Abstract
OBJECTIVE
To describe metastasis-free survival (MFS) and overall survival (OS) among men with prostate-specific antigen (PSA)-recurrent prostate cancer after radical prostatectomy who did not receive additional therapy until metastasis, using a multicentre database capturing a wide ethnic mix.
PATIENTS AND METHODS
A retrospective analysis of the Center for Prostate Disease Research National Database (comprised of five US military hospitals and one civilian centre) was performed for patients with PSA relapse (≥0.2 ng/mL) after radical prostatectomy who had no additional therapy until the time of radiographic metastatic disease.
We investigated factors influencing metastasis and all-cause mortality using univariate and multivariate Cox regression analysis.
RESULTS
There were a total of 346 men who underwent radical prostatectomy between May 1983 and November 2008 and fulfilled the entry criteria. All patients had information on survival and 190 men had information on metastasis. Among patients with survival data (n = 346), 10-year OS was 79% after a median follow-up of 8.6 years from biochemical recurrence.
Among men with metastasis data (n = 190), 10-year MFS was 46% after a median follow-up of 7.5 years.
In Cox regressions, four clinical factors (Gleason score, pathological stage, time to PSA relapse and PSA doubling time), as well as age, were predictive of OS and/or MFS in univariate analysis, although only PSA doubling time (≥9 vs 3–8.9 vs <3 months) remained independently predictive of these outcomes in multivariate analysis (P < 0.001).
CONCLUSIONS
This multicentre multi-ethnic dataset shows that OS and MFS can be extensive for men with PSA-recurrent prostate cancer, even in the absence of further therapy before metastasis.
This unique patient cohort, the second largest of its type after the Johns Hopkins cohort, confirms that PSA doubling time is the strongest determinant of OS and MFS in men with PSA-recurrent disease.
Longer follow-up and more events will be required to determine whether other variables may also contribute to these outcomes.
Keywords: metastasis-free survival, natural history, overall survival, prostate cancer, PSA recurrence
INTRODUCTION
Prostate cancer is the most prevalent malignancy in men worldwide, and accounts for over 200 000 new cases in the USA each year [1]. Although radical prostatectomy is curative for most patients with localized prostate cancer, ≈20–40% will experience disease recurrence by 10 years after surgery [2]. As a result of the universal availability and high sensitivity of PSA testing, most patients with disease relapse after prostatectomy present with a rising PSA in the absence of detectable local or distant recurrence. Once PSA progression has occurred, the condition is often incurable, although the clinical course of the disease may be extensive.
There is currently no consensus on the optimal management of non-metastatic PSA-recurrent prostate cancer [3,4]. Treatment options include continuous androgen deprivation therapy (ADT) initiated at the time of PSA recurrence [5,6], deferred ADT reserved until after the development of metastases [7,8], intermittent ADT [9,10] or enrollment in clinical trials [11]. A subset of patients with PSA-recurrent disease may be candidates for salvage pelvic irradiation [12]. An understanding of the factors that predict metastatic progression and death is crucial when choosing optimal risk-based therapies for these patients.
To help inform treatment decisions for such patients, investigators from Johns Hopkins previously described the natural history of a cohort of patients with PSA-recurrent disease after prostatectomy who did not receive additional therapies (such as ADT) until the development of metastases. This unique cohort has been used to estimate metastasis-free survival (MFS) [13,14], prostate cancer-specific survival [15] and overall survival (OS) [16] in this patient population. In addition, clinical factors influencing these outcomes have been described and can be used to risk-stratify patients. These factors include the surgical Gleason score, the PSA doubling time (PSADT) after biochemical recurrence, and possibly the time from surgery to biochemical recurrence. However, these findings represent data from a single institution and have not been validated elsewhere, primarily because most men with PSA relapse were historically treated with immediate ADT (thus precluding a study of the natural history of these patients). Furthermore, the number of men in the Johns Hopkins cohort that were non-white was very small (≈10%) [14,15], and this may not reflect the true racial mix of prostate cancer patients.
In an effort to confirm and validate the Johns Hopkins natural history data, the present study aimed to describe MFS and OS in a similar population of men with hormone-naïve PSA-recurrent prostate cancer after radical prostatectomy using information from the Center for Prostate Disease Research (CPDR) National Database [17]. This repository contains patient information from five US military hospitals and one civilian centre, and it includes a larger proportion of non-white men (28%). In addition, the present study aimed to identify risk factors for metastasis and all-cause mortality using this patient population. The ultimate goal was to identify those patients with PSA-recurrent prostate cancer who may benefit from early systemic therapy and to distinguish them from those in whom a conservative approach may be reasonable.
