Abstract
The forkhead box M1b (FoxM1b) transcription factor is over-expressed in human cancers, and its expression often correlates with poor prognosis. Previously, using conditional knockout strains, we showed that FoxM1b is essential for hepatocellular carcinoma (HCC) development. However, over-expression of FoxM1b had only marginal effects on HCC progression. Here we investigated the effect of FoxM1b expression in the absence of its inhibitor Arf. We show that transgenic expression of FoxM1b in an Arf-null background drives hepatic fibrosis and metastasis of HCC. We identify novel mechanisms of FoxM1b that are involved in epithelial–mesenchymal transition, cell motility, invasion and a pre-metastatic niche formation. FoxM1b activates the Akt-Snail1 pathway and stimulates expression of Stathmin, lysyl oxidase, lysyl oxidase like-2 and several other genes involved in metastasis. Furthermore, we show that an Arf-derived peptide, which inhibits FoxM1b, impedes metastasis of the FoxM1b-expressing HCC cells. The observations indicate that FoxM1b is a potent activator of tumour metastasis and that the Arf-mediated inhibition of FoxM1b is a critical mechanism for suppression of tumour metastasis.
Keywords: Arf, FoxM1, metastasis
INTRODUCTION
Metastasis is the leading cause of cancer-related death. Understanding the molecular mechanisms of metastasis and genetic changes required for metastasis are essential to develop novel therapeutic strategies and improve their efficacy for treatment of the metastatic disease. During tumour progression, cancer cells undergo dramatic changes in cytoskeletal organization to adopt invasive phenotype and eventually metastasize to other organs. Epithelial–mesenchymal transition (EMT), which is associated with reduced cell-surface expression of E-cadherin and altered expression of several cytoskeletal proteins, plays a significant role towards the migratory activity of the tumour cells (Kalluri & Weinberg, 2009). A recent study indicated that the microtubule (MT) destabilizing protein Stathmin/Oncoprotein 18 plays a pivotal role in cell migration/invasion. MT stability controls migration of cells through extracellular matrix, and destabilization of MT by Stathmin increases motility (Baldassarre et al, 2005). Stathmin is often over-expressed in cancer and associated with increased metastasis (Singer et al, 2007). However, the mechanism by which Stathmin is over-expressed in cancer remains elusive.
Changes in cancerous cells alone are not sufficient to promote metastasis (Kopfstein & Christofori, 2006). Tumour microenvironment also plays a pivotal role in tumour progression and metastasis (Joyce & Pollard, 2009). The importance of a pre-metastatic niche or local microenvironment at metastatic sites has emerged (Psaila & Lyden, 2009). Increased expression of lysyl oxidase (LOX) has been implicated in tumour cell invasiveness and tumour metastasis (Akiri et al, 2003; Erler et al, 2006; Sakai et al, 2009). LOX is a copper dependent amine oxidase that causes covalent crosslinking of collagen by catalysing oxidative deamination of lysine and hydroxylysine residues in collagen, leading to both inter- and intramolecular crosslinking (Payne et al, 2007). Secreted LOX induces collagen crosslinking at pre-metastatic organs to generate sites that attract Cd11b+ myeloid cells to form a receptive niche for arriving tumour cells (Erler et al, 2009).
Forkhead box M1 (FoxM1) is a proliferation-specific transcription factor and plays a critical role in cell cycle progression (Laoukili et al, 2007; Wierstra & Alves, 2007). Gene expression profiling in a number of carcinomas revealed that elevated FoxM1 expression is a general phenomenon and likely to be a crucial step of disease progression in all carcinomas examined (Pilarsky et al, 2004). Moreover, over-expression of FoxM1 strongly correlates with disease progression and poor prognosis in various human malignancies (Calvisi et al, 2009; Chandran et al, 2007; Dai et al, 2007; Gialmanidis et al, 2009; Liu et al, 2006; Yang et al, 2009). The role of FoxM1 in tumour progression has been studied extensively in mice using liver carcinogenesis models. Alb-Cre-mediated deletion of FoxM1 in the livers of FoxM1 fl/fl strain rendered the mice resistant to development of hepatocellular carcinomas (HCCs) induced by the diethylnitrosamine/phenobarbital (DEN/PB) liver carcinogenesis protocol (Kalinichenko et al, 2004). Also, deletion of FoxM1 in pre-existing HCC inhibited tumour growth, indicating a persistent requirement for FoxM1 in tumour progression (Gusarova et al, 2007). Although the increased expression of FoxM1 correlates with tumour grade and metastasis in various human malignancies (Chandran et al, 2007; Dai et al, 2007), its contribution to the metastasis process remains undefined.
Interestingly, there is growing evidence that the tumour suppressor p19Arf (p14Arf in human) can inhibit cell invasion and tumour metastasis (Aguirre et al, 2003; Chen et al, 2008). Deletion of p19Arf in p53-null background further facilitated malignant tumour conversion and metastasis, indicating p53-independent tumour suppressor functions of p19Arf (Kelly-Spratt et al, 2004). In fact, mounting evidence suggests that p19Arf (p14Arf in human) confers its tumour suppression activity through p53-dependent and -independent pathways (Eischen et al, 1999; Kamijo et al, 1999; Lin & Lowe, 2001; Schmitt et al, 1999). Previously, we reported that p19Arf targets FoxM1b, a splice variant of FoxM1, to the nucleolus and inhibits the transcriptional activity of FoxM1b (Kalinichenko et al, 2004). An 18 amino acid sequence between residues 26 and 44 of p19Arf is sufficient to bind and inhibit its activity. Intraperitoneal injection of a peptide corresponding to the 26–44 residues of p19Arf inhibited the proliferation of the HCC cells and stimulated their apoptosis (Gusarova et al, 2007).
In this study, using a liver carcinogenesis model, we show that transgenic (Tg) expression of FoxM1b combined with the loss of its inhibitor p19Arf induces liver fibrosis and metastasis, which resemble the pathology of human HCC. In addition, we show that an Arf-derived peptide that inhibits FoxM1b inhibits metastasis of HCC cells. This work reveals novel mechanisms of FoxM1b in tumour metastasis and provides evidence that inhibition of FoxM1b will be significant in therapy for metastatic tumours.
RESULTS
Transgenic expression of FoxM1b in an Arf-deficient background drives aggressive HCC, hepatic fibrosis and pulmonary metastasis of liver cancer
Elevated expression of FoxM1 has been reported in human HCC and its expression is indispensable for the development and progression of HCC (Calvisi et al, 2009; Gusarova et al, 2007; Kalinichenko et al, 2004; Okabe et al, 2001). However, over-expression of FoxM1 alone in a Tg mouse model had only marginal effects on liver cancer development and progression (Kalinina et al, 2003). Since the tumour suppressor p19Arf inhibits FoxM1 transcriptional activity (Kalinichenko et al, 2004), we considered the possibility that p19Arf might be a dominant inhibitor of FoxM1 in tumour development and progression. Therefore, we sought to determine the effects of FoxM1 over-expression in the absence of p19Arf. We bred p19Arf-null mice with FoxM1b Tg mice, which ubiquitously express FoxM1b driven by Rosa26 promoter. The bi-transgenic heterozygotes (Arf+/−Rosa26-FoxM1b Tg) were backcrossed for six to nine generations into the C57BL/6 background. Fourteen-day old pups from the Arf+/−Rosa26-FoxM1b Tg crosses were subjected to the DEN/PB tumour induction protocol, a well-established method that effectively generates HCC with greater than 90% penetrance. As expected, the majority of the mice developed HCC (Fig 1A and C). FoxM1b Tg;Arf−/− mice exhibited markedly increased mortality and tumour burden (Fig S1A and B of Supporting Information). Moreover, FoxM1b Tg;Arf−/− mouse livers appeared fibrotic (Fig 1A). Robust collagen deposition along with strong alpha-smooth muscle actin (α-SMA) expression was observed in a significant number (5 out of 12) of FoxM1b Tg;Arf−/− mice (Fig 1B). No collagen deposition or α-SMA staining was detected in the parenchyma of Arf−/− or FoxM1b Tg mice (Fig 1B).
