Abstract
Background
The choice between lower limit of normal or fixed value of forced expiratory volume in one second/forced vital capacity ratio (FEV1/FVC) < 0.70 as the criterion for confirming airway obstruction is an open issue. In this study, we compared the criteria of lower limit of normal and fixed FEV1/FVC for diagnosis of airway obstruction, with a focus on healthy elderly people.
Methods
We selected 367 healthy nonsmoking subjects aged 65–93 years from 1971 participants in the population-based SARA (Salute Respiratoria nell’Anziano, Italian for “Respiratory Health in the Elderly”) study, analyzed their spirometric data, and tested the relationship between spirometric indices and anthropometric variables. The lower limit of normal for FEV1/FVC was calculated as the fifth percentile of the normal distribution for selected subjects.
Results
While FEV1 and FVC decreased significantly with aging, the relationship between FEV1/FVC and age was not statistically significant in men or women. The lower limit of normal for FEV1/FVC was 0.65 in men and 0.67 in women. Fifty-five participants (15%) had FEV1/FVC < 0.70 and would have been inappropriately classified as obstructed according to the Global Initiative for Obstructive Lung Disease, American Thoracic Society/European Respiratory Society, and Canadian guidelines on chronic obstructive pulmonary disease. By applying different FEV1/FVC thresholds for the different age groups, as previously proposed in the literature, (0.70 for <70 years, 0.65 for 70–80 years, and 0.60 for >80 years) the percentage of patients classified as obstructed decreased to 6%. No subjects older than 80 years had an FEV1/FVC < 0.60.
Conclusion
The present results confirm the inadequacy of FEV1/FVC < 0.70 as a diagnostic criterion for airway obstruction after the age of 65 years. FEV1/FVC < 0.65 and <0.67 (for men and women, respectively) could identify subjects with airway obstruction in such a population. Further reduction of the threshold after 80 years is not justified.
Keywords: aging, airflow obstruction, chronic obstructive pulmonary disease, forced expiratory volume, lung function tests, spirometry
Introduction
The most appropriate way to diagnose airway obstruction is currently the subject of heated debate.1–10 Most national and international chronic obstructive pulmonary disease (COPD) guidelines recommend to use a forced expiratory volume in one second/forced vital capacity ratio (FEV1/FVC) of 0.70 as a suitable threshold value to define the presence of an obstructive ventilatory defect. Commonly used guidelines include those of the Global Initiative for Obstructive Lung Disease (GOLD),11 the American Thoracic Society/European Respiratory Society,12 the British Thoracic Society,13 the Canadian Thoracic Society,14 and the National Institute for Health and Clinical Excellence.15
However, there is evidence that the fixed cut-off value of 0.70 for FEV1/FVC becomes less specific in males aged > 40 years and females > 50 years, implying a risk of overestimation of airway obstruction. This is due to an age-related decline in pulmonary volumes, particularly in FEV1, which is observed even in healthy people with no history of exposure to noxious particles or gases.16–19 Proposed strategies for reducing the misclassification of airway obstruction include use of the lower limit of normal for FEV1/FVC, calculated as the fifth percentile of the normal distribution in a healthy population,20–22 or the use of different FEV1/FVC thresholds for different age groups (eg, 0.70 for subjects aged < 70 years, 0.65 for those aged 70–80 years, and 0.60 for those aged > 80 years).19
On this basis, a group of colleagues involved in respiratory research and/or the diagnosis and treatment of lung diseases recently wrote an open letter to members of the GOLD committee inviting them to change the method by which airway obstruction is defined and asking for retraction of the fixed ratio in favor of the lower limit of normal.1
The aim of the present study was to provide additional information for determination of the most appropriate spirometric criteria for confirming airway obstruction in the elderly, by describing lung function and calculating the lower limit of normal for FEV1/FVC in healthy nonsmoking elderly subjects (age > 65 years) who participated in the Italian multicenter SARA (acronym of Salute Respiratoria nell’Anziano, Italian for “Respiratory Health in the Elderly”) study. The degree of potential misclassification relative to use of 0.70 or other proposed fixed thresholds for FEV1/FVC was also evaluated.