PATIENTS AND METHODS
The CPDR database is a Department of Defense-sponsored multicentre national repository containing clinical information on patients with prostatic diseases representing five US military hospitals and a civilian centre. In this cohort of patients, the contributing centres were Walter Reed Army Medical Center, Brooke Army Medical Center, Naval Medical Center of San Diego, Eisenhower Army Medical Center, Madigan Army Medical Center and Virginia Mason Medical Center. A detailed description of this database has been provided previously [17,18]. This research was approved by the appropriate Institutional Review Boards and met the requirements of the Health Insurance Portability and Accountability Act.
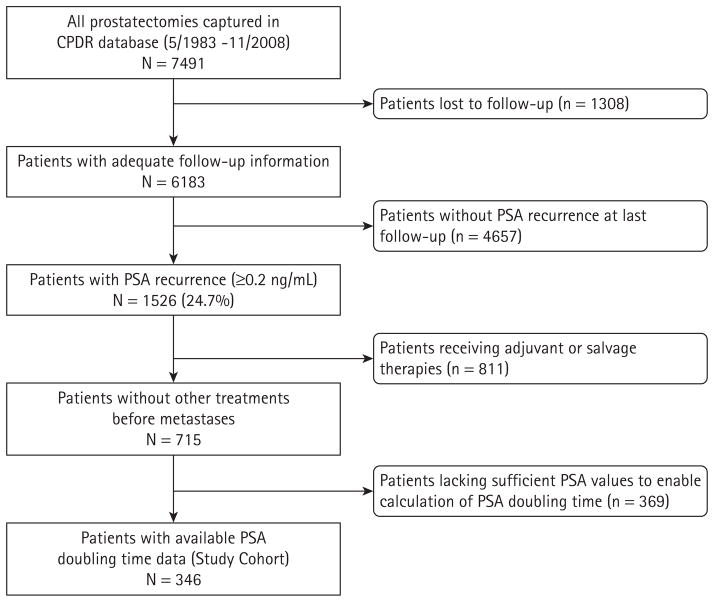
We retrospectively examined the records of men undergoing radical prostatectomy for localized disease between May 1983 and November 2008. In this analysis, 715 patients were identified with biochemical recurrence who had not received further therapy unless distant metastasis occurred (Fig. 1). Biochemical recurrence was defined as a postoperative PSA value of ≥0.2 ng/mL. Patients receiving neoadjuvant or adjuvant radiation or hormonal therapy, and those receiving salvage radiation or hormonal therapy after PSA recurrence but before metastases, were excluded. Only 346 men had sufficient postoperative PSA data enabling calculation of PSADT, and these patients alone formed the present study cohort. All 346 men had information on OS status, whereas only 190 men had information on metastasis. Patients were followed up until October 2009.
FIG. 1.
Consort diagram.
After radical prostatectomy, patients were generally followed with PSA measurements every 2–4 months for the first year, every 3–6 months for the second year, and every 6–12 months thereafter. After biochemical recurrence, PSA was measured every 3–6 months. Distant metastases were identified using radiographic studies (CT, MRI) and/or nuclear imaging (bone scans). Imaging studies were ordered at the discretion of the treating physician based on increasing PSA values and/or clinical signs and symptoms. Because the database captured information from many treating physicians at multiple institutions, follow-up protocols were not always uniform.
Metastatic disease was defined as the presence of osseous metastases visualized on bone scan (or MRI scan); and/or visceral (liver, lung, brain) or extrapelvic nodal metastases visualized on CT scans. MFS was defined as the interval from PSA recurrence to initial metastasis or death. Patients were captured at the time of their first positive scan or censored at the time of their last confirmed negative scan. OS was defined as the interval from PSA recurrence to death from any cause. PSADT was calculated using the natural log of 2 divided by the slope of the linear regression line of the natural log of PSA against time (months) [13]. All PSA values ≥0.2 ng/mL obtained within 24 months after biochemical recurrence were used. A minimum of two PSA values collected ≥3 months apart were required. Because no patient received salvage therapy upon biochemical recurrence, PSADT determinations were not influenced by treatment.
Comparisons between patient subgroups were performed using chi-squared tests for categorical data and t-tests for continuous data. Age at prostatectomy, preoperative PSA (logarithmically transformed) and time to PSA recurrence were treated as continuous variables. Race (white, African American, other), pathological stage (T1–T2, T3–T4), Gleason sum (4–7, 8–10), degree of local extension (organ-confined, extracapsular extension, seminal vesicle invasion), surgical margin status (positive, negative) and PSADT (<3, 3–8.9, ≥9 months) were considered as categorical variables.
Risk factors for metastasis and death were examined using Cox proportional hazards models. Univariate exploratory analyses showed that grouping Gleason score as 4–7 vs 8–10, and time from surgery to biochemical recurrence as ≤3 vs >3 years, maximized the likelihood ratio chi-squared for both metastasis and death; therefore, these groupings were used in the multivariate models. For multivariate analyses, Cox proportional hazards regressions were used. Variables considered for entry into the model included age, race, preoperative PSA, pathological stage, operative Gleason score, surgical margin status, time to biochemical recurrence and PSADT. Only those variables with P-values from the univariate model of ≤0.20 entered the multivariate analysis. The proportional hazards assumption of the Cox model was tested by graphical examination of the log minus-log plots of the variables used in the model. These plots formed approximately parallel straight lines as required.