Figure 1. FoxM1b Tg;Arf−/− mice developed fibrosis and metastases of HCC.
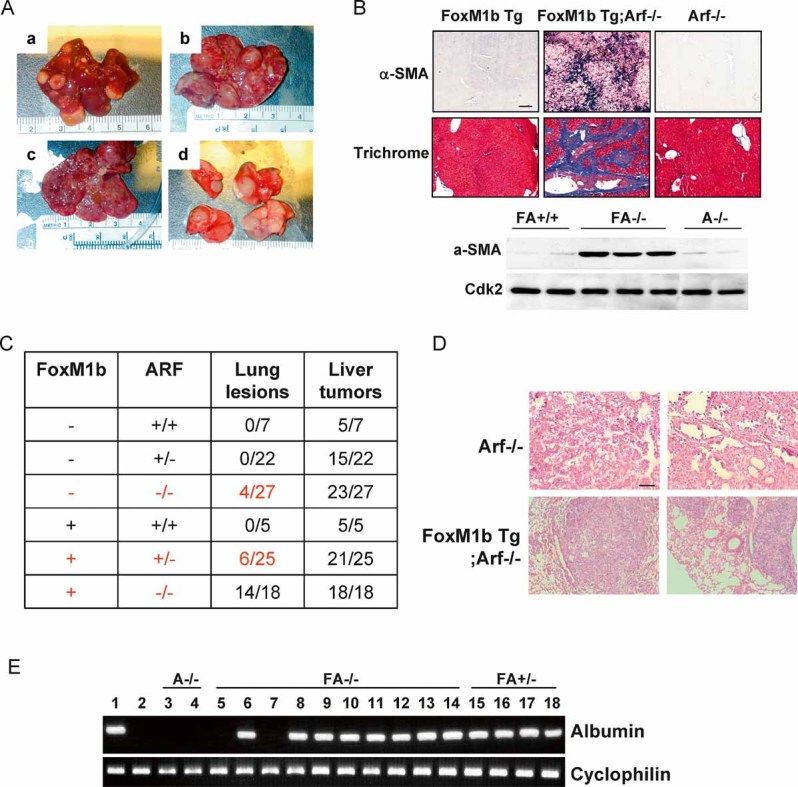
- We sacrificed the mice and harvested liver tissues after 33 weeks of DEN/PB exposure. FoxM1b Tg;Arf−/− liver tumours (a–c) and lung lesions (d) are shown.
- Liver sections were stained for the activated HSC marker α-SMA or Masson's trichrome for collagen deposition. Magnification: ×200. Lower panel: Liver extracts were subjected to Western blotting assay using α-SMA antibody.
- Numbers of mice bearing lung lesions and liver tumours are shown. For all panels of (B) and (D), the scale bar (indicated in the upper left panel) = 200 µm.
- Lung lesions were stained with hematoxylin and eosin (H&E). Magnification: ×100.
- Total RNA from the lung was extracted for RT-PCR using primers specific for albumin. 1. liver; 2. lung; 3–4. Arf−/− (A: lung); 5–13. FoxM1b Tg;Arf−/− (FA−/−: lung); 14. FoxM1b Tg;Arf−/− (FA−/−: spleen); 15–18. FoxM1b Tg;Arf+/− (FA+/−: lung).
Interestingly, we found that approximately 75% (14/18) of the FoxM1b Tg;Arf−/− mice developed lesions in the lung, as shown in Fig 1A (part d) and C. We also found that one of the FoxM1b Tg;Arf−/− mice displayed nodules in the spleen. A significantly lower percentage (less than 30%) of the FoxM1b Tg;Arf+/− mice and 13% (4/27) of the Arf−/− mice exhibited nodules in the lung. Histologically, the lung nodules from Arf−/− mice resembled lung adenocarcinomas (Fig 1D). Since the Arf−/− mice are known to develop lung adenocarcinomas (Kamijo et al, 1999) and, in the DEN/PB model, lung metastasis is relatively rare, we determined the origin of the lung nodules. We assayed for albumin mRNA expression, which is only synthesized in the liver. A significant number (7 out of 9) of the lung tumour samples (and one tumour sample from spleen) from the FoxM1b Tg;Arf−/− mice expressed albumin mRNA (Fig 1E), confirming the presence of HCC-derived cells in the lung. Also, the lung lesions from FoxM1b Tg;Arf+/− mice were positive for albumin-mRNA, while the lung samples from the Arf−/− mice were negative for albumin mRNA (Fig 1E). Therefore, it is likely that the albumin-negative lung nodules observed in Arf−/− mice were indeed lung adenocarcinomas (Kamijo et al, 1999). Fewer metastases in Arf+/− background (less than 30% compared to 75% in Arf−/− background) provide strong evidence that Arf inhibits metastasis driven by FoxM1b expression.
HCC cells derived from the FoxM1b Tg;Arf−/− mice are highly tumourigenic and metastatic
To determine whether FoxM1b in the Arf−/− background could support metastasis in a cell-autonomous fashion, we studied the HCC cells derived from the FoxM1b Tg;Arf−/− mice and compared them with the HCC cells from the Arf−/− mice, as there was no evidence of metastasis in Arf+/+ or FoxM1b Tg; Arf+/+ mice. Also, it was difficult to obtain stable lines in the Arf +/+ background. We isolated HCC cells from the livers of three independent mice of each genotype. As expected, all three FoxM1b Tg;Arf−/− HCC lines expressed higher levels of FoxM1 than Arf−/− HCC cells (Fig S2A of Supporting Information). Also, no expression of p19Arf was observed in the isolated HCC cells (Fig S2A of Supporting Information). We then examined anchorage-independent growth ability of HCC cells. FoxM1b Tg;Arf−/− cells formed significantly more and larger colonies compared to the Arf−/− cells in soft agar (Fig 2A). The increased tumourigenicity was further confirmed by xenograft experiments. Arf−/− or FoxM1b Tg;Arf−/− HCC cells were subcutaneously injected into the flank of athymic nude mice. The Arf−/− HCC cells failed to develop tumours in 3 weeks after injection, whereas the FoxM1b Tg;Arf−/− cells started to grow exponentially with a short latency period and all mice injected developed palpable tumours (Fig 2B).
Figure 2. Cells from FoxM1b Tg;Arf−/− HCC are highly tumourigenic and metastatic.
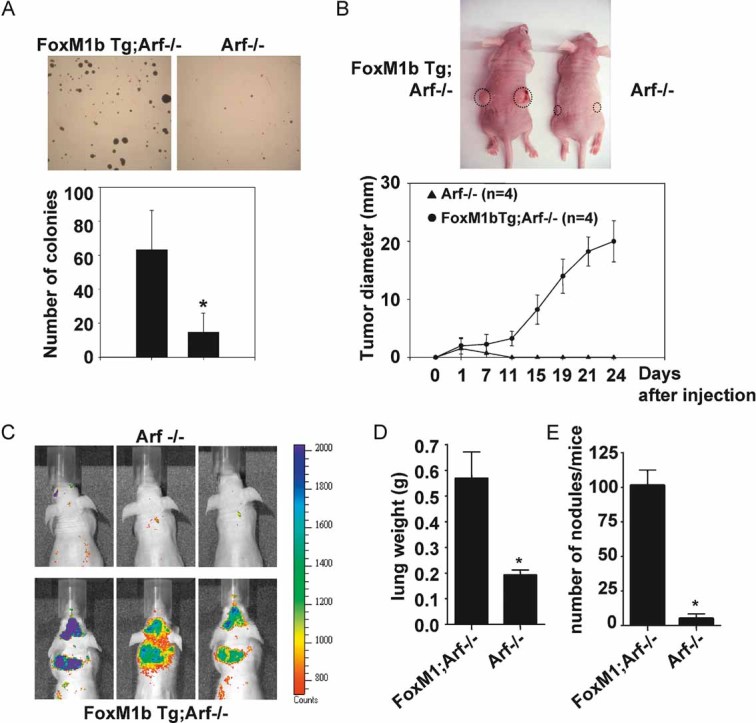
- A. Soft agar assays were performed to compare anchorage-independent growth ability. Bar graph presents quantification of the representative data of three independent experiments. Data are expressed as mean ± SD (*p < 0.05).
