Abstract
Background
There is a likely genetic component to transsexualism, and genes involved in sex steroidogenesis are good candidates. We explored the specific hypothesis that male-to-female transsexualism is associated with gene variants responsible for undermasculinization and/or feminization. Specifically, we assessed the role of disease-associated repeat length polymorphisms in the androgen receptor (AR), estrogen receptor β (ERβ), and aromatase (CYP19) genes.
Methods
Subject-control analysis included 112 male-to-female transsexuals and 258 non-transsexual males. Associations and interactions were investigated between CAG repeat length in the AR gene, CA repeat length in the ERβ gene, and TTTA repeat length in the CYP19 gene and male-to-female transsexualism.
Results
A significant association was identified between transsexualism and the AR allele, with transsexuals having longer AR repeat lengths than non-transsexual male control subjects (p = .04). No associations for transsexualism were evident in repeat lengths for CYP19 or ERβ genes. Individuals were then classified as short or long for each gene polymorphism on the basis of control median polymorphism lengths in order to further elucidate possible combined effects. No interaction associations between the three genes and transsexualism were identified.
Conclusions
This study provides evidence that male gender identity might be partly mediated through the androgen receptor.
Keywords: Androgen receptor, AR, aromatase, CYP19, ERβ, estrogen receptor β, gender identity disorder, transsexualism
From an early age, people develop an inner sense of being male or female. Transsexuals however, identify with a physical sex opposite to their biological sex. Such individuals might seek to alleviate their distress by altering their bodies through hormone therapy and sex reassignment surgery (1). The prevalence of transsexualism ranges from 1:2,900 to 1:100,000; and little is known about the etiology of this condition (2–4). Some theories have suggested that psychosocial factors— including dysfunctional family dynamics (5) and traumatic childhood experiences (6)—lead to the development of a transsexual identity.
Increasingly, biomedical research is implicating biological factors. Co-occurrence among twin pairs, father-son pairs, and brother-sister pairs (7,8) raises the question of whether gender dysphoria is heritable. Anatomical studies show that certain brain structures in male-to-female transsexuals are more “female-like” in volume and neuronal density (9,10). Furthermore, the response to the odor of male and female steroids in male-to-female transsexuals was more similar to that of control women than control men (11). Other studies suggest that sex steroids influence gender identity. Female-to-male transsexuality has been associated with polycystic ovary syndrome and hyperandrogenemia (12). Moreover, female subjects with the disorder of sex development called congenital adrenal hyperplasia are exposed to high levels of androgens prenatally and seem to be at much higher risk of gender identity disorder than the general population (13). A significant association was identified between female-to-male transsexualism and the CYP17 gene (which encodes 17α-hydroxylase, the enzyme deficient in some virilized congenital adrenal hyperplasia patients) (14). Aromatase (CYP19), the enzyme that converts testosterone to estrogen, has also been implicated in female gender identity. A 46, XX woman with congenital adrenal hyperplasia carried a null CYP19 mutation, was born with phallic enlargement, a uterus, and ovaries, and exhibited a persistent male gender identity and male gender role behavior (15).
There are few genetic association studies of male-to-female transsexualism. A study of 29 Swedish male-to-female transsexuals identified a significant association with a dinucleotide CA polymorphism in the estrogen receptor β (ERβ) gene (p = .03) (16). It has been suggested that ERβ has a defeminization role in male brain and behavior, on the basis of knockout mouse studies (17). Altogether, genetic studies on transsexuals suggest that both androgen and estrogen might play a role in gender identity.
We sought to investigate whether sex steroidogenesis genes are associated with male-to-female transsexualism in the largest cohort collected to date. We analyzed the variable polymorphism lengths of three genes—androgen receptor (AR), ERβ, and CYP19—in Caucasian transsexuals and compared these with non-transsexual male control subjects.
Methods and Materials
Participants
One hundred and twelve Caucasian male-to-female transsexuals, pre- and post-operative, were recruited from Monash Medical Centre (MMC), Victoria, Australia (n = 76) and from University of California, Los Angeles (UCLA) (n = 36) as per criteria in the DSM-IV—some of whom had reports of gender dysphoria in childhood. Almost all transsexual individuals were receiving hormone treatment. Two hundred and fifty-eight Caucasian male control subjects were also recruited from MMC. Ethical approvals for this study were obtained from MMC and UCLA, and consent procedures adhered to the tenets of the Declaration of Helsinki. The sexuality is only known for approximately 40% of patients, because some patients did not wish to discuss or disclose this information or the patient's sexuality was flexible and not easily classified.
Genotyping
Genomic DNA was extracted from whole blood (18) or saliva (OrageneT). Androgen receptor exon 1 CAG repeat was amplified with polymerase chain reaction with VIC-labelled 5′-TCTGGAT-CACTTCGCGCAC-3′ and 5′-GTTCCTCATCCAGGACCAGGTA-3′. The ERβ intron 5 CA repeat was amplified with FAM-labelled 5′-GGTACAGACCATGGTTTACC-3′, and 5′-AACAAAATGTT-GAATGAGTGGG-3. The CYP19 intron 4 TTTA repeat was amplified with NED-labelled 5′-GGTACTTAGTTAGCTACAATC-3′, and 5′-GGGTGATAGAGTCAGAGCCT-3′. Polymerase chain re action was 95°C for 30 sec, 30 sec at 59°C for AR, 55°C for ERβ, and 58°C for CYP19, and extension at 72°C for 30 sec for 35 cycles. The polymerase chain reaction products from the three genes were then mixed for each individual with Genescan LIZ-500 size standard and analyzed on an ABI Prism 3130xl (Applied Biosystems, Foster City, California). Successful genotyping was achieved for at least 101 of the 112 transsexual individuals across the three gene polymorphisms (101 for AR, 111 for ERβ, and 104 for CYP19) and 258 control subjects.
Statistics
To evaluate the repeat length polymorphism data for possible associations with male-to-female transsexualism, independent samples t tests were used. Interactions between the three gene polymorphisms were evaluated with a binary logistic regression model. Analyses were performed with Statistical Package for the Social Sciences 12.0 software (SPSS, Chicago, Illinois). A p value < .05 was considered significant. The primary analysis performed was of the association between male-to-female transsexualism and AR, ERβ, and CYP19 genotypes.
Results
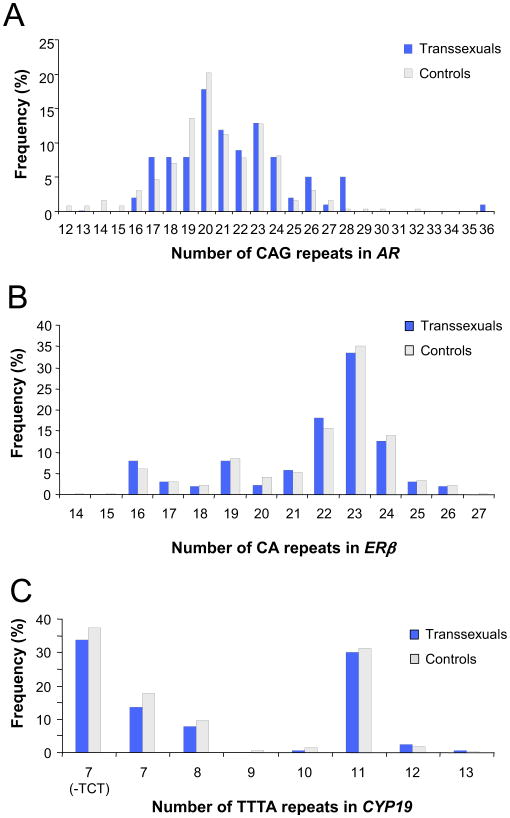
Polymorphic fragment lengths for 258 male subjects and 112 transsexuals were obtained. Twenty-one different alleles were identified for the AR gene polymorphism, 14 for the ERβ gene polymorphism, and 8 for the CYP19 gene polymorphism. The percentages of each allele in the control and transsexual populations are shown in Figure 1. For the AR gene, a difference in the mean repeat length was identified, with transsexuals having significantly longer mean repeat lengths (243.2 base pairs) than control subjects (245.1 base pairs, p = .04).
Figure 1.