Materials and methods
We performed a cross-sectional analysis of data from the SARA study, the design of which, along with technical characteristics of instruments as well as training of operators and results of quality control of spirometry, have been described in detail elsewhere.23 Briefly, the study involved 24 pulmonary or geriatric institutions distributed throughout Italy. A total of 1971 subjects aged 65–100 years were recruited as consecutive outpatients referred between January 1996 and December 1997 to the participating centers (see Appendix). The study design was approved by the ethics committee of the University of Palermo. Patients gave their written consent to participate in the study.
Lung function was measured using an identical fully computerized water-sealed Stead-Wells spirometer (Baires System, Biomedin, Padua, Italy) by specifically trained and certified personnel supervised by a rigorous real-time control of acceptability and repeatability23 according to American Thoracic Society recommendations.24 FVC maneuvers were performed with the patient sitting. The largest FVC and FEV1 were selected from a minimum of two acceptable tests. The FEV1/FVC ratio was calculated on the basis of the highest values of individual parameters obtained in the acceptable curves for each subject. Analyses were conducted only on patients with good repeatability of the above-mentioned indices (difference between two best values < 150 mL).25
Of the 1870 subjects who performed spirometry, we selected only those without any previous or present diagnosis or any sign or symptom suggestive of respiratory diseases according to the modified International Union against Tuberculosis and Lung Disease bronchial symptoms questionnaire.25,26 Current smokers and previous smokers with a smoking exposure > 5 pack/year were excluded, since the <5 pack/year smoking exposure was not significantly associated with decreased lung function.27 Additional exclusion criteria were: severe hepatic failure; severe renal failure; severe cardiac failure; cognitive and/or sensory impairment severe enough to affect a multidimensional assessment; severe kyphoscoliosis with occiput wall distance (distance between the occiput and the wall when the patient stands with heels and shoulder against the wall with the back straight) >10; occurrence of a major psychosocial event (eg, bereavement) within the past 6 months; and hospitalization for any reason within the past 6 months. We further excluded individuals who had hypertension (diastolic pressure ≥90 mmHg and/or systolic pressure ≥160 mmHg), diabetes, and/or major electrocardiographic abnormalities.
Statistical analyses were performed using SPSS (SPSS Inc, Chicago, IL) and Stata (Stata Corporation, College Station, TX) software packages. Regression models were used for testing the relationship between spirometric indices and anthropometric variables. Based on the recommendations from the American Thoracic Society/European Respiratory Society task force,22 the lower limit of normal for FEV1/FVC was estimated as the fifth percentile of its frequency distribution. To evaluate the effect of aging on spirometric measures independently of body height, FEV1 and FVC were normalized for height at the third power.28,29
Results
After applying the above-mentioned selection criteria, our final data set consisted of 367 healthy, nonsmoking subjects. One hundred and one subjects were excluded for lack of availability of lung function testing, a further 709 subjects because of a history of respiratory disease, 445 for significant smoking exposure (>5 pack/year), 262 for inadequate quality of spirometry, and 87 for the above-mentioned additional exclusion criteria.
Tables 1 and 2 show anthropometric and functional data for the sample and distribution of the participants according to age and gender. Although the most advanced ages were less represented, a total of 73 subjects aged 80 years and over were included. The sample consisted of 314 never-smokers (85.6%) and 53 former-smokers with a smoking exposure from 0.15 to 5 pack-years (mean ± standard deviation, 2.75 ± 1.5).
Table 1.
Anthropometric and functional characteristics of the study sample, with data expressed as the mean ± standard deviation
| Women | Men | |
|---|---|---|
| Age, years | 73.3 ± 6.2 | 74.2 ± 6.9 |
| Height, cm | 154.8 ± 7.0 | 167.8 ± 6.4 |
| Weight, kg | 63.1 ± 11.2 | 73.7 ± 11.2 |
| FEV1, mL | 1907 ± 474 | 2694 ± 586 |
| LVC, mL | 2532 ± 595 | 3688 ± 736 |
| FVC, mL | 2456 ± 604 | 3618 ± 743 |
Abbreviations: FEV1, forced expiratory volume in one second; FVC, forced vital capacity; LVC, lung volume change.
