Abstract
Increasing evidence suggests that Human epidermal growth factor receptor 2 (HER2/neu) is involved in progression of prostate cancer. Recently, sarcosine was reported to be highly increased during prostate cancer progression, and exogenous sarcosine induces an invasive phenotype in benign prostate epithelial cells. The aim of this work was to investigate the effect of sarcosine on HER2/neu expression in prostate cancer cell lines LNCaP (androgen dependent), PC-3 and DU145(both androgen independent).
Relative amounts of HER2/neu and androgen receptor (AR) transcripts were determined using RT-qPCR. Total expression of HER2/neu was confirmed by Western blot (WB). HER2/neu protein on the surface of living LNCaP cells was visualized by confocal microscopy using a HER2/neu-specific fluorescent probe. Exposure of LNCaP cells to 50 μM sarcosine for 24 h resulted in a 58% increase of the HER2/neu mRNA level (p<0.001) indicating that sarcosine effects HER2/neu expression on the level of transcription. Control experiments with alanine, an isomer of sarcosine, showed no significant effect on HER2/neu transcription. The upregulation of HER2/neu mRNA preceded the corresponding increment of the protein level after the 48-h exposure to sarcosine as shown by WB and confocal microscopy. Interestingly, sarcosine had no effect on the activated (phosphorylated) form of HER2/neu. No significant change in AR expression was observed after exposure to sarcosine. This is the first report indicating that sarcosine is involved in the regulation of the oncoprotein HER2/neu. Thus, sarcosine may induce prostate cancer progression by increased HER2/neu expression. However, detailed information on cellular mechanisms remains to be elucidated.
Keywords: HER2/neu, LNCaP, prostate cancer, sarcosine
INTRODUCTION
Human epidermal growth factor receptor 2 (HER2/neu or HER2), a tyrosine kinase receptor, is a potent oncoprotein in various cancers including breast, ovarian, lung, and bladder cancer. Overexpression of the HER2/neu protein and amplification of the HER2/neu gene have been implicated in tumor development and progression and associated with a poor prognosis in several types of cancers [1,2]. HER2/neu is able to activate both the nuclear factor kappa-beta (NF-κB) and phosphoinositide 3-kinase (PI3K) pathways [3], leading to changes in invasion and proliferation of breast cancer cells. There is increasing evidence that HER2/neu is also involved in prostate cancer [4,5,6,7,8]. Recently, Minner et al. demonstrated in a large study that the level of HER2/neu expression is variable in prostate cancer and that an increased HER2/neu expression level is associated with unfavorable tumor phenotype, rapid tumor cell proliferation, and poor prognosis [6]. Furthermore, preoperative plasma HER2/neu levels are associated with prostate cancer progression after radical prostatectomy and predict poor outcome after chemotherapy [9]. The pathways downstream HER2/neu are, however, not well characterized, but recently it has been demonstrated that NF-κB and PI3K also are involved in HER2/neu signaling in prostate cancer [5].
Recently, Sreekumar et al. demonstrated that sarcosine, an N-methyl derivative of the amino acid glycine, is a differential metabolite that appears highly increased during prostate cancer progression to metastasis [10]. Furthermore, the authors demonstrated that addition of exogenous sarcosine or knockdown of the enzyme that leads to sarcosine degradation, sarcosine dehydrogenase, induced an invasive phenotype in benign prostate epithelial cells. Although sarcosine is indicated as a potentially important metabolic intermediary involved in cancer progression, its downstream cellular signaling leading to increased invasiveness was, however, not investigated.
The growing knowledge on HER2/neu in aggressive cancer, including prostate cancer [4], together with the recently suggested role of sarcosine in progression of prostate cancer [10], prompted us to investigate a possible relation between sarcosine and expression of HER2/neu in androgen dependent (LNCaP) and androgen independent (PC3 and DU145) prostate cancer cells.