To identify clinically relevant patient subpopulations, we investigated several thresholds of Gleason score and time to PSA relapse for their differential effects on MFS and OS. Strongest statistical separation between men who did and did not develop metastasis or death was observed when the Gleason sum was dichotomized at ≤7 vs 8–10, and when the time to biochemical relapse was dichotomized at ≤3 vs >3 years. We also explored various PSADT thresholds by dividing patients into subgroups based on 3-month PSADT increments: <3.0, 3.0–5.9, 6.0–8.9, 9.0–11.9 months, etc. Men with a PSADT ≥24 months were all combined into one group. PSADT groups were then examined in multivariate Cox analysis and groups with statistically similar hazard ratios were combined. This resulted in three distinct PSADT categories: <3.0, 3.0–8.9 and ≥9.0 months.
MFS and OS probabilities were estimated for the whole cohort and within strata of prognostic factors using unadjusted Kaplan–Meier analysis. Survival curve differences were evaluated using the log-rank test. Kaplan–Meier plots used the time of PSA recurrence as time zero. Median, 5-, 10- and 15-year MFS and OS estimates were derived from Kaplan–Meier curves. The 95% CIs were calculated as described previously [19]. In some subgroups, the upper confidence limit for median survival was not computable and was represented as >tmax, where tmax was the maximum survival time observed for that subgroup. P < 0.05 was considered statistically significant and analyses were performed using SAS, version 9.1 (SAS Institute Inc., Cary, NC, USA).
RESULTS
The clinical features of all patients with PSA-recurrent prostate cancer forming the cohort in the present study are summarized in Table 1. Most patients had pathologically organ-confined disease and Gleason scores ≤7. Of the 346 men with OS data, mean (median) follow-up after biochemical recurrence was 9.0 (8.6) years, and mean (median) time from surgery to PSA recurrence was 3.1 (2.0) years. At last follow-up, 63 of 346 patients (18.2%) had died. Among the 190 men with MFS data, the mean (median) follow-up after biochemical recurrence was 7.9 (7.5) years, and mean (median) time from surgery to PSA recurrence was 2.9 (2.1) years. At last follow-up, 39 of 190 patients (20.5%) had developed metastasis. The median PSA value at the time of initial metastasis was 27.6 ng/mL.
TABLE 1.
Patient characteristics of men with PSA-recurrent prostate cancer after radical prostatectomy, representing a study period from May 1983 to November 2008
| Characteristic | Men with OS data (n = 346) | Men with MFS data (n = 190) |
|---|---|---|
| Age at surgery (years) | ||
| Mean | 63.7 | 63.9 |
| SD | 6.7 | 6.5 |
| Race, n (%) | ||
| White | 249 (72.0) | 134 (70.5) |
| African-American | 69 (20.0) | 43 (22.6) |
| Other | 28 (8.0) | 13 (6.8) |
| Preoperative PSA (ng/mL) | ||
| Median | 6.7 | 6.7 |
| Range | 0.1–499.0 | 0.1–499.0 |
| Pathological stage, n (%) | ||
| T2 | 156 (45.1) | 77 (40.5) |
| T3–T4 | 190 (54.9) | 113 (59.5) |
| Pathological Gleason sum, n (%) | ||
| 4–7 | 302 (87.3) | 161 (84.7) |
| 8–10 | 44 (12.7) | 29 (15.3) |
| Local extension, n (%) | ||
| Organ-confined disease | 156 (45.1) | 77 (40.5) |
| Extraprostatic extension | 150 (43.4) | 88 (46.3) |
| Seminal vesicle invasion | 40 (11.6) | 25 (13.2) |
| Surgical margin status, n (%) | ||
| Positive | 169 (48.8) | 93 (48.9) |
| Negative | 177 (51.2) | 97 (51.1) |
| Time to PSA recurrence (years) | ||
| Mean | 3.1 | 2.9 |
| Range | 0.1–17.0 | 0.1–17.0 |
| PSA doubling time, n (%) | ||
| <3.0 months | 7 (2.0) | 7 (3.7) |
| 3.0–8.9 months | 59 (17.1) | 38 (20.0) |
| ≥9.0 months | 280 (80.9) | 145 (76.3) |
Data on overall survival (OS) were available for 346 men, and data on metastasis-free survival (MFS) were available for 190 men.
