Abstract
Hypoxia inducible factor (HIF)-1α-mediated gene activation in the renal medulla in response to high salt intake plays an important role in the control of salt sensitivity of blood pressure. High salt-induced activation of HIF-1α in the renal medulla is blunted in Dahl S rats. The present study determined whether the impairment of the renal medullary HIF-1α pathway was responsible for salt sensitive hypertension in Dahl S rats. Renal medullary HIF-1α levels were induced by either transfection of HIF-1α expression plasmid or chronic infusion of CoCl2 into the renal medulla, which was accompanied by increased expressions of anti-hypertensive genes, cyclooxygenase-2 and heme oxygenase-1. Overexpression of HIF-1α transgenes in the renal medulla enhanced the pressure natriuresis, promoted the sodium excretion and reduced sodium retention after salt overload. As a result, hypertension induced by 2-week high salt was significantly attenuated in rats treated with HIF-1α plasmid or CoCl2. These results suggest that an abnormal HIF-1α in the renal medulla may represent a novel mechanism mediating salt-sensitive hypertension in Dahl S rats and that induction of HIF-1α levels in the renal medulla could be a therapeutic approach for the treatment of salt-sensitive hypertension.
Keywords: pressure natriuresis, heme oxygenase-1, cyclooxygenase-2, sodium excretion
1. Introduction
Salt-sensitive hypertension accounts for 50% of hypertensive population[1–2]. Importantly, the salt sensitivity of blood pressure is closely associated with a much greater propensity to develop organ injuries in hypertension [2–4]. Mechanism for salt-sensitive hypertension is not fully understood. It is well documented that renal medullary function play an important role in the regulation of renal sodium excretion and arterial blood pressure, and that dysfunction in the renal medulla is involved in salt-sensitive hypertension[5–10]. We have recently demonstrated that the transcription factor hypoxia inducible factor (HIF)-1α-mediated gene activation in the renal medulla is an important adaptive mechanism in response to high salt intake, which leads to inductions of various protective factors in the renal medulla and promotes extra sodium excretion [11].
HIF-1α and some of its target genes, such as nitric oxide synthase (NOS), cyclooxygenase-2 (COX-2) and hemeoxygenase-1 (HO-1), are highly expressed in the renal medulla [6, 10, 12–15]. These HIF-1α target genes in the renal medulla are up-regulated in response to high salt intake [6, 13–16]. The products of these genes play critical roles in regulating renal medullary blood flow and tubular activity, and thereby maintaining the constancy of body fluid volume and arterial blood pressure [6, 10, 13–14, 17–19]. Interestingly, inhibition of these genes and/or the enzymes encoded by these genes within the renal medulla reduces sodium excretion and increases salt sensitivity of arterial blood pressure [6, 10, 13–14, 17–19]. We previously showed that high salt intake increased HIF-1α levels in the renal medulla [11], and that inhibition of HIF-1α blocked the activation of its target genes in the renal medulla in response to high salt intake and promoted sodium retention, consequently producing salt-sensitive hypertension[11]. This previous study was carried out in normotensive animals and suggested that HIF-1α-mediated gene regulation in the renal medulla represents an important molecular adaptive mechanism in response to high salt intake and plays a crucial role in the maintenance of sodium balance. However, it remains unknown whether renal medullary HIF-1α pathway is involved in the development of hypertension in salt-sensitive individuals.
Dahl salt sensitive hypertensive rat is a widely used genetic model of human salt-sensitive hypertension that exhibits many phenotypic characteristics in common with human hypertension [3, 20–23]. Renal medullary dysfunction is one of the major mechanisms for this rat strain to develop hypertension [7–10]. Most interestingly, the above protective genes regulated by HIF-1α has been shown to be impaired this animal model and deficiencies of these HIF-1α target genes in the renal medulla are considered to be responsible for the development of hypertension in Dahl S rats [9–10, 24–27]. We recently showed that upregulation of HIF-1α levels in response to high salt intake was blunted in the renal medulla in Dahl S rats [28]. We therefore hypothesized the abnormal responses of the above protective genes are due to a defect in renal medullary HIF-1α and that impairment in HIF-1α-mediated gene activation in the renal medulla is responsible for salt sensitive hypertension in Dahl S rats. In the present study, we induced the expression of HIF-1α levels in the renal medulla by overexpression of HIF-1α transgenes or infusion of CoCl2, a HIF-1α inducer, into the renal medulla and then determined the improvement of renal sodium handling and salt-sensitive hypertension in this animal model. Our results suggested that restoration of the deficit in HIF-1α-mediated gene activation in the renal medulla attenuated salt-sensitive hypertension through the improvement of sodium excretion in Dahl S rats.
2. Materials and Methods
2.1. Animal
Experiments were performed in male Dahl S rats (Charles River, Wilmington, MA), weighing 250–350 g. Animals were kept on a low salt diet (0.4%NaCl) and some of them were fed with a high salt diet (4% NaCl) during experiments as indicated in the results section. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Virginia Commonwealth University.
2.2. Transfection of DNA into the renal medulla
Rats were uninephrectomized one week before, and the remaining left kidney was transfected with plasmids encoding human HIF-1α (50 μg) (Addgene, Cambridge, MA) into the renal medulla using in vivo-jetPEI™ (Polyplus-transfection, New York, NY), a polyethylenimine derivative, in combination with ultrasound radiation as we described before [28]. We used human HIF-1α plasmids because that rat HIF-1α plasmids were not available and that the hypoxia response elements in HIF-1α target genes are conservative among human-rat-mouse [29–30]. We also confirmed that transfection of these human HIF-1α plasmids into cultured rat cells remarkably increased the expression of HIF-1α target genes HO-1 and COX-2 in preliminary experiment. Plasmids expressing luciferase were used in control animals.