- B. 5 × 105 cells were subcutaneously injected into nude mice. The tumour diameter was measured at the indicated time points. Data are expressed as mean ± SD.
- C–E. 106 RFP labelled cells were injected into 8-week-old male nude mice intravenously via tail vein. (C) Mice were subjected to fluorescence imaging. (D and E) Mice were then sacrificed and lung tissues were harvested. Lungs were weighed (D) and fixed with Bouin's solution for 24 h. Macroscopic surface tumour nodules were counted (E). Data are expressed as mean ± SD (*p < 0.05).
To determine the metastatic potential of the HCC cells, cells stably expressing the Red fluorescence protein (RFP) were injected into nude mice via tail vein. The mice were imaged using a fluorescence imager after injection of the cells. As shown in Fig 2C, the mice injected with FoxM1b Tg;Arf−/− cells displayed stronger fluorescence signals in lungs compared to the mice injected with tumour cells isolated from Arf−/− HCCs. To obtain a more quantitative measure of the tumour burden, we removed lungs, weighed to compare tumour burden between two groups, and fixed the lungs in Bouin's solution to count macroscopic surface metastases. Clearly, the tumour burden and the number of tumour nodules were much higher in the mice injected with FoxM1b Tg;Arf−/− HCC cells compared to the mice injected with Arf−/−HCC cells (Fig 2D and E), providing further evidence that FoxM1b, in the absence of its inhibitor Arf, drives metastasis of the tumour cells.
FoxM1b activates AKT and induces an EMT-like phenotype
The FoxM1b Tg;Arf−/− HCC cells show phenotypic changes reminiscent of EMT. The cells exhibited a spindle-shape morphology (Fig 3A) and expressed low levels of E-cadherin and high levels of the mesenchymal markers vimentin and α-SMA (Fig 3B). These differences were observed in all three independent lines. Also, similar results were observed in Maden–Darby Canine kidney (MDCK) epithelial cells, which are wildly used to study EMT and express negligible amount of Arf (Fig S2B of Supporting Information). We observed, however, that MDCK cells express Arf upon ultraviolet irradiation, suggesting that these cells do have the ability to induce Arf in response to stress signalling (Fig S2B of Supporting Information). Ectopic FoxM1 expression induced dramatic changes in cell morphology (Fig S2C of Supporting Information). Interestingly, we observed increased Akt kinase activity in FoxM1b Tg;Arf−/− cells compared to Arf−/− cells (Fig 3C). Akt has been shown to induce EMT by transcriptional repression of E-cadherin (Grille et al, 2003). glycogen synthase kinase-3 beta (GSK-3β) is a target of Akt and inactivates the transcriptional repressor Snail, a potent inducer of EMT (Zhou et al, 2004). As expected, we observed increased inhibitory phosphorylation of GSK-3β and increased Snail expression in FoxM1b Tg;Arf−/− cells (Fig 3C). Similar results were observed in the MDCK cells (Fig S2D of Supporting Information). Our observations suggest that FoxM1b induces an EMT-like phenotype by activating the Akt-Snail pathway.
Figure 3. FoxM1b Tg;Arf−/− cells exhibit morphological changes reminiscent of EMT.
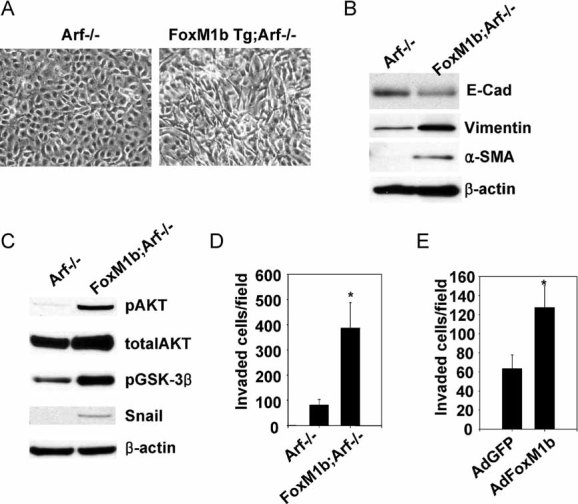
- Representative phase-contrast images of the cells are shown. Magnification: ×200.
- Expression of EMT markers was determined by immunoblotting. β-Actin served as a loading control.
- Activation of AKT/GSK-3β/Snail pathway was assessed by immunoblotting.
- Invasion assays were performed as described in the Materials and Methods Section.
- Arf−/− HCC cells were infected with adenovirus-expressing GFP or FoxM1b for 48 h and subjected to invasion assay. Data are expressed as mean ± SD (*p < 0.05).
Previous in vitro studies indicated that over-expression of FoxM1b could stimulate cell migration/invasion (Dai et al, 2007; Liu et al, 2006). However, the mechanism remains to be elucidated. Recent studies indicate that invasion of cancer cells are critically associated with the acquisition of EMT phenotypes (Kang & Massague, 2004; Thiery et al, 2009; Yang et al, 2004). Therefore, it is likely that the EMT-like changes induced by FoxM1b contribute to tumour cell invasion. As expected, the HCC cells derived from the FoxM1b Tg;Arf−/− mice were significantly more invasive compared to those from the Arf−/− mice (Fig 3D). Moreover, over-expression of FoxM1b in the Arf−/− HCC cells stimulated EMT-like changes in cells (Fig S2E and F of Supporting Information) and increased invasiveness of the tumour cells (Fig 3E).
FoxM1b stimulates Stathmin and destabilizes microtubule
To further investigate the mechanism of the increased cell invasiveness, we considered Stathmin because it was demonstrated that the MT-destabilizing activity of Stathmin plays significant roles in cell migration and invasion (Baldassarre et al, 2005). All of the six FoxM1b Tg;Arf−/− tumour samples displayed greatly elevated expression of Stathmin compared to the samples from the Arf−/− tumours (Fig S3A of Supporting Information). Moreover, we found that FoxM1 directly bound to the promoter of the Stathmin gene, as judged by chromatin-IP assays (Fig S3B of Supporting Information). Stathmin was shown to increase cell migration by destabilizing MTs. Therefore, we compared the MT-destabilizing activity in the HCC cells using a tubulin dilution assay. While they express comparable levels of total tubulin, FoxM1b Tg;Arf−/− cells contained much lower levels of MT after the tubulin dilution (Fig S3C and D of Supporting Information), which is consistent with the increased expression of Stathmin. Thus, FoxM1b stimulates multiple mechanisms to increase invasiveness of the tumour cells.
FoxM1b stimulates lysyl oxidase expression and pre-metastatic niche formation
We determined expression of various metastasis genes in FoxM1b Tg;Arf−/− and Arf−/− liver tumours. Total RNA extracted from tumour samples was analysed by semi-quantitative reverse transcriptase polymerase chain reaction (RT-PCR). Previous in vitro studies demonstrated that FoxM1b could stimulate expression of matrix metalloprotease MMP2 and the pro-angiogenic factor vascular endothelial growth factor (VEGF) (Dai et al, 2007; Joyce & Pollard, 2009; Li et al, 2009; Zhang et al, 2008). We observed increased expression of those genes, as well as increased expression of MT1-MMP in FoxM1b Tg;Arf−/− liver samples (Fig S4 of Supporting Information). Interestingly, we also observed elevated expression of PDGF-C in FoxM1b Tg;Arf−/− tumour samples. PDGF-C has been reported to be a potent mitogen for hepatic stellate cells (HSCs), which leads to hepatic fibrosis and HCC (Campbell et al, 2005). Therefore, it is conceivable that sustained production of high level PDGF-C in FoxM1b Tg;Arf−/− mice activates HSC proliferation, causing increase in collagen deposition and subsequent hepatic fibrosis. In addition, we found that the chemokine receptor CXCR4, which is involved in tumour cell-homing, was up-regulated in the FoxM1b Tg;Arf−/− tumour samples.