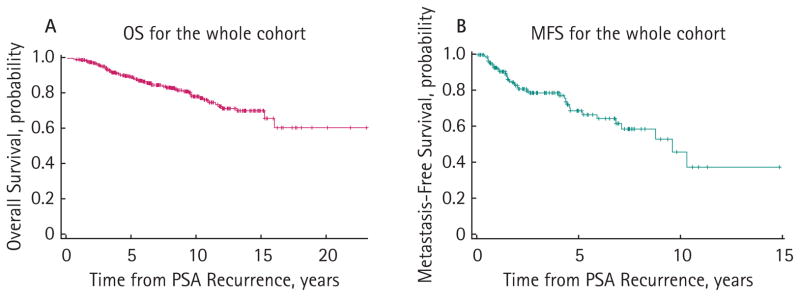
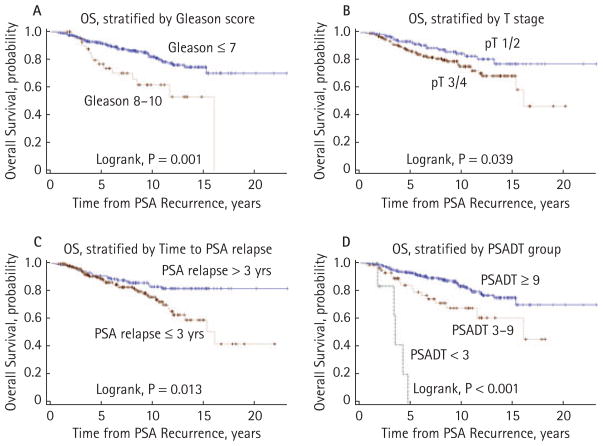
Median OS after PSA recurrence was >23.0 years (95% CI, 16.1 to >23.0 years) for the overall cohort (n = 346; Fig. 2). Five-, 10-, and 15-year OS probabilities were 89.7% (95% CI, 85.8% to 92.6%), 79.3% (95% CI, 73.4% to 84.0%) and 71.5% (95% CI, 63.8% to 77.9%), respectively. Univariate proportional hazards models showed significant associations with OS for age at prostatectomy, surgical Gleason sum, pathological stage, time to biochemical recurrence and PSADT (Table 2). Figure 3 shows unadjusted Kaplan–Meier OS curves stratified by Gleason score (≤7 vs 8–10, log-rank P = 0.001), pathological stage (T1–T2 vs T3–T4, log-rank P = 0.039), time to PSA relapse (>3 vs ≤3 years, log-rank P = 0.013), and PSADT (<3.0 vs 3.0–8.9 vs ≥9.0 months, log-rank P < 0.001). On multivariate analysis, only age at surgery (P = 0.001) and PSADT (<3.0 vs 3.0–8.9 vs ≥9.0 months, P < 0.001) emerged as significant independent predictors of OS (Table 2).
FIG. 2.
Kaplan–Meier estimates of (A) overall survival (OS) and (B) metastasis-free survival (MFS) following PSA recurrence after radical prostatectomy. OS status was available for 346 patients, and MFS status was available for 190 patients.
TABLE 2.
Cox proportional hazards models for predicting overall survival (OS) and metastasis-free survival (MFS) after PSA recurrence following radical prostatectomy
| Variables | OS
|
MFS
|
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate model
|
Multivariate model
|
Univariate model
|
Multivariate model
|
|||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age at surgery (years) (continuous) | 1.10 (1.05–1.15) | <0.001 | 1.09 (1.03–1.14) | 0.001 | 1.02 (0.97–1.07) | 0.493 | – | – |
| Race | ||||||||
| White | 1 (reference) | 1 (reference) | 1 (reference) | |||||
| Non-white | 0.66 (0.35–1.23) | 0.189 | 0.88 (0.45–1.70) | 0.704 | 0.71 (0.31–1.61) | 0.409 | – | – |
| Preoperative PSA (ng/mL) (continuous) | 1.00 (0.99–1.01) | 0.770 | – | – | 0.99 (0.95–1.02) | 0.450 | – | – |
| Pathological Gleason sum | ||||||||
| ≤7 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| 8–10 | 2.56 (1.43–4.58) | 0.002 | 1.42 (0.73–2.77) | 0.297 | 2.17 (1.09–4.30) | 0.027 | 1.76 (0.85–3.67) | 0.129 |
| Pathological stage | ||||||||
| T2 | 1 (reference) | 1 (reference) | 1 (reference) | |||||
| T3–T4 | 1.75 (1.02–2.98) | 0.042 | 1.62 (0.93–2.83) | 0.088 | 1.94 (0.49–1.78) | 0.841 | – | – |
| Surgical margin status | ||||||||
| Negative | 1 (reference) | 1 (reference) | ||||||
| Positive | 1.23 (0.75–2.02) | 0.420 | – | – | 1.15 (0.61–2.15) | 0.674 | – | – |
| Time to PSA recurrence | ||||||||
| >3 years | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| ≤3 years | 1.93 (1.14–3.27) | 0.015 | 1.50 (0.85–2.63) | 0.158 | 1.89 (0.99–3.60) | 0.052 | 1.28 (0.66–2.50) | 0.470 |
| PSA doubling time | ||||||||
| ≥9.0 months | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| 3.0–8.9 months | 2.18 (1.22–3.89) | 0.008 | 1.95 (1.04–3.66) | 0.039 | 6.69 (3.33–13.45) | <0.001 | 6.16 (3.00–12.64) | <0.001 |
| <3.0 months | 17.90 (6.71–47.75) | <0.001 | 7.77 (2.65–22.76) | <0.001 | 33.6 (11.1–101.8) | <0.001 | 27.4 (8.70–86.38) | <0.001 |
HR, hazard ratio. Bold indicates statistically significant P-value.
FIG. 3.
Kaplan–Meier estimates of overall survival (OS), stratified by (A) surgical Gleason sum, (B) pathological T stage, (C) time to PSA relapse and (D) PSA doubling time.
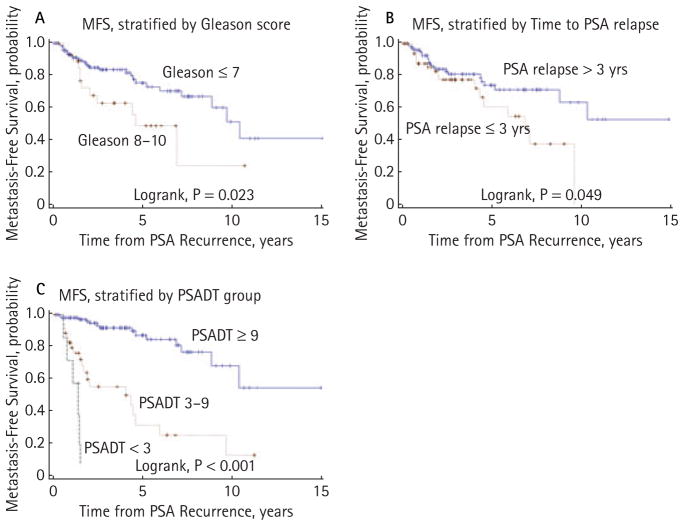
Median MFS after PSA recurrence was 9.6 years (95% CI, 7.1 to >15.0 years) for the overall cohort (n = 190; Fig. 2). Five- and 10-year MFS probabilities were 70.1% (95% CI, 59.4% to 78.5%) and 40.0% (95% CI, 19.8% to 59.6%), respectively. Univariate Cox analysis showed significant associations with MFS for pathological Gleason sum, time to PSA relapse and PSADT (Table 2). Figure 4 shows unadjusted Kaplan–Meier MFS curves stratified by Gleason score (≤7 vs 8–10, log-rank P = 0.023), time to PSA recurrence (>3 vs ≤3 years, log-rank P = 0.049) and PSADT (<3.0 vs 3.0–8.9 vs ≥9.0 months, log-rank P = 0.001). However, only PSADT (<3.0 vs 3.0–8.9 vs ≥9.0 months, P < 0.001) remained independently predictive of MFS in multivariate regressions (Table 2).
FIG. 4.
Kaplan–Meier estimates of metastasis-free survival (MFS), stratified by (A) surgical Gleason sum, (B) time to PSA relapse and (C) PSA doubling time.
Table 3 lists OS and MFS estimates (median, 5-year, 10-year, and 15-year rates) stratified by the three statistically discreet PSADT categories. Patients with a PSADT after biochemical recurrence of ≥9.0 months are at lowest risk of metastatic progression and death, whereas those with a PSADT of 3.0–8.9 months are at intermediate risk of metastasis and death. Those with a PSADT of <3.0 months are at highest risk for both. Notably, men with a PSADT of 3.0–8.9 months have an almost twofold increased risk of death and a sixfold increased risk of metastatic progression compared to men with a PSADT of ≥9.0 months. More strikingly, men with a PSADT of <3.0 months have an almost eightfold increased risk of death and a 27-fold increased risk of metastasis than men with a PSADT of ≥9.0 months.
TABLE 3.
Overall survival (OS) and metastasis-free survival (MFS) after PSA recurrence, stratified by PSA doubling time
| Overall survival (OS)
|
Metastasis-free survival (MFS)
|
||||||
|---|---|---|---|---|---|---|---|
| PSADT <3 months (n = 7) | PSADT 3–9 months (n = 59) | PSADT ≥9 months (n = 280) | PSADT <3 months (n = 7) | PSADT 3–9 months (n = 38) | PSADT ≥9 months (n = 145) | ||
| Median OS (years) | 3.4 | 16.1 | >23.0* | Median MFS (years) | 1.4 | 4.0 | >15.0* |
| 95% CI | (3.3–4.7) | (11.5, >23.0) | (NA, >23.0) | 95% CI | (0.7, 1.5) | (1.7–5.9) | (8.8, >15.0) |
| OS rate (%) at 5 years | 0 | 82 | 93 | MFS rate (%) at 5 years | 0 | 31 | 87 |
| 95% CI | (0.00–0.60) | (0.67–0.90) | (0.89–0.95) | 95% CI | (0.0–0.56) | (0.12–0.52) | (0.76–0.94) |
| OS rate (%) at 10 years | NA | 68 | 83 | MFS rate (%) at 10 years | NA | 12 | 54 |
| 95% CI | NA | (0.51–0.80) | (0.77–0.88) | 95% CI | NA | (0.1–0.38) | (0.24–0.78) |
| OS rate (%) at 15 years | NA | 46 | 75 | MFS rate (%) at 15 years | NA | NA | NA |
| 95% CI | NA | (0.17–0.71) | (0.66–0.82) | 95% CI | NA | NA | NA |
In each subgroup, the median OS/MFS as well as the 5-, 10- and 15-year probability of OS/MFS from the time of PSA recurrence are shown. PSADT, prostate-specific antigen doubling time; NA, not applicable.