MATERIALS AND METHODS
Cell culture and experimental set ups
Prostate cancer cell lines LNCaP, PC3 and DU145, were obtained from American Type Culture Collection (ATCC, Rockville, MD). Culture medium consisted of RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA), 100 units/ml penicillin and 100 μg/ml streptomycin (this medium is referred to as growth medium). Cells were grown in 6-well plates to 70–80% confluency, starved for 24 h in starvation medium [growth medium in which FBS was substituted with 0.1% bovine serum albumin (BSA, Sigma) and 100 ng/ml epidermal growth factor (EGF, Invitrogen, Carlsbad, CA)], and exposed to 50 μM sarcosine [10] in starvation medium for 24, 48 or 72 h. The sarcosine analogue alanine (50 μM) was used as a control. For microscopic observations and image acquisition, cells were grown in 35-mm IbiTreat dishes (Ibidi GmbH, Martinsried, Germany) with cover slip-like bottom. In case of treatments exceeding 24 h, fresh sarcosine-containing starvation medium was added to the cells every 24 h. For each condition studied, both treated and control cells were grown in duplicate or triplicate wells, and the whole experiment was performed at least three times. The figures were prepared using Adobe Photoshop.
Reverse transcription real-time quantitative PCR (RT-qPCR)
Total RNA was purified by RNeasy Miniprep Kit (QIAGEN GmbH, Hilden, Germany) and its concentration was determined by measuring UV absorption. RNA preparations with A260/280 > 1.8 were used for further PCR analysis. After conversion to cDNA (Affinity Script™ Master Mix Kit, Stratagene, La Jolla, CA), the following PCR primer pairs were used in relative quantitative PCR (Brilliant® SYBR® Green QPCR master Mix Kit, Stratagene, La Jolla, CA): HER2/neu: forward 5’-CCAGCAGGGCTTCTTCTGT-3’, and reverse 5’-TCCAGCCCTAGTGTCAGGTC-3’; AR: forward 5’-TACCAGCTCACCAAGCTCCT-3’, and reverse 5’-AAAGTCCACGCTCACCATGT-3’; and β-actin: forward 5’-ACTCTTCCAGCCTTCCTTCC-3’, and reverse 5’-AGCACTGTGTTGGCGTACAG-3’ (TAGC Copenhagen A/S, Denmark). All primers were designed using Primer 3 and synthesized by TAGC Copenhagen A/S (Copenhagen, Denmark). PCR was performed using a thermal cycler (Mx3000P QPCR System, Stratagene, La Jolla, CA) and the MxPro™ QPCR Software. The relative amount of HER2/neu and AR transcripts in each sample were determined using the standard curve method and by normalizing for the β-actin mRNA expression levels as described elsewhere [11]. Throughout the experiments with sarcosine treatment, the reference gene β-actin was found stably expressed.
Protein extraction and Western blot analysis
Cells were scraped and harvested (500 g for 5 minutes), washed once with PBS, and lysed in RIPA buffer (Sigma) containing a protease inhibitor cocktail (Complete Mini, Roche Diagnostics GmbH, Mannheim, Germany). Cell debris was removed by centrifugation (10,000 g for 20 minutes) and total protein concentration was determined by the Bio-Rad Protein Assay (BioRad, Hercules, CA). Seventy μg of total protein extract was loaded on 4–12% gradient polyacrylamide gels, and separated proteins were blotted using the XCell SureLock™ Mini-Cell system (Invitrogen, La Jolla, CA) according to the manufacturer’s guidelines. Blotting of the reference protein β-actin was used as a loading control. Briefly, membranes were blocked with 5% nonfat dry milk in PBS-T (PBS containing 0.05% Tween-20) and then probed over night at 4°C with the following primary antibodies (all from Santa-Cruz Biotechnology, Santa Cruz, CA) in blocking buffer: anti-HER2/neu (C-18; 1 μg/ml), anti-phospho-HER2/neu (Tyr 1248-R; 2 μg/ml), anti-AR (N-20; 1 μg/ml) and anti-β-actin (ACTBD11B7; 1 μg/ml). Target proteins were detected using a secondary antibody (anti-rabbit-HRP or anti-mouse-HRP; 1:10,000; BioRad, Hercules, CA) together with an enhanced chemiluminescence detection system (SuperSignal® West Pico, Pierce Biotechnology, Inc., Rockford, IL) and the MultiGauge software.