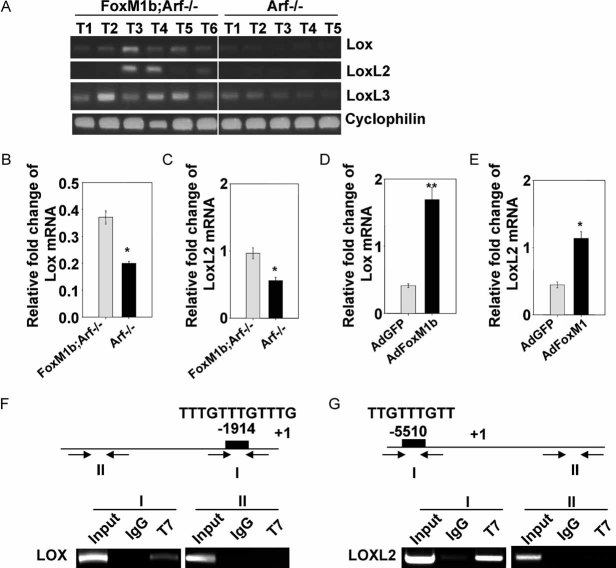
Increased expression of LOX has been implicated in tumour cell invasiveness and tumour metastasis (Akiri et al, 2003; Erler et al, 2006; Sakai et al, 2009). Interestingly, we found that LOX, LOXL2 and LOXL3 were expressed at higher levels in FoxM1 Tg;Arf−/− liver tumours than in Arf−/− tumours (Fig 4A). Also, FoxM1b Tg;Arf−/− HCC cells expressed substantially higher levels of LOX and LOXL2 than Arf−/− cells (Fig 4B and C). Moreover, over-expression of FoxM1b stimulated expression of LOX and LOXL2 mRNA (Fig 4D and E). Chromatin immunoprecipitation (ChIP) assays further confirmed that FoxM1b directly binds to the promoter region of LOX and LOXL2 (Fig 4F and G). A more detailed ChIP experiment is included in Fig S5 of Supporting Information.
Figure 4. FoxM1b Tg;Arf−/− HCCs express LOX at higher levels compared to Arf−/− HCCs.
- A. Semi-quantitative RT-PCR was performed using total RNA from liver tumours.
- B-E. Quantitative RT-PCR was performed to determine mRNA levels of LOX and LOXL2.
- D,E. Arf−/− HCC cells were infected with adenovirus expressing GFP or FoxM1b for 48 h. Data are expressed as mean ± SD (*p < 0.05; **p < 0.01).
- F,G. Chromatin-immunoprecipitation (ChIP) assays were performed using a monoclonal antibody against T7-epitope to detect specific binding of FoxM1b to LOX (F) and LOXL2 (G) promoters in T7-FoxM1b-transfected Sk-hep1 cells.
It was shown that LOX secreted from primary tumours accumulates at target organs and recruits Cd11b+ bone marrow derived cells to form pre-metastatic niche (Erler et al, 2009). Immunohistochemical staining of the lung sections with Cd11b and c-Kit antibodies showed that Cd11b+ myeloid cells as well as c-Kit+ myeloid progenitor cells had infiltrated into metastatic lung tumours of FoxM1b Tg;Arf−/− mice, but not in lung sections of Arf−/− mice (Fig 5A–C). Also, a substantial number of Cd11b+ cells was recruited to the tumour-free lungs of the FoxM1b Tg;Arf−/− mice (Fig 5D and E). To investigate whether LOX induced by FoxM1b is responsible for the recruitment of Cd11b+ cells in lung, we over-expressed FoxM1b in the Arf−/−HCC cells using retrovirus. The FoxM1b-expressing Arf−/− HCC cells were used to determine the role of LOX. LOX in those cells was inhibited by shRNA or by a specific irreversible inhibitor, beta-aminopropionitrile (BAPN) (Sigma–Aldrich). A recent study indicated that BAPN also is a potent inhibitor of LOXL2 (Rodriguez et al, 2010). We found that LOX/LOXL2 inhibition had only marginal effects on the tumour growth resulting from subcutaneous injection of the FoxM1b-expressing Arf−/− HCC cells (Fig 6A). Interestingly, FoxM1b expressing tumours increased recruitment of Cd11+ cells in the lung of the animals (panel 2 in Fig 6B), a marker for pre-metastatic niche. Moreover, in contrast to tumour growth, LOX-shRNA or BAPN substantially decreased the recruitment of Cd11b+ cells to the lung (panels 3 and 4 in Fig 6B). Together, these observations indicate that FoxM1b induces pre-metastatic niche formation by stimulating the expression of LOX and LOXL2.
Figure 5. Infiltration of the Cd11b+ myeloid cells into the lungs of FoxM1b Tg;Arf−/− mice.
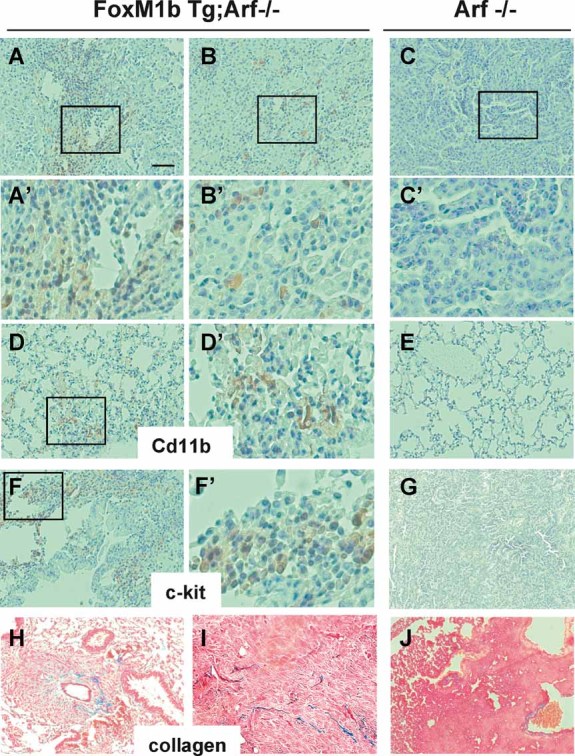
- A–C. Lung tumour sections were stained for Cd11b+ cells.
- A'–C'. Higher magnification images of the boxed regions in panels A, B and C respectively.
- D, E. Tumour-free lung sections were stained for CdL11b+ cells.
- D'. Higher magnification image of the boxed region in panel D.
- F, G. Lung tumour sections were stained for c-Kit+ cells.
- F'. Higher magnification image of the boxed region in panel F.
- H–J. Collagen accumulation was assessed by Masson's trichrome staining (blue). Scale bar (indicated in panel A) for all lower magnification images = 200 µm.
Figure 6. LOX, LOXL2 and Stathmin play important roles in FoxM1b-mediated metastasis.
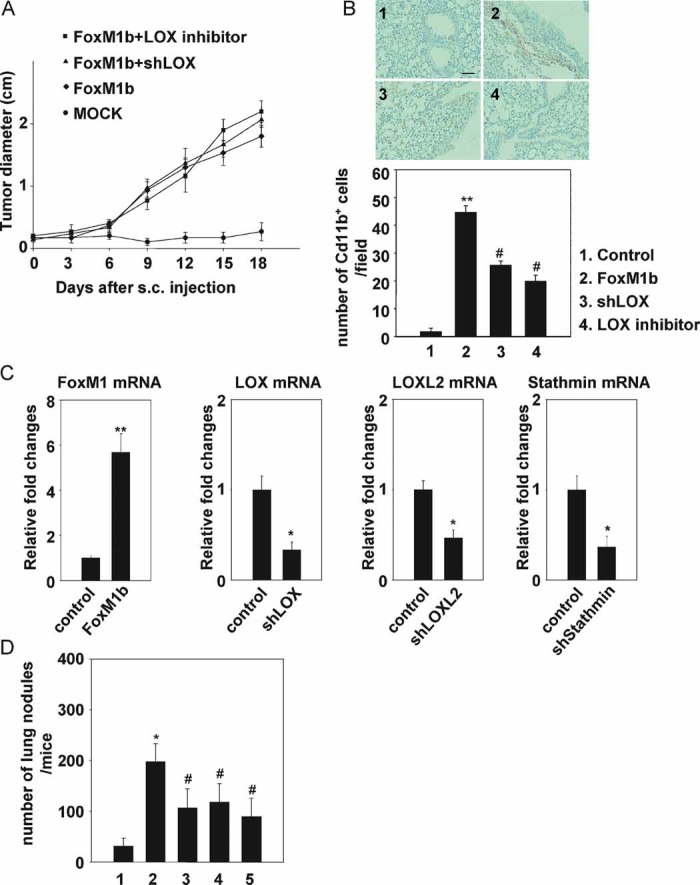
- A–D. Arf−/− HCC cells were infected with retrovirus expressing FoxM1b and lentivirus expressing shRNA specific to LOX, LOXL2 or Stathmin. Where indicated (in panels A & B) a total of 100 µg/g body weight of a LOX inhibitor, β-aminoproprionitrile (BAPN), was administered daily by i.p. injection starting 1 day prior to injection for the duration of the experiment (18 days).
