Abstract
Previously, we have published that pharmacological induction of oxidative stress causes anxiety-like behavior in rats and also is associated with hypertension in these animals. Here, we report that sub-chronic induction of oxidative stress via pharmacological induction leads to i) reduction in glyoxalase (GLO)-1 and glutathione reductase (GSR)-1 expression; ii) calpain mediated reduction of brain derived neurotrophic factor (BDNF) levels; iii) NFκB mediated upregulation of proinflammatory factors interleukin (IL)-6 and tumor necrosis factor (TNF)-α and elevated angiotensin (AT)-1 receptor levels in hippocampus, amygdala and locus coeruleus regions of the brain. Acute oxidative stress has opposite effects. We speculate that regulation of GLO1, GSR1, BDNF, NFκB and AT-1 receptor may contribute to anxiety-like behavior and hypertension in rats.
Keywords: Oxidative stress, Anxiety, Antioxidants, Hypertension, inflammation
1. Introduction
Recently, we and others have reported role of oxidative stress in anxiety-like behavior in rodents (Salim et al. 2010a; Salim et al. 2010b; Hovatta et al. 2005; Masood et al. 2008; de Oliveira et al. 2007). Masood et al. (2008) reported that induction of oxidative stress in hypothalamus and amygdala occurs in parallel with anxiety in mice. Consumption of high sucrose diet was reported to increase protein oxidation in frontal cortex and induce anxiety in rats (Souza et al. 2007). Increased anxiety has been found to be positively correlated with increases in reactive oxygen species in granulocytes (Bouayed et al. 2007). In another study, oxidative stress in the adult rat hippocampus was reported to be anxiogenic (de Oliveira et al. 2007). Our own recent publications suggest that treatment with two separate oxidative stress inducers, L-Buthionine-(S,R)-sulfoximine (BSO) and xanthine plus xanthine oxidase (X+XO) both cause anxiety-like behavior (Salim et al. 2010a; Salim et al. 2010b) and also increase blood pressure in rats (Salim et al. 2010b). Interestingly, induction of oxidative stress via a non-pharmacological method also leads to anxiety-like behavior in rats (Craig et al. 2011). Despite a causal role of oxidative stress in anxiety, (Salim et al. 2010a) as well as a positive correlation between oxidative stress and high blood pressure, (Salim et al. 2010b) underlying neuronal mechanisms and specific molecules that potentially regulate anxiety and hypertensive behaviors remain unclear. In this study we provide novel mechanistic insights by revealing specific molecular targets that may be critical to the etiology of anxiety and hypertension, two of the highly prevalent comorbid diseases (McLaughlin et al. 2003; Roy-Byrne et al. 2008). By drawing attention to specific molecular targets, this study would open new avenues for research into the causal role of these molecules in anxiety and hypertension that may be amenable to pharmacological intervention.
Increasing evidence suggests a central role for oxidative and nitrosative stress elicited via generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS), in a variety of congenital and acquired disorders of the central nervous system (CNS) (Calabrese et al. 2000; Halliwell B. 2006; Saye et al. 2008). This association is largely attributed to high vulnerability of brain to oxidative load (Ng et al. 2008). To cope with harmful effects of ROS and RNS, many detoxifying or antioxidant enzymes are expressed in the CNS (Ishii et al. 2000). Currently, two such enzymes have received great attention. Overexpression of glyoxalase (GLO)-1 and glutathione reductase (GSR)-1 in mouse brain increased while inhibition of GLO1 expression decreased anxiety-like behavior of mice (Hovatta et al. 2005). A negative relationship between anxiety-like behavior and GLO1 expression also has been reported (Kromer et al. 2005; Ditzen et al. 2006; Landgraf et al. 2007; Thornalley et al. 2006). This disagreement most likely is due to genetic variation or different models studied (Williams et al. 2009). Regulation of GLO1 and GSR1 seems important for anxiety-like behavior no matter which studies we tend to consider. This is particularly intriguing considering the important role this enzyme system plays with regards to dicarbonyl glycation. Oxidative stress leads to formation of advanced glycation end-products (AGEs) (Thornalley P.J. 2005) and contributes to cytotoxicity and inflammation. GLO-GSH system detoxifies dicarbonylation (Thornalley P.J. 2003) and methylglyoxal (MG)-mediated cytotoxicity (Di Loreto et al. 2008). In connection to this, brain derived neurotrophic factor (BDNF) is protective against MG challenge, protects neurons from oxidative stress and promotes neurogenesis (Duman et al. 1997). An antioxidant role of BDNF also has been proposed (Chan et al. 2010) and BDNF is considered critical to the pathophysiology of anxiety disorders (Martinowich et al 2007).
Relevant to this, increased intraneuronal calcium in the brain accompanies oxidative stress and activates calcium-activated proteases, the calpains (Ghosh et al 1995; Kim et al. 2007). Ca2+/calmodulin protein kinase (CaMK)-IV is a substrate for calpain (Temper-Wells et al. 2005) and catalyzes phosphorylation of cAMP response element-binding protein (CREB) (Corcoran et al. 2000). CREB is known to regulate gene expression of BDNF (Pandey et al. 2003; Einat et al. 2003). This pathway involving calpains, CAMKIV and CREB therefore seem important for regulation of BDNF. Moreover, activation of inflammatory pathways has been observed in patients with anxiety disorders (O’Donovan et al. 2010) as well as with hypertension (Saavedra et al. 2011). NFκB is activated upon oxidative stress and reported to intensify oxidative stress leading to inflammation and neuronal damage (Mattson et al. 2001; Elks et al. 2009). NF-kappaB p50-deficient mice have been reported to show reduced anxiety-like behaviors (Kassed and Herkenham, 2004). Calpain dependent NFκB activation is also known (Shumway et al. 1999). NFκB binding sites are located on angiotensin II AT1 receptor promoter region (Cowling et al. 2005). Brain angiotensin system plays a significant role in cardiovascular control (Armando et al. 2003; Saavedra JM. 2005). In this study we provide a putative mechanism involving the above molecules that may be critical to the etiology of anxiety and hypertension and their comorbid phenotype.
2. Results
2.1. Differential regulation of GLO1 and GSR1 expression upon induction of acute and sub-chronic oxidative stress
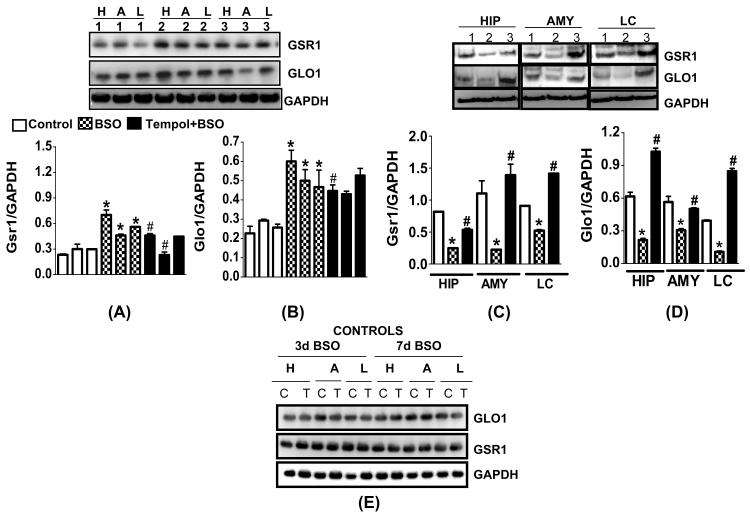
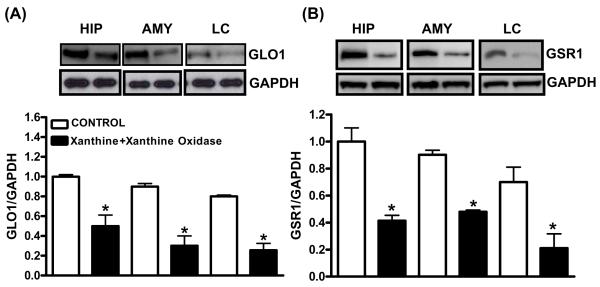
GSR1 and GLO1 protein expression significantly increased after 3 day BSO treatment in the hippocampus (+150%:GSR1; +172%:GLO1), amygdala (+66%:GSR1; 67%:GLO1) and LC (+93%:GSR1; +61%:GLO1) (Fig. 1A,B) while their levels decreased after 7 day BSO treatment in hippocampus (−75%:GSR1; −58% GLO1), amygdala (−85%:GSR1; −%48:GLO1) and LC (−44%:GSR1; −71%:GLO1) (Fig. 1C,D). GAPDH (loading control) remained unchanged. GSR1 and GLO1 levels were normalized in the tempol supplemented groups. GLO1 and GSR1 levels were not significantly altered in cerebellum, cortex, striatum and hypothalamus (data not shown). Tempol treatment alone did not alter GSR1 or GLO1 at 3 or 7d BSO (Fig. 1E). X+XO treatment for 7 days significantly decreased levels of GLO1 and GSR1 proteins in the hippocampus (−50%:GLO1; −60%:GSR1), amygdala (−72%: GLO1; −53%:GSR1) and LC (−73%:GLO1; −71%:GSR1) as compared to the vehicle treated control samples. GAPDH remained unchanged (Fig. 2).
Fig. 1. Effect of 3 and 7 day BSO treatment on GLO1 and GSR1 protein expression in hippocampus (HIP), amygdala (AMY) and locus coeruleus (LC).
Tissue homogenates (15-25μg proteins) were subjected to western blotting using GLO1, GSR1 and GAPDH (loading control) antibodies. Panels A and B indicate 3 day BSO treatment while panels C and D indicate 7 day BSO treatment. Left hand side of panel E indicates 3 day BSO treatment while right hand side shows 7 day BSO treatment. Lanes 1: control; 2: BSO; 3: Tempol+BSO. Protein samples in C and D were loaded on a standard 10-well 8-16% SDS-PAGE gel while samples in A, B and E were loaded on a 48-well E-PAGE™ 48 Protein electrophoresis System (Invitrogen Corp. #EP048-08). E: western blotting for GlO1 and GSR1 indicate no change in controls, vehicle: C and tempol alone: T. H: hippocampus, A: amygdala, L: locus coeruleus. *Significantly different from control, # from BSO p<0.05, ANOVA, N=12 rats.
Fig. 2. Effect of X+XO treatment on GLO1 and GSR1 protein expression in hippocampus (HIP), amygdala (AMY) and locus coeruleus (LC).
Tissue homogenates (25μg proteins) were subjected to western blotting, using GLO1, GSR1 and GAPDH (loading control) antibodies. Shown are representative blots and densitometric ratios of proteins normalized to GAPDH, respectively. *Significantly different from control, # from BSO p<0.05, ANOVA, N=6 rats.
2.2. Induction of sub-chronic oxidative stress activates calpain pathway and decreases CAMKIV, CREB and BDNF protein expression
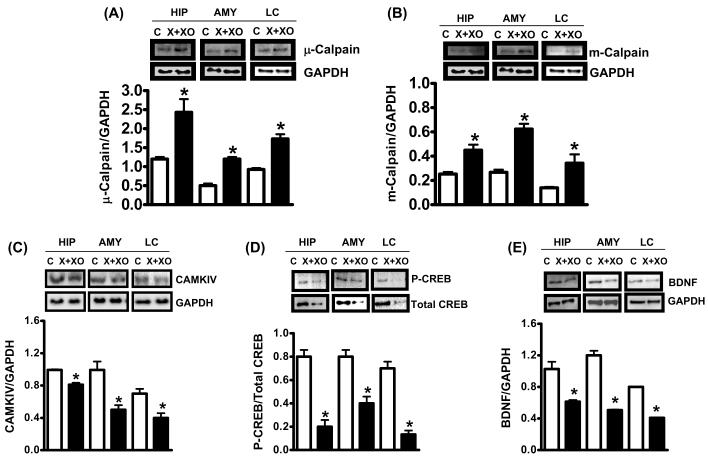
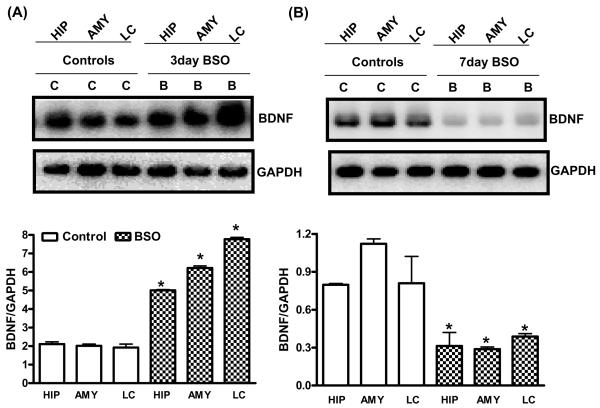
X+XO treatment for 7 days significantly increased micro (μ) and millimolar (m)-calpain expression (indicators of calpain activation) as compared to the vehicle treated control samples of tissue homogenates prepared from the hippocampus (+98%:μ-calpain; +91% m-calpain), amygdala (+100%:μ-calpain; +140% m-calpain) and LC (+102%:μ-calpain; +101% m-calpain) (Fig. 3A,B). X+XO treatment significantly decreased levels of CAMKIV (Fig. 3C), P-CREB/total CREB (Fig. 3D) and BDNF (Fig. 3E) proteins in the hippocampus (−26%:CAMKIV; −75%:p/totalCREB, −100%:BDNF) amygdala (−57%:CAMKIV;-49%: p/total CREB, −65%:BDNF) and LC (−49%:CAMKIV; −72%:p/total CREB, −51%:BDNF) as compared to the controls. Levels of BDNF were increased with 3d BSO treatment in hippocampus (+135%), amygdala (+211%) and LC (+309%) (Fig. 4A) while 7d BSO decreased BDNF in the hippocampus (−61%), amygdala (−74%) and LC (−53%) as compared to the vehicle treated controls (Fig. 4B). GAPDH remained unchanged
Fig. 3. Effect of X+XO treatment on expression of calpains, CAMKIV, CREB and BDNF.
Brain tissue homogenates (25-35 μg proteins) were subjected to western blotting using μ-calpain (A) m-calpain (B), CAMKIV (C), P-CREB, total CREB (D), BDNF (E) and GAPDH (loading control) antibodies. Shown are representative blots and densitometric ratios of proteins normalized to GAPDH, respectively. *Significantly different from control, # from X+XO p<0.05, ANOVA, N=6 rats.
Fig. 4. Effect of 3 and 7 days of BSO treatment on BDNF protein expression in hippocampus (HIP), amygdala (AMY) and locus coeruleus (LC).
Tissue homogenates (25μg proteins) were subjected to western blotting, using anti-BDNF and GAPDH (loading control) antibodies. Panel A indicate 3 day BSO treatment while panel B indicates 7 day BSO treatment. Shown are representative blots and densitometric ratios of proteins normalized to GAPDH, respectively. *Significantly different from control, # from BSO (B) p<0.05, ANOVA, N=6 rats.
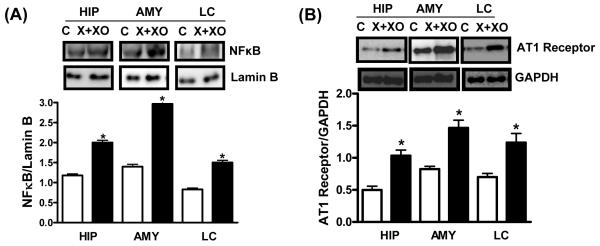
2.3. Induction of sub-chronic oxidative stress activates NFκB and increases AT1 receptor expression
X+XO treatment significantly increased levels of NFκB in the nuclear extracts prepared from hippocampus (+69%), amygdala (+111%) and LC (+81%) (Fig. 5A). This treatment increased AT1 receptor expression (Fig. 5B) in the hippocampus (+107%), amygdala (+62%) and LC homogenates (+77%) as compared to the vehicle treated controls. Lamin B and GAPDH used as a loading control for NFκB and AT1 receptor respectively remained unchanged.
Fig. 5. Effect of X+XO treatment on NFkB and AT1 receptor protein levels in hippocampus (HIP), amygdala (AMY) and locus coeruleus (LC).
Tissue homogenates (25μg proteins) or nuclear extracts of different brain areas were subjected to western blotting, using anti-NFkB, AT1, lamin B (loading control for nuclear proteins) and GAPDH (loading control) antibodies. Shown are representative blots and densitometric ratios of proteins normalized to lamin B or GAPDH, respectively. *Significantly different from control, # from X+XO p<0.05, ANOVA, N=6 rats.
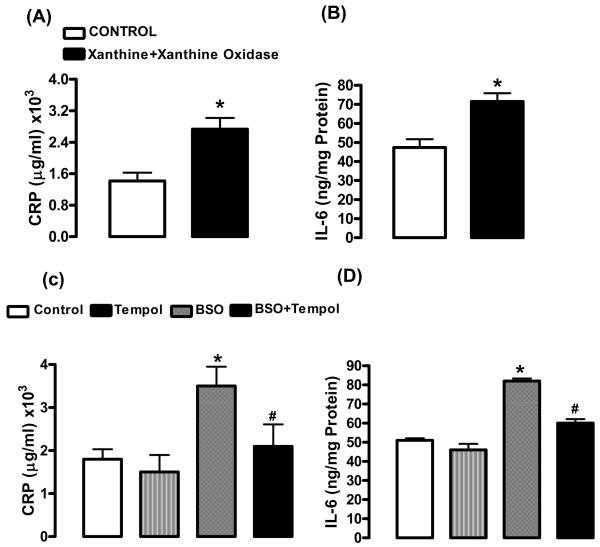
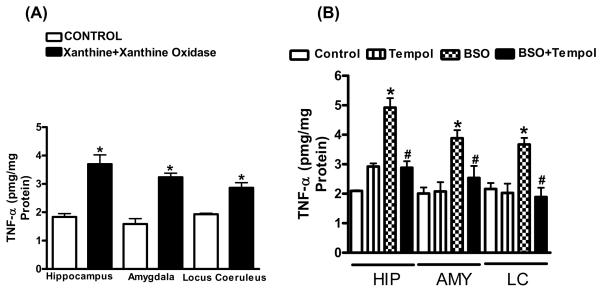
2.4. Induction of sub-chronic oxidative stress increases pro-inflammatory markers
The levels of CRP and IL-6, markers of inflammation, in the plasma increased in rats treated with X+XO (+93%:CRP; +54%:IL-6) (Fig. 6A,B) or with 7d BSO (+97%:CRP; +60%:IL-6) when compared to their controls. Tempol treated rats had CRP and IL-6 comparable to controls (Fig. 6C,D). Levels of TNF-α also were evaluated. TNF-α significantly increased in hippocampus (+103%), amygdala (+107%) and LC (+47%) in X+XO rats as compared to controls (Fig.7A). TNF-α levels also increased in hippocampus (+137%), amygdala (+95%) and LC (+71%) in 7d BSO treated rats. Tempol treatment normalized TNF-α (Fig. 7B).
Fig. 6. Effect of 7 day X+XO and BSO treatment on plasma levels of CRP and IL-6 in rats.
CRP and IL-6, markers of inflammation were examined using kit based ELISA assays in rat plasma of control, X+XO and BSO-treated rats. *Significantly different from control rats p < 0.05, ANOVA, N = 6–10 rats.
Fig. 7. Effect of X+XO or BSO treatment on TNF-α in brain homogenates of rats.
Brain homogenates were subjected to ELISA for measurement of TNF-α levels according to the manufacturer’s protocol (R&D Systems, Minneapolis, MN). *Significantly different from control rats p < 0.05, ANOVA, N = 6–8 rats.
3. Discussion
Recently, we published two studies reporting behavioral consequences of pharmacological induction of oxidative stress. In one, we established that oxidative stress produced via sub-chronic BSO treatment (7 days) caused anxiety-like behavior in rats while acute BSO treatment (3 days) did not (Salim et al. 2010a). In another study we demonstrated that oxidative stress produced via X+XO treatment caused anxiety-like behavior in rats. This treatment also was associated with hypertension in these animals (Salim et al. 2010b). Here we provide some biochemical results using blood and brain tissue homogenates from the rats utilized in the above studies.
In the present study we report that GLO1, GSR1 and BDNF levels were differentially regulated upon acute versus sub-chronic oxidative stress with BSO or X+XO treatments. Reduced levels of GLO1, GSR1 and BDNF were observed in hippocampus, amygdala and LC upon sub-chronic induction of oxidative stress with BSO or with X+XO treatments. Interestingly, GLO1, GSR1 and BDNF protein expression increased in these brain areas upon acute treatment. It seems that amygdala, LC and hippocampus is somewhat equally responsive to changes in oxidative stress (Salim et al. 2010a, Salim et al. 2010b). Involvement of these brain areas in anxiety disorders is known (Shin et al. 2006; Lopez et al. 1999; Dell’Osso et al. 2010; Charney and Drevets 2002) but their exact role in regulation of anxiety remains unclear (Shin et al. 2006, Menard and Treit 1999). Although there is enough consensus in the field regarding the role of amygdala in anxiety (Menard and Treit 1999), involvement of other brain regions is still a matter of debate (Davis M 2006; Maren S 2008; Quirk and Mueller 2008). It is believed that gamma amino butyric acid (GABA) projections transmit anxiety-related information from the amygdala to various centers in the brainstem, including, LC (LeDoux et al., 1988; Dong et al., 2001) and LC provides primary noradrenergic projections to the hippocampus and amygdala. Thus a critical inter-connection made within these regions may be critical for regulation of oxidative stress-mediated anxiety. It is clear that high anxiety (Salim et al. 2010a; Salim et al. 2010b) corresponds to low GSR1, GLO1 and BDNF while no anxiety (Salim et al. 2010a) with high levels of these proteins in these models. In agreement with these findings, our recent publication using a neuronal cell culture model of acute oxidative stress, suggests that GLO1 and GSR1 are transcriptionally up-regulated via Regulator of G-protein Signaling protein (RGS)-2 dependent mechanisms (Salim et al. 2011). Sub-chronic oxidative stress in this cell line reduced RGS2, GLO1 and GSR1 mRNA and proteins (unpublished observations).
Furthermore, X+XO mediated sub-chronic induction of oxidative stress increased calpain expression in the hippocampus, amygdala and LC, accompanied with a concomitant reduction in CAMKIV and CREB levels. CAMKIV is a substrate of calpain and regulates CREB levels which in turn modulate BDNF expression (Pandey et al. 2003; Einat et al. 2003). Thus BDNF levels are down-regulated perhaps due to calpain dependent degradation of its upstream regulators, CAMKIV and CREB. These are interesting observations considering that induction of oxidative stress causes protein glycation and GLO-GSH system detoxifies dicarbonylation (Thornalley PJ 2003). And, BDNF is reported to be upregulated upon MG challenge. Perhaps BDNF levels increase during acute oxidative stress (3d BSO) as an immediate protective response against glycation. As a result of this compensatory response antioxidant defense (GLO1 and GSR1 expression) also is upregulated. Upon chronic oxidative stress (7d BSO or 7d X+XO) this adaptive mechanism is lacking due to calpain dependent degradation of BDNF. It is reasonable to suggest that decline in GLO1 and GSR1 leads to excessive protein glycation. BDNF can no longer compensate for this defect and impairment in this detoxification mechanism occur inducing irreversible alterations in the antioxidant homeostasis in the brain. Moreover, BDNF is not only considered as protective against oxidative stress but is also known to promote neurogenesis (Duman et al. 1997) and synaptic plasticity (Schaaf et al. 2001). Thus it raises an intriguing possibility that decreased BDNF favors a condition reflective of high oxidative stress and reduced plasticity, all leading to a potentially high anxiety state.
Furthermore, our results demonstrate increased levels of transcription factor, NFκB in nuclear extracts of hippocampus, amygdala and LC samples obtained from X+XO treated rats, accompanied with increased angiotensin II AT1 receptor protein levels. Calpain dependent NFκB activation has been reported (Shumway et al. 1999). NFκB binding sites are located on the AT1 receptor promoter region (Cowling et al. 2005). Therefore, we postulate that oxidative stress activates calpains, which via NFκB lead to transcriptional activation of AT1 receptor thus increasing AT1 expression. Role of amygdala, LC and hippocampus is proposed in central control of blood pressure (Saha S. 2005; Elam et al. 1985; Pietranera et al. 2008) but information regarding precise neural mechanisms operated within these regions that enable such control is lacking. Which out of the three regions is lesser, equal or more effective than the other in regulation of hypertension via oxidative stress mechanisms is an interesting topic for future studies. Many studies have linked central angiotensin activity with elevated blood pressure. In fact, blockade of the brain angiotensin II AT1 receptors contribute to blood pressure decrease in hypertension, in several animal models (Armando et al. 2003; Saavedra JM 2005). Furthermore, NFκB activation is reported to promote inflammation leading to neuronal damage (Mattson et al. 2001; Elks et al. 2009). Increased pro-inflammatory cytokines including interleukin-6 (IL-6) and TNF-α have been detected in anxiety disorder (O’Donovan et al. 2010) and hypertensive patients (Saavedra et al. 2011). Our results are in agreement with these reports. We observed increased CRP-1 and IL-6 in the serum and elevated TNF-α in the brain of 7d X+XO as well as 7d BSO treated rats.
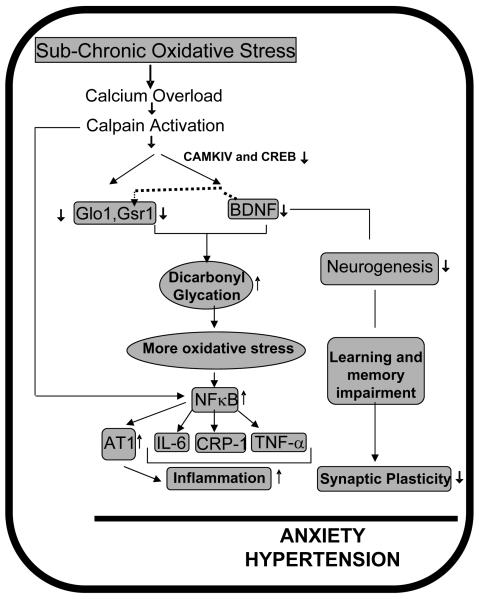
Taken together, our observations have prompted the following speculation (Fig. 8). Sub-chronic oxidative stress i) decreases GLO1 and GSR1 increasing dicarbonyl glycation; ii) activates calpain to degrade BDNF causing detoxification impairment, contributing to greater oxidative stress, reducing neurogenesis and plasticity; iv) promotes NFκB-dependent inflammation; all of these factors contribute to co-morbidity of anxiety-like behavior and hypertension in rats.
Fig. 8. Schematic representation of our hypothesis.
GLO1, GSR1, BDNF, NFκB and AT1 receptor are potential molecular targets that collectively influence anxiety-like behavior and hypertension.
4. Experimental Procedures
4.1. Animal model of oxidative stress
All experiments were conducted in accordance with the NIH guidelines using approved protocols from the University of Houston Animal Care and Use Committee. Male Sprague-Dawley rats (200-250 g) 7-8 weeks of age were utilized, oxidative stress produced as described (Salim et al. 2010a; Salim et al. 2010b) and brains harvested as below.
4.2. Brain Dissections and Preparation of Homogenates
After conducting anxiety-like behavior tests (Salim et al. 2010a; Salim et al. 2010b), rats were anesthetized, brains removed, frozen and brain stem removed (Salim et al. 2010b). Hippocampus, amygdala and locus coeruleus (LC) were identified according to Paxinos and Watson (1986) and isolated as before (Salim et al. 2010a; Salim et al. 2010b). Homogenates were prepared as published (Salim et al. 2010a; Salim et al. 2010b).
4.3. Western Blot Analysis
Homogenates were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and western blotting using primary (GLO1, GSR1, calpains, BDNF, CAMKIV, phospho and total CREB, NFκB, AT1 receptor, lamin B and GAPDH) (overnight, 4°C) and horseradish peroxidase (HRP)-conjugated secondary (room temperature, 1h) antibodies. Band intensities were determined on Alpha Ease FC 4.0 (Alpha Innotech Corp., San Leandro, CA).
4.4. C-reactive protein (CRP) measurement
C-reactive protein is an acute phase protein, the levels of which increase in inflammation and is considered as a marker of inflammation (Schaap et al. 2006). CRP was measured by CRP-ELISA kit using purified rat CRP as standard supplied with the kit (Immunology Consultants Labs., Inc., Newberg, OR).
4.5. Interleukin-6 (IL-6) and TNF-α measurement
Rat plasma was coated on a 96-well plate (overnight, 4°C), washed, incubated with anti-rat IL-6 primary (cat# ARC0062, Biosource, Camarillo, