Abstract
Background
Sexually transmitted infections (STIs) are associated with an increased risk of HIV infection. To model the interaction between STIs and HIV infection, we evaluated the capacity of the pigtail macaque model to sustain triple infection with Trichomonas vaginalis, Chlamydia trachomatis, and SHIVSF162P3.
Methods
Seven SHIVSF162P3-infected pigtail macaques were inoculated with T. vaginalis only (n = 2), C. trachomatis only (n = 1), both T. vaginalis and C. trachomatis (n = 2), or control media (no STI; n = 2). Infections were confirmed by culture and/or nucleic acid testing. Genital mucosa was visualized by colposcopy.
Results
Characteristic gynecologic signs were observed for both STIs, but not in control animals. Manifestations were most prominent at days 7–10 post-infection. STIs persisted between 4 and 6 weeks and were cleared with antibiotics.
Conclusions
These pilot studies demonstrate the first successful STI-SHIV triple infection of pigtail macaques, with clinical presentation of genital STI symptoms similar to those observed in humans.
Keywords: coinfection, genital, mucosal, non-human primate
Introduction
Sexually transmitted infections (STIs) are highly prevalent in HIV-positive individuals and in populations at high risk of contracting HIV [1–4]. Additionally, multiple epidemiologic studies have associated STIs with an increased risk of HIV infection [2, 3, 5–8]. The precise underlying mechanism of this association, particularly in the case of non-ulcerative infections, remains unknown. A behavioral component is plausible; however, the recruitment of HIV target cells to the genital mucosa or a shift in the immune milieu of genital secretions because of STI-induced inflammation may also enhance susceptibility to HIV [6, 9, 10]. An animal model appropriate to analyze these susceptibility factors would greatly improve our understanding of the role of STIs in facilitating HIV infection.
In this current study, we describe the development of a pigtail macaque model of STI–HIV coinfection utilizing the bacterium Chlamydia trachomatis and the protozoan Trichomonas vaginalis. Chlamydia trachomatis is one of the most prevalent STIs worldwide [4] and is the most commonly reported bacterial STI in the United States. Incidence of C. trachomatis infection in the United States has risen significantly over the past several years and rates are highest in African Americans, particularly in reproductive-age women [1]. Trichomonas vaginalis is the most common non-viral STI, with approximately 170 million worldwide cases reported annually [4, 8]. Sutton et al., report a T. vaginalis prevalence rate of 3.1% among women in the United States, with African American women having the highest prevalence (13.3%) [1, 11]. Chlamydia trachomatis and T. vaginalis are commonly found to coinfect the female genital tract, and both are also associated with increased risk of HIV infection [5, 7, 8, 12]. Sorvillo et al., [7] suggests trichomoniasis may be a key component driving the increase in HIV incidence among African American women in the United States. We chose to model T. vaginalis and C. trachomatis infections in the setting of SHIV infection in the female pigtail macaque because of reproductive tract similarities to human females and our expertise in genital tract studies in this species [13–18]. Additionally, previous studies by Patton et al. with C. trachomatis and T. vaginalis have demonstrated the pig-tailed macaque is susceptible to infections with these pathogens, alone or in combination [19–24]. Our long-term goal is to evaluate the impact of these STIs on acquisition of SHIV.
In women, C. trachomatis infects the columnar epithelium of the endocervix and cervical transformation zone, with risk of the infection ascending into the upper reproductive tract [10, 25]. In contrast, T. vaginalis is tropic for the stratified epithelium of the vaginal mucosa and is rarely invasive; however, cervical inflammation may be observed [7, 26]. Both infections elicit an inflammatory response in the genital compartment, resulting in the trafficking of inflammatory and immune effector cells to the tissues and the upregulation of inflammatory cytokines [9, 10, 26–28]. Classical clinical/gynecologic presentation of C. trachomatis includes the presence of cervical mucosal erythema, mucopurulent discharge, friability, and edema. The presence of elevated numbers of polymorphonuclear cell infiltrates, as detected by microscopy, and in severe cases, epithelial erosion are also symptomatic of C. trachomatis infection [10, 29, 30]. Classic presentation of T. vaginalis infection includes erythematous, or ‘strawberry’ cervix and a foamy, yellow-green vaginal discharge [26, 31]. We aimed to replicate C. trachomatis– T. vaginalis coinfection in pigtail macaques as reported by Patton et al. [21, 22, 24] and demonstrate genital tract symptoms and manifestations similar to humans.
In this pilot study for model development, we have confirmed not only C. trachomatis–T. vaginalis dual coinfection, but also demonstrated STI-SHIV triple infections in macaques with an established SHIVSF162P3 infection. Data from this novel and relevant triple coinfection model will be utilized in future studies to evaluate mechanisms of enhanced susceptibility to HIV in SHIV-naïve animals and test biomedical HIV prevention strategies in the context of STIs.
Materials and methods
Macaques
Seven female, SHIVSF162P3-positive pigtail macaques (Macaca nemestrina) were utilized in this study. All seven animals were of breeding age, with weights ranging from 6 to 10 kg, and were housed at the Centers for Disease Control and Prevention (CDC). The Institutional Animal Care and Use Committee of the CDC approved all procedures described in this study; procedures were in accordance with standards established in the Guide for the Care and Use of Laboratory Animals (published by the National Academy of Science, National Academy Press, Washington, DC). Macaques were anesthetized with ketamine (10 mg/kg) prior to all procedures. After the study’s conclusion, animals were humanely euthanized with an intravenous dose of pentobarbital (>100 mg/kg). One animal, 96Po17, was euthanized prior to the conclusion of the study because of bacterial hepatitis, a condition deemed unrelated to the study by the attending veterinarian.