Live cell imaging using confocal microscopy
HER2/neu molecules displayed on the cell surface were probed using 50μg/ml of the HER2/neu specific fluorescent Affiprobe, Z(HER2)-red [12] in RPMI 1640 medium (without phenol red) for 30 minutes at room temperature. Unbound Affiprobe was removed and living cells were kept in RPMI 1640 medium (without phenol red) during image acquisition (< 30 minutes per culture dish), performed using a Leica TCS SP5 confocal laser scanning microscope (Leica Microsystems CMS GmbH, Mannheim, Germany) with an HCX PL APO CS 63 × /1.4 oil objective. SYTO13 (Invitrogen, La Jolla, CA) was used for counterstaining of nuclei. Differential Interference Contrast (DIC) images of cells were collected to check cell morphology. Acquired images were processed for display using the LAS AF software (Leica Microsystems CMS GmbH, Mannheim, Germany) and Adobe Photoshop.
RESULTS
Effect of sarcosine on HER2/neu mRNA levels in LNCaP cells
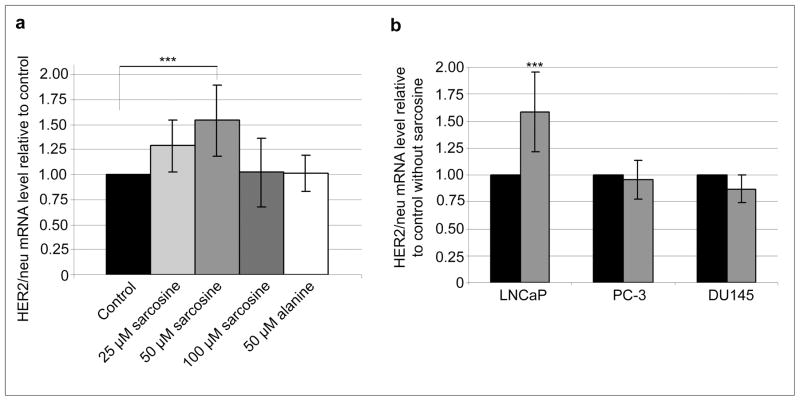
First, we evaluated the effect of increasing concentrations of sarcosine on HER2/neu expression in the LNCaP cells incubated for 24 h. As shown in Figure 1A LNCaP cells exposed to 25, 50 and 100 μM sarcosine showed a dose-dependent upregulation of HER2/neu mRNA with the strongest effect observed for 50 μM. This concentration was therefore used in the following experiments. Alanine, an isomer of sarcosine, was used as a control. As can be seen in figure 1A alanine showed no significant effect on HER2/neu mRNA level.
Fig. 1.
Effect of sarcosine on relative HER2/neu mRNA levels in prostate cancer cells. (a) Exposure of LNCaP cells to 25–100 μM sarcosine or 50 μM alanine for 24 h; (b) exposure of androgen-dependent (LNCaP) and androgen-independent (PC-3 and DU145) to 50 μM sarcosine for 24 h. Black, grey and white bars represent control experiments without addition of sarcosine, sarcosine and alanine experiments, respectively. Mean values of at least three independent experiments are shown. Error bars show SD and the asterisks indicate significant fold change (P < 0.001, as determined by the student’s t-test)
Effect of sarcosine on HER2/neu mRNA levels in other prostate cancer cell lines
To determine whether sarcosine is relevant in other prostate cancer cell lines we incubated two androgen independent cell lines PC-3 and DU145 with 50 μM sarcosine and compared these with LNCaP cells. A highly significant increase (p<0.001) of HER2/neu expression in LNCaP cells was observed following incubation of LNCaP cells with sarcosine. Exposure of LNCaP cells to 50 μM sarcosine for 24 hrs resulted in a 58% increase of the relative HER2/neu mRNA level (Figure1B), indicating that sarcosine influences HER2/neu expression on the level of transcription. In contrast, no significant alteration of the relative HER2/neu mRNA expression in the two androgen independent cell lines PC-3 and DU145 was observed (Figure 1B).