Table 2.
Distribution of subjects according to gender and age group, with data presented as n (%)
| Age (years) | Females | Males |
|---|---|---|
| All | 246 (67.0) | 121 (33.0) |
| 65–69 | 77 (31.3) | 35 (28.9) |
| 70–74 | 76 (30.9) | 34 (28.1) |
| 75–79 | 50 (20.3) | 22 (18.2) |
| ≥80 | 43 (17.5) | 30 (24.8) |
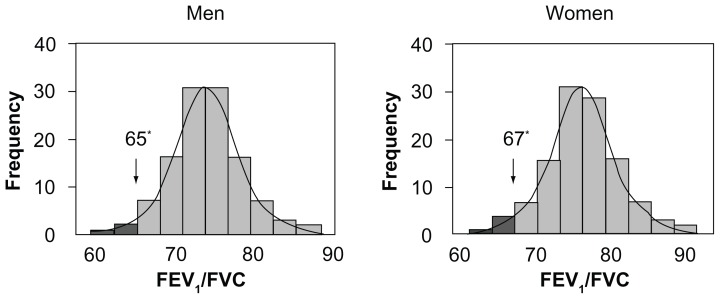
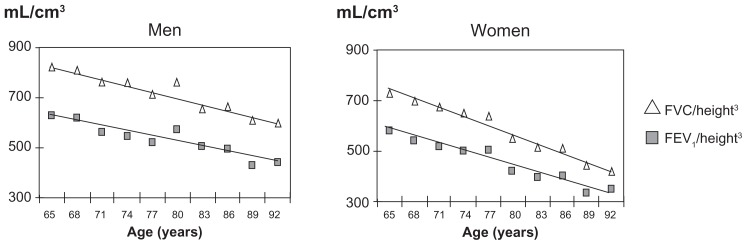
As shown in Figure 1, the values of FEV1/FVC showed a normal frequency distribution. The mean FEV1/FVC was 0.75 ± 0.6 in males and 0.78 ± 0.6 in females, whereas the corresponding fifth percentiles were 0.65 and 0.67, respectively, in males and females. FEV1 and FVC significantly decreased with age (r = −0.38 and −0.35; P < 0.001), while FEV1/FVC, was not significantly correlated with age or height in either gender group over the considered range of age (lowest P = 0.103). As a consequence, there was no rationale to develop reference equations for FEV1/FVC, with age or height as independent variables in this restricted range of age. Figure 2 shows the decline in FEV1 and FVC with increasing age observed in the study sample in men and women, after normalization for height.
Figure 1.
Distribution and fifth percentile of FEV1/FVC by gender (Caucasian Southern Europeans > 65 years).
Note: Asterisks indicate the 5th percentile.
Abbreviations: FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
Figure 2.
Decline of FEV1 and FVC with aging, normalized for height.3
Abbreviations: FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
In the sample of healthy subjects, 15% had a FEV1/FVC < 0.70, and would have been inappropriately classified as obstructed according to GOLD criteria. Table 3 describes the proportion of participants with the ratio < 70 in the different age groups. By applying the FEV1/FVC thresholds proposed by Hardie et al19 for different age groups (ie, 0.70 for <70 years, 0.65 for 70–80 years, and 0.60 for >80 years), the percentage of obstructed subjects decreased to 6% (men 11%, women 4%). In particular, the proportion of subjects aged 65–70 years with a ratio below 0.70 was 11% (men 16%, women 8%); the proportion of subjects between 70 and 80 years of age with the ratio below 0.65 was 5% (men 12%, women 2%), whereas none of the subjects aged 80 years or more had FEV1/FVC < 0.60.
Table 3.