The macaques were infected during previous titration studies of SHIVSF162P3 stocks (unpublished data). SHIVSF162P3 (SIVmac239 backbone with HIV-1 clade B envelope [32]) was provided by the NIH AIDS Research and Reference Reagent Program (NARRRP, catalog #6526) and propagated at CDC. Animals were infected using an established repeat low-dose (RLD), intravaginal challenge model [14, 15, 18]. With the exception of one animal, TD6, (challenged with 10 TCID50) animals were inoculated with 50 TCID50 of SHIVSF162P3 virus stock. Median time frame between SHIV infection and STI inoculation was 278 days (approximately 40 weeks; range: 246–358 days).
SHIV RNA levels
To quantify plasma SHIV RNA levels, virions in 1-ml plasma aliquots were pelleted by high-speed centrifugation, and viral RNA was extracted using the NucliSens system, according to manufacturer’s protocol. RNA levels were quantified using an internally controlled and normalized reverse-transcription real-time TaqMan© PCR (Applied Biosystems, Carlsbad, CA, USA) assay with a threshold sensitivity level of 50 copies per sample [33]. Plasma SHIV RNA levels were reported as copies per ml plasma. Secretions for genital SHIV RNA levels were collected from the endocervix and vaginal vault on polyester-tipped swabs, submerged in RNAlater (Applied Biosystems, Carslbad, CA, USA), and processed as previously reported [34]. Viral RNA extraction and quantitation proceeded as described above. Genital SHIV RNA levels were divided by 2 (to account for double swab collection) and evaluated as copies per swab (as much as 400 μl of genital secretions were collected per swab).
CD4+ T cell levels
Blood was obtained from the femoral vein using mononuclear cell preparation tubes (CPT) (Becton Dickinson Biosciences, San Jose, CA, USA), and peripheral blood mononuclear cells (PBMCs) were collected from mononuclear cell fractions after centrifugation. CD4+ T cells were enumerated in freeze–thawed cells by flow cytometry using a FACS Calibur (Becton Dickinson Biosciences, San Jose, CA, USA) and a previously described protocol and antibody panel [35].
Trichomonas vaginalis culture, challenge, and detection
Trichomonas vaginalis strain Balt-42 was utilized in this study because it had previously been shown to enhance HIV infection in an in vitro model [36]. Cultures were propagated in Diamond’s media to high concentration and viability. Macaques were atraumatically inoculated with 6 × 106 viable trichomonads. Because the growth media contains antibiotics potentially disruptive to the vaginal microflora, just prior to challenge, trichomonads were resuspended in 1 ml of 37°C Roswell Park Memorial Institute (RPMI) media and drawn into a 3-cc syringe. Inoculums were then applied to the vaginal mucosa using a sized gastric feeding tube (similar to RLD virus challenge, [14, 15]). Control animals received mock inoculations of 1 ml plain RPMI.
To detect and monitor T. vaginalis infection, vaginal secretion samples were collected twice-weekly on cotton- tipped swabs and used to inoculate an InPouch© Trichomonas culture packet (BioMed Diagnostics, White City, OR, USA) [37, 38]. Cultures were incubated at 37°C and examined by microscopy every 24 hours post-collection for the presence of motile trichomonads. Cultures void of motile trichomonads after 72 hours were deemed negative [37, 38]. After completion of trichomoniasis analyses (14 weeks for TD6; 8 weeks for FH3 and 96Po78), animals were treated with metronidazole (35 mg/kg, once per day, for 3 days). Treatment was not administered to the euthanized animal. Test of cure was conducted 1 week posttreatment to confirm clearance of infection.
Chlamydia trachomatis culture, challenge, and detection
Two serovars of C. trachomatis were utilized. Serovar E (strain UWR109, clinical isolate) was obtained from the Chlamydia Laboratory at the University of Washington. Serovar D (strain D-LC, NCBI accession #CP002054) [39] was provided by Dr. Harlan Caldwell (NIH/NIAID Rocky Mountain Laboratories, MT). For both serovars, macaques were inoculated twice, at once-weekly intervals, with 1 × 106 inclusion-forming units (IFU) in 1 ml sucrose–phosphate–glutamate (SPG) media. Inocula were drawn into a 3-cc syringe and atraumatically applied to the surface of the ectocervix using a sized gastric feeding tube. Control animals received mock inoculations of 1 ml plain SPG media. Chlamydia trachomatis infection was detected and monitored using the APTIMA GenProbe system (San Diego, CA, USA) and confirmed with culture (ReadyCells© Chlamydia Detection System, Athens, OH, USA). After the 8-week study period of Chlamydia cervicitis, animals were cleared of C. trachomatis with azithromycin (14 mg/kg, once per day, for 7 days). Test of cure was conducted 1 week post-treatment via APTIMA testing and confirmed 3 weeks post-treatment by culture.
Characterization of genital tract inflammation
A pediatric speculum and colposcopy were utilized twice-weekly to visualize the genital tract mucosa [40]. Using a standard questionnaire, trained personnel documented the presence/severity of vaginal and cervical erythema, presence/severity, color, and consistency of vaginal and cervical discharge, and presence/severity of cervical erosion in each macaque. In the C. trachomatis- only- and T. vaginalis + C. trachomatis–infected macaques, colposcopic images of the cervix were captured using a Carl Zeiss colposcope (Model # LR66238).