- A,B. 5 × 105 cells were subcutaneously injected into 8-week-old male nude mice.
- A. The tumour diameter was measured at the indicated time points. Data are expressed as mean ± SD (n = 3).
- B. The mice were sacrificed 21 days after s.c. injection. Lungs were fixed and lung sections were stained with anti-Cd11b antibodies. Upper panels show representative images. Magnification: ×100. Nuclei were stained with hematoxylin (panel 1, control; panel 2, FoxM1b; panel 3, FoxM1b + LOXshRNA; panel 4, FoxM1b + BAPN). Scale bar indicated in panel 1 is same for all panels and equals to 200 µm. Number of Cd11b positive cells per microscopic field was counted (lower panel). A total of 10 fields were randomly chosen. Bar graph presents quantification of the representative data of three independent experiments. Data are expressed as mean ± SD (**p < 0.01 vs. control; #p < 0.05 vs. FoxM1b).
- C. Total RNA was extracted and quantitative RT-PCR was performed to determine mRNA levels of FoxM1, LOX, LOXL2 and Stathmin. Data are expressed as mean ± SD (*p < 0.05; **p < 0.01).
- D. 106 HCC cells were injected into nude mice intravenously via tail vein. Mice were sacrificed and lung tissues were harvested. Lungs were fixed and macroscopic surface tumour nodules were counted. Data are expressed mean ± SD (*p < 0.05 vs. 1; #p < 0.05 vs. 2). 1. MOCK (n = 8); 2. FoxM1b + control (n = 12); 3. FoxM1b + shLOX (n = 5); 4. FoxM1b + shLOX2 (n = 6); 5. FoxM1b + shStathmin (n = 6).
We further examined the significance of LOX, LOXL2 and Stathmin in FoxM1b-induced metastasis. We reduced expression of those genes using lentivirus-delivered shRNAs (Fig 6C). The shRNA-expressing HCC cells were injected into nude mice intravenously through tail vein. Three weeks after injection, the lungs were harvested and tumour nodules were counted. Clearly, we found that decrease in LOX, LOXL2 or Stathmin expression inhibited metastasis of the FoxM1b-expressing Arf−/− HCC cells (Fig 6D). However, we did not rule out the possibility that depletion of Stathmin affects other events in tumour progression, such as tumourigenicity.
An Arf-derived peptide inhibits metastatic growth of the HCC cells
Previously, we reported that the transcriptional activity of FoxM1 is inhibited by p19Arf. The amino acid residues between 26 and 44 of p19Arf are sufficient to sequester FoxM1 to the nucleolus and subsequently inhibit its transcriptional activity (Kalinichenko et al, 2004). Moreover, a cell-penetrating form of the Arf-derived peptide was able to induce apoptosis in mouse HCCs (Gusarova et al, 2007). We found that Arf peptide treatment significantly reduced expression of LOX, LOXL2 and Stathmin in mouse HCC cells (Fig 7A) as well as in Sk-hep1 (Fig 7B), a metastatic line derived from human liver cancer that lacks Arf expression (Sayan et al, 2001). Therefore, we sought to investigate whether this Arf-derived peptide could prevent lung metastasis of HCC cells. We injected the fluorescently labelled FoxM1b Tg;Arf−/− HCC cells into nude mice intravenously via the tail vein. The following day, the mice were subjected to intraperitoneal (i.p.) injection of Arf-derived peptide (WT), a mutant peptide (Mut), or phosphate-buffered saline (PBS) every other day for 3 weeks. The mice were then subjected to fluorescence imaging to evaluate metastasis in distant organs. Tumour nodules were counted after fixation to quantify the tumour burden. We found that the Arf peptide treatment significantly inhibited lung metastases (Fig 7C and D). Some difference in the number of tumour nodules between the PBS versus Mut peptide injected mice was also observed. This could be due to some non-specific cytotoxicity from an arginine-rich peptide (Moulton et al, 2004). Nevertheless, our data suggests that the Arf-peptide has strong therapeutic potential for treatment of metastasis.
Figure 7. Cell-penetrating ARF peptide inhibitor of FoxM1 prevented pulmonary metastasis of HCC cells.
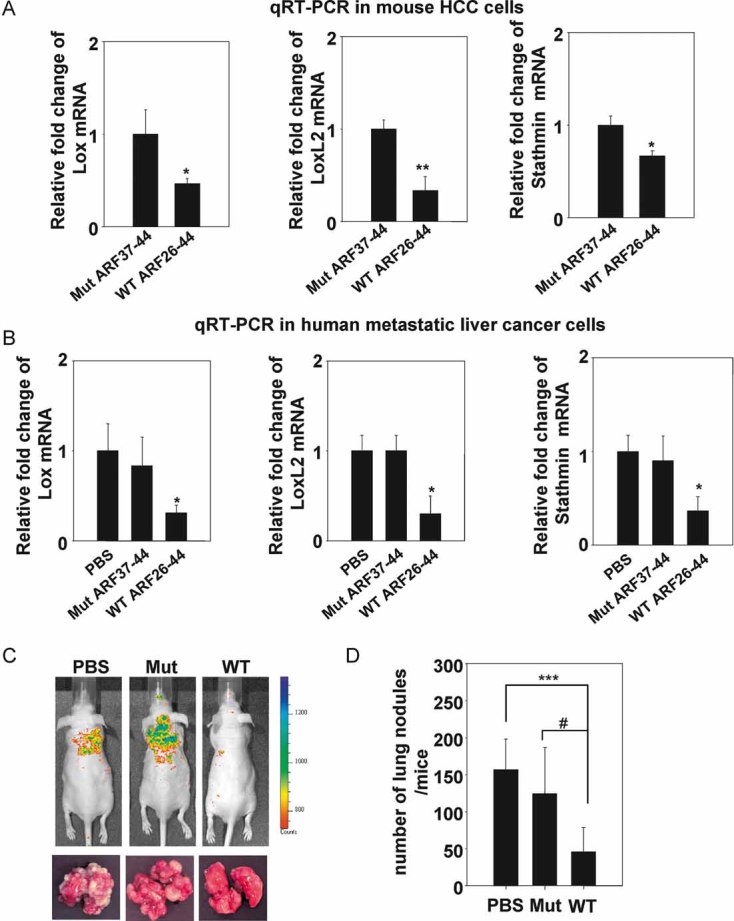
- A,B. FoxM1b Tg;Arf−/− HCC cells (A) or Sk-hep1, a human metastatic cell line (B) were treated with WT ARF26–44 peptide (10 µM) or Mut37–44 peptide (10 µM) for 72 h. Total RNA was extracted and quantitative RT-PCR was performed to determine mRNA levels of LOX, LOXL2 and stathmin. Data are expressed as mean ± SD (*p < 0.05; **p < 0.01).
- C,D. 106 cells labelled with RFP were injected into nude mice intravenously via tail vein. From the next day, mice were i.p. injected with WT ARF peptide, Mut peptide or PBS every other day for 3 weeks at 5 mg/kg body weight. On day 22 from inoculation, mice were subjected to in vivo imaging (C). Mice were then sacrificed and lung tissues were harvested for further quantification. Lungs were fixed with Bouin's solution for 24 h. Macroscopic surface tumour nodules were counted (D). Data are expressed mean ± SD (***p < 0.001; #p < 0.05).