Proportion of healthy elderly subjects with FEV1/FVC ratio < 0.70 in different age groups, with data presented as n (%)
| Age (years) | |||
|---|---|---|---|
|
|
|||
| 65–79 n = 294 | ≥80 n = 73 | All n = 367 | |
| FEV1/FVC ≥ 0.70 | 254 (86) | 59 (81) | 313 (85) |
| FEV1/FVC < 0.70 FEV1% ≥ 80 | 28 (10) | 8 (11) | 36 (10) |
| FEV1/FVC < 0.70 FEV1% < 80 | 12 (4) | 6 (8) | 18 (5) |
Abbreviations: FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
Discussion
This study provides additional evidence helpful for determining the most appropriate spirometric criteria to define airway obstruction in elderly subjects. The fifth percentile of FEV1/FVC observed in the considered sample of healthy subjects aged > 65 years was lower than 0.70 in both men and women, thus confirming the inadequacy of this threshold for FEV1/FVC after the age of 65 years. Moreover, the findings of the present study suggest that, in such a population, FEV1/FVC < 0.65 and <0.67 (for males and females, respectively) could represent valid criteria that are simple to use and incorporate the known physiological decline in lung function with aging.
The present findings are in agreement with observations made by other authors,16–18 who have emphasized that the use of the FEV1/FVC threshold proposed by GOLD leads to a risk of overdiagnosis of COPD in geriatric subjects. Furthermore, the current results suggest that the inaccuracy of using a threshold of 0.70 for FEV1/FVC already exists in subjects aged 65 years and older. This differs to some extent from what has been suggested by the most recent GOLD guidelines11 (that recognize some imprecision of the threshold of 0.70 for FEV1/FVC in people over 70 years of age) and by Medbo et al,10 who suggested the use of a threshold of FEV1/FVC < 0.65 in subjects over the age of 70 years on the basis of prebronchodilator spirometry data from a population-based study in Norway.
Interestingly, in our study, the rate of decline in the FEV1/FVC ratio with aging was not statistically significant in men or women older than 65 years, because of a concomitant decline in both FEV1 and FVC. Thus, in contrast with the findings of Hardie et al,19 our results do not support the need to decrease the lower limit of normal for FEV1/FVC to 0.60 in elderly subjects (>80 years). For the same reason, no predictive equation for FEV1/FVC could be derived; accordingly, the mean value and the fifth percentile of FEV1/FVC from this healthy population can be used as the predicted value and lower limit of normal, respectively, for people aged 65 years and over.
All reference equations derived from samples with a wide age range describe a progressive decline in the FEV1/FVC ratio with aging; the age-related decrease involves both FEV1 and FVC, seems to be nonlinear, and accelerates with aging.30–32 In 1982, Crapo et al33 found that between 20 and 70 years of age, vital capacity decreases to approximately 75% of the best values achieved previously. According to the present observations, presumably in the oldest people the decline of vital capacity accelerates more than in the younger age groups and such a decline is similar to the reduction of FEV1, so that the FEV1/FVC ratio could undergo minimal variations in the last decades. Recently, Langhammer et al34 and Falaschetti et al35 observed that FEV1/FVC reaches a near plateau phase in elderly subjects. The authors emphasized that, although the sample consisted of subjects with a wide range of height and age, the extremes did not influence the equations.
In a reference study specifically designed for elderly residents in Madrid (age range 65–85 years), Garcia et al36 found a significant relationship between FEV1/FVC and age in men and between FEV1/FVC, age and height in women. Even in this study, the predicted equation for FEV1/FVC had a very low R2 (0.048 and 0.083 for men and women, respectively) and the authors highlighted the strong negative relationship of FVC with age. By applying predictive equations recently derived from Kuster et al37 in a Swiss population, the lower limit of normal for FEV1/FVC does not show important decreases with aging; for example, in men with a height of 170 cm, the lower limit of normal for FEV1/FVC ranges from 0.66 at the age of 65 years to 0.64 for people aged 95 years. All these results support a position in favor of almost stable predicted values and lower limits of normal for FEV1/FVC in elderly people aged 65 years and over.
A clear position in favor of the fixed threshold of FEV1/FVC < 0.70 has been recently reported by other authors, with the claim that it is easy to use, thus helping to remove barriers to widespread use of spirometry.3,6–8 Probably the use of the two lower fixed values for the elderly, suggested by data from the SARA study, does not add elements of particular complexity for physicians involved in interpretation of pulmonary function tests. However, this approach would diminish the number of false diagnoses of COPD, with significant cost savings due to reduction of inappropriately prescribed drugs. Thus, more resources could be redirected to primary prevention of COPD (smoking cessation) and treatment of more severe COPD.