Graphical representation of the allele frequency distributions of (A) androgen receptor (AR), (B) estrogen receptor B (ERB), and (C) aromatase (CYP19) genes showing repeat number for each gene polymorphism. Individuals with 7 TTTA repeats with further classified into those with or without the three base pair TCT insertion polymorphism within this polymorphism.
The repeat lengths were then sub-classified to compare genotypes. For the control population, equally sized genotype groups were generated on the basis of the median repeat length for all three genes whereby alleles below this length were assigned as “short” and above this length assigned as “long” (AR short ≤ 20 repeats, long > 20 repeats; ERβ: short ≤ 22 repeats, long > 22 repeats; and CYP19 short ≤ 7 repeats, long > 7 repeats). The number of short and long repeat length alleles for AR, ERβ, and CYP19 are shown in Table 1. The genotypes of CYP19 and ERβ for all individuals were determined as SS (two short alleles), SL (one short allele and one long), or LL (two long alleles). The AR genotype, being X-linked, is hemizygous, and thus the comparison undertaken was between short and long genotypes. An independent samples ttest revealed no significant association for the AR gene when sub-classified (p > .05). Logistic regression revealed that no significant associations were identified for ERβ or CYP19 genotypes between the two populations (p> .05; Table 2). With binary logistic regression analysis we observed no significant interactions for any of the variable combinations for the three genes (p > .05, Table 3).
Table 1. Allele Frequency Numbers for Short and Long Alleles in Both the Transsexual and Control Populations Across all Three Genes.
| Gene | Polymorphism | Transsexuals n (%) | Control Subjects n (%) |
|---|---|---|---|
| AR | Short | 45 (44.6) | 135 (52.3) |
| Long | 56 (55.4) | 123 (47.6) | |
| ERβ | Short | 106 (47.7) | 229 (44.7) |
| Long | 116 (52.3) | 283 (55.3) | |
| CYP19 | Short | 113 (54.3) | 285 (55.2) |
| Long | 95 (45.7) | 231 (44.8) |
AR, androgen receptor; ERB, estrogen receptor β; CYP19, aromatase.
Table 2. Genotype Frequency ORs of Transsexualism Among ERβ and CYP19 Genes.
| Locus | Repeat | Genotype | Transsexuals n = 112 (%) | Control Subjects n = 258 (%) | OR (95% CI) | p |
|---|---|---|---|---|---|---|
| ERβ | CA | SS | 75 (67.6) | 165 (64.0) | 1.00 (reference) | .77 |
| SL | 32 (28.8) | 83 (32.2) | 1.15 (.35–3.79) | .81 | ||
| LL | 4 (3.6) | 10 (3.8) | .96 (.28–3.30) | .95 | ||
| CYP19 | TTTA | SS | 31 (29.8) | 76 (29.5) | 1.00 (reference) | .87 |
| SL | 51 (49.0) | 133 (51.5) | .91 (.47–1.75) | .77 | ||
| LL | 22 (21.2) | 49 (19.0) | .45 (.47–1.55) | .60 |
OR, odds ratio; CI, confidence interval; ERβ, estrogen receptor beta; CYP19, aromatase; LL, two long alleles; SL, one short and one long allele; SS, two short alleles.
Table 3. Logistic Regression Analysis Results for Possible Gene Polymorphism Interactions and Their Association with the Occurrence of Transsexualism.
| Gene Repeat | p | OR | CI |
|---|---|---|---|
| AR | .38 | 1.70 | (.53–5.48) |
| CYP19 | .93 | 1.09 | (.17–6.94) |
| ERβ | .97 | .98 | (.37–2.60) |
| AR-CYP19 | .69 | .59 | (.04–7.93) |
| AR-ERβ | .72 | .78 | (.20–3.02) |
| ERfj-CYP19 | .88 | .85 | (.11–6.73) |
| AR-ERQ-CYP19 | .87 | 1.28 | (.07–22.43) |
Discussion
To date, this is the largest genetic study of transsexualism conducted. We observed a significant association between longer AR gene polymorphisms and male-to-female transsexualism. Longer CAG repeats in the AR gene lead to reduced binding of the AR protein to co-activator, due to its inhibitory interaction with the receptor, resulting in less effective testosterone signalling (19), a mechanism typically involved in masculinization of the brain during early development (1). Female subjects typically lack the gonadal testosterone surge that occurs in male subjects. Consequently, the AR gene is not activated (20). It is possible that a decrease in testosterone levels in the brain during development might result in incomplete masculinization of the brain in male-to-female transsexuals, resulting in a more feminized brain and a female gender identity.
A recent study on female-to-male transsexuals identified a CYP17 single nucleotide polymorphism that was significantly associated with the occurrence of transsexualism (14). These individuals have a higher serum testosterone level than control female subjects, the converse effect of what is suggested in our study of male-to-female transsexualism. The effect we identified was weak; thus it seems highly likely that male-to-female transsexualism is due to multiple genetic factors.
We were unable to replicate the significant association between longer CA repeat lengths in the ERβ gene and male-to-female transsexualism, contrary to previous findings in 29 Swedish male-to-female transsexuals (16), even though we undertook the same statistical analysis that they used. Our sample size was approximately four times larger than that of the Swedish study, so it is possible that the former study was underpowered to detect a false positive. Alternatively, there might be differences between Swedish and non-Swedish populations in this polymorphism. In the Swedish study, the long repeat occurred in 51.8% of control subjects and 67.1% of transsexuals (16), whereas in the present study the long repeat occurred in 36.5% of control subjects and 44.1% of transsexuals. Thus, although there was a trend in the same direction in both studies, there are major differences in prevalence of these long repeats between the two populations.
In conclusion, our findings indicate a significant association between male-to-female transsexualism and the long polymorphism for the AR repeat. This finding links the androgen receptor and further implicates genes in the steroidogenesis pathway as playing a role in male-to-female transsexualism. We speculate that reduced androgen and androgen signalling might contribute to the female gender identity of male-to-female transsexuals. Further studies including replication in other populations, larger patient collections, and analysis of other polymorphisms, both for the genes studied here and other sex steroidogenesis genes, should be undertaken.
Acknowledgments
Support was provided by the National Health and Medical Research Council Program Grant 33414 (to VRH) and the National Institutes of Health training Grant 5 T32 HD07228 (to FS). All authors have reported no biomedical financial interests or potential conflicts of interest.
We thank Vivien Vasic for assisting with genotyping, research nurse Elise Forbes for collecting samples, Professor Don Bowden for the donation of control DNAs, and Dr. Izabella Czajka for criticism. We also thank the individuals who voluntarily participated and who are an integral part of this research. This manuscript is dedicated to Dr. Herbert Bower, who inspired us.
References
- 1.Hulshoff Pol HE, Cohen-Kettenis PT, Van Haren NEM, Peper JS, Brans RGH, Cahn W, et al. Changing your sex changes your brain: Influences of testosterone and estrogen on adult human brain structure. Eur J Endocrinol. 2006;155:S107–S114. [Google Scholar]
- 2.Kockett G, Fahrner EM. Transsexuals who have not undergone surgery: A follow up study. Arch Sex Behav. 1987;16:511–522. doi: 10.1007/BF01541715. [DOI] [PubMed] [Google Scholar]
- 3.Bakker A, van Kesteren PJ, Gooren LJ, Bzemer PD. The prevalence of transsexualism in the Netherlands. Acta Psychiatr Scand. 1993;87:237–238. doi: 10.1111/j.1600-0447.1993.tb03364.x. [DOI] [PubMed] [Google Scholar]
- 4.Landen M, Walinder J, Lundstrom B. Prevalence, incidence and sex ratio of transsexualism. Acta Psychiatr Scand. 1996;93:221–223. doi: 10.1111/j.1600-0447.1996.tb10638.x. [DOI] [PubMed] [Google Scholar]
