Abstract
Rationale
Prolonged exposure (PE) therapy has been found to reduce symptoms of posttraumatic stress disorder (PTSD); however, it is difficult for many patients to engage fully in the obligatory retelling of their traumatic experiences. This problem is compounded by the fact that habituation and cognitive restructuring – the main mechanisms through which PE is hypothesized to work – are not instantaneous processes, and often require several weeks before the distress associated with imaginal exposure abates.
Case reports
Two cases are described that respectively illustrate the use of hydrocortisone and placebo, in combination with PE, for the treatment of combat-related PTSD. Based on known effects of glucocorticoids on learning and memory performance, we hypothesized that augmentation with hydrocortisone would improve the therapeutic effects of PE by hastening “new” learning and facilitating decreases in the emotional impact of fear memories during the course of treatment. The veteran receiving hydrocortisone augmentation of PE displayed an accelerated and ultimately greater decline in PTSD symptoms than the veteran receiving placebo.
Conclusions
While no general conclusion can be derived from comparison of two patients, the findings are consistent with the rationale for augmentation. These case reports support the potential for an appropriately designed and powered clinical trial to examine the efficacy of glucocorticoids in augmenting the effects of psychotherapy for PTSD.
Keywords: Glucocorticoids, PTSD, cortisol, treatment, prolonged exposure, learning, memory
Cognitive behavioral psychotherapy has emerged as a first line treatment for posttraumatic stress disorder (PTSD), with the evidence for efficacy of prolonged exposure (PE) therapy being particularly strong (Bisson & Andrew, 2005; Eisenman et al., 2006; Foa, 2006). PE is a cognitive behavioral intervention that is designed to reduce the emotional impact of traumatic memories (Foa & Kozak, 1986). The therapy requires that patients confront, rather than avoid, traumatic memories, mostly by revisiting the “imaged” scene while constructing a sequential narrative in structured therapy sessions. By accessing the memory in session there is an opportunity for patients to experience abreaction in relative safety, and then to begin a process of habituation to the memory. Because the initial recounting of traumatic events is psychologically and often physiologically distressing, a patient may engage in avoidance before habituation can begin. Habituation, in this context, refers to the process of gradually decreasing the person's arousal response to his/her traumatic memory through repeated exposures. Avoidance can be manifested by only partial engagement in the therapeutic work (e.g., the patient does not allow him/herself to become sufficiently distressed), or in more severe cases, avoidance of the treatment altogether (e.g., by not showing up for a session or dropping out of therapy completely). Methods that increase distress tolerance while allowing for full engagement with traumatic material during the initial phases of therapy may accelerate habituation and can therefore be of great value to those seeking relief from PTSD symptoms.
Combat veterans with PTSD likely pose an additional difficulty with respect to successful PE treatment, due to the likelihood of post-deployment cognitive impairments, manifested by difficulties in attention, as well as episodic and working memory (e.g., Golier & Yehuda, 2002; Vasterling et al., 2002). Such impairments are not typically as prominent or severe in civilian PTSD (Jenkins, Langlais, Delis, & Cohen, 1998; Stein, Kennedy, & Twamley, 2002). The presence of cognitive deficits may pose a challenge in the context of PE, or any therapy, that depends on cognitive processing and relearning. Indeed, in clinical trials with combat veterans, effect sizes have not been as large as those observed in civilian PTSD treatment studies, nor have gains been as consistently sustained (Schnurr et al., 2007). Therefore, in combat veterans there is a clinically and theoretically important rationale for an attempt to augment the effects of PE treatment with agents that might facilitate new learning, including extinction learning. The need for augmentation of cognitive behavioral therapy has now prompted several studies (e.g., Hofmann, 2007; Ressler et al., 2004), for instance, focusing on D-cycloserine in PTSD and other anxiety disorders.