Authors advocating retaining FEV1/FVC < 0.70 often quote the results of Mannino et al, who found that such a threshold is very good for identifying patients at risk of death and COPD-related hospitalizations.38 However, although this indicates that FEV1/FVC < 0.70 may recognize a proportion of individuals at risk, it does not mean that this is the best way to diagnose the disease. On the other hand, Vas Fragoso et al39 found elevated risk of death and respiratory symptoms in adults with FEV1/FVC less than the lower limit of normal. Sorino et al40 recently confirmed that FEV1/FVC less than the lower limit of normal, FEV1/FEV6 less than the lower limit of normal, and FEV1 less than the lower limit of normal are all significant predictors of all-cause and cardiopulmonary mortality in older individuals. The strongest spirometric predictor of all-cause mortality remains the appropriately named vital capacity, because the majority of deaths in adult smokers, with or without COPD, are caused by cardiovascular disease.
Two recent studies investigated subjects in between the two definitions of airway obstruction (ie, FEV1/FVC < 0.70 but ≥ lower limit of normal), showing that their clinical profile is characterized by relevant comorbid disease and poor health-related quality of life, but similar exercise, frequency of exacerbations, and indices of systemic effects.41,42 The investigators emphasized that these subjects might be at risk and should be followed carefully; we should be aware that fewer than one in five smokers with mild airway obstruction ever develop clinically important COPD, and that today we are not yet able to identify which smokers will be rapid fallers.2,4
The present study has some limitations. First, the cohort was recruited for a cross-sectional investigation, whereas the effect of aging on respiratory function would be better assessed in a longitudinal study. It is plausible that FEV1/FVC decreases significantly even after the age of 65 years, but this can be less reliable when derived by a cross-sectional observation of older people. In fact, they could represent individuals who had higher spirometric values at a younger age, and FEV1/FVC similar to those of younger subjects at the time of recruitment. Second, subjects participating in the SARA project were not randomly selected from the population, but consisted mainly of subjects with nonrespiratory illnesses attending outpatient clinics; this might have resulted in some selection bias, although it would not explain the higher pulmonary volumes than in other studies. The authors wish to emphasize that FEV1/FVC less than the lower limit of normal should not be the only criterion used for diagnosis of airway obstruction, but should always be combined with evaluation of FEV1 as percent of predicted. Indeed, patients with severe airflow limitation could have an important reduction both in FEV1 and FVC, with a sustained FEV1/FVC ratio. Thus, in doubtful cases, measurement of residual volume and total lung capacity is recommended.
In conclusion, the present findings confirm the inadequacy of FEV1/FVC < 0.70 for diagnosis of airway obstruction in elderly people, and we propose other easy to remember thresholds for FEV1/FVC after 65 years of age, ie, 0.65 in men and 0.67 women. Further studies are needed to assess both the classificatory and prognostic properties of such a threshold as well as epidemiological surveys to confirm it.
Appendix
Respiratory Health in the Elderly study group
Coordinators: V Bellia (Palermo) and F Rengo (Napoli). Scientific committee members: R Antonelli Incalzi (Taranto), V Grassi (Brescia), S Maggi (Padua), G Masotti (Florence), G Melillo (Naples), D Olivieri (Parma), M Palleschi (Rome), R Pistelli (Rome), M Trabucchi (Rome), S Zuccaro (Rome).