2.3. Chronic renal medullary infusion of CoCl2
The rats were uninephrectomized and anesthetized as above. To implant the renal medullary infusion catheter, the left kidney was exposed by a flank incision (1–1.5 cm), and a medullary interstitial catheter (tapered tip, 4–5mm) was implanted into the kidney. The catheter was made with a number of circular “pig-tail” bends, which prevented the catheter from being pulled out of the kidney during normal movement of the animal. The catheter was anchored into place on the kidney surface with Vetbond Tissue Adhesive (3M) and a small piece of fat tissue. These catheters were tunneled to the back of neck and connected to an osmotic pump (ALZET, model 2ML2), which contained CoCl2 (2 mmol/L) and was implanted subcutaneously. This technique has been successfully used for chronic infusion into the kidneys previously [31–34]. We also confirmed the successfully chronic infusion using this method by visualizing the delivery of a red dye into the renal medulla and checking no solution left in the pump in preliminary experiments. At the end of experiment, kidneys were removed and rapidly dissected into the renal cortex and medulla and then frozen in liquid N2. The precise location of interstitial infusion catheter was determined when dissecting kidney tissue. No solution remained in the osmotic pump was also checked and confirmed at the end.
2.4. Measurement of pressure natriuresis in response to the elevations of renal perfusion pressure
Animals were transfected with HIF-1α or control plasmids as described above and maintained on low salt diet. Ten days after transfection, pressure natriuresis studies were performed as described previously [6, 35].
2.5. Measurement of urinary sodium excretion in response to acute sodium loading
Additional groups of animals transfected with HIF-1α or control plasmids as above were surgically prepared similar to that in the pressure natriuresis studies and received a continuous infusion of 0.9% NaCl solution containing 2% albumin at a rate of 1ml/hr/100 g BW throughout the experiment to replace fluid loss. After 1-hour equilibration and two 10-min control period sample collections, a 5% body weight isotonic saline load was administered intravenously and three 10-min samples were collected over 30 minutes [11, 36], and then three more 10-min post-control samples were taken. Urinary volume and sodium excretion were measured.
2.6. Measurement of daily sodium balance
Additional groups of animals the same as above were housed in metabolic cages and daily indexes of sodium balance were computed by subtracting urinary sodium excretion from total sodium intake. After 1 day of control measurements, the animals were switched from tap water to 2% NaCl water and experimental measurements were continued for 3 days [37–38].
2.7. Chronic monitoring of arterial blood pressure in conscious rats
A telemetry transmitter (Data Sciences International) was implanted for the measurement of mean arterial blood pressure (MAP) as we described previously [6]. After baseline MAP was recorded on 3 consecutive control days while the rats remained on low salt diet, animals were switched to high salt diet (Dyets, Inc) and MAP was recorded for additional 2 weeks. Four groups of animals, including rats treated with control plasmids (Ctrl) + low salt diet (LS), Ctrl + high salt diet (HS), HIF-1α plasmids + HS and CoCl2 + HS, were examined. At the end of experiment, renal tissues were collected for protein and RNA isolation later.
2.8. Preparation of tissue homogenate and nuclear extracts and Western blot analyses for protein levels of HIF-1α
Renal tissue homogenates and nuclear protein were prepared, and Western blot analyses were performed as described previously [39–40]. Species reactivity of the primary antibody used in the present study included both rat and human (monoclonal, Novus Biologicals, 1:300 dilution). The intensities of the blots were determined using an imaging analysis program (ImageJ, free download from http://rsbweb.nih.gov/ij/).
2.9. RNA extraction and quantitative RT-PCR analysis of heme oxygenase (HO)-1 and cyclooxygenase (COX)-2 mRNA
Total RNA from renal medulla was extracted using TRIzol solution (Life Technologies, Inc. Rockville MD) and then reverse-transcribed (RT) (cDNA Synthesis Kit, Bio-Rad, Hercules, CA). The RT products were amplified using TaqMan Gene Expression Assays kits (Applied Biosystems). The level of 18S ribosomal RNA was used as an endogenous control. The relative gene expressions were calculated in accordance with the Δ ΔCt method. Relative mRNA levels were expressed by the values of 2−Δ Δ Ct.
2.10. Statistics
Data are presented as means ± SE. The significance of differences in mean values within and between multiple groups was evaluated using an ANOVA followed by a Duncan’s multiple range test. Student’s t-test was used to evaluate statistical significance of differences between two groups. P<0.05 was considered statistically significant.