Effect of sarcosine on HER2/neu protein expression and phosphorylation in LNCaP cells
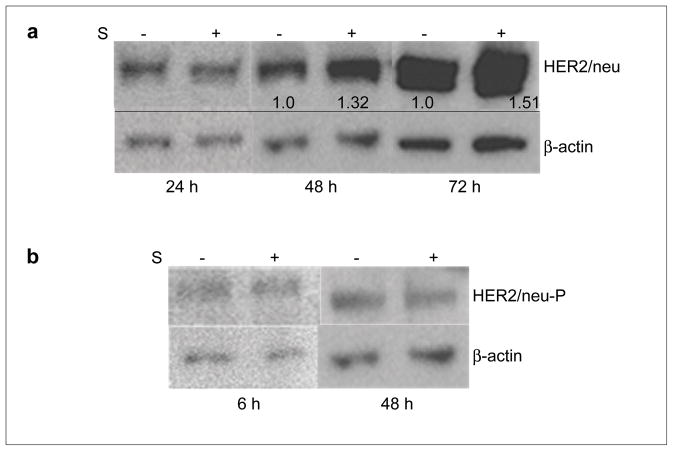
Treatment of LNCaP cells with 50 μM sarcosine for 48 and 72 h resulted in increased amounts of HER2/neu protein expression (Figure 2A). The upregulation of HER2 mRNA preceded the corresponding increment on the protein level as shown by Western blot (Figure 2A). The band intensity of the loading control β-actin appears to differ between the sampling points (Figure 2A). However, the expression of β-actin within each experiment (control and treated cells after 24, 48 or 72 h, respectively) was stable. Next, to investigate whether sarcosine also activates the HER2/neu receptor we looked for the presence of its phosphorylated form, HER2/neu-P, in sarcosine treated LNCaP using Western blot. In contrast to the noticeable upregulation of HER2/neu found after 48 h of sarcosine treatment, this was not the case for HER2/neu-P neither after 6 h nor 48 h of treatment (Figure 2B).
Fig. 2.
Effect of sarcosine on HER2/neu protein expression and phosphorylation in LNCaP cells. Sarcosine (50 μM) treatment for 48 and 72 h results in upregulated expression of HER2/neu protein in LNCaP cells (a). The numbers indicate the relative increase in detected intensity of the HER2/neu protein bands. No increase in the phosphorylated form, HER2/neu-P, was observed (b). Western blot analysis of total protein extracts from LNCaP after 6, 24, 48 or 72 h of 50 μM sarcosine exposure; ± S indicates addition of sarcosine and control cells, respectively
Membrane localization of HER/neu receptors in LNCaP cells and effect of sarcosine
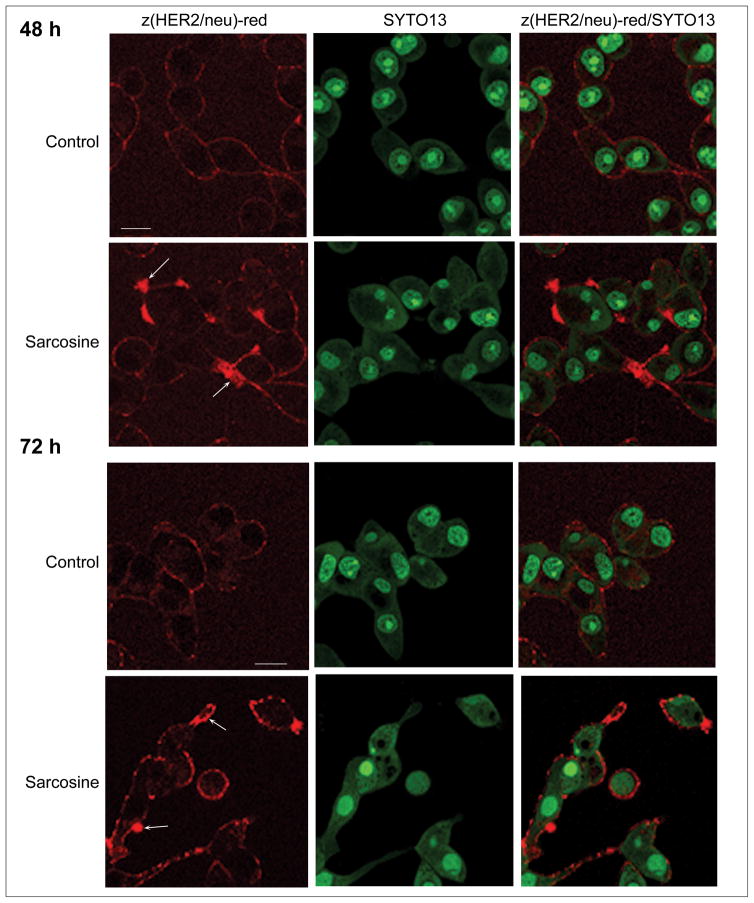
The total expression of HER2/neu on living cells was visualized using confocal microscopy. HER2/neu molecules on the surface of living LNCaP cells were labeled with the red fluorescent Affiprobe and images were acquired. The presence of HER2/neu on the surface of living LNCaP cells appeared to be more pronounced after 48 h of sarcosine treatment. This effect was also observed after a 72-h exposure (Figure 3).