Participating centers, principal investigator, and associated investigators (the latter in brackets): Divisione di Medicina I, Ospedale Geriatrici INRCA, Ancona, DL Consales (D Lo Nardo, P Paggi); Divisione di Geriatria, Ospedale Civile, Asti, F Goria (P Fea, G Iraldi, R Corradi); Cattedra di Gerontologia e Geriatria, Policlinico Universitario, Bari, A Capurso (R Flora, S Torres, G Venezia, M Mesto); Divisione di Geriatria, Ospedale Malpighi, Bologna, S Semeraro (L Bellotti, A Tansella); Divisione di Medicina Generale, Ospedale Civile, Brescia, V Grassi (S Cossi, G Guerini, C Fantoni, M De Martinis, L Pini); Clinica Pneumologica, Fondazione “E Maugeri”, Telese, G Melillo (R Battiloro, C Gaudiosi, S De Angelis); Istituto di Medicina Interna e Geriatria, Ospedale Cannizzaro, Catania, L Motta (I Alessandria, S Savia); Istituto di Gerontologia e Geriatrici, Ospedale Ponte Nuovo, Università Florence, Florence, G Masotti (M Chiarlone, S Zacchei); Divisione di Geriatria, Ospedale Morgagni, Forli, V Pedone (D Angelini, D Cilla); Divisione di Geriatria, Ospedale Galliera, Genova, E Palummeri (M Agretti, P Costelli, D Torriglia); Grouppo Ricerca Geriatrica Ricerca Geriatrica, Ospedale Richiedei, Gussago, M Trabucchi (P Barbisoni, F Guerini, P Ranieri); Divisione di Geriatria, Ospedale Generale, L’Aquila, F Caione (D Caione, M La Chiara); Divisione di Geriatria, Ospedale San Gerardo, Monza, G Galetti (A Cantatore, D Casarotti, G Anni); Cattedra di Gerontologia e Geriatria, Università Federico II, Napoli, F Rengo (F Cacciatore, AI Pisacreta, C Calabrese); Istituto di Medicina Interna, Ospedale Geriatrico, Padova, G Enzi (P Dalla Montà, S Peruzza, P Albanese, F Tiozzo); Istituto di Clinica delle Malattie dell’Apparato Respiratorio, Ospedale Rasori, Parma, D Olivieri (V Bocchino, A Comel, N Barbarito); Istituto di Gerontologia e Geriatria, Policlinico Monteluce, Perugia, U Senin (F Arnone, L Camilli, S Peretti); Divisione di Geriatria, Ospedale Israelitico, Roma, SM Zuccaro (M Marchetti, L Palleschi); Divisione di Geriatria, Ospedale Generale Addolorata, Roma, M Palleschi (C Cieri, F Vetta); Istituto di Medicina Interna e Geriatria, Policlinico Gemelli, Roma, PU Carbonin (F Pagano, P Ranieri); Istituto di Semeiotica Medicina e Geriatria, Policlinico Le Scotte, Siena, S Forconi (G Abate, G Marotta, E Pagni); Fondazione San Raffaele, Cittadella della Carità, Taranto, R Antonelli-Incalzi (C Imperiale, C Spada); Cattedra di Gerontologia e Geriatria, Ospedale Maggiore, Milano, C Vergani (G Giardini, MC Sandrini, I Dallera); Cattedra di Malattie dell’Apparato Respiratorio, Ospedale V Cervello, Palermo, V Bellia (F Catalano, N Scichilone, S Battaglia).