3. Results
3.1. Effect of renal medullary transfection of HIF-1α or CoCl2 infusion on the levels of HIF-1α and its target genes in the renal medulla
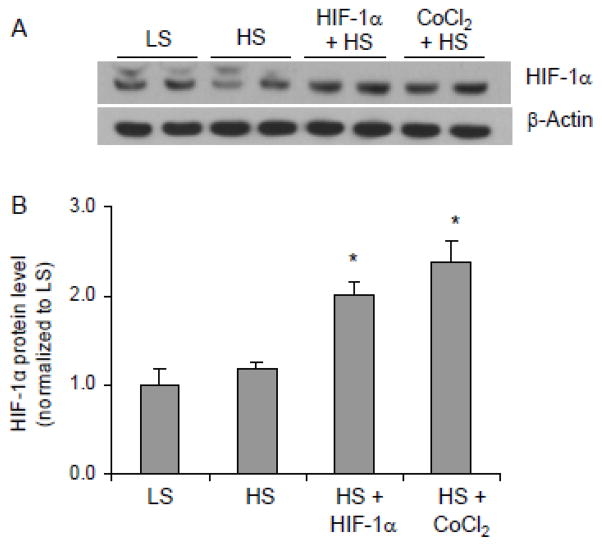
As shown in figure 1, high salt intake failed to significantly upregulate HIF-1α protein levels in the renal medulla in this animal strain, which was consistent with previous report [28]. However, HIF-1α levels were significantly increased in the renal medulla from rats treated with HIF-1α plasmids or CoCl2 after high salt diet (Fig. 1). The mRNA levels of two important HIF-1α target genes, HO-1 and COX2, in the renal medulla were shown in figure 2. Similar to the patterns of HIF-1α protein levels, both HO-1 and COX2 transcriptions were remarkably activated in rats treated with HIF-1α plasmids or CoCl2. Although the increases of these HIF-1α target genes in high salt alone group were statistically significant, these responses to high salt intake were marginal and considerably blunted compared with the responses of 3–4 fold increases in normal rats [11]. Treatments with HIF-1α plasmids and CoCl2 recovered the impaired responses of these protective genes transcriptions after high salt challenge. These results verified the successful induction of HIF-1α-mediated gene activation in the renal medulla by HIF-1α plasmids or CoCl2.
Figure 1. Effects of renal medullary transfection of HIF-1α transgene or CoCl2 infusion on HIF-1α levels in the renal medulla.
A: Representative ECL gel documents of Western blot analyses depicting the protein levels of HIF-1α. B: Summarized intensities of the HIF-1α blots (ratio to β-actin). * P < 0.05 vs. control (n=6). LS = low salt + control vectors, HS = high salt + control vectors, HIF-1α = HIF-1α expression vectors, CoCl2 = CoCl2 infusion..
Figure 2. Effect of HIF-1α transgene overexpression or CoCl2 infusion on the mRNA levels of HIF-1α target genes HO-1 and COX2 in the renal medulla.
* P < 0.05 vs. others including LS group (n=6).
3.2 Effects of renal medullary transfection of HIF-1α transgenes on pressure natriuresis in response to the elevations of renal perfusion pressure (RPP)
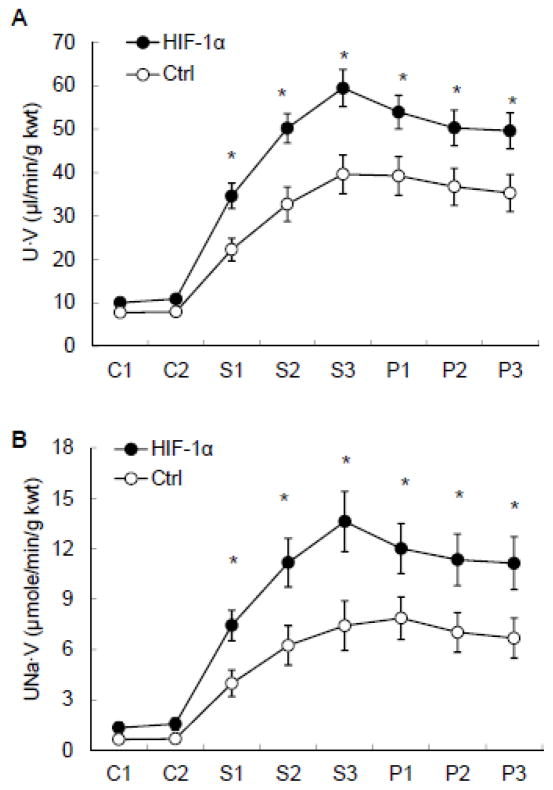
Both the urine flow and urinary sodium excretion rates were increased in response to the elevation of RPP. However, these pressure diuretic and natriuretic responses were significantly enhanced in HIF-1α plasmids-transfected rats compared with the control group (Fig. 3).
Figure 3. Effects of renal medullary transfection of HIF-1α transgene on pressure natriuresis.
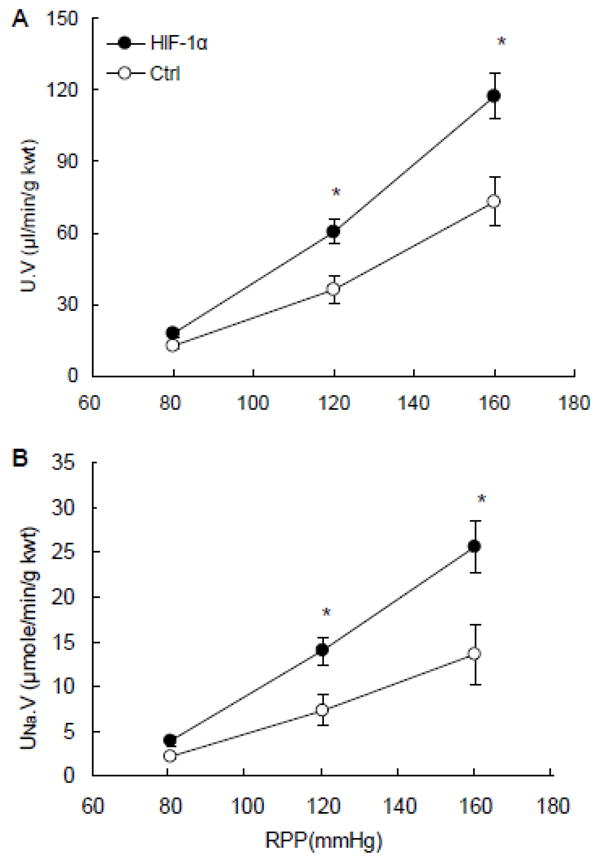
A: urine flow rates (U·V) in response to the elevations of renal perfusion pressure (RPP). B: urinary sodium excretion rates (UNa·V) in response to the elevations of RPP. * P < 0.05 vs. control (n=6).