Fig. 3.
Expression of HER2/neu on the surface of living LNCaP cells. The extracellular part of HER2/neu on the surface of living cells after (a) 48 and (b) 72 h of sarcosine treatment was probed with 50 μg/ml Z(HER2)-red [10] (red); arrows indicate local increase of HER2/neu-bound probe. Scale bars = 20 μm. Representative images from five independent experiments are shown
Effect of sarcosine on the androgen receptor expression in LNCaP cells
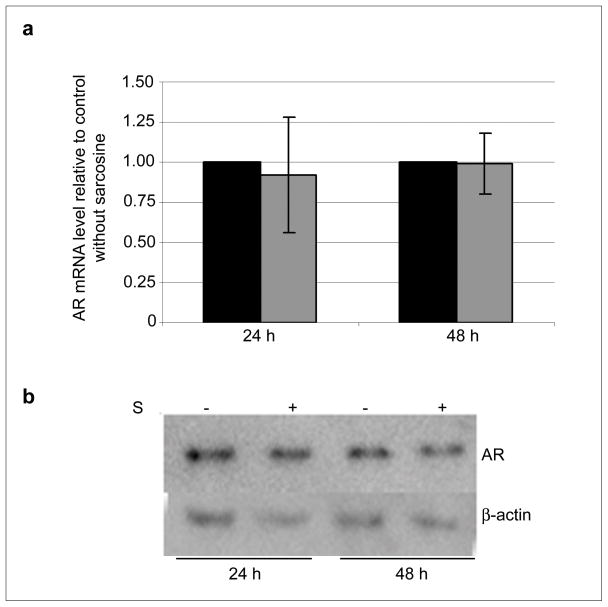
Finally, we evaluated the effect of sarcosine on the expression of the androgen receptor (AR) in the androgen dependent LNCaP cells. Exposure to sarcosine for 24 or 48 h did not show any significant effect either on AR or HER2/neu mRNA as judged by RT-qPCR and Western blot (Figure 4A and B, respectively). Additionally, incubation of LNCaP cells with bicalutamide, a pure non-steroidal antiandrogen which by binding to the AR prevents its activation, showed no effect on the regulation of the HER2/neu expression by sarcosine (data not shown).
Fig. 4.
Sarcosine has no apparent effect on the expression of the androgen receptor (AR) in LNCaP cells. (a) Relative AR mRNA levels in LNCaP cells after 24 and 48 h of sarcosine treatment. Black and grey bars represent control cells and treated cells, respectively. Mean values of at least three independent experiments are shown; error bars show SD; (b) Western blot analysis of whole cell extracts from LNCaP cells exposed to sarcosine for 24 and 48 h
DISCUSSION
In this study, we investigated the possible link between sarcosine and HER2/neu expression in prostate cancer progression. In order to mimic the transformation from local to metastatic prostate cancer we used LNCaP cell line as a model for androgen dependent prostate cancer [13,14]. The experiments were supplemented by two other prostate cancer cell lines i. e. the androgen-independent cells lines PC-3 and DU145. We found that LNCaP cells exposed to sarcosine show a significant upregulation of HER2/neu both on the level of transcription and the expressed protein, as analyzed by RT-qPCR, Western blot and confocal microscopy. The techniques utilized provide correlating results supporting the idea that sarcosine might be involved in the HER2/neu pathway triggering prostate cancer progression.
In the work by Sreekumar et al., the sarcosine concentration for treatment of cells was set to 50 μM [10]. The increase in intracellular sarcosine concentration observed in cells exposed to this concentration corresponds to the relative increase in sarcosine level found when comparing benign and metastatic biopsies. Even though it was not further explained by the authors, interpreting the presented figures led us to continue the use of 50 μM sarcosine, supplemented with evaluation of lower and higher concentrations (Fig. 1A). The downregulation of HER2/neu mRNA found for the highest concentration might be due to cytotoxic effects.