Coordinating center: Dipartimento di Medicina, Pneumologia, Fisiologia e Nutrizione Umana, Università degli Studi di Palermo.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- Quanjer PH, Enright PL, Miller MR, et al. The need to change the method for defining mild airway obstruction. Eur Respir J. 2011;37:720–722. doi: 10.1183/09031936.00135110. [DOI] [PubMed] [Google Scholar]
- 2.Enright P, Brusasco V. Counterpoint: Should we abandon FEV1/FVC < 0.70 to detect airway obstruction? Yes. Chest. 2010;138:1040–1042. doi: 10.1378/chest.10-2052. [DOI] [PubMed] [Google Scholar]
- 3.Celli BR, Halbert RJ. Point: should we abandon FEV1/FVC < 0.70. No. Chest. 2010;138:1037–1040. doi: 10.1378/chest.10-2049. [DOI] [PubMed] [Google Scholar]
- 4.Pellegrino R, Brusasco V, Viegi G, et al. Definition of COPD: based on evidence or opinion? Eur Respir J. 2008;31:681–690. doi: 10.1183/09031936.00154307. [DOI] [PubMed] [Google Scholar]
- 5.Enright PL. Are GOLDen slumbers drug induced? Am J Respir Crit Care Med. 2008;177:1291. doi: 10.1164/ajrccm.177.11.1291a. [DOI] [PubMed] [Google Scholar]
- 6.Fabbri LM, Boschetto P, Mapp CE. Time to wake up! Am J Respir Crit Care Med. 2008;177:1291–1292. [Google Scholar]
- 7.Fabbri LM. FEV1/FVC fixed ratio again! Chest. 2011;139:1252–1253. doi: 10.1378/chest.10-3148. [DOI] [PubMed] [Google Scholar]
- 8.Mannino DM. Defining chronic obstructive pulmonary disease … and the elephant in the room. Eur Respir J. 2007;30:189–190. doi: 10.1183/09031936.00058707. [DOI] [PubMed] [Google Scholar]
- 9.Bhatt NY, Wood KL. What defines abnormal lung function in older adults with chronic obstructive pulmonary disease? Drugs Aging. 2008;25:717–728. doi: 10.2165/00002512-200825090-00001. [DOI] [PubMed] [Google Scholar]
- 10.Medbo A, Melbye H. Lung function testing in the elderly – can we still use FEV1/FVC < 70% as a criterion of COPD? Respir Med. 2007;101:1097–1105. doi: 10.1016/j.rmed.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of COPD. 2011. [Accessed May 20, 2012]. Available from: http://www.goldcopd.org/
- 12.Qaseem A, Wilt TJ, Weinberger SE, et al. American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 13.British Thoracic Society. BTS guidelines for the management of chronic obstructive pulmonary disease. Thorax. 2004;59(Suppl):1–232. [PMC free article] [PubMed] [Google Scholar]
- 14.O’Donnell D, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease: 2007 update. Can Respir J. 2007;14:5B–32B. doi: 10.1155/2007/830570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institute for Health and Clinical Excellence. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. National Clinical Guideline Centre; 2010. [Accessed June 27, 2011]. Available from: http://guidance.nice.org.uk/CG101/Guidance/pdf/English. [Google Scholar]
- 16.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 17.Enright PL, Kronmal RA, Higgins M, et al. Spirometry reference values for women and men 65 to 85 years of age. Cardiovascular Health study. Am Rev Respir Dis. 1993;147:125–133. doi: 10.1164/ajrccm/147.1.125. [DOI] [PubMed] [Google Scholar]
- 18.Roberts SD, Farber MO, Knox KS, et al. FEV1/FVC ratio of 70% misclassifies patients with obstruction at the extremes of age. Chest. 2006;130:200–206. doi: 10.1378/chest.130.1.200. [DOI] [PubMed] [Google Scholar]
- 19.Hardie JA, Buist AS, Vollmer WM, et al. Risk of over-diagnosis of COPD in asymptomatic elderly never-smokers. Eur Respir J. 2002;20:1117–1122. doi: 10.1183/09031936.02.00023202. [DOI] [PubMed] [Google Scholar]
- 20.Swanney MP, Ruppel G, Enright PL, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008;63:1046–1051. doi: 10.1136/thx.2008.098483. [DOI] [PubMed] [Google Scholar]
- 21.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 22.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 23.Bellia V, Pistelli R, Catalano F, et al. Quality control of spirometry in the elderly. The SARA study. SAlute Respiration nell’Anziano, Respiratory Health in the Elderly. Am J Respir Crit Care Med. 2000;161:1094–1100. doi: 10.1164/ajrccm.161.4.9810093. [DOI] [PubMed] [Google Scholar]
- 24.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 25.Abramson MJ, Hensley MJ, Saunders NA, et al. Evaluation of a new asthma questionnaire. J Asthma. 1991;28:129–139. doi: 10.3109/02770909109082737. [DOI] [PubMed] [Google Scholar]
- 26.Burney PG, Laitinen LA, Perdrizet S, et al. Validity and repeatability of the IUATLD (1984) Bronchial Symptoms Questionnaire: an international comparison. Eur Respir J. 1989;2:940–945. [PubMed] [Google Scholar]
- 27.Pistelli R, Bellia V, Catalano F, et al. Spirometry reference values for women and men aged 65–85 living in southern Europe: the effect of health outcomes. Respiration. 2003;70:484–489. doi: 10.1159/000074204. [DOI] [PubMed] [Google Scholar]
- 28.Peat JK, Woolcock AJ, Cullen K. Rate of decline of lung function in subjects with asthma. Eur J Respir Dis. 1987;70:171–179. [PubMed] [Google Scholar]
- 29.Cibella F, Cuttitta G, Bellia V, et al. Lung function decline in bronchial asthma. Chest. 2002;122:1944–1948. doi: 10.1378/chest.122.6.1944. [DOI] [PubMed] [Google Scholar]
- 30.Dockery DW, Ware JH, Ferris BG, et al. Distribution of forced expiratory volume in one second and forced vital capacity in healthy, white, adult never-smokers in six US cities. Am Rev Respir Dis. 1985;131:511–520. doi: 10.1164/arrd.1985.131.4.511. [DOI] [PubMed] [Google Scholar]
- 31.Glindmeyer HW, Lefante JJ, McColloster C, et al. Blue-collar normative spirometric values for Caucasian and African-American men and women aged 18 to 65. Am J Respir Crit Care Med. 1995;151:412–422. doi: 10.1164/ajrccm.151.2.7842200. [DOI] [PubMed] [Google Scholar]
- 32.Brandli O, Schindler C, Kunzli N, et al. Lung function in healthy never smoking adults: reference values and lower limits of normal of a Swiss population. Thorax. 1996;51:277–283. doi: 10.1136/thx.51.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crapo RO, Morris AH, Clayton PD, et al. Lung volumes in healthy nonsmoking adults. Bull Eur Physiopathol Respir. 1982;18:419–425. [PubMed] [Google Scholar]
- 34.Langhammer A, Johnsen R, Gulsvik A, et al. Forced spirometry reference values for Norwegian adults: the Bronchial Obstruction in Nord-Trondelag Study. Eur Respir J. 2001;18:770–779. doi: 10.1183/09031936.01.00255301. [DOI] [PubMed] [Google Scholar]
- 35.Falaschetti E, Laiho J, Primatesta P, et al. Prediction equations for normal and low lung function from the Health Survey for England. Eur Respir J. 2004;23:456–463. doi: 10.1183/09031936.04.00055204. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Rio F, Pino JM, Dorgham A, et al. Spirometric reference equations for European females and males aged 65–85 years. Eur Respir J. 2004;24:397–405. doi: 10.1183/09031936.04.00088403. [DOI] [PubMed] [Google Scholar]
- 37.Kuster SP, Kuster D, Schindler C, et al. Reference equations for lung function screening of healthy never-smoking adults aged 18–80 years. Eur Respir J. 2008;31:860–868. doi: 10.1183/09031936.00091407. [DOI] [PubMed] [Google Scholar]
- 38.Mannino DM, Buist AS, Petty TL, et al. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax. 2003;58:388–393. doi: 10.1136/thorax.58.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vas Fragoso CA, Concato J, McAvay G, et al. The ratio of FEV1/FVC as a basis for establishing chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:446–451. doi: 10.1164/rccm.200909-1366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorino C, Sherrill D, Guerra S, et al. Prognostic value of FEV1/FEV6 in elderly people. Clin Physiol Funct Imaging. 2011;31:101–107. doi: 10.1111/j.1475-097X.2010.00984.x. [DOI] [PubMed] [Google Scholar]
- 41.Lamprecht B, Schirnhofer L, Kaiser B, et al. Subjects with discordant airways obstruction: lost between spirometric definitions of COPD. Pulm Med. 2011;2011:780215. doi: 10.1155/2011/780215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.García-Rio F, Soriano JB, Miravitlles M, et al. Overdiagnosing subjects with COPD using the 0.7 fixed ratio: correlation with a poor health-related quality of life. Chest. 2011;139:1072–1080. doi: 10.1378/chest.10-1721. [DOI] [PubMed] [Google Scholar]