3.3. Effects of renal medullary transfection of HIF-1α transgenes on urinary sodium excretion in response to acute sodium loading
Acute sodium loading increased urine volume (U·V) and urinary sodium excretion (UNa·V). These increases in U·V and UNa·V were considerably enhanced in rats treated with HIF-1α plasmids compared with control (Fig. 4).
Figure 4. Effects of renal medullary transfection of HIF-1α transgene on U·V (A) and UNa·V (B) in response to acute Na+ loading.
* P < 0.05 vs. control (n=6). S=sodium loading; P=post sodium loading.
3.4. Effects of renal medullary transfection of HIF-1α transgeness on salt balance
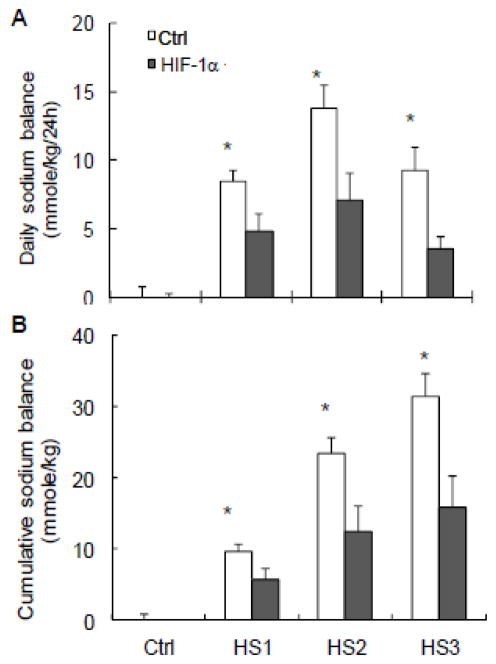
High salt intake induced a positive daily and cumulative salt balance. The daily positive salt balances were progressively increased in the first two days and decreased on the third day of high salt intake. The high salt-induced positive salt balance was significantly attenuated in rats treated with HIF-1α plasmids compared with control rats (Fig. 5).
Figure 5. Effects of renal medullary transfection of HIF-1α transgene on salt balances.
A: daily sodium balance. B: cumulative sodium balance. * P < 0.05 vs. HIF-1α (n=6).
3.5. Effects of renal medullary transfection of HIF-1α transgenes or infusion of CoCl2 on arterial blood pressure
There was no difference in baseline mean arterial pressure (MAP) among animas treated with control and HIF-1α plasmids as well as CoCl2 when the animals were fed with a low salt diet. After the rats were challenged with a high salt diet, the MAP were progressively increased from 113 ± 0.9 to 150 ± 7.03 mmHg in control rats. Both treatments of HIF-1α plasmids and CoCl2 remarkably blocked the HS-induced increase in MAP. MAP was only increased to 129 ± 3.1 mmHg in both HIF-1α plasmid-and CoCl2-treated groups by the end of the experiment (Fig. 6).
Figure 6. Effects of renal medullary transfection of HIF-1α transgene or CoCl2 infusion.

on mean arterial pressure (MAP). * P < 0.05 vs. others. (n=6).
4. Discussion
The present study demonstrated that induction of HIF-1α-mediated gene activation in the renal medulla stimulated the expression of anti-hypertensive genes in the renal medulla, and consequently enhanced the urinary sodium excretion in response to the elevations of RPP and sodium overloading, reduced sodium retention, as a result, attenuated the salt-sensitive hypertension in Dahl S rats.
Our results showed that local delivery of HIF-1α plasmids or CoCl2 substantially up-regulated the levels of HIF-1α and enhanced the transcription of its target genes in the renal medulla, which validated the manipulation of HIF-1α-mediated gene regulation in the renal medulla and allowed us to evaluate the contribution of HIF-1α-mediated gene activation in the development of hypertension in response to high salt intakes in Dahl S rats.
We first determined the effects of up-regulation in HIF-1α-mediated gene activation on pressure natriuresis. Renal medullary function plays an important role in the regulation of pressure natriuresis [8, 41–43] and several HIF-1α target genes such as HO-1, COX-2 and NOS have been reported to be crucial regulators in renal medullary function and sodium excretion, as well as pressure natriuresis [12, 43–46]. Pressure natriuresis has been shown to be significantly blunted in Dahl S rats [47–50]. Interestingly, renal medullary levels of the above enzymes are much lower [9, 24–25] and their responses to high salt diet are diminished [9–10, 26–27] in Dahl S rats. Increase of HIF-1α levels would be expected to activate the transcriptions of these HIF-1α target genes in the renal medulla, thereby improving pressure natriuresis relationship. Our data showed that transfection of HIF-1α plasmids into the renal medulla significantly enhanced the pressure natriuresis, suggesting that impaired HIF-1α pathway may be responsible for the renal medullary dysfunction in Dahl S rats. Since the products of the enzymes encoded by these HIF-1α target genes have been shown to dilate the medullary vasculature and inhibit the tubular activities [8, 41–42, 51], the effect of HIF-1α-mediated pathway on pressure natriuresis may be through both vascular and tubular actions.