Cervical infiltrate
Cervical cells were collected by inserting a cytobrush (Cooper Surgical, Trumbull, CT, USA) into the cervical os and gently rotating 360° twice. Samples were placed in RPMI 1640 supplemented with gentamicin and amphotericin B and processed within 4 hours of collection [41, 42]. Cells were eluted from the cyto-brush and enumerated by microscopy utilizing an Endtz-trypan stain to differentiate polymorphonuclear cells (PMNs), mononuclear cells, epithelial cells, and red blood cells [43]. Greater than or equal to 100 cells were enumerated from each sample, and each cell type was normalized to a percentage of the total population [41].
Mucosal IFN-gamma analyses
Endocervical secretions were collected on Merocel ophthalmic sponges (Medtronic, Jacksonville, FL, USA) and extracted using a protocol and buffer described by Castle et al. [44]. IFN-gamma levels were determined using the Monkey Cytokine 5-Plex Panel, according to manufacturer’s protocol (Invitrogen, Cat # LPC0001, Carlsbad, CA, USA). Levels were normalized to collected secretion weight and evaluated as pg IFN-gamma per ml secretion.
Histopathology
Using standard biopsy forceps, vaginal and cervical pinch biopsies were collected pre-/post-STI inoculation and stored in 10% buffered formalin. Tissues were paraffin-embedded, thin-sectioned, H&E stained, and analyzed by a veterinary pathologist for epithelial integrity and infiltrate intensity using an Olympus BX41 light microscope. Biopsies were collected at baseline and then at 7 weeks post-inoculation from macaques FH3, 96Po25, 96Po58, and 96Po78. From macaques TD6 and 96Po26, biopsies were collected 4.5 weeks post-inoculation. Biopsies were collected from macaque 96Po17 at necropsy, 3.8 weeks post-inoculation.
Results
The median time from SHIVSF162P3 infection to STI (or mock) inoculation was 278 days (40 weeks), and the time frame of STI infection follow-up ranged from 32 to 98 days (4.5–14 weeks) (Table 1). At the time of STI inoculation, CD4+ T cells levels were within normal levels of SHIV-infected and uninfected animals in our colony; CD4+ T cell levels did not change significantly throughout the course of the study (data not shown).
Table 1.
Study overview: time frame of infection and gynecologic sign presentation
| Experiment Arm/Macaque | Time (days) between SHIV+ and STI inoculation | STI infection time frame (days) | Gynecologic sign presentation1
|
||
|---|---|---|---|---|---|
| Cervical | Vaginal | Time frame2 | |||
| Trichomonas vaginalis only | |||||
| TD6 | 358 | 983 | Erythematous mucosa; ‘strawberry cervix’ | Yellow-green, foamy discharge |
Initial: 4 dpi Peak: 7 dpi Persist: until treatment (gradual decrease in severity) |
| 96Po17 | 278 | 324 | Erythematous mucosa; ‘strawberry cervix’ | Yellow-green, foamy discharge |
Initial: 4 dpi Peak: 7 dpi Persist: n/a4 |
| Chlamydia trachomatis only | |||||
| 96Po58 (E) | 281 | 563 | Erythematous mucosa with punctate erosion; thick, semi-transparent purulent discharge | Mild-to-moderate clear discharge |
Initial: 7 dpi Peak: 10 dpi Persist: until treatment; persistent erythema, gradual decrease in discharge |
| T. vaginalis + C. trachomatis | |||||
| FH3 (E) | 2915 | 563 | Edematous and severely erythematous mucosa with blistering erosion; thick, opaque bubbling discharge originating from os | Moderate mucosal erythema; moderate semi-transparent, foamy discharge |
Initial: 7 dpi Peak: 10 dpi Persist: until treatment; persistent erythema, gradual decrease in discharge |
| 96Po78 (D) | 2775 | 563 | Edematous and severely erythematous mucosa with blistering erosion; thick, opaque bubbling discharge originating from os | Severe mucosal erythema; severe, milky, foamy discharge |
Initial: 4 dpi Peak: 10 dpi Persist: until treatment; both erythema and discharge consistent until treatment |
| Controls | |||||
| 96Po25 | 2636 | n/a | Pink (normal) mucosa; normal, menstrual cycle-related discharge | Pink-to-pale pink (normal) mucosa; scant, clear discharge (normal) | n/a |
| 96Po26 | 2466 | n/a | Pink (normal) mucosa; normal, menstrual cycle-related discharge | Pink (normal) mucosa; scant, clear discharge (normal) | n/a |
| Median = 278 | Median = 56 | ||||
dpi, days post-infection; STI, sexually transmitted infection.
Signs most predominant at 7–10 dpi.
Time frame—assessments limited by biweekly access to animals.
Appropriate treatment (T. vaginalis—metronidazole, C. trachomatis—azithromycin) administered to resolve infection.
Animal euthanized.
With respect to 1st C. trachomatis inoculation (2nd C. trachomatis + T. vaginalis given 1 week later).
Between SHIV+ and mock inoculation.
All STI-inoculated macaques (n = 5) were successfully infected with T. vaginalis alone (n = 2), C. trachomatis alone (n = 1), or C. trachomatis and T. vaginalis (n = 2). Two macaques were utilized as STI-negative controls, mock-infected as previously described. Colposcopy was used to visualize the cervicovaginal compartment and characterize the genital mucosa and discharge. In macaques infected with T. vaginalis only, characteristic manifestations of ‘strawberry cervix’ and green, foamy discharge were observed (Table 1). Similarly, characteristic gynecologic signs were observed in the macaque infected with C. trachomatis only, such as cervical erythema, blistering and erosion of the cervical mucosa, and mucopurulent discharge (Table 1). Signs were most overt in the dual STI-infected macaques. In addition to foamy vaginal discharge, increased severity of erythema and erosion, as well as mucosal edema, was also noted (Table 1). Manifestations were most prominent at days 7–10 post-inoculation, although erythema and discharge persisted to varying degrees in all STI-infected animals throughout the study course. Discharge persisted longer and was more consistently observed than mucosal erythema. These observations were compared with mock media-inoculated animals, in which normal genital mucosa and discharge were observed (Table 1).
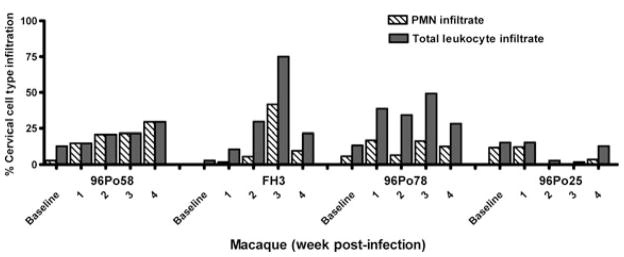
Cervical cell infiltrate collected by cytobrush sampling was examined at different time points throughout the study in the C. trachomatis-only-, dual STI-infected animals, and mock media-inoculated animal. (Fig. 1). An increase in PMNs, relative to baseline, was observed in all STI-infected macaques, rising as high as 42% in one animal (FH3), and peaked at approximately 2–3 weeks post-infection. A similar increase was observed in the percentage of total leukocyte infiltrate (PMNs + mononuclear cells). Increased levels of cell infiltrate were observed only in STI-infected macaques. The number of animals in this pilot study was not powered to achieve statistical significance in these analyses.
Fig. 1.
Relative increase in inflammatory cells in cervical cell collections of sexually transmitted infection (STI)-infected macaques. Analyses were conducted in Chlamydia trachomatis-only (96Po58)- and C. trachomatis + Trichomonas vaginalis–infected macaques (FH3 and 96Po78). 96Po25 received mock media inoculations (control). Cell populations were collected by cervical cytobrush sampling and enumerated by microscopy utilizing an Endtz-trypan differential stain. The graph longitudinally depicts the percent of cervical infiltrate cell types present at baseline and weeks 1–4 post-infection (week 1, relative to first C. trachomatis inoculum). An increase in the percentage of inflammatory cells consisting of polymorphonuclear (PMN) cells and/or total leukocyte infiltrate, relative to baseline, was observed in all three sexually transmitted infection (STI)-infected macaques over the course of the study.
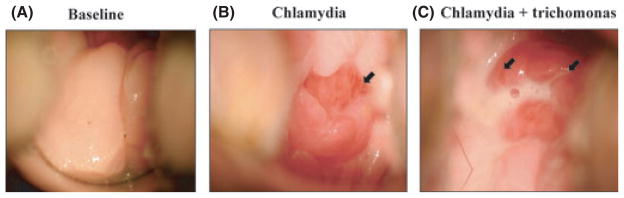
Cervical colposcopic images from a representative animal (FH3), which was dual STI-infected, are shown in Fig. 2A–C. Compared with baseline, vascularization and edema of the cervix visible by day 7 (post-administration of 1st C. trachomatis inoculums, Fig. 2B) and manifestations worsen by day 10 (post-administration of T. vaginalis and the 2nd C. trachomatis inoculums, Fig. 2C). Pronounced edema and discharge, severe erythema, and points of blistering erosion are visible (Fig. 2). These gynecologic signs are analogous to those documented in women for both C. trachomatis and T. vaginalis infections.
Fig. 2.
Cervical colposcopic images of a representative macaque (FH3) (10 × magnification). (A) Cervical mucosa (ectocervix) at baseline, characterized by normal, pink coloration, and presence of scant, clear normal menstrual-related discharge. (B) Cervical mucosa 7 days after administration of 1st Chlamydia trachomatis inoculum (1 × 106 IFU). Erythema, tissue vascularization (arrow), edema, and presence of mucopurulent discharge noted during visual inspection. (C) Cervical mucosa 4 days after administration of second C. trachomatis (1 × 106 IFU) and Trichomonas vaginalis (6 × 106 trichomonads) inoculums; 10 days post-infection, relative to 1st C. trachomatis inoculation. Findings include severe erythema and edema, areas of blistering erosion (arrows), and opaque, bubbly, mucopurulent discharge originating from the cervical os. IFU, inclusion-forming units.
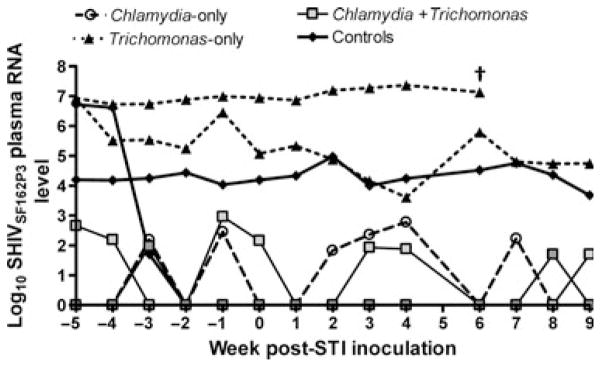
Plasma SHIV RNA levels were monitored throughout the study course, but did not fluctuate in response to STI inoculation/infection (Fig. 3). Genital SHIV RNA analysis was also undertaken, but conclusions were limited because of infrequent sample collection (data not shown).
Fig. 3.
No impact of sexually transmitted infections on plasma SHIVSF162P3 RNA levels. No differences in plasma SHIV RNA levels (y-axis) was observed in response to Chlamydia trachomatis (96Po58), Trichomonas vaginalis (TD6 and 96Po17), or C. trachomatis + T. vaginalis infection (FH3 and 96Po78) (Controls: 96Po25 and 96Po26) (x-axis). Threshold sensitivity level = 50 copies per sample. SHIV RNA levels reported at copies per ml plasma. †Animal euthanized for reasons unrelated sexually transmitted infection (STI) inoculation/infection.
Infections were observed a minimum of 32 days (4.6 weeks) to 98 days (14 weeks) before STI treatments were administered. One animal, 96Po17, was euthanized on day 32 of the study, prior to treatment, with a diagnosis of bacterial hepatitis; however, the attending veterinarian determined cause of death was unrelated to T. vaginalis infection. The remaining STIinfected macaques successfully cleared infections with the respective, appropriate treatment(s).
Infections with both C. trachomatis serovars D and E were tested and compared in this study, albeit with small animal numbers. Macaque 96Po58 was infected with serovar E only, whereas macaques 96Po78 and FH3 were both dual-infected with T. vaginalis and C. trachomatis serovars D and E, respectively (Table 1). More prolonged erythema and discharge were noted in the serovar D/Trichomonas-infected animal compared with the serovar E/Trichomonas-infected animal. Additionally, up to 500-fold higher levels of mucosal IFN-gamma were observed in the serovar D-infected animal between days 4 and 14 post- STI infection, compared with the serovar E-infected animal (data not shown). From the pinch biopsy sampling, increased vaginal histopathology (leukocyte infiltrate and epithelial sloughing) was also noted in the serovar D-infected animal (data not shown). Cervical histopathologic changes were not detected in any of the STI-infected animals (data not shown).
Discussion
These analyses are the first to demonstrate the triple infection of pigtail macaques with C. trachomatis, T. vaginalis, and SHIV. Chlamydia trachomatis and T. vaginalis infections in this study demonstrated characteristic profiles of erythema, discharge, inflammatory cervical infiltrate, and/or cervical mucosal disruption. Such gynecologic symptoms and manifestations are analogous to those documented in women diagnosed with Chlamydia cervicitis and/or trichomoniasis. [10, 26, 29, 31]. These pathogens were chosen because of their high prevalence and association with increased risk of HIV infection, as reported particularly in minority populations in the United States [1, 11]. Thus, we maintain this is a relevant model of STI coinfection which can later be adapted to study the effects of these infections on SHIV susceptibility.
Although C. trachomatis may cause cervical mucosal erosion, both C. trachomatis and T. vaginalis are considered non-ulcerative pathogens. However, their infections are characterized by increased cell infiltration to the genital mucosa [29, 31]. An advantage of a non-ulcerative model is that it will primarily address mechanistic issues related to lymphocyte and inflammatory cell infiltrates in the genital mucosa without the mechanical disruption of the epithelium, as has previously been modeled with herpes viral infections [45]. Thus, this model will allow us to focus on the question of whether STI-induced lymphocyte infiltration increases susceptibility to HIV.
We aim to utilize C. trachomatis serovar D in the next phase of analyses utilizing SHIV-naïive animals. Our preliminary data (IFN-gamma levels, histopathology, persistence of erythema and discharge, and consistency in levels of PMN/leukocyte infiltrate) from these pilot studies suggest C. trachomatis serovar D may elicit a more heightened inflammatory response than serovar E. This cannot be concluded with certainty because comparisons were made between only two animals. However, these data are consistent with previous work characterizing the same serovar D strain, which yielded similar results in mice [39].
This pilot study was conducted in a small number of SHIV-infected animals, but was necessary to develop methodologies and train personnel in new laboratory techniques and approaches. Because of limited availability and cost of SHIV-naïve female pigtail macaques, we chose to utilize SHIV-positive animals that had been used in previous virus titration studies to demonstrate that pigtail macaques may be triply infected with C. trachomatis, T. vaginalis, and SHIVSF162P3. SHIV infection is a confounding factor, and it is possible these infections may have been less likely in SHIV-negative macaques. However, we contend this is unlikely because of the previous published work of Patton et al., indicating these infections are achievable in SHIV-negative/naïve macaques, with similar inoculation doses, both singly and as a dual coinfection [21, 23, 24]. It is also possible the gynecologic symptoms may be more pronounced in these animals than what might be observed in SHIV-negative macaques. Indeed, a strawberry cervix was observed in both T. vaginalis-only-infected macaques, whereas this is seen in <50% of T. vaginalis-infected women [46]. Also, despite these triple infections, the animals were clinically well, with localized infections, no alterations in CD4+ or plasma SHIV RNA levels, and the STIs were easily treated with metronidazole or azithromycin.
Our model of STI followed by SHIV infection will incorporate a repeat low-dose intravaginal SHIV challenge modality which closely mimics mucosal HIV transmission [14–16]. This study design requires that STIs would need to persist throughout the period of repeated virus challenge. In these pilot analyses, infections were maintained for a minimum of 4.6 weeks and for as long as 14 weeks in one T. vaginalis-infected macaque (Table 1). This time frame, even at the minimum, would provide ample time for repeat virus challenges.