In this report we describe the experience of a patient treated with PE that was augmented with hydrocortisone (Cortef®, Pfizer, Inc.), a synthetic glucocorticoid that mimics the effects of cortisol. The rationale for augmenting with glucocorticoids has recently been described in connection with anxiety disorders (de Quervain, Aerni, Schelling, & Roozendaal, 2009) and illustrated in patients with specific and social phobia (Soravia et al., 2006), and is based on well-established, albeit complex, effects of these hormones on the regulation of learning and memory. For example, it has been demonstrated that glucocorticoids enhance memory consolidation of emotionally arousing experiences (de Quervain et al., 2009) and promote extinction learning. These effects may be well-exploited in the context of a therapy session in which emotionally arousing experiences are discussed for the purpose of providing corrective information (e.g., cognitive restructuring) that permits habituation or extinction. Glucocorticoids have also been found to impair delayed memory retrieval of emotionally arousing information (de Quervain, Aerni, & Roozendaal, 2007; de Quervain, Roozendaal, & McGaugh, 1998; de Quervain, Roozendaal, Nitsch, McGaugh, & Hock 2000; Kuhlmann, Piel, & Wolf, 2005). This action, in tandem with the aforementioned ones, may allow for full exposure during a session, with the additional benefit of reduced distress (i.e., increased tolerance for disturbing recollections) after the psychotherapy, which might in turn minimize avoidance and enhance adherence to homework and attendance at therapy. In that this action might also obstruct the recounting of traumatic memories necessary for PE, it is essential to test, rather than anticipate, the effects of hydrocortisone. Were glucocorticoids to be beneficial, this might implicate actions in decreasing the emotional impact of conditioned fear memories based on their actions in potentiating the effects of glutamatergic N-methyl-d-aspartate (NMDA) receptors in the amygdala (Abrari, Rashidy-Pour, Semnanian, & Fathollahi, 2007; Cai, Blundell, Han, Greene, & Powell, 2006; Cordero, Kruyt, Merino, & Sandi, 2002; Kohda et al., 2007; Tronel & Alberini, 2007). This effect is analogous to the effects of d-cycloserine. Observations of lower basal cortisol levels in PTSD, as well as increased glucocorticoid receptor responsivity (Bremner, Vermetten, & Kelley, 2007; Yehuda, 2002), provide further support for the use of glucocorticoids as an augmentation strategy to psychotherapeutic interventions in PTSD. Further proof of this concept is provided by a recent case report describing positive effects of repeated low dose (10mg) hydrocortisone administration in the treatment of PTSD even in the absence of psychotherapy (Aerni et al., 2004), and in a study showing reduced subsequent intensive care unit-related PTSD symptoms in post-surgical patients treated with stress-doses of hydrocortisone (Schelling et al., 2004). Consistent with this effect, we recently reported that a single i.v. bolus of hydrocortisone reversed the previously observed difference between aging combat veterans with PTSD and control subjects in laterality of the amygdala and hippocampus (Yehuda et al., 2010) and in regional glucose utilization of the prefrontal cortex (Collins et al., in preparation). Interestingly, hydrocortisone also improved episodic and working memory performance compared to performance observed on the placebo day in this group (Yehuda, Harvey, Buchsbaum, Tischler, Schmeidler, 2007).
We reasoned therefore that a moderate dose of hydrocortisone, if administered prior to each therapy session that included imaginal exposure, would lead to earlier and more successful amelioration of PTSD symptoms than placebo administration. We also thought that hydrocortisone would be accompanied by improvements in memory performance at treatment completion. The following cases are presented for the purpose of providing a clinical illustration of the rationale presented above.
Case reports
Description of treatment procedures
The study was approved by the IRB at the James J Peters Bronx VA. The procedures, including randomization to placebo or hydrocortisone were explained, and patients signed written informed consent. PE therapy was performed according to the published manual (Foa, Hembree, & Rothbaum, 2007) except that Patient A received 30mg of oral hydrocortisone approximately 30min prior to sessions 3 through 10, and Patient B received a placebo pill on the same time schedule. No pill was administered prior to Sessions 1 and 2 as no imaginal exposure occurs in these preliminary sessions.