To further evaluate the impact of renal medullary HIF-1α defect on salt handling, we examined the sodium excretion after acute sodium loading and salt balance after chronic sodium challenge. Because high salt-induced up-regulation of HIF-1α levels in the renal medulla is blunted in Dahl S rats, the impairment in those anti-hypertensive factors, such as COX2 and HO-1, in the renal medulla is probably attributed to the defect in HIF-1α response after high salt challenge in this animal model. Correction of the defect in HIF-1α-mediated gene activation in the renal medulla would improve the salt handling in Dahl S rats. The results from these extra sodium loading experiments demonstrated that restoration of renal medullary HIF-1α levels remarkably improved the capability of the kidneys to remove extra sodium load, which reduced sodium retention. These data additionally suggest that deficiency in enal medullary HIF-1α pathway may contribute to the impaired regulation of sodium excretion in Dahl S rats.
Since pressure-natriuresis and normal renal medullary function are key determinants to the long-term control of arterial blood pressure [7–8, 41–42, 52], the improvement in sodium excretions in responses to RPP and extra sodium loading would lead to an decrease in MAP in response to high salt intake in Dahl S rats. To test this hypothesis, we compared MAPs between animals transfected with HIF-1α and control plasmids into the renal medulla. It was found that high salt-induced increase in MAP was significantly blocked in HIF-1α plasmids-treated rats. An alternative way to induce HIF-1α by CoCl2 infusion achieved a similar result to block high salt-induced hypertension. It has been shown that high salt-induced activation of HIF-1α-regualted pathways is considered as an adaptive mechanism to high salt intake, which leads to an induction of various protective factors and promotes extra sodium excretion [11]. Therefore, deficiency of HIF-1α-mediated gene transcription in the renal medulla may decrease the production of various protective factors, impair renal medullary function, damage the capability of the kidneys to remove extra sodium load, consequently disrupt salt adaptation and increase the salt sensitivity of arterial blood pressure in Dahl S rats. This deficiency in HIF-1α-mediated gene activation may represent an important mechanism for the development of salt sensitive hypertension. Induction of HIF-1α in the renal medulla may restore the molecular adaptation to high salt intake and stimulate the production of different renal medullary protective or antihypertensive factors, thereby, attenuate salt-sensitive hypertension.
The present study did not attempt to explore the mechanisms that caused the impaired HIF-1α response to high salt in Dahl rats. In this regard, HIF-prolyl hydroxylases, the enzymes that promote the degradation of HIF-1α, may be accountable for it. HIF prolyl-hydroxylases catalyze site-specific proline hydroxylation of HIF-1α and then the hydroxylated HIF-1α is recognized and targeted for degradation by the ubiquitin-proteasome pathway [53–54]. Three isoforms of HIF prolyl-hydroxylase, including prolyl hydroxylase domain-containing proteins 1, 2, and 3 (PHD1, 2, and 3), have been identified [53, 55–56]. PHDs are present in the kidneys with PHD2 as the predominant isoform [39, 57–60] and PHD2 is most abundantly expressed in the renal medulla [39, 60]. It has been shown that high salt-induced inhibition of PHD2 in the renal medulla may be the upstream mediator for the activation of renal medullary HIF-1α in response to high salt challenge [28]. A defect in renal medullary PHD2 could be responsible for the impairment of HIF-1α-mediated salt adaptive pathway in Dahl S rats [28]. Detailed mechanisms associated with renal medullary PHD2 in salt adaptation need to be clarified in future investigations.
In summary, the present study demonstrated that up-regulation of HIF-1α levels in the renal medulla stimulated the transcription of enzymes that produce anti-hypertensive factor in the renal medulla, which corrected the defect in HIF-1α-mediated renal adaptation in response to high salt intake. As a result, this correction improved sodium excretion and attenuated salt-sensitive hypertension in Dahl S rats. It is concluded that deficiency in HIF-1α-mediated gene activation may be responsible for the hypertension in Dahl S rats and correction of this defect may be used to as a therapeutic strategy for salt-sensitive hypertension.
Highlights.
High salt-induced HIF-1α activation in the renal medulla is blunted in Dahl S rat.
Induction of HIF-1α increased the expressions of anti-hypertensive genes.
Induction of HIF-1α in the renal medulla enhanced sodium excretion.
Induction of HIF-1α in the renal medulla attenuated salt-sensitive hypertension.
Abnormal HIF-1α in the renal medulla is the cause for salt-sensitive hypertension.