Interestingly, the androgen independent prostate cancer cell lines PC3 and DU145 did not display an increase in HER2/neu level upon sarcosine exposure. This is in contrast to the results obtained in the androgen dependent LNCaP cells, indicating a role of AR in the sarcosine pathway as suggested by Sreekumar et al. [10].
In our study, activation, i. e. phosphorylation, of HER2/neu seems unaffected by sarcosine treatment, as indicated by the results of Western blot analysis. Considering that absence of the phosphorylated form might be due to rapid degradation [15], we also analyzed cell samples after a 6-h exposure to sarcosine but no effect was seen at this early stage. The apparent inability of sarcosine to directly activate HER2/neu might be explained by lack of ligands with ability to directly activate this receptor [16,17]. Additionally, visualization of living LNCaP cells using confocal microscopy confirmed the increase in HER2/neu expression; addition of sarcosine in our study did not seem to impair on cell proliferation or morphology, as judged by inspection using differential interference contrast (DIC) (not shown).
Sreekumar et al. demonstrated that activation of the sarcosine pathway is directly linked to AR and ETS gene fusion regulation [10]. Berger et al. demonstrated that regulation of HER2/neu in LNCaP cells is androgen dependent: in an androgen deficient environment, HER2/neu is upregulated [13]. The postulated connections between AR and HER2/neu [13], together with our findings, prompted us to study the effect of sarcosine on the expression of AR in the androgen-dependent LNCaP cells. No effect was found on AR expression. Furthermore, inhibition of the AR with the antiandrogen bicalutamide did not influence the increased HER2/neu expression. Thus, the exact cellular mechanisms by which sarcosine induces increased HER2 expression in androgen-dependent prostate cells remains to be explained, and is currently under investigation.
The ongoing discussion regarding sarcosine as a possible new biomarker in prostate cancer screening questions the promising results of the initial study by Sreekumar in 2009 [10]. Very recently, Jentzmik et al. re-evaluated the potential of sarcosine as a biomarker for early prostate cancer detection [18]. In contrast to Sreekumar et al., the authors found that sarcosine in urine after rectal digital examination cannot be considered as a suitable marker to differentiate between patients with and without prostate cancer [18]. However, these two studies differ at several levels which may explain the conflicting results [19]. Moreover, regardless the evaluation of sarcosine as a biomarker of prostate cancer, our results on the effect of sarcosine on HER2/neu expression, together with the findings in the Sreekumar study, indicates a role for sarcosine in prostate cancer progression.
In the recent years, much focus has been on personalized medicine, whereby pharmaceutical therapies are tailored to the particular characteristics of the individual patient. Our results support a role of HER2/neu in prostate cancer, and increased HER2/neu expression in prostate cancer represents a potential target for both molecular imaging and targeted therapy [20,6]. However, considerable research is needed for personalized medicine to be routinely used in prostate cancer.
CONCLUSIONS
We herein report on a highly significant upregulation of HER2/neu in androgen-dependent prostate cancer cells upon exposure to exogenous sarcosine, indicating that sarcosine may be involved in regulation of HER2/neu. However, detailed information on downstream cellular mechanisms remains to be elucidated. Our results are in line with the previously suggested role of HER/neu in prostate cancer. Increased HER2/neu expression may be a potential target for future treatment strategies in prostate cancer patients.