DISCUSSION
Human HCC is the fifth most common cancer, and it is among the most lethal cancers worldwide because of late detection and high frequency of tumour recurrence rendering therapy ineffective (Bruix et al, 2004). Although HCC patients frequently develop intrahepatic or/and distal metastasis, only a few mouse studies have reported development of metastatic liver cancer (Boissan et al, 2005; Lewis et al, 2005). We have developed a new mouse model of aggressive metastatic liver cancer. After 33 weeks of DEN/PB exposure, FoxM1b Tg;Arf−/− mouse livers appeared fibrotic and developed aggressive HCC that metastasised to lungs, whereas neither hepatic fibrosis nor metastasis of liver tumours was observed in Arf−/− or FoxM1b Tg mice.
FoxM1b is a proliferation specific transcription factor, which is critical for orderly progression of the cell cycle. Our data suggest that in addition to its well-characterized function in proliferation, deregulation of FoxM1b is a major driving force for multiple steps of tumour metastasis (see model in Fig 8). The HCC cells that we isolated from FoxM1b Tg;Arf−/− mice expressed lower levels of E-cadherin and higher levels of the mesenchymal markers, suggesting that FoxM1b contributes to EMT-like changes associated with aggressive phenotypes of tumour cells. Interestingly, we found that FoxM1b increases AKT activity, suggesting that aberrant FoxM1 activation may contribute to the hyperactivation of AKT observed in various tumours (Biggs et al, 1999; Brunet et al, 1999; Carnero et al, 2008). AKT inhibits GSK-3β, which suppresses Snail, a well-established EMT inducer. It is likely that FoxM1b induces EMT-like changes in part by activating AKT and subsequently increasing Snail expression. Also, given that AKT activation is one of the key survival mechanisms of tumour cells, increased activity of AKT might contribute to FoxM1b-mediated tumour metastasis through survival of the HCC cells in the lung.
Figure 8. A schematic diagram depicting the various steps of tumour metastasis activated by FoxM1b.
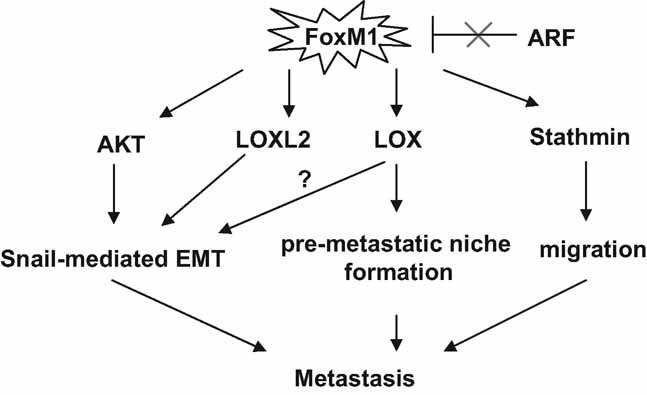
FoxM1b activates the Akt pathway and increases expression of LOX and LOXL2 to bring about an EMT-like change. Secreted LOX induces a pre-metastatic niche in the lung. FoxM1-induced expression of Stathmin increases flexibility of the cytoskeleton to enhance cell migration. These mechanisms of FoxM1 contribute to metastasis of HCC.
Emerging evidence indicates that increased LOX contributes to multistage metastasis progression. It has been shown that LOX secreted from primary tumours accumulates at target organs making it more amenable to subsequent tumour cell invasion. We found that deregulated FoxM1b stimulates expression of LOX and LOXL2 in vivo and in vitro, suggesting that FoxM1b may promote tumour metastasis by facilitating a pre-metastatic niche formation. Notably, LOXL2 is also known to induce EMT by attenuating GSK-3β-dependent degradation of Snail (Peinado et al, 2005). These observations indicate that FoxM1 promotes metastasis not only by inducing changes in tumour cells to increase motility and invasiveness, but also cultivating target organs to be congenial to the arriving tumour cells.
Previously, we developed a cell-penetrating form of Arf-derived peptide, which inhibits transcriptional activity of FoxM1 (Gusarova et al, 2007; Kalinichenko et al, 2004). In this study, we show that the Arf peptide treatment significantly inhibited pulmonary growth of tumour cells injected into the blood stream. Although this experimental metastasis assay does not exactly recapitulate pathological metastasis, it has been observed that millions of tumour cells are released from primary tumours yet a few of them actually develop overt metastasis, making this model useful to study the later and perhaps rate-limiting steps of metastasis. Therefore, our observation that inhibition of FoxM1b prevents pulmonary metastasis after intravenous injection of tumour cells makes FoxM1b an attractive target of cancer therapy, which could kill two birds with one stone, treating primary tumours and preventing metastasis. Recently, FoxM1b has been implicated in tumour dormancy, the process by which cells disseminated from the primary tumours are unable to proliferate yet survive for extended periods (Adam et al, 2009). This unpredictability makes cancer an even more devastating disease. Therefore, it will be worthwhile to study whether long-term FoxM1b inhibition could effectively prevent the late-developing metastasis.
MATERIALS AND METHODS
Animals
Dr Charles J. Sherr (St Jude Children's Research Hospital, Memphis, TN, USA) provided the Arf−/− C57BL/6 mice (Kamijo et al, 1999). The Arf−/− C57BL/6 mice were bred with Rosa26-FoxM1b FVBN Tg mice, which ubiquitously express the human FoxM1b cDNA to generate Arf+/− Rosa26-FoxM1b Tg mice. Then the Arf+/− Rosa26-FoxM1b Tg mice were backcrossed for six to nine generations into the C57BL/6 background. The backcrossed strain was used to generate the FoxM1b Tg;Arf−/− and other genotypes used in this study.
DEN/PB-induced liver tumour protocol
At 14 days after birth each male mice received a single i.p. injection of the tumour initiator DEN (5 µg/g body weight; Sigma–Aldrich). Two weeks later, mice were given water containing 0.05% of the tumour promoter, phenobarbital for the duration of experiments. All protocols were approved by the animal protocol committee at the University of Illinois at Chicago.
RT-PCR
RT-PCR was performed as described previously (Gusarova et al, 2007). Total RNA was isolated using Trizol (Invitrogen). Following DNaseI treatment, we used cDNA synthesis kit (BioRad) to synthesize cDNA from 2 µg of total RNA. For quantitative RT-PCR, we used SYBR Green Supermix (BioRad) and the reaction was amplified and analysed in triplicate using a MyiQ Single Colour Real-Time PCR Detection System (BioRad). For semi-quantitative RT-PCR, the linear ranges for the amplicon of each PCR primer were determined. The number cycles used in the PCR are included in Supporting Information S6. The PCR products were normalized to cyclophilin. The primers and conditions used to amplify and measure mRNA are listed in Supporting Information S6A.
ChIP assay
Sk-hep1 cells were transfected with T7-FoxM1b and subjected to ChIP assay as described previously (Park et al, 2009). The primers used for ChIP assay are listed in Supporting Information S6B.
Establishment of HCC cell lines
HCC tumours from mice were excised, minced and enzymatically dissociated with type I collagenase (200 U/ml) and DNase (270 U/ml). Cells were then rinsed with serum free medium three times and maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% foetal bovine serum, l-glutamine and nonessential amino acid. We isolated HCC cells from at least three mice of each genotype. Early passage (less than 5) cells were used.
Adenoviral infection
Arf−/− HCC cells were infected with green fluorescence protein (GFP) or FoxM1-expressing adenovirus for 48 or 72 h at a multiplicity of infection approximately 500 pfu/cell. Adenovirus expressing FoxM1 was a gift from Dr Guy Adami (UIC—College of Dentistry).
Invasion assay
Cells in 0.5% serum free DMEM were plated into Matrigel-coated transwells. Transwells were then placed in 0.5 ml of DMEM containing 10% FBS. After 16 h cells were fixed and stained with crystal violet. Invaded cells were counted from three fields per transwell. All experiments were performed in triplicates and repeated three times at a minimum. Data are shown for representative experiments.