Acknowledgments
Support: National Institutes of Health Grant HL89563 and HL106042
Footnotes
Conflict of Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8:II127–134. doi: 10.1161/01.hyp.8.6_pt_2.ii127. [DOI] [PubMed] [Google Scholar]
- 2.Chrysant GS, Bakir S, Oparil S. Dietary salt reduction in hypertension--what is the evidence and why is it still controversial? Prog Cardiovasc Dis. 1999;42:23–38. doi: 10.1016/s0033-0620(99)70007-1. [DOI] [PubMed] [Google Scholar]
- 3.Campese V. Salt sensitivity in hypertension. Renal and cardiovascular implications [clinical conference] Hypertension. 1994;23:531–550. doi: 10.1161/01.hyp.23.4.531. [DOI] [PubMed] [Google Scholar]
- 4.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt Sensitivity, Pulse Pressure, and Death in Normal and Hypertensive Humans. Hypertension. 2001;37:429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 5.Cowley AWMD, Jr, Lu S, Roman RJ. The renal medulla and hypertension. Hypertension. 1995;25:663–673. doi: 10.1161/01.hyp.25.4.663. [DOI] [PubMed] [Google Scholar]
- 6.Li N, Yi F, dos Santos EA, Donley DK, Li P-L. Role of Renal Medullary Heme Oxygenase in the Regulation of Pressure Natriuresis and Arterial Blood Pressure. Hypertension. 2007;49:148–154. doi: 10.1161/01.HYP.0000250086.06137.fb. [DOI] [PubMed] [Google Scholar]
- 7.Bergstrom G, Evans RG. Mechanisms underlying the antihypertensive functions of the renal medulla. Acta Physiologica Scandinavica. 2004;181:475–486. doi: 10.1111/j.1365-201X.2004.01321.x. [DOI] [PubMed] [Google Scholar]
- 8.Mattson DL. Importance of the renal medullary circulation in the control of sodium excretion and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2003;284:R13–27. doi: 10.1152/ajpregu.00321.2002. [DOI] [PubMed] [Google Scholar]
- 9.Szentivanyi M, Jr, Zou A-P, Mattson DL, Soares P, Moreno C, Roman RJ, Cowley AW., Jr Renal medullary nitric oxide deficit of Dahl S rats enhances hypertensive actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2002;283:R266–272. doi: 10.1152/ajpregu.00461.2001. [DOI] [PubMed] [Google Scholar]
- 10.Tan DY, Meng S, Cason GW, Manning RD., Jr Mechanisms of salt-sensitive hypertension: role of inducible nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2297–2303. doi: 10.1152/ajpregu.2000.279.6.R2297. [DOI] [PubMed] [Google Scholar]
- 11.Li N, Chen L, Yi F, Xia M, Li P-L. Salt-Sensitive Hypertension Induced by Decoy of Transcription Factor Hypoxia-Inducible Factor-1{alpha} in the Renal Medulla. Circ Res. 2008;102:1101–1108. doi: 10.1161/CIRCRESAHA.107.169201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou A-P, Billington H, Su N, Cowley AW., Jr Expression and Actions of Heme Oxygenase in the Renal Medulla of Rats. Hypertension. 2000;35:342–347. doi: 10.1161/01.hyp.35.1.342. [DOI] [PubMed] [Google Scholar]
- 13.Mattson DL, Higgins DJ. Influence of Dietary Sodium Intake on Renal Medullary Nitric Oxide Synthase. Hypertension. 1996;27:688–692. doi: 10.1161/01.hyp.27.3.688. [DOI] [PubMed] [Google Scholar]
- 14.Zewde T, Mattson DL. Inhibition of Cyclooxygenase-2 in the Rat Renal Medulla Leads to Sodium-Sensitive Hypertension. Hypertension. 2004;44:424–428. doi: 10.1161/01.HYP.0000140924.91479.03. [DOI] [PubMed] [Google Scholar]
- 15.Yang T, Singh I, Pham H, Sun D, Smart A, Schnermann JB, Briggs JP. Regulation of cyclooxygenase expression in the kidney by dietary salt intake. Am J Physiol Renal Physiol. 1998;274:F481–489. doi: 10.1152/ajprenal.1998.274.3.F481. [DOI] [PubMed] [Google Scholar]
- 16.Harris RC, Breyer MD. Physiological regulation of cyclooxygenase-2 in the kidney. Am J Physiol Renal Physiol. 2001;281:F1–11. doi: 10.1152/ajprenal.2001.281.1.F1. [DOI] [PubMed] [Google Scholar]
- 17.Yao B, Harris RC, Zhang M-Z. Interactions between 11{beta}-hydroxysteroid dehydrogenase and COX-2 in kidney. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1767–1773. doi: 10.1152/ajpregu.00786.2004. [DOI] [PubMed] [Google Scholar]
- 18.Mattson DL, Maeda CY, Bachman TD, Cowley AW., Jr Inducible Nitric Oxide Synthase and Blood Pressure. Hypertension. 1998;31:15–20. doi: 10.1161/01.hyp.31.1.15. [DOI] [PubMed] [Google Scholar]
- 19.Nakanishi K, Hara N, Nagai Y. Salt-sensitive hypertension in conscious rats induced by chronic nitric oxide blockade. American Journal of Hypertension. 2002;15:150–156. doi: 10.1016/s0895-7061(01)02267-1. [DOI] [PubMed] [Google Scholar]
- 20.Rapp JP. Dahl salt-susceptible and salt-resistant rats. A review. Hypertension. 1982;4:753–763. doi: 10.1161/01.hyp.4.6.753. [DOI] [PubMed] [Google Scholar]
- 21.Tobian L. Salt and hypertension. Lessons from animal models that relate to human hypertension. Hypertension. 1991;17:I52–58. doi: 10.1161/01.hyp.17.1_suppl.i52. [DOI] [PubMed] [Google Scholar]