Acknowledgments
We thank Majken Madvig Jansen (Cancer & Molecular Imaging Unit, Dept. of Clin. Biochemistry, Koege Hospital, Denmark) for expert technical assistance. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. This work was sponsored by the Foundation of Holger K. Christiansen Foundation, Denmark, Grant number: 74272KB; Foundation of Region of Zealand, Denmark, Grant number: 1-01-83-0011-07. The contribution of JC and GKM to this work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Reference List
- 1.Slamon D, Clark G, Wong S, Levin W, Ullrich A, McGuire W. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Slamon D, Godolphin W, Jones L, Holt JA, Wong S, Keith D, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 3.Merkhofer EC, Cogswell P, Baldwin AS. Her2 activates NF-kappaB and induces invasion through the canonical pathway involving IKKalpha. Oncogene. 2010;29:1238–1248. doi: 10.1038/onc.2009.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlsson J. EGFR-family expression and implications for targeted radionuclide therapy. In: Stigbrand T, Carlsson J, Adamas G, editors. Targeted Radionuclide Tumor Therapy. Springer; 2008. pp. 25–58. [Google Scholar]
- 5.Koumakpayi I, Le Page C, Mes-Masson A, Saad F. Hierarchical clustering of immunohistochemical analysis of the activated ErbB/PI3K/Akt/NF-kappaB signalling pathway and prognostic significance in prostate cancer. Br J Cancer. 2010;102:1163–1173. doi: 10.1038/sj.bjc.6605571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minner S, Jessen B, Stiedenroth L, Burandt E, Kollermann J, Mirlacher M, et al. Low level HER2 overexpression is associated with rapid tumor cell proliferation and poor prognosis in prostate cancer. Clin Cancer Res. 2010;16:1553–1560. doi: 10.1158/1078-0432.CCR-09-2546. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y, Brands F, Chatterjee S, Feng A, Groshen S, Schewe J, et al. Her-2/neu expression in prostate cancer: high level of expression associated with exposure to hormone therapy and androgen independent disease. J Urol. 2001;166:1514–1519. doi: 10.1016/s0022-5347(05)65822-3. [DOI] [PubMed] [Google Scholar]
- 8.Signoretti S, Montironi R, Manola J, Altimari A, Tam C, Bubley G, et al. Her-2-neu expression and progression toward androgen independence in human prostate cancer. Cancer Inst. 2000;92:1918–1925. doi: 10.1093/jnci/92.23.1918. [DOI] [PubMed] [Google Scholar]
- 9.Shariat S, Bensalah K, Karam J, Roehrborn C, Gallina A, Lotan Y, et al. Preoperative plasma HER2 and epidermal growth factor receptor for staging and prognostication in patients with clinically localized prostate cancer. Clin Cancer Res. 2007;13:5377–5384. doi: 10.1158/1078-0432.CCR-07-0330. [DOI] [PubMed] [Google Scholar]
- 10.Sreekumar A, Poisson L, Rajendiran T, Khan A, Cao Q, Yu J, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–915. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Lyakhov I, Zielinski R, Kuban M, Kramer-Marek G, Fisher R, Chertov O, et al. HER2-and EGFR-specific Affiprobes: novel recombinant optical probes for cell imaging. ChemBioChem. 2009;10:1–7. doi: 10.1002/cbic.200900532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger R, Lin DI, Nieto M, Sicinska E, Garraway LA, Adams H, et al. Androgen-dependent regulation of Her-2/neu in prostate cancer cells. Cancer Res. 2006;66:5723–5728. doi: 10.1158/0008-5472.CAN-05-3928. [DOI] [PubMed] [Google Scholar]
- 14.Horoszewics J, Leong S, Kawinski E, Karr J, Rosenthal H, Chu T, et al. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–1818. [PubMed] [Google Scholar]
- 15.Cai C, Portnoy D, Wang H, Jiang X, Chen S, Balk S. Androgen receptor expression in prostate cancer cells is suppressed by activation of epidermal growth factor receptor and ErbB2. Cancer Res. 2009;69:5202–5209. doi: 10.1158/0008-5472.CAN-09-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubin I, Yarden Y. The basic biology of HER2. Ann Onc. 2001;12:S3–S8. doi: 10.1093/annonc/12.suppl_1.s3. [DOI] [PubMed] [Google Scholar]
- 17.Shepard H, Brdlik C, Schreiber H. Signal integration: a framework for understanding the efficacy of therapeutics targeting the human EGFR family. J Clin Inv. 2008;118:3574–3581. doi: 10.1172/JCI36049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jentzmik F, Stephan C, Miller K, Schrader M, Erbersdobler A, Kristiansen G, Lein M, Jung K. Sarcosine in Urine after Digital Rectal Examination Fails as a Marker in Prostate Cancer Detection and Identification of Aggressive Tumours. Curr Opin Oncol. 2010 doi: 10.1016/j.eururo.2010.01.035. In press. [DOI] [PubMed] [Google Scholar]
- 19.Schalken J. Is urinary sarcosine useful to identify patients with significant prostate cancer? The trials and tribulations of biomarker development. Curr Opin Oncol. 2010 doi: 10.1016/j.eururo.2010.02.025. In press. [DOI] [PubMed] [Google Scholar]
- 20.Bouchelouche K, Capala J. ‘Image and treat’: an individualized approach to urological tumors. Curr Opin Oncol. 2010 doi: 10.1097/CCO.0b013e3283373d5c. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]