The two patients were evaluated at pre- and post-treatment with respect to severity of PTSD using Clinician-Administered PTSD Scale (CAPS) (Blake et al., 1995) and the self-rated PTSD Symptom Scale (PSS-SR) (Foa, Riggs, Dancu, & Rothbaum, 1993) – administered additionally at weeks 2, 4, 6, 8, and 10. At pretreatment only, the Structured Clinical Interview for the DSM-IV (SCID) (Spitzer, Gibbon, & Williams, 1995) was administered in order to determine psychiatric comorbidities, and the CAPS was used to determine the presence of current and lifetime PTSD. Clinical severity of current PTSD was obtained by summing the frequency and intensity scores of the 17 symptoms of PTSD.
Brief cognitive testing prior to and following study-related treatment used three subtests from the Wechsler Memory Scale – Third Edition (WMS-III) (Weschler, 1997). These were selected to assess retention, attention and working memory (the Logical Memory Test [I and II], Digit Span, and Letter-Number Sequencing [LNS] tasks) based on prior findings in combat veterans with PTSD (Yehuda et al., 2007).
Trauma histories and clinical presentation of cases
Patient A (hydrocortisone)
Patient A was a 42-year old, Ecuadorian male veteran who served for one year as part of Operation Iraqi Freedom (OIF), 2 years prior to initiating treatment. He identified his focal trauma, from among many critical incidents, as an event in which he was on tower duty, and his friend's truck flipped over. He could hear his friend screaming in pain from the tower, but could not leave his post to help. During the time it took for help to arrive he worried that his friend would die. The injury to the friend resulted in a loss of two fingers.
Patient B (placebo)
Patient B was a 42-year old African American male veteran who served in OIF for 1-year, 5 years prior to initiating treatment. During his deployment he was exposed to numerous events including mortar explosions and firefights. He identified his focal trauma as an incident in which he and his “battle buddy” shot a civilian who continued to approach their convoy despite being warned several times to stop. The shooting occurred out of suspicion that the civilian was a suicide bomber.
At initiation of treatment, both patients met criteria for both PTSD and Major Depressive Disorder (MDD) and had similar levels of overall PTSD symptom severity as assessed by both the CAPS (Patient A: 97; Patient B: 94) and PSS-SR (Patient A: 46; Patient B: 46). As assessed by CAPS and PSS-SR subscale scores, both participants showed similar pre-treatment levels of re-experiencing, avoidance, and hyperarousal symptoms, accounting for their similar total scores. In regard to cognitive tests (see Table 1), the patients had comparable performance on tests of working memory (Digit Span and LNS) and delayed recall (Logical Memory II). However, Patient A had poorer performance on immediate recall (Logical Memory I).
Table 1.
Comparison of pre- and post-treatment cognitive assessments for hydrocortisone and placebo treated PE participants.
| Patient A (hydrocortisone) | Patient B (placebo) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Cognitive measures | Pre-a | Post-a | Λb | Pre-a | Post-a | Λb |
| Immediate recall | ||||||
| Logical Memory (I) recall Units | 9 | 14 | +5 | 14 | 15 | +1 |
| Logical Memory (I) thematic units | 3 | 6 | +3 | 5 | 5 | 0 |
| Logical Memory (I) total3 | 12 | 20 | +8 | 19 | 20 | +1 |
| Delayed recall | ||||||
| Logical Memory (II) recall units | 8 | 10 | +2 | 10 | 14 | +4 |
| Logical Memory (II) thematic units | 3 | 5 | +2 | 4 | 5 | +1 |
| Logical Memory (II) totalc | 11 | 15 | +4 | 14 | 19 | +5 |
| Working memory & attention | ||||||
| Digit span total | 9 | 7 | −2 | 9 | 8 | −1 |
| Letter-number sequencing | 9 | 4 | −5 | 12 | 7 | −5 |
Pre- and post-treatment evaluation scores.
Pre- to post-treatment change in scores.
Because we ensured that alternate story forms were presented at pre- and post-treatment evaluations, the Logical Memory tests were not scaled according to age norms. Subjects were the same age, however, permitting direct comparison of test scores.
Treatment course and response
Patient A (hydrocortisone)
During session 2, the patient constructed a ‘hierarchy’ of in-vivo exposures in which he engaged regularly between sessions. In session 3, the participant began a first imaginal exposure but, after 10 minutes, discontinued the exercise complaining of extreme distress, and was not willing to continue the exerise within the session. He did however engage in imaginal exposures in future sessions for the specified time.