The development and future utilization of this model provides opportunities to better understand the role of STIs in HIV transmission. However, this model will also be valuable for areas of HIV prevention research. The efficacy of intervention strategies currently under investigation, such as vaginal rings, topical microbicides, and even oral PrEP modalities, could potentially be affected by the presence of STIs or STI-induced inflammation in the genital tract mucosa. This is a key consideration given the high prevalence of STIs in individuals at risk of HIV infection [2, 4]. The availability of an STI–HIV coinfection model will provide another platform for the rigorous evaluation of biomedical interventions safety and efficacy and enhance their ability to be translated into clinical practice.
Acknowledgments
We acknowledge Peter Augostini and Chen Cheng for their expertise and assistance with T. vaginalis culture and trichomonad analyses, respectively. We appreciate the input of Dr. Ronald Ballard on these STIs and study design. We also thank Carol Farshy for her assistance with C. trachomatis Gen-Probe APTIMA assays. We are grateful for the input of Drs. Katherine Paul and Lindsay Livingston in designing the macaque experiments and the assistance of Shanon Bachman in performing the procedures. Many thanks are given to Wei Luo, Debra Adams, Patricia Guenthner, and Dr. Ronald Otten for technical assistance and expertise in generating the SHIVSF162P3 stock. We acknowledge Eileen Breding and Dr. Prachi Sharma of the Yerkes National Primate Center for their assistance with histopathologic tissue analyses. We also thank Dr. James Smith for insightful scientific discussion of these experiments. This work was funded by the Centers for Disease Control and Prevention and partially supported by Interagency Agreement Y1-AI-0681-02 between CDC and the National Institutes of Health. T. H. was funded by a fellowship by the American Society for Microbiology (ASM)/CDC Program in Infectious Disease and Public Health Microbiology.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2009. Prevention. Atlanta: U.S. Department of Health and Human Services; 2010. [Google Scholar]
- 2.Laga M, Manoka A, Kivuvu M, Malele B, Tuliza M, Nzila N, Goeman J, Behets F, Batter V, Alary M, Heyward WL, Ryder RW, Piot P. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993;7:95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Sexton J, Garnett G, Rottingen JA. Metaanalysis and metaregression in interpreting study variability in the impact of sexually transmitted diseases on susceptibility to HIV infection. Sex Transm Dis. 2005;32:351–7. doi: 10.1097/01.olq.0000154504.54686.d1. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Global Prevalence and Incidence of Selectable Curable Sexually Transmitted Infections Overview and Estimates. Geneva: World Health Organization; 2001. [Google Scholar]
- 5.Chalmet K, Staelens D, Blot S, Dinakis S, Pelgrom J, Plum J, Vogelaers D, Vandekerckhove L, Verhofstede C. Epidemiological study of phylogenetic transmission clusters in a local HIV-1 epidemic reveals distinct differences between subtype B and non-B infections. BMC Infect Dis. 2010;10:262. doi: 10.1186/1471-2334-10-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen CR, Plummer FA, Mugo N, Maclean I, Shen C, Bukusi EA, Irungu E, Sinei S, Bwayo J, Brunham RC. Increased interleukin- 10 in the endocervical secretions of women with non-ulcerative sexually transmitted diseases: a mechanism for enhanced HIV-1 transmission? AIDS. 1999;13:327–32. doi: 10.1097/00002030-199902250-00004. [DOI] [PubMed] [Google Scholar]
- 7.Sorvillo F, Smith L, Kerndt P, Ash L. Trichomonas vaginalis, HIV, and African-Americans. Emerg Infect Dis. 2001;7:927–32. doi: 10.3201/eid0706.010603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Der Pol B, Kwok C, Pierre-Louis B, Rinaldi A, Salata RA, Chen PL, van de Wijgert J, Mmiro F, Mugerwa R, Chipato T, Morrison CS. Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. J Infect Dis. 2008;197:548– 54. doi: 10.1086/526496. [DOI] [PubMed] [Google Scholar]
- 9.Ahn MH, Song HO, Ryu JS. Trichomonas vaginalis-induced neutrophil apoptosis causes antiinflammatory cytokine production by human monocyte-derived macrophages. Parasite Immunol. 2008;30:410–6. doi: 10.1111/j.1365-3024.2008.01037.x. [DOI] [PubMed] [Google Scholar]
- 10.Marrazzo JM, Martin DH. Management of women with cervicitis. Clin Infect Dis. 2007;44(Suppl 3):S102–10. doi: 10.1086/511423. [DOI] [PubMed] [Google Scholar]
- 11.Sutton M, Sternberg M, Koumans EH, McQuillan G, Berman S, Markowitz L. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis. 2007;45:1319–26. doi: 10.1086/522532. [DOI] [PubMed] [Google Scholar]
- 12.Feldblum PJ, Lie CC, Weaver MA, Van Damme L, Halpern V, Adeiga A, Bakare R, Schwartz J, Becker M, Solomon S. Baseline factors associated with incident HIV and STI in four microbicide trials. Sex Transm Dis. 2010;37:594–601. [PubMed] [Google Scholar]
- 13.Blakley GB, Beamer TW, Dukelow WR. Characteristics of the menstrual cycle in nonhuman primates. IV. Timed mating in Macaca nemestrina. Lab Anim. 1981;15:351–3. doi: 10.1258/002367781780953059. [DOI] [PubMed] [Google Scholar]
- 14.Otten RA, Adams DR, Kim CN, Jackson E, Pullium JK, Lee K, Grohskopf LA, Monsour M, Butera S, Folks TM. Multiple vaginal exposures to low doses of R5 simian-human immunodeficiency virus: strategy to study HIV preclinical interventions in nonhuman primates. J Infect Dis. 2005;191:164– 73. doi: 10.1086/426452. [DOI] [PubMed] [Google Scholar]
- 15.Otten RA, Smith DK, Adams DR, Pullium JK, Jackson E, Kim CN, Jaffe H, Janssen R, Butera S, Folks TM. Efficacy of postexposure prophylaxis after intravaginal exposure of pig-tailed macaques to a humanderived retrovirus (human immunodeficiency virus type 2) J Virol. 2000;74:9771–5. doi: 10.1128/jvi.74.20.9771-9775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parikh UM, Dobard C, Sharma S, Cong ME, Jia H, Martin A, Pau CP, Hanson DL, Guenthner P, Smith J, Kersh E, Garcia-Lerma JG, Novembre FJ, Otten R, Folks T, Heneine W. Complete protection from repeated vaginal simianhuman immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J Virol. 2009;83:10358–65. doi: 10.1128/JVI.01073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Promadej-Lanier N, Smith JM, Srinivasan P, McCoy CF, Butera S, Woolfson AD, Malcolm RK, Otten RA. Development and evaluation of a vaginal ring device for sustained delivery of HIV microbicides to non-human primates. J Med Primatol. 2009;38:263–71. doi: 10.1111/j.1600-0684.2009.00354.x. [DOI] [PubMed] [Google Scholar]
- 18.Promadej-Lanier N, Srinivasan P, Curtis K, Adams DR, Kim C, Luo W, Jia H, Subbarao S, Otten RA, Butera S. Systemic and mucosal immunological responses during repeated mucosal SHIV(162P3) challenges prior to and following infection in pigtailed macaques. Virology. 2008;375:492–503. doi: 10.1016/j.virol.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 19.Lichtenwalner AB, Patton DL, Cosgrove Sweeney YT, Gaur LK, Stamm WE. Evidence of genetic susceptibility to Chlamydia trachomatis-induced pelvic inflammatory disease in the pig-tailed macaque. Infect Immun. 1997;65:2250–3. doi: 10.1128/iai.65.6.2250-2253.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patton DL. Immunopathology and histopathology of experimental chlamydial salpingitis. Rev Infect Dis. 1985;7:746–53. doi: 10.1093/clinids/7.6.746. [DOI] [PubMed] [Google Scholar]
- 21.Patton DL, Sweeney YT, Agnew KJ, Balkus JE, Rabe LK, Hillier SL. Development of a nonhuman primate model for Trichomonas vaginalis infection. Sex Transm Dis. 2006;33:743–6. doi: 10.1097/01.olq.0000218871.89901.61. [DOI] [PubMed] [Google Scholar]
- 22.Patton DL, Sweeney YT, Stamm WE. Significant reduction in inflammatory response in the macaque model of chlamydial pelvic inflammatory disease with azithromycin treatment. J Infect Dis. 2005;192:129–35. doi: 10.1086/431365. [DOI] [PubMed] [Google Scholar]
- 23.Van Voorhis WC, Barrett LK, Sweeney YT, Kuo CC, Patton DL. Repeated Chlamydia trachomatis infection of Macaca nemestrina fallopian tubes produces a Th1-like cytokine response associated with fibrosis and scarring. Infect Immun. 1997;65:2175–82. doi: 10.1128/iai.65.6.2175-2182.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patton DL, Cosgrove-Sweeney Y, Agnew K, Hillier SL. Concurrent STI: development of a macaque model for C. trachomatis and T. vaginalis infections. Chlamydial Infections Proceedings of the Eleventh International Symposium on Human Chlamydial Infections; Niagara-on-the-Lake, ON. International Symposium on Human Chlamydial Infections; 2006. pp. 417–20. [Google Scholar]
- 25.Division of Sexually Transmitted Diseases Program and Training Branch NCfHA. Clinical Manifestations and Sequellae of Chlamydia trachomatis. Atlanta: Centers for Disease Control and Prevention; 2009. Viral Hepatitis, STD and TB Prevention. [Google Scholar]
- 26.Swygard H, Sena AC, Hobbs MM, Cohen MS. Trichomoniasis: clinical manifestations, diagnosis and management. Sex Transm Infect. 2004;80:91–5. doi: 10.1136/sti.2003.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darville T, Hiltke TJ. Pathogenesis of genital tract disease due to Chlamydia trachomatis. J Infect Dis. 2010;201(Suppl 2):S114–25. doi: 10.1086/652397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zariffard MR, Harwani S, Novak RM, Graham PJ, Ji X, Spear GT. Trichomonas vaginalis infection activates cells through tol-like receptor 4. Clin Immunol. 2004;111:103–7. doi: 10.1016/j.clim.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Stamm WE. Chlamydia trachomatis infections in the adult. In: Holmes, editor. Sexually Transmitted Diseases. New York: McGraw Hill; 1999. pp. 407–22. [Google Scholar]
- 30.Workowski KA, Stevens CE, Suchland RJ, Holmes KK, Eschenbach DA, Pettinger MB, Stamm WE. Clinical manifestations of genital infection due to Chlamydia trachomatis in women: differences related to serovar. Clin Infect Dis. 1994;19:756–60. doi: 10.1093/clinids/19.4.756. [DOI] [PubMed] [Google Scholar]
- 31.Krieger JA. Trichomonas vaginalis and trichomoniasis. In: Holmes, editor. Sexually Transmitted Diseases. New York: McGraw Hill; 1999. pp. 587–604. [Google Scholar]
- 32.Harouse JM, Gettie A, Eshetu T, Tan RC, Bohm R, Blanchard J, Baskin G, Cheng-Mayer C. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIV(SF162P3) J Virol. 2001;75:1990–5. doi: 10.1128/JVI.75.4.1990-1995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subbarao S, Otten RA, Ramos A, Kim C, Jackson E, Monsour M, Adams DR, Bashirian S, Johnson J, Soriano V, Rendon A, Hudgens MG, Butera S, Janssen R, Paxton L, Greenberg AE, Folks TM. Chemoprophylaxis with tenofovir disoproxil fumarate provided partial protection against infection with simian human immunodeficiency virus in macaques given multiple virus challenges. J Infect Dis. 2006;194:904–11. doi: 10.1086/507306. [DOI] [PubMed] [Google Scholar]
- 34.Henning TR, Lacour N, Amedee AM. Efficient methodologies for sensitive HIV-1 RNA quantitation from plasma and vaginal secretions. J Clin Virol. 2009;46:309–13. doi: 10.1016/j.jcv.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kersh EN, Luo W, Adams DR, Mitchell J, Garcia-Lerma JG, Butera S, Folks T, Otten R. Evaluation of the lymphocyte trafficking drug FTY720 in SHIVSF162P3- infected rhesus macaques. J Antimicrob Chemother. 2009;63:758–62. doi: 10.1093/jac/dkp008. [DOI] [PubMed] [Google Scholar]
- 36.Guenthner PC, Secor WE, Dezzutti CS. Trichomonas vaginalis-induced epithelial monolayer disruption and human immunodeficiency virus type 1 (HIV-1) replication: implications for the sexual transmission of HIV-1. Infect Immun. 2005;73:4155–60. doi: 10.1128/IAI.73.7.4155-4160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borchardt KA, Zhang MZ, Shing H, Flink K. A comparison of the sensitivity of the InPouch TV, Diamond’s and Trichosel media for detection of Trichomonas vaginalis. Genitourin Med. 1997;73:297–8. doi: 10.1136/sti.73.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.el-Naga IF, Khalifa AM, el-Azzouni MZ. In-pouch TV culture system in diagnosis of Trichomonas vaginalis infection. J Egypt Soc Parasitol. 2001;31:647–56. + 641p plate. [PubMed] [Google Scholar]
- 39.Sturdevant GL, Kari L, Gardner DJ, Olivares-Zavaleta N, Randall LB, Whitmire WM, Carlson JH, Goheen MM, Selleck EM, Martens C, Caldwell HD. Frameshift mutations in a single novel virulence factor alter the in vivo pathogenicity of Chlamydia trachomatis for the female murine genital tract. Infect Immun. 2010;78:3660–8. doi: 10.1128/IAI.00386-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patton DL, Sweeney YC, Tsai CC, Hillier SL. Macaca fascicularis vs. Macaca nemestrina as a model for topical microbicide safety studies. J Med Primatol. 2004;33:105–8. doi: 10.1111/j.1600-0684.2004.00059.x. [DOI] [PubMed] [Google Scholar]
- 41.Henning TR, Kissinger P, Lacour N, Meyaski-Schluter M, Clark R, Amedee AM. Elevated cervical white blood cell infiltrate is associated with genital HIV detection in a longitudinal cohort of antiretroviral therapy-adherent women. J Infect Dis. 2010;202:1543–52. doi: 10.1086/656720. [DOI] [PubMed] [Google Scholar]
- 42.Quayle AJ, Kourtis AP, Cu-Uvin S, Politch JA, Yang H, Bowman FP, Shah M, Anderson DJ, Crowley-Nowick P, Duerr A. T-lymphocyte profile and total and virus-specific immunoglobulin concentrations in the cervix of HIV-1- infected women. J Acquir Immune Defic Syndr. 2007;44:292–8. doi: 10.1097/QAI.0b013e31802c5b3a. [DOI] [PubMed] [Google Scholar]
- 43.Endtz AW. A rapid staining method for differentiating granulocytes from “germinal cells” in Papanicolaou-stained semen. Acta Cytol. 1974;18:2–7. [PubMed] [Google Scholar]
- 44.Castle PE, Rodriguez AC, Bowman FP, Herrero R, Schiffman M, Bratti MC, Morera LA, Schust D, Crowley-Nowick P, Hildesheim A. Comparison of ophthalmic sponges for measurements of immune markers from cervical secretions. Clin Diagn Lab Immunol. 2004;11:399– 405. doi: 10.1128/CDLI.11.2.399-405.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crostarosa F, Aravantinou M, Akpogheneta OJ, Jasny E, Shaw A, Kenney J, Piatak M, Lifson JD, Teitelbaum A, Hu L, Chudolij A, Zydowsky TM, Blanchard J, Gettie A, Robbiani M. A macaque model to study vaginal HSV-2/immunodeficiency virus co-infection and the impact of HSV-2 on microbicide efficacy. PLoS ONE. 2009;4:e8060. doi: 10.1371/journal.pone.0008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolner-Hanssen P, Krieger JN, Stevens CE, Kiviat NB, Koutsky L, Critchlow C, DeRouen T, Hillier S, Holmes KK. Clinical manifestations of vaginal trichomoniasis. JAMA. 1989;261:571–6. doi: 10.1001/jama.1989.03420040109029. [DOI] [PubMed] [Google Scholar]