DOI] [PubMed] [Google Scholar]
- Ritossa FA. New puffing pattern induced by temperature shoch and DNP in Drosophila. Experientia. 1962;18:571–573. [Google Scholar]
- Taman M, Liu Y, Tolbert E, Dworkin LD. Increase urinary hepatocyte growth factor excretion in human acute renal failure. Clin Nephrol. 1997;48:241–245. [PubMed] [Google Scholar]
- Tsutsumi S, Neckers L. Extracellular heat shock protein 90: a role for a molecular chaperone in cell motility and cancer metastasis. Cancer Sci. 2007;98:1536–1539. doi: 10.1111/j.1349-7006.2007.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turman MA, Rosenfeld SL. Heat shock protein 70 overexpression protects LLC-PK1 tubular cells from heat shock but not hypoxia. Kidney Int. 1999;55:189–197. doi: 10.1046/j.1523-1755.1999.00251.x. [DOI] [PubMed] [Google Scholar]
- Uchida K, Gotoh A. Measurement of cystatin-C and creatinine in urine. Clin Chim Acta. 2002;323:121–128. doi: 10.1016/s0009-8981(02)00177-8. [DOI] [PubMed] [Google Scholar]
- Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290:F517–F529. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- Vaidya VS, Waikar SS, Ferguson MA, Collings FB, Sunderland K, Gioules C, Bradwin G, Matsouaka R, Betensky RA, Curhan GC, et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci. 2008;1:200–208. doi: 10.1111/j.1752-8062.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya VS, Ford GM, Waikar SS, Wang Y, Clement MB, Ramirez V, Glaab WE, Troth SP, Sistare FD, Prozialeck WC, et al. A rapid urine test for early detection of kidney injury. Kidney Int. 2009;76:108–114. doi: 10.1038/ki.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, Bobadilla NA, Marrer E, et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010;28:478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagener G, Jan M, Kim M, Mori K, Barasch JM, Sladen RN, Lee HT. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology. 2006;105:485–491. doi: 10.1097/00000542-200609000-00011. [DOI] [PubMed] [Google Scholar]
- Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006;17:1143–1150. doi: 10.1681/ASN.2005091017. [DOI] [PubMed] [Google Scholar]
- Wu I, Parikh CR. Screening for kidney diseases: older measures versus novel biomarkers. Clin J Am Soc Nephrol. 2008;3:1895–1901. doi: 10.2215/CJN.02030408. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Noiri E, Ono Y, Doi K, Negishi K, Kamijo A, Kimura K, Fujita T, Kinukawa T, Taniguchi H, et al. Renal L-type fatty acid-binding protein in acute ischemic injury. J Am Soc Nephrol. 2007;18:2894–2902. doi: 10.1681/ASN.2007010097. [DOI] [PubMed] [Google Scholar]
- Yu Y, Jin H, Holder D, Ozer JS, Villarreal S, Shughrue P, Shi S, Figueroa DJ, Clouse H, Su M, et al. Urinary biomarkers trefoil factor 3 and albumin enable early detection of kidney tubular injury. Nat Biotechnol. 2010;28:470–477. doi: 10.1038/nbt.1624. [DOI] [PubMed] [Google Scholar]
- Zhipeng W, Li L, Qibing M, Linna L, Yuhua R, Rong Z. Increased expression of heat shock protein (HSP)72 in a human proximal tubular cell line (HK-2) with gentamicin-induced injury. J Toxicol Sci. 2006;31:61–70. doi: 10.2131/jts.31.61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.