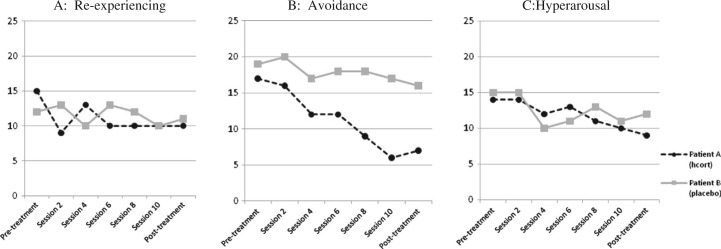
When assessed at the end of treatment, Patient A showed a 54 point reduction in CAPS and a 20 point reduction on PSS-SR, reflecting a substantial decrease in PTSD symptom severity. As illustrated in Fig. 1 (panels A, B, and C), the patient experienced some reduction in all symptoms clusters, but his reduction in avoidance symptoms was greater (58.8% reduction as assessed by PSS-SR) than the reduction he experienced in either re-experiencing or hyperarousal symptoms. This result was echoed by a 50% reduction in severity of avoidance symptoms as assessed by CAPS.
Fig. 1.
Comparison of self-reported PTSD Symptom Scale clusters for hydrocortisone and placebo treated PE participants.
Patient A also demonstrated improved performance on the Logical Memory tests I and II of immediate recall and delayed recall (an 8 point increase and 4 point increase respectively; see Table 1). However, he showed slight deterioration in Digit-Span and Letter-Number Sequencing tests of working memory.
Patient B (placebo)
During session 2, this veteran constructed a hierarchy of in-vivo exposures. Initially, he engaged in one exposure exercise two or three times between sessions, and stopped doing even these by session 6. In session 3, the patient was able to complete his first imaginal exercise despite extreme distress but showed little habituation across sessions. In session 8, patient reported that although he understood the rationale for imaginal exposures he had not disclosed an even more disturbing element of his focal trauma which he proceeded to report. During sessions 9 and 10, Patient B engaged in an imaginal exercise relating the entire event.
Patient B also showed some progress in terms of symptom reduction following treatment. His post-treatment CAPS score was 42 points lower than at pre-treatment and his PSS-SR showed a 7 point reduction which, as shown in Fig. 1 (panels A, B, and C), reflected a small decrease within each symptom cluster. He also demonstrated improved performance on the Logical Memory tests I and II of immediate recall and delayed recall (a 1 point increase and 5 point increase respectively; see Table 1). However, he showed slight deterioration in Digit-Span and Letter-Number Sequencing tests of working memory.
Discussion
The current case report illustrates the treatment response of two participants who completed PE treatment with either the addition of pre-session hydrocortisone or placebo to augment the effects of PE to reduce symptoms of PTSD. These two treatments illustrate that although both participants experienced some reduction in their symptoms of PTSD, the participant who received hydrocortisone augmentation experienced substantially greater improvement. This was evident as assessed by CAPS (a 54 point reduction compared to a 42 point reduction achieved by Patient B) and by self-report with the PSS-SR (a 20 point reduction, compared to a 7 point reduction achieved by Patient B). Although no conclusions can be drawn from a comparison of two cases, these examples illustrate our reasoning for glucocorticoid augmentation.
An examination of both clinician-assessed and self-reported severity of specific symptom clusters indicates that this discrepancy is largely accounted for by a substantially greater reduction in avoidance symptoms in the hydrocortisons-treated patient. Clinician-rated CAPS assessment indicated that Patient A experienced a 50% (17 point) reduction in avoidance as compared to a 22.9% (8 point) reduction achieved by Patient B. Self-report indices showed similar results with a 58.8% (10 point) reduction in avoidance ratings by Patient A compared to a 15.8% (3 point) reduction by Patient B. Fig. 1 (panel B) demonstrates a consistent downward trajectory of avoidance symptoms beginning between sessions 2 and 4 for Patient A that is not observed for Patient B. This is consistent with PE's hypothesized action of targeting and thereby reducing avoidance by activating pathological fear structures, and in so doing, providing opportunities for integration of corrective information (Foa & Kozak, 1986; Kozak, Foa, & Steketee, 1988). The veteran who received hydrocortisone experienced more rapid gains in treatment – again, specifically with respect to symptoms of avoidance. In addition to self-report measures indicating this, Patient A's ability to adhere to homework assignments can be interpreted as a reduction in behavioral avoidance.