- 22.Rapp JP. Genetic Analysis of Inherited Hypertension in the Rat. Physiol Rev. 2000;80:135–172. doi: 10.1152/physrev.2000.80.1.135. [DOI] [PubMed] [Google Scholar]
- 23.Cowley AW., Jr The genetic dissection of essential hypertension. Nat Rev Genet. 2006;7:829–840. doi: 10.1038/nrg1967. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda Y, Saito K, Kim J-I, Yokoyama M. Nitric Oxide Synthase Isoform Activities in Kidney of Dahl Salt-Sensitive Rats. Hypertension. 1995;26:1030–1034. doi: 10.1161/01.hyp.26.6.1030. [DOI] [PubMed] [Google Scholar]
- 25.Cowley AW, Jr, Mori T, Mattson D, Zou A-P. Role of renal NO production in the regulation of medullary blood flow. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1355–1369. doi: 10.1152/ajpregu.00701.2002. [DOI] [PubMed] [Google Scholar]
- 26.Yoshihara F, Suga S, Yasui N, Horio T, Tokudome T, Nishikimi T, Kawano Y, Kangawa K. Chronic administration of adrenomedullin attenuates the hypertension and increases renal nitric oxide synthase in Dahl salt-sensitive rats. Regul Pept. 2005;128:7–13. doi: 10.1016/j.regpep.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 27.Tojo A, Onozato ML, Kobayashi N, Goto A, Matsuoka H, Fujita T. Antioxidative effect of p38 mitogen-activated protein kinase inhibitor in the kidney of hypertensive rat. J Hypertens. 2005;23:165–174. doi: 10.1097/00004872-200501000-00027. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Zhu Q, Xia M, Li PL, Hinton SJ, Li N. Hypoxia-inducible factor prolyl-hydroxylase 2 senses high-salt intake to increase hypoxia inducible factor 1alpha levels in the renal medulla. Hypertension. 2010;55:1129–1136. doi: 10.1161/HYPERTENSIONAHA.109.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu J, Stiehl DP, Setzer C, Wichmann D, Shinde DA, Rehrauer H, Hradecky P, Gassmann M, Gorr TA. Molecular Cancer Research. 2011. Interaction of HIF and USF Signaling Pathways in Human Genes Flanked by Hypoxia-Response Elements and E-box Palindromes. [DOI] [PubMed] [Google Scholar]
- 31.Moore AF, Heiderstadt NT, Huang E, Howell NL, Wang Z-Q, Siragy HM, Carey RM. Selective Inhibition of the Renal Angiotensin Type 2 Receptor Increases Blood Pressure in Conscious Rats. Hypertension. 2001;37:1285–1291. doi: 10.1161/01.hyp.37.5.1285. [DOI] [PubMed] [Google Scholar]
- 32.Yamada M, Katsuma S, Adachi T, Hirasawa A, Shiojima S, Kadowaki T, Okuno Y, Koshimizu T-a, Fujii S, Sekiya Y, Miyamoto Y, Tamura M, Yumura W, Nihei H, Kobayashi M, Tsujimoto G. Inhibition of protein kinase CK2 prevents the progression of glomerulonephritis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7736–7741. doi: 10.1073/pnas.0409818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanada H, Yatabe J, Midorikawa S, Katoh T, Hashimoto S, Watanabe T, Xu J, Luo Y, Wang X, Zeng C, Armando I, Felder RA, Jose PA. Amelioration of Genetic Hypertension by Suppression of Renal G Protein-Coupled Receptor Kinase Type 4 Expression. Hypertension. 2006;47:1131–1139. doi: 10.1161/01.HYP.0000222004.74872.17. [DOI] [PubMed] [Google Scholar]
- 34.Yoneda M, Sanada H, Yatabe J, Midorikawa S, Hashimoto S, Sasaki M, Katoh T, Watanabe T, Andrews PM, Jose PA, Felder RA. Differential Effects of Angiotensin II Type-1 Receptor Antisense Oligonucleotides on Renal Function in Spontaneously Hypertensive Rats. Hypertension. 2005;46:58–65. doi: 10.1161/01.HYP.0000171587.44736.ba. [DOI] [PubMed] [Google Scholar]
- 35.Dos Santos EA, Dahly-Vernon AJ, Hoagland KM, Roman RJ. Inhibition of the formation of EETs and 20-HETE with 1-aminobenzotriazole attenuates pressure natriuresis. Am J Physiol Regul Integr Comp Physiol. 2004;287:R58–68. doi: 10.1152/ajpregu.00713.2003. [DOI] [PubMed] [Google Scholar]
- 36.DiBona GF, Sawin LL. Effect of Metoprolol Administration on Renal Sodium andling in Experimental Congestive Heart Failure. Circulation. 1999;100:82–86. doi: 10.1161/01.cir.100.1.82. [DOI] [PubMed] [Google Scholar]
- 37.Dibona GF, Jones SY, Sawin LL. Angiotensin receptor antagonist improves cardiac reflex control of renal sodium handling in heart failure. Am J Physiol Heart Circ Physiol. 1998;274:H636–641. doi: 10.1152/ajpheart.1998.274.2.H636. [DOI] [PubMed] [Google Scholar]
- 38.Li P, Morris M, Ferrario CM, Barrett C, Ganten D, Callahan MF. Cardiovascular, endocrine, and body fluid-electrolyte responses to salt loading in mRen-2 transgenic rats. Am J Physiol Heart Circ Physiol. 1998;275:H1130–1137. doi: 10.1152/ajpheart.1998.275.4.H1130. [DOI] [PubMed] [Google Scholar]