Tubulin dilution assay
The tubulin stability was assayed as described previously (Khawaja et al, 1988). Cells were adhered to FN coated coverslip for 2 h and then incubated in 0.1% Triton X-100 containing PEM buffer (100 mM piperazine-N,N′-bis(2-ethane sulfonic acid) (PIPES) pH 6.9, 1 mM ethylene glycol tretaacetic acid (EGTA), 2 mM MgCl2) in 37°C for 1 min. Cells were then washed and incubated in complete medium for 15 min to allow dilution of soluble tubulin into medium. Cells were fixed in PFA and subjected to immunofluorescnce assay using anti-α-tubulin and fluorescein isothiocyanate (FITC)-conjugated secondary antibodies.
Lentiviral shRNA transduction
We used Mission shRNA lentiviral transduction system, which includes sequence-verified shRNA lentiviral plasmids (Sigma–Aldrich). LOX (NM_010728), LOXL2 (NM_033325) and Stathmin1 (NM_019641) mission shRNA pLKO.1-puro plasmids were used to establish stable knockdown cell lines. The parental plasmid, pLKO.1-puro, was used as a negative control. Lentiviral particle production and transduction were performed following the manufacturer's instructions.
The paper explained
PROBLEM
Metastasis of tumour cells is the major cause of cancer-related deaths worldwide. However, the molecular mechanisms and pathways that encourage tumour cells to leave the primary site of tumour development and undergo metastasis are poorly understood. A greater understanding of the mechanisms involved in tumour cell metastasis will allow us to identify the molecular targets and then design effective therapy against metastatic tumours.
RESULTS
Using liver cancer as a model, we identify that a combination of over-expression of the transcription factor FoxM1 and a loss of the tumour suppressor Arf causes primary liver cancer cells to undergo metastasis to the lung. We show that the metastatic transformation of the liver cancer cells is associated with EMT-like (epithelial to mesenchymal-like) changes. Moreover, expression of FoxM1 in Arf-deficient background induces pre-metastatic niche formation at the site of metastasis (lung). Also, we show that a treatment with an Arf-derived peptide-inhibitor of FoxM1 blocks metastasis of the tumour cells.
IMPACT
Over-expression of FoxM1 and loss of Arf expression are common features in many aggressive cancers. Our observations suggest that those changes in cancer cells are related their metastatic activity. Moreover, our observations provide proof-of-principle that inhibition of FoxM1 using an Arf-derived peptide could have strong therapeutic implications.
Xenograft cancer model
5 × 105 cells in 100 µl of PBS were subcutaneously injected into the flank side of nude mice at 6–8 weeks of age. We measured the diameter of the tumours every day.
Experimental metastasis assay
106 cells in 100 µl of PBS were intravenously injected into the lateral tail vein of nude mice at 6–8 weeks of age. After 3 weeks post injection, mice were sacrificed and lungs were removed for further examination. Lungs were rinsed in tap water and fixed with Bouin's solution. After 24 h, the surface tumour colonies were counted for each mouse.
Treatment of mice with peptide
Genemed Synthesis, Inc. manufactured the WT ARF26–44 peptide (rrrrrrrrrKFVRSRRPRTASCALAFVN) or mutant ARF37–44 peptide (rrrrrrrrrSCALAFVN) containing nine d-Arg (r) residues at the N terminus to enhance cellular uptake of polypeptide. We i.p. injected peptide (5 mg/kg body weight) or PBS every 2 days for 3 weeks.
Acknowledgments
We dedicate this work to the memory of Dr Robert H. Costa. Also, we thank Dr G. Adami (UIC-College of Dentistry) for the FoxM1b adenovirus. This work was supported by US Public Health Service (PHS) Grants CA 124488, CA 100035 and AG02438 to PR, and by a Merit Review Grant (IO1BX000131) from the Veteran's Administration to PR. LFL is supported by PHS grant AG021842. ALT is supported by the PHS grants DK44525 and DK068503 to ALT. NH is supported by the PHS grants CA090764, AG016927 and AG025953.
Supporting information is available at EMBO Molecular Medicine online.
The authors declare that they have no conflict of interest.
Author Contributions
HJP, GG, RHC and PR designed the experiments. HJP, GG, ZW, JRC, JL, KHK, JQ and YDP performed the experiments. HJP, PRW, NH, ALT, LFL and PR analysed the data. HJP and PR wrote the paper.
For more information
FoxM1:
http://www.ncbi.nlm.nih.gov/IEB/Research/Acembly/av.cgi?db=human&l=FOXM1
Tumour metastasis:
http://www.cancer.gov/cancertopics/factsheet/Sites-Types/metastatic
Pradip Raychaudhuri's web page:
Supplementary material
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
References
- Adam AP, George A, Schewe D, Bragado P, Iglesias BV, Ranganathan AC, Kourtidis A, Conklin DS, Aguirre-Ghiso JA. Computational identification of a p38SAPK-regulated transcription factor network required for tumor cell quiescence. Cancer Res. 2009;69:5664–5672. doi: 10.1158/0008-5472.CAN-08-3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, Redston MS, DePinho RA. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiri G, Sabo E, Dafni H, Vadasz Z, Kartvelishvily Y, Gan N, Kessler O, Cohen T, Resnick M, Neeman M, et al. Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo. Cancer Res. 2003;63:1657–1666. [PubMed] [Google Scholar]
- Baldassarre G, Belletti B, Nicoloso MS, Schiappacassi M, Vecchione A, Spessotto P, Morrione A, Canzonieri V, Colombatti A. p27(Kip1)–stathmin interaction influences sarcoma cell migration and invasion. Cancer Cell. 2005;7:51–63. doi: 10.1016/j.ccr.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Biggs WH, III, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci USA. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissan M, Wendum D, Arnaud-Dabernat S, Munier A, Debray M, Lascu I, Daniel JY, Lacombe ML. Increased lung metastasis in transgenic NM23-Null/SV40 mice with hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:836–845. doi: 10.1093/jnci/dji143. [DOI] [PubMed] [Google Scholar]
- Bruix J, Boix L, Sala M, Llovet JM. Focus on hepatocellular carcinoma. Cancer Cell. 2004;5:215–219. doi: 10.1016/s1535-6108(04)00058-3. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Calvisi DF, Pinna F, Ladu S, Pellegrino R, Simile MM, Frau M, De Miglio MR, Tomasi ML, Sanna V, Muroni MR, et al. Forkhead box M1B is a determinant of rat susceptibility to hepatocarcinogenesis and sustains ERK activity in human HCC. Gut. 2009;58:679–687. doi: 10.1136/gut.2008.152652. [DOI] [PubMed] [Google Scholar]
- Campbell JS, Hughes SD, Gilbertson DG, Palmer TE, Holdren MS, Haran AC, Odell MM, Bauer RL, Ren HP, Haugen HS, et al. Platelet-derived growth factor C induces liver fibrosis, steatosis, and hepatocellular carcinoma. Proc Natl Acad Sci USA. 2005;102:3389–3394. doi: 10.1073/pnas.0409722102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets. 2008;8:187–198. doi: 10.2174/156800908784293659. [DOI] [PubMed] [Google Scholar]
- Chandran UR, Ma C, Dhir R, Bisceglia M, Lyons-Weiler M, Liang W, Michalopoulos G, Becich M, Monzon FA. Gene expression profiles of prostate cancer reveal involvement of multiple molecular pathways in the metastatic process. BMC Cancer. 2007;7:64. doi: 10.1186/1471-2407-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Paliwal S, Draheim K, Grossman SR, Lewis BC. p19Arf inhibits the invasion of hepatocellular carcinoma cells by binding to C-terminal binding protein. Cancer Res. 2008;68:476–482. doi: 10.1158/0008-5472.CAN-07-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai B, Kang SH, Gong W, Liu M, Aldape KD, Sawaya R, Huang S. Aberrant FoxM1B expression increases matrix metalloproteinase-2 transcription and enhances the invasion of glioma cells. Oncogene. 2007;26:6212–6219. doi: 10.1038/sj.onc.1210443. [DOI] [PubMed] [Google Scholar]
- Eischen CM, Weber JD, Roussel MF, Sherr CJ, Cleveland JL. Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 1999;13:2658–2669. doi: 10.1101/gad.13.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gialmanidis IP, Bravou V, Amanetopoulou SG, Varakis J, Kourea H, Papadaki H. Overexpression of hedgehog pathway molecules and FOXM1 in non-small cell lung carcinomas. Lung Cancer. 2009;66:64–74. doi: 10.1016/j.lungcan.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Grille SJ, Bellacosa A, Upson J, Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN, Larue L. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003;63:2172–2178. [PubMed] [Google Scholar]
- Gusarova GA, Wang IC, Major ML, Kalinichenko VV, Ackerson T, Petrovic V, Costa RH. A cell-penetrating ARF peptide inhibitor of FoxM1 in mouse hepatocellular carcinoma treatment. J Clin Invest. 2007;117:99–111. doi: 10.1172/JCI27527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinichenko VV, Major ML, Wang X, Petrovic V, Kuechle J, Yoder HM, Dennewitz MB, Shin B, Datta A, Raychaudhuri P, et al. Foxm1b transcription factor is essential for development of hepatocellular carcinomas and is negatively regulated by the p19ARF tumor suppressor. Genes Dev. 2004;18:830–850. doi: 10.1101/gad.1200704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinina OA, Kalinin SA, Polack EW, Mikaelian I, Panda S, Costa RH, Adami GR. Sustained hepatic expression of FoxM1B in transgenic mice has minimal effects on hepatocellular carcinoma development but increases cell proliferation rates in preneoplastic and early neoplastic lesions. Oncogene. 2003;22:6266–6276. doi: 10.1038/sj.onc.1206640. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial–mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo T, Bodner S, van de Kamp E, Randle DH, Sherr CJ. Tumor spectrum in ARF-deficient mice. Cancer Res. 1999;59:2217–2222. [PubMed] [Google Scholar]
- Kang Y, Massague J. Epithelial–mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Kelly-Spratt KS, Gurley KE, Yasui Y, Kemp CJ. p19Arf suppresses growth, progression, and metastasis of Hras-driven carcinomas through p53-dependent and -independent pathways. PLoS Biol. 2004;2:E242. doi: 10.1371/journal.pbio.0020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khawaja S, Gundersen GG, Bulinski JC. Enhanced stability of microtubules enriched in detyrosinated tubulin is not a direct function of detyrosination level. J Cell Biol. 1988;106:141–149. doi: 10.1083/jcb.106.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopfstein L, Christofori G. Metastasis: cell-autonomous mechanisms versus contributions by the tumor microenvironment. Cell Mol Life Sci. 2006;63:449–468. doi: 10.1007/s00018-005-5296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laoukili J, Stahl M, Medema RH. FoxM1: at the crossroads of ageing and cancer. Biochim Biophys Acta. 2007;1775:92–102. doi: 10.1016/j.bbcan.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Lewis BC, Klimstra DS, Socci ND, Xu S, Koutcher JA, Varmus HE. The absence of p53 promotes metastasis in a novel somatic mouse model for hepatocellular carcinoma. Mol Cell Biol. 2005;25:1228–1237. doi: 10.1128/MCB.25.4.1228-1237.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhang N, Jia Z, Le X, Dai B, Wei D, Huang S, Tan D, Xie K. Critical role and regulation of transcription factor FoxM1 in human gastric cancer angiogenesis and progression. Cancer Res. 2009;69:3501–3509. doi: 10.1158/0008-5472.CAN-08-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AW, Lowe SW. Oncogenic ras activates the ARF-p53 pathway to suppress epithelial cell transformation. Proc Natl Acad Sci USA. 2001;98:5025–5030. doi: 10.1073/pnas.091100298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Dai B, Kang SH, Ban K, Huang FJ, Lang FF, Aldape KD, Xie TX, Pelloski CE, Xie K, et al. FoxM1B is overexpressed in human glioblastomas and critically regulates the tumorigenicity of glioma cells. Cancer Res. 2006;66:3593–3602. doi: 10.1158/0008-5472.CAN-05-2912. [DOI] [PubMed] [Google Scholar]
- Moulton HM, Nelson MH, Hatlevig SA, Reddy MT, Iversen PL. Cellular uptake of antisense morpholino oligomers conjugated to arginine-rich peptides. Bioconjug Chem. 2004;15:290–299. doi: 10.1021/bc034221g. [DOI] [PubMed] [Google Scholar]
- Okabe H, Satoh S, Kato T, Kitahara O, Yanagawa R, Yamaoka Y, Tsunoda T, Furukawa Y, Nakamura Y. Genome-wide analysis of gene expression in human hepatocellular carcinomas using cDNA microarray: identification of genes involved in viral carcinogenesis and tumor progression. Cancer Res. 2001;61:2129–2137. [PubMed] [Google Scholar]
- Park HJ, Carr JR, Wang Z, Nogueira V, Hay N, Tyner AL, Lau LF, Costa RH, Raychaudhuri P. FoxM1, a critical regulator of oxidative stress during oncogenesis. EMBO J. 2009;28:2908–2918. doi: 10.1038/emboj.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne SL, Hendrix MJ, Kirschmann DA. Paradoxical roles for lysyl oxidases in cancer—a prospect. J Cell Biochem. 2007;101:1338–1354. doi: 10.1002/jcb.21371. [DOI] [PubMed] [Google Scholar]
- Peinado H, Del Carmen Iglesias-de la Cruz M, Olmeda D, Csiszar K, Fong KS, Vega S, Nieto MA, Cano A, Portillo F. A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. EMBO J. 2005;24:3446–3458. doi: 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilarsky C, Wenzig M, Specht T, Saeger HD, Grutzmann R. Identification and validation of commonly overexpressed genes in solid tumors by comparison of microarray data. Neoplasia. 2004;6:744–750. doi: 10.1593/neo.04277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez HM, Vaysberg M, Mikels A, McCauley S, Velayo AC, Garcia C, Smith V. Modulation of lysyl oxidase-like 2 enzymatic activity by an allosteric antibody inhibitor. J Biol Chem. 2010;285:20964–20974. doi: 10.1074/jbc.M109.094136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai M, Kato H, Sano A, Tanaka N, Inose T, Kimura H, Sohda M, Nakajima M, Kuwano H. Expression of lysyl oxidase is correlated with lymph node metastasis and poor prognosis in esophageal squamous cell carcinoma. Ann Surg Oncol. 2009;16:2494–2501. doi: 10.1245/s10434-009-0559-5. [DOI] [PubMed] [Google Scholar]
- Sayan AE, Sayan BS, Findikli N, Ozturk M. Acquired expression of transcriptionally active p73 in hepatocellular carcinoma cells. Oncogene. 2001;20:5111–5117. doi: 10.1038/sj.onc.1204669. [DOI] [PubMed] [Google Scholar]
- Schmitt CA, McCurrach ME, de Stanchina E, Wallace-Brodeur RR, Lowe SW. INK4a/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev. 1999;13:2670–2677. doi: 10.1101/gad.13.20.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S, Ehemann V, Brauckhoff A, Keith M, Vreden S, Schirmacher P, Breuhahn K. Protumorigenic overexpression of stathmin/Op18 by gain-of-function mutation in p53 in human hepatocarcinogenesis. Hepatology. 2007;46:759–768. doi: 10.1002/hep.21736. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial–mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Wierstra I, Alves J. FOXM1, a typical proliferation-associated transcription factor. Biol Chem. 2007;388:1257–1274. doi: 10.1515/BC.2007.159. [DOI] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Yang DK, Son CH, Lee SK, Choi PJ, Lee KE, Roh MS. Forkhead box M1 expression in pulmonary squamous cell carcinoma: correlation with clinicopathologic features and its prognostic significance. Hum Pathol. 2009;40:464–470. doi: 10.1016/j.humpath.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang N, Dai B, Liu M, Sawaya R, Xie K, Huang S. FoxM1B transcriptionally regulates vascular endothelial growth factor expression and promotes the angiogenesis and growth of glioma cells. Cancer Res. 2008;68:8733–8742. doi: 10.1158/0008-5472.CAN-08-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial–mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.