Indeed, many patients have difficulty in overcoming the strong urge to avoid trauma-specific reminders which has a direct impact on the progress of therapy. Within sessions, avoidance impacts the ability to engage in imaginal exposure exercises and, between sessions, it influences adherence to exposure homework assignments – both of which can influence therapy outcomes. In comparing the progress of therapy for these two participants, it is clear that both experienced some difficulties with completing in-session imaginal assignments as well as listening to recordings of the imaginal exposure between sessions. Patient B, however, experienced comparatively more difficulties with continued adherence – completely omitting a significant detail of the trauma memory and therefore not truly completing a single imaginal exposure until session 9, and completing few in-vivo exposures between sessions 2 and 6 before stopping them completely – despite voicing a good understanding of the rationale for these exercises. Their identical symptom severity and comparable levels of avoidance at treatment outset allows for speculation that some element of the process of exposure was more tolerable for Patient A than Patient B.
Although conclusions regarding the efficacy of augmenting with hydrocortisone cannot be drawn by comparing these two cases, if these results were maintained in the context of a larger trial, they would warrant interpretation. We have chosen to present these reports for their illustrative and heuristic value. If the relatively quicker and ultimately greater outcome in Patient A relates to the effects of hydrocortisone, this would be consistent with the rationale for augmentation – to promote recovery by enhancing extinction learning, facilitating new learning, or by increasing tolerance of fear memories, and by hastening these processes. These effects would almost certainly contribute to the experience of exposures feeling more tolerable. Indeed, Soravia and colleagues (2006) observed that individuals whose exposures were augmented by cortisone reported less subjective distress although there were no significant differences in measures of physiological arousal, suggesting that the administered cortisone may have operated to enhance tolerance of exposure-related distress. The experience of a reduction in distress associated with repeated exposures early in treatment (as occurred here for the patient who received hydrocortisone) could certainly reinforce the exercise as helpful and emotionally manageable, while also producing some of the self-efficacy necessary for continuing with the exposures. In addition, the hypothesized reduction in fear associated with the experience of revisiting the trauma memory would also allow for more opportunities to identify unhelpful cognitions and provide corrective information to promote cognitions that are more compatible with recovery. If glucocorticoids indeed act primarily to decrease avoidance, this would allow patients to make better gains in treatment – even compared to patients in therapy for an equivalent duration.
Differences in cognitive performance are somewhat more difficult to interpret given that the participant who received hydrocortisone showed comparatively poorer performance on immediate recall at the outset of treatment. While the differential improvement in immediate recall evidenced by Patient A (hydrocortisone) may relate to an effect of the hydrocortisone augmentation, it is also possible that the cognitive findings in this patient are an indirect consequence of a reduction in PTSD symptom severity. Additionally, both patients inexplicably performed more poorly on working memory measures at post-treatment. This decline is interesting and is worthy of further study, but does not appear to be relevant to the hydrocortisone administration per se as both patients declined equally.