- 39.Li N, Yi F, Sundy CM, Chen L, Hilliker ML, Donley DK, Muldoon DB, Li P-L. Expression and actions of HIF prolyl-4-hydroxylase in the rat kidneys. Am J Physiol Renal Physiol. 2007;292:F207–216. doi: 10.1152/ajprenal.00457.2005. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Q, Wang Z, Xia M, Li PL, Van Tassell BW, Abbate A, Dhaduk R, Li N. Silencing of Hypoxia-Inducible Factor-1{alpha} Gene Attenuated Angiotensin II-Induced Renal Injury in Sprague-Dawley Rats. Hypertension. 2011;58:657–664. doi: 10.1161/HYPERTENSIONAHA.111.177626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Granger JP, Alexander BT, Llinas M. Mechanisms of pressure natriuresis. Curr Hypertens Rep. 2002;4:152–159. doi: 10.1007/s11906-002-0040-3. [DOI] [PubMed] [Google Scholar]
- 42.Evans RG, Majid DS, Eppel GA. Mechanisms mediating pressure natriuresis: what we know and what we need to find out. Clin Exp Pharmacol Physiol. 2005;32:400–409. doi: 10.1111/j.1440-1681.2005.04202.x. [DOI] [PubMed] [Google Scholar]
- 43.Gross JM, Dwyer JE, Knox FG. Natriuretic Response to Increased Pressure Is Preserved With COX-2 Inhibitors. Hypertension. 1999;34:1163–1167. doi: 10.1161/01.hyp.34.5.1163. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez F, Kemp R, Balazy M, Nasjletti A. Effects of Exogenous Heme on Renal Function: Role of Heme Oxygenase and Cyclooxygenase. Hypertension. 2003;42:680–684. doi: 10.1161/01.HYP.0000085785.40581.1A. [DOI] [PubMed] [Google Scholar]
- 45.Majid DS, Navar LG. Nitric oxide in the control of renal hemodynamics and excretory function. Am J Hypertens. 2001;14:74S–82S. doi: 10.1016/s0895-7061(01)02073-8. [DOI] [PubMed] [Google Scholar]
- 46.Majid DS, Navar LG. Nitric oxide in the mediation of pressure natriuresis. Clin Exp Pharmacol Physiol. 1997;24:595–599. doi: 10.1111/j.1440-1681.1997.tb02098.x. [DOI] [PubMed] [Google Scholar]
- 47.Roman RJ. Abnormal renal hemodynamics and pressure-natriuresis relationship in Dahl salt-sensitive rats. Am J Physiol Renal Physiol. 1986;251:F57–65. doi: 10.1152/ajprenal.1986.251.1.F57. [DOI] [PubMed] [Google Scholar]
- 48.Takenaka T, Suzuki H, Sakamaki Y, Itaya Y, Saruta T. Contribution of prostaglandins to pressure natriuresis in Dahl salt-sensitive rats. Am J Hypertens. 1991;4:489–493. doi: 10.1093/ajh/4.6.489. [DOI] [PubMed] [Google Scholar]
- 49.Patel A, Layne S, Watts D, Kirchner KA. L-arginine administration normalizes pressure natriuresis in hypertensive Dahl rats. Hypertension. 1993;22:863–869. doi: 10.1161/01.hyp.22.6.863. [DOI] [PubMed] [Google Scholar]
- 50.Kirchner KA, Crosby BA, Patel AR, Granger JP. Segmental chloride transport in the Dahl-S rat kidney during L-arginine administration. J Am Soc Nephrol. 1995;5:1567–1572. doi: 10.1681/ASN.V581567. [DOI] [PubMed] [Google Scholar]
- 51.Wang T, Sterling H, Shao WA, Yan Q, Bailey MA, Giebisch G, Wang W-H. Inhibition of heme oxygenase decreases sodium and fluid absorption in the loop of Henle. Am J Physiol Renal Physiol. 2003;285:F484–490. doi: 10.1152/ajprenal.00135.2003. [DOI] [PubMed] [Google Scholar]
- 52.Hall JE. The Kidney, Hypertension and Obesity. Hypertension. 2003;41:625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]
- 53.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 54.Jaakkola P, Mole DR, Tian Y-M, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim Av, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 55.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 56.Bruick RK, McKnight SL. A Conserved Family of Prolyl-4-Hydroxylases That Modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 57.Takeda K, Cowan A, Fong G-H. Essential Role for Prolyl Hydroxylase Domain Protein 2 in Oxygen Homeostasis of the Adult Vascular System. Circulation. 2007;116:774–781. doi: 10.1161/CIRCULATIONAHA.107.701516. [DOI] [PubMed] [Google Scholar]
- 58.Rosenberger C, Rosen S, Shina A, Frei U, Eckardt K-U, Flippin LA, Arend M, Klaus SJ, Heyman SN. Activation of hypoxia-inducible factors ameliorates hypoxic distal tubular injury in the isolated perfused rat kidney. Nephrol Dial Transplant. 2008;23:3472–3478. doi: 10.1093/ndt/gfn276. [DOI] [PubMed] [Google Scholar]
- 59.Takeda K, Aguila HL, Parikh NS, Li X, Lamothe K, Duan L-J, Takeda H, Lee FS, Fong G-H. Regulation of adult erythropoiesis by prolyl hydroxylase domain proteins. Blood. 2008;111:3229–3235. doi: 10.1182/blood-2007-09-114561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schodel J, Klanke B, Weidemann A, Buchholz B, Bernhardt W, Bertog M, Amann K, Korbmacher C, Wiesener M, Warnecke C, Kurtz A, Eckardt KU, Willam C. HIF-Prolyl Hydroxylases in the Rat Kidney: Physiologic Expression Patterns and Regulation in Acute Kidney Injury. Am J Pathol. 2009;174:1663–1674. doi: 10.2353/ajpath.2009.080687. [DOI] [PMC free article] [PubMed] [Google Scholar]