Median survival not reached.
DISCUSSION
The management of PSA-recurrent prostate cancer after local therapy remains a grey area in urological oncology. Although it is generally accepted that treatment with ADT is the standard of care for metastatic disease [20,21], optimal management of patients with a rising PSA alone is unclear [3,4,8]. Therefore, identifying and treating only those patients at highest risk of metastasis and death would likely have the greatest impact on patient outcomes. However, to best describe the natural history of the disease in these patients, it is necessary to study a cohort of men whose course is not altered by additional therapies between the time of biochemical recurrence and the development of metastasis. This type of natural history analysis has only been conducted by one other group, using the Johns Hopkins Master Prostatectomy Database [13–16].
The results of the present study serve to confirm and validate the data reported by the Johns Hopkins investigators. In the present study, we show that in a population of patients with PSA relapse after prostatectomy, 5- and 10-year MFS rates are 70% and 40%, respectively, whereas 5- and 10-year OS rates are 90% and 79%, respectively. These estimates closely match those from the Johns Hopkins database, where 5- and 10-year MFS rates were reportedly 68% and 50%, respectively [14], whereas 5- and 10-year OS rates were reportedly 94% and 72%, respectively [16]. Although the present cohort is slightly smaller than the Johns Hopkins cohort (346 vs 379 patients) [16], it still represents the second largest population of men with untreated PSA-recurrent disease until metastasis. In addition, the length of follow-up after biochemical recurrence in the present study is slightly longer than that reported by the Johns Hopkins investigators (median 8.6 vs 7.3 years) [16].
When examining risk factors for metastasis and death in the present study, only the PSADT emerged as an independently prognostic indicator for both outcomes on multivariate analysis. This finding was in contrast to the Johns Hopkins data, where pathological Gleason score and time to PSA relapse were also independently associated with MFS [13] (although the latter was not confirmed in the updated analysis [14]) and OS [15]. This discrepancy may be a true difference or it may be the result of a smaller sample size in the present study and fewer events (metastases and deaths). However, it should be noted that other studies have also reported on the prognostic utility of Gleason score [2,22,23] and the time to PSA recurrence with respect to MFS and OS [24–27], in addition to the PSADT [22,24,28–30]. In all of these studies, patients treated with ADT before the development of metastases were not excluded from analysis; therefore, these cohorts are not directly comparable to the present population.
A notable strength of the present study is that the patients captured in the CPDR database were very ethnically diverse: 72% were white, 20% were African American and 8% were of other ethnicities. This demographic diversity is in contrast to the Johns Hopkins cohort, which was comprised of only 10% non-white patients [14,15]. In addition, the CPDR database reflects contributions from six separate institutions harbouring a variety of practice patterns. By contrast, the Johns Hopkins database contains only information on patients treated at a highly specialized academic institution and may not reflect the range of practice patterns across the USA. For these reasons, we deemed it important to validate the Johns Hopkins natural history data using a large multicentre database that is perhaps more representative of the broader population.
The results obtained in the present study are important because they confirm that, even in the absence of additional therapy before metastasis, men with PSA-recurrent prostate cancer generally have prolonged MFS and OS. These outcomes are greatly influenced by PSADT. In addition, we show that, in this population of men, PSADT is the strongest predictor of metastasis and death compared to all other known clinical variables. Moreover, stratifying men according to PSADT categories may help determine those patients with PSA relapse who may require immediate systemic therapy vs those who may be managed with initial observation alone. For example, a man with biochemical recurrence and a PSADT of ≥9 months may reasonably be managed without intervention, whereas a man with a PSADT of <9 months may benefit from early ADT. Men with a PSADT of <3 months may be offered even more aggressive therapy, such as a combination of ADT plus chemotherapy as part of a clinical trial. In addition to their clinical relevance, the data obtained in the present study may also provide the framework for the rational selection of patients, treatments and endpoints for clinical trials involving men with biochemically-relapsed disease. Such clinical trials have been difficult to design as a result of uncertainty about the length of follow-up required. With these data, the duration of follow-up in such studies may now be estimated more reliably, especially if metastatic progression or survival are used as putative endpoints.