The observed improvement in cognitive performance, however, is consistent with the actions of glucocorticoids in modulating cognitive processing by increasing attention, resulting in improved learning (Lupien et al., 2002). More recent basic and clinical cognitive neuroscience research continues to identify effects of glucocorticoids that might be relevant to the actions of single doses of cortisol. For example, a single administration of glucocorticoids was recently shown to enhance attention in a threat-selective attention task, building on previous work showing that a single dose of glucocorticoids acutely reduces selective attention to fearful faces and, likewise, reduces preferential processing of fearful faces in a spatial working memory task (Putman, Hermans, & van Honk, 2010). Furthermore, a single intravenous cortisol infusion before retrieval testing suppressed the number of false recognitions as determined by using a modified Deese-Roediger-McDermott paradigm designed to evaluate non-semantic memory for shapes and objects (Diekelmann, Wilhelm, Wagner, & Born, in press). The improved effects on cognition may indeed relate to the administration of single, rather than continuous, doses of cortisol. Single doses have not generally been observed to disrupt memory acquisition or lead to prominent changes of cerebral metabolites (Scheel, Strohle, & Bruhn, 2010). Rather, investigations of molecular pathways continue to uncover mechanisms that are more consistent with facilitated learning. A particularly relevant mechanism may be through glucocorticoid mediated synaptic trafficking (Conboy & Sandi, 2010). Indeed, glucocorticoids may, through modulation of AMPA (alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-prioprionate) receptor function, promote overall memory consolidation (Krugers, Hoogenraad, & Groc, in press). These effects of glucocorticoids may be synergistic with other glucocorticoid-mediated actions in the amygdala on dampening the effects of fear memories.
Acknowledgements
This work was supported by funding from the following sources: the VISN3 MIRECC, the Lightfighter Trust, and DOD award #W81XWH-10-2-007. The authors wish to acknowledge Melissa Altman Stein, Ph.D., who was the psychotherapist for one of the participants receiving Prolonged Exposure therapy, and Ann E. Smith, Ph.D., who was an evaluator. Drs. Stein and Smith are affiliated with the James J. Peters Veterans Affairs Medical Center, Bronx, NY.
For the abstract or full text in other languages, please see Supplementary files (under Reading Tools online).
Conflict of interest and funding
The authors have not received any funding or benefits from industry to conduct this study.
References
- Abrari K., Rashidy-Pour A., Semnanian S., Fathollahi Y. Administration of corticosterone after memory reactivation disrupts subsequent retrieval of a contextual conditioned fear memory: Dependence upon training intensity. Neurobiology of Learning and Memory. 2007;89(2):178–184. doi: 10.1016/j.nlm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Aerni A., Traber R., Hock C., Roozendaal B., Schelling G., Papassotiropoulos A., et al. Low-dose cortisol for symptoms of posttraumatic stress disorder. American Journal of Psychiatry. 2004;161:1488–1490. doi: 10.1176/appi.ajp.161.8.1488. [DOI] [PubMed] [Google Scholar]
- Bisson J., Andrew M. Psychological treatment of post-traumatic stress disorder (PTSD) Cochrane Database of Systematic Reviews. 2005;18(2) doi: 10.1002/14651858.CD003388.pub2. [DOI] [PubMed] [Google Scholar]
- Blake D. D., Weathers F. W., Nagy L. M., Kaloupek D. G., Gusman F. D., Charney D. S., et al. The development of a clinician-administered PTSD scale. Journal of Trauma Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bremner D., Vermetten E., Kelley M. E. Cortisol, dehydroepiandrosterone, and estradiol measured over 24 hours in women with childhood sexual abuse-related posttraumatic stress disorder. Journal of Nervous and Mental Disorders. 2007;195(11):919–927. doi: 10.1097/NMD.0b013e3181594ca0. [DOI] [PubMed] [Google Scholar]
- Cai W. H., Blundell J., Han J., Greene R. W., Powell C. M. Postreactivation glucocorticoids impair recall of established fear memory. The Journal of Neuroscience. 2006;26(37):9560–9566. doi: 10.1523/JNEUROSCI.2397-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins K., Hazlett E. A., Golier J., Bierer L. M., Buchsbaum M. S., Yehuda R. Normalization of frontal lobe glucose metabolism in PTSD with hydrocortisone; in preparation. [Google Scholar]
- Conboy L., Sandi C. Stress at learning facilitates memory formation by regulating AMPA receptor trafficking through a glucocorticoid action. Neuropsychopharmacology. 2010;35(3):674–685. doi: 10.1038/npp.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero M. I., Kruyt N. D., Merino J. J., Sandi C. Glucocorticoid involvement in memory formation in a rat model for traumatic memory. Stress. 2002;5(1):73–79. doi: 10.1080/1025389029000124404. [DOI] [PubMed] [Google Scholar]
- de Quervain D., Aerni A., Roozendaal B. Preventive effect of beta-adrenoceptor blockade on glucocorticoid-induced memory retrieval deficits. American Journal of Psychiatry. 2007;164:967–969. doi: 10.1176/ajp.2007.164.6.967. [DOI] [PubMed] [Google Scholar]
- de Quervain D., Aerni A., Schelling G., Roozendaal B. Glucocorticoids and the regulation of memory in health and disease. Frontiers in Neuroendocrinology. 2009;30:358–370. doi: 10.1016/j.yfrne.2009.03.002. [DOI] [PubMed] [Google Scholar]
- de Quervain D., Roozendaal B., McGaugh J. L. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- de Quervain D., Roozendaal B., Nitsch R. M., McGaugh J. L., Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nature Neuroscience. 2000;3(4):313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- Diekelmann S., Wilhelm I., Wagner U., Born J. Journal of Cognitive Neuroscience. Elevated cortisol at retrieval suppresses false memories in parallel with correct memories. (in press) Manuscript submitted for publication. [DOI] [PubMed] [Google Scholar]
- Eisenman D., Weine S., Green B., de Jong J., Rayburn N., Ventevogel P., et al. The ISTSS/Rand guidelines on mental health training of primary healthcare providers for trauma-exposed populations in conflict-affected countries. Journal of Trauma Stress. 2006;19(1):5–17. doi: 10.1002/jts.20094. [DOI] [PubMed] [Google Scholar]
- Foa E. B. Psychosocial therapy for posttraumatic stress disorder. Journal of Clinical Psychiatry. 2006;67(Suppl. 2):40–45. [PubMed] [Google Scholar]
- Foa E. B., Hembree E. A., Rothbaum B. O. Prolonged exposure therapy for PTSD. New York: Oxford University Press; 2007. [Google Scholar]
- Foa E. B., Kozak M. J. Emotional processing of fear: Exposure to corrective information. Psychology Bulletin. 1986;99:20–35. [PubMed] [Google Scholar]
- Foa E. B., Riggs D. S., Dancu C. V., Rothbaum B. O. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. Journal of Traumatic Stress. 1993;6:459–473. [Google Scholar]
- Golier J., Yehuda R. Neuropsychological processes in post-traumatic stress disorder. Psychiatry Clinics of North America. 2002;25(2):295–315. doi: 10.1016/s0193-953x(01)00004-1. [DOI] [PubMed] [Google Scholar]
- Hofmann S. G. Enhancing exposure-based therapy from a translational research perspective. Behaviour Research and Therapy. 2007;45(9):1987–2001. doi: 10.1016/j.brat.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M. A., Langlais P. J., Delis D., Cohen R. Learning and memory in rape victims with posttraumatic stress disorder. American Journal of Psychiatry. 1998;155(2):278–279. doi: 10.1176/ajp.155.2.278. [DOI] [PubMed] [Google Scholar]
- Kohda K., Harada K., Kato K., Hoshino A., Motohashi J., Yamaji T., et al. Glucocorticoid receptor activation is involved in producing abnormal phenotypes of single-prolonged stress rats: A putative post-traumatic stress disorder model. Neuroscience. 2007;148(1):22–33. doi: 10.1016/j.neuroscience.2007.05.041. [DOI] [PubMed] [Google Scholar]
- Kozak M. J., Foa E. B., Steketee G. Process and outcome of exposure treatment with obsessive-compulsives: Psychophysiological indicators of emotional processing. Behavior Therapy. 1988;19:157–169. [Google Scholar]
- Krugers H. J., Hoogenraad C. C., Groc L. Nature Reviews Neuroscience. (in press) Manuscript submitted for publication. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S., Piel M., Wolf O. T. Impaired memory retrieval after psychosocial stress in healthy young men. Journal of Neuroscience. 2005;25(11):2977–2982. doi: 10.1523/JNEUROSCI.5139-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S. J., Wilkinson C. W., Brière S., Ménard C., Ng Ying Kin N. M., Nair N. P. The modulatory effects of corticosteroids on cognition: Studies in young human populations. Neuroendocrinology. 2002;27(3):401–416. doi: 10.1016/s0306-4530(01)00061-0. [DOI] [PubMed] [Google Scholar]
- Putman P., Hermans E. J., van Honk J. Cortisol administration acutely reduces threat-selective spatial attention in healthy young men. Physiology & Behavior. 2010;99(3):294–300. doi: 10.1016/j.physbeh.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Ressler K. J., Rothbaum B. O., Tannenbaum L., Anderson P., Graap K., Zimand E., et al. Cognitive enhancers as adjuncts to psychotherapy: Use of d-cycloserine in phobic individuals to facilitate extinction of fear. Archives of General Psychiatry. 2004;61(11):1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Scheel M., Strohle A., Bruhn H. Effects of short-term stress-like cortisol on cerebral metabolism: A proton magnetic resonance spectroscopy study at 3.0 T. Journal of Psychiatric Research. 2010;44(8):521–526. doi: 10.1016/j.jpsychires.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Schelling G., Kilger E., Roozendaal B., de Quervain D. J., Briegel J., Dagge A., Rothenhäsler H. B., Krauseneck T., Nollert G., Kapfhammer H. P. Stress doses of hydrocortisone, traumatic memories, and symptoms of posttraumatic stress disorder in patients after cardiac surgery: a randomized study. Biological Psychiatry. 2004;55(6):627–633. doi: 10.1016/j.biopsych.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Schnurr P. P., Friedman M. J., Engel C. C., Foa E. B., Shea M. T., Chow B. K., et al. Cognitive behavioral therapy for posttraumatic stress disorder in women: A randomized controlled trial. The Journal of the American Medical Association. 2007;297(8):820–830. doi: 10.1001/jama.297.8.820. [DOI] [PubMed] [Google Scholar]
- Soravia L. M., Heinrichs M., Aerni A., Maroni C., Schelling G., Ehlert U., et al. Glucocorticoids reduce phobic fear in humans. Proceedings of the National Academy of Sciences. 2006;103(14):5585–5590. doi: 10.1073/pnas.0509184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer R. L., Gibbon M., Williams J. B. W. Structured clinical interview for DSM-IV axis 1 disorders (SCID) New York: Biometrics Research, New York State Psychiatric Institute; 1995. [Google Scholar]
- Stein M. B., Kennedy C. M., Twamley E. W. Neuropsychological function in female victims of intimate partner violence with and without posttraumatic stress disorder. Biological Psychiatry. 2002;52(11):1079–1088. doi: 10.1016/s0006-3223(02)01414-2. [DOI] [PubMed] [Google Scholar]
- Tronel S., Alberini C. M. Persistent disruption of a traumatic memory by postretrieval inactivation of glucocorticoid receptors in the amygdala. Biological Psychiatry. 2007;62(1):33–39. doi: 10.1016/j.biopsych.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasterling J. J., Duke L. M., Brailey K., Constans J. I., Allain A. N., Jr., Sutker P. B. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16(1):5–14. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler memory scale. 3rd ed. San Antonio, TX: The Psychological Corp; 1997. [Google Scholar]
- Yehuda R. Current status of cortisol findings in post-traumatic stress disorder. Psychiatric Clinics of North America. 2002;25(2):341–368. doi: 10.1016/s0193-953x(02)00002-3. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Golier J. A., Bierer L.M., Mikhno A., Pratchett L. C., Burton C. L., et al. Hydrocortisone responsiveness in Gulf War veterans with PTSD: effects on ACTH, declarative memory hippocampal [(18)F]FDG uptake on PET. Psychiatry Research. 2010;184(2):117–127. doi: 10.1016/j.pscychresns.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Harvey P. D., Buchsbaum M., Tischler L., Schmeidler J. Enhanced effects of cortisol administration on episodic and working memory in aging veterans with PTSD. Neuropsychopharmacology. 2007;32(12):2581–2591. doi: 10.1038/sj.npp.1301380. [DOI] [PubMed] [Google Scholar]