- 5.Loeb L, Shane M. The resolution of a transsexual with in a five-year-old boy. J Am Psychoanal Assoc. 1982;30:419–434. doi: 10.1177/000306518203000205. [DOI] [PubMed] [Google Scholar]
- 6.Devor H. Transsexualism, dissociation, and child abuse: An initial discussion based on non-clinical data. J Psychol Hum Sexuality. 1994;6:49–72. [Google Scholar]
- 7.Hyde C, Kenna JC. A male MZ twin pair, concordant for transsexualism, discordant for schizophrenia. Acta Psychiatr Scand. 1977;56:265–275. doi: 10.1111/j.1600-0447.1977.tb00227.x. [DOI] [PubMed] [Google Scholar]
- 8.Green R. Family cooccurrence of “gender dysphoria”: Ten sibling or parent-child pairs. Arch Sex Behav. 2000;29:499–507. doi: 10.1023/a:1001947920872. [DOI] [PubMed] [Google Scholar]
- 9.Zhou JN, Hofman MA, Gooren LJG, Swaab DF. A sex difference in the human brain and its relation to transsexuality. Nature. 1995;378:68–70. doi: 10.1038/378068a0. [DOI] [PubMed] [Google Scholar]
- 10.Kruijver FP, Zhou JN, Pool CW, Hofman MA, Gooren LJG, Swaab DF. Male-to-female transsexuals have female neuron numbers in a limbic nucleus. J Clin Endocrinol Metab. 2000;85:2034–2041. doi: 10.1210/jcem.85.5.6564. [DOI] [PubMed] [Google Scholar]
- 11.Berglund H, Lindström P, Dhejne-Helmy C, Savic I. Male-to-female transsexual show sex-atypical hypothalamus activation when smelling odorous steroids. Cereb Cortex. 2007;18:1900–1908. doi: 10.1093/cercor/bhm216. [DOI] [PubMed] [Google Scholar]
- 12.Baba T, Endo T, Honnma H, Kitajima Y, Hayashi T, Ikeda H, et al. Association between polycystic ovary syndrome and female-to-male transsexuality. Hum Reprod. 2007;22:1011–1016. doi: 10.1093/humrep/del474. [DOI] [PubMed] [Google Scholar]
- 13.Meyer-Bahlburg HFL, Gruen RS, New MI, Bell JJ, Morishima A, Shimshi M, et al. Gender change from female to male in classical congenital adrenal hyperplasia. Horm Behav. 1996;30:319–332. doi: 10.1006/hbeh.1996.0039. [DOI] [PubMed] [Google Scholar]
- 14.Bentz EK, Hefler LA, Kaufman U, Huber JC, Kolbus A, Tempfer CB. A polymorphismofthe CYP17 gene relatedtosex steroid metabolism is associated with female-to-male but not male-to-female transsexualism. Fertil Steril. 2008;90:56–59. doi: 10.1016/j.fertnstert.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 15.Lin L, Ercan O, Raza J, Burren CP, Creighton SM, Auchus RJ, et al. Variablephenotypes associated with aromatase (CYP19) insufficiency in humans. J Clin Endocrinol Metab. 2007;92:982–990. doi: 10.1210/jc.2006-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henningsson S, Westberg L, Nilsson S, Lundström B, Ekselius L, Bodlund O, et al. Sex steroid-related genes and male-to-female transsexu-alism. Psychoneuroendocrinology. 2005;30:657–664. doi: 10.1016/j.psyneuen.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Kudwa AE, Bodo C, Gustafsson J-Å, Rissman EF. A previously uncharacterized role for estrogen receptor B: Defeminization of male brain and behaviour. Proc Nat Acad Sci U S A. 2005;102:4608–4612. doi: 10.1073/pnas.0500752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kazemi-Esfarjani P, Trifiro M, Pinsky L. Evidence for a repressive function ofthe long polyglutamine tract in the human androgen receptor: Possible pathogenetic relevance for the (CAG)n-expanded Neuronopathies. Hum Mol Genet. 1995;4:523–527. doi: 10.1093/hmg/4.4.523. [DOI] [PubMed] [Google Scholar]
- 20.Westberg L, Baghaei F, Rosmond R, Hellstrand M, Landen M, Jansson M, et al. Polymorphisms of the androgen receptor gene and the estrogen beta gene are associated with androgen levels in women. J Clin Endocrinol Metab. 2001;86:2562–2568. doi: 10.1210/jcem.86.6.7614. [DOI] [PubMed] [Google Scholar]