The present study has several limitations. First, the patterns of follow-up care were not uniform across all institutions and for all patients, resulting in different intervals between PSA measurements and different scanning frequencies. In addition, some patients did not have any documented postoperative PSA values, and these men were excluded from the analysis. This exclusion may have introduced bias if patients with available PSA information were somehow different from those without PSA data. Second, the database did not document the cause of death for each patient. Therefore, it was not possible to distinguish between cancer-related deaths and non-cancer-related deaths. For this reason, prostate cancer-specific survival could not be estimated. Third, the overall numbers of patients and events (metastases and deaths) in the present dataset are small, especially at longer follow-up times. Consequently, estimates of median, 5-, 10- and 15-year MFS and OS may lack precision and often have wide confidence intervals (especially in several subgroups shown in Table 3). These facts should be taken into consideration by physicians when using these estimates in clinical practice. Finally, stratifications of the clinical prognostic variables were based on analysis of optimal thresholds (i.e. thresholds that maximized the survival difference). This approach is known to be associated with increased type-I error and the possibility of over-fitting [31].
In summary, the present analysis of the CPDR database shows that, among patients with PSA-recurrent prostate cancer after prostatectomy, MFS and OS may be extensive but variable. These outcomes are most strongly influenced by PSA doubling time, a variable that can be used to risk-stratify patient into different prognostic groups. These findings validate the Johns Hopkins data, and represent the second largest known cohort of men whose therapy was delayed until after the development of metastasis.
What’s known on the subject? and What does the study add?
Patients who develop biochemical (PSA) recurrence after prostatectomy may subsequently develop metastases leading to their eventual death. However, many such patients receive additional therapies before developing metastases, so the natural history of disease progression in these patients is poorly described. We aimed to report metastasis-free survival and overall survival for such patients using a multicentre database.
We present data on the natural history of metastatic progression and survival for men treated with radical prostatectomy who did not receive additional adjuvant or salvage therapies before developing metastases. Even in the absence of subsequent therapies, we demonstrate that metastasis-free survival and overall survival may be prolonged but are variable. We confirm that PSA doubling time is the strongest prognostic factor determining risk of metastasis and death in men with biochemically-recurrent prostate cancer.
Acknowledgments
The present study was supported by the Department of Defense and the Congressional Special Interest biomedical research programme. Views and opinions of, and endorsements by, the author(s) do not reflect those of the US Army or the Department of Defense.
Abbreviations
- ADT
androgen deprivation therapy
- CPDR
Center for Prostate Disease Research
- MFS
metastasis-free survival
- OS
overall survival
- PSADT
prostate-specific antigen doubling time
Footnotes
CONFLICT OF INTEREST
None declared. Source of Funding: the present study was supported by the Department of Defense and the Congressional Special Interest (CSI) biomedical research programme.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910–4. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 3.Boccon-Gibod L, Djavan WB, Hammerer P, et al. Management of prostate-specific antigen relapse in prostate cancer: a European Consensus. Int J Clin Pract. 2004;58:382–90. doi: 10.1111/j.1368-5031.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 4.Sandler HM, Eisenberger MA. Assessing and treating patients with increasing prostate specific antigen following radical prostatectomy. J Urol. 2007;178:S20–4. doi: 10.1016/j.juro.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 5.Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472–9. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- 6.Tenenholz TC, Shields C, Ramesh VR, Tercilla O, Hagan MP. Survival benefit for early hormone ablation in biochemically recurrent prostate cancer. Urol Oncol. 2007;25:101–9. doi: 10.1016/j.urolonc.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Moul JW, Wu H, Sun L, et al. Early versus delayed hormonal therapy for prostate specific antigen only recurrence of prostate cancer after radical prostatectomy. J Urol. 2004;171:1141–7. doi: 10.1097/01.ju.0000113794.34810.d0. [DOI] [PubMed] [Google Scholar]
- 8.Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596–605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 9.Opfermann KJ, Lai Z, Essenmacher L, Bolton S, Ager J, Forman JD. Intermittent hormone therapy in nonmetastatic prostate cancer. Clin Genitourin Cancer. 2006;5:138–43. doi: 10.3816/CGC.2006.n.030. [DOI] [PubMed] [Google Scholar]
- 10.Prapotnich D, Cathelineau X, Rozet F, et al. A 16-year clinical experience with intermittent androgen deprivation for prostate cancer: oncological results. World J Urol. 2009;27:627–35. doi: 10.1007/s00345-009-0393-1. [DOI] [PubMed] [Google Scholar]
- 11.Scher HI, Eisenberger M, D’Amico AV, et al. Eligibility and outcomes reporting guidelines for clinical trials for patients in the state of a rising prostate-specific antigen: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 2004;22:537–56. doi: 10.1200/JCO.2004.07.099. [DOI] [PubMed] [Google Scholar]
- 12.Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy versus observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299:2760–9. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 14.Antonarakis ES, Trock BJ, Feng Z, et al. The natural history of metastatic progression in men with PSA-recurrent prostate cancer after radical prostatectomy: 25-year follow-up. J Clin Oncol. 2009;27(Suppl):abstract 5008. [Google Scholar]
- 15.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–9. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 16.Freedland SJ, Humphreys EB, Mangold LA, et al. Death in patients with recurrent prostate cancer after radical prostatectomy: prostate-specific antigen doubling time subgroups and their associated contributions to all-cause mortality. J Clin Oncol. 2007;25:1765–71. doi: 10.1200/JCO.2006.08.0572. [DOI] [PubMed] [Google Scholar]
- 17.Brassell SA, Dobi A, Petrovics G, Srivastava S, McLeod D. The Center for Prostate Disease Research (CPDR): a multidisciplinary approach to translational research. Urol Oncol. 2009;27:562–9. doi: 10.1016/j.urolonc.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Sun L, Gancarczyk K, Paquette EL, et al. Introduction to Department of Defense Center for Prostate Disease Research (CPDR) multicenter national prostate cancer database, and analysis of changes in the PSA-era. Urol Oncol. 2001;6:203–9. [Google Scholar]
- 19.Dorey FJ, Korn EL. Effective sample sizes for confidence intervals for survival probabilities. Stat Med. 1987;6:679–87. doi: 10.1002/sim.4780060605. [DOI] [PubMed] [Google Scholar]
- 20.Crawford ED, Eisenberger MA, McLeod DG, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321:419–24. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 21.Eisenberger MA, Blumenstein BA, Crawford ED, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339:1036–42. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 22.Okotie OT, Aronson WJ, Wieder JA, et al. Predictors of metastatic disease in men with biochemical failure following radical prostatectomy. J Urol. 2004;171:2260–4. doi: 10.1097/01.ju.0000127734.01845.99. [DOI] [PubMed] [Google Scholar]
- 23.Kim-Sing C, Pickles T Prostate Cohort Outcomes Initiative. Intervention after PSA failure: examination of intervention time and subsequent outcomes from a prospective patient database. Int J Radiat Oncol Biol Phys. 2004;60:463–9. doi: 10.1016/j.ijrobp.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Patel A, Dorey F, Franklin J, deKernion JB. Recurrence patterns after radical retropubic prostatectomy: clinical usefulness of prostate specific antigen doubling times and log slope prostate specific antigen. J Urol. 1997;158:1441–5. doi: 10.1016/s0022-5347(01)64238-1. [DOI] [PubMed] [Google Scholar]
- 25.Chang SL, Freedland SJ, Terris MK, et al. Freedom from a detectable ultrasensitive prostate-specific antigen at two years after radical prostatectomy predicts a favorable clinical outcome: analysis of the SEARCH database. Urology. 2010;75:439–44. doi: 10.1016/j.urology.2009.06.089. [DOI] [PubMed] [Google Scholar]
- 26.Hanlon AL, Diratzouian H, Hanks GE. Post-treatment prostate-specific antigen nadir highly predictive of distant failure and death from prostate cancer. Int J Radiat Oncol Biol Phys. 2002;53:297–303. doi: 10.1016/s0360-3016(02)02717-7. [DOI] [PubMed] [Google Scholar]
- 27.Buyyounouski MK, Hanlon AL, Horwitz EM, Pollack A. Interval to biochemical failure highly prognostic for distant metastasis and prostate cancer-specific mortality after radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:59–66. doi: 10.1016/j.ijrobp.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 28.Zagars GK, Pollack A. Kinetics of serum prostate-specific antigen after external beam radiation for clinically localized prostate cancer. Radiother Oncol. 1997;44:213–21. doi: 10.1016/s0167-8140(97)00123-0. [DOI] [PubMed] [Google Scholar]
- 29.D’Amico AV, Cote K, Loffredo M, Renshaw AA, Schultz D. Determinants of prostate cancer-specific survival after radiation therapy for patients with clinically localized prostate cancer. J Clin Oncol. 2002;20:4567–73. doi: 10.1200/JCO.2002.03.061. [DOI] [PubMed] [Google Scholar]
- 30.D’Amico AV, Moul JW, Carroll PR, Sun L, Lubeck D, Chen MH. Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Natl Cancer Inst. 2003;95:1376–83. doi: 10.1093/jnci/djg043. [DOI] [PubMed] [Google Scholar]
- 31.Keegan PE, Maththews JNS, Lunec J, Neal DE. Statistical problems with ‘optimal’ thresholds in studies of new prognostic factors in urology. BJU Int. 2000;85:392–7. doi: 10.1046/j.1464-410x.2000.00491.x. [DOI] [PubMed] [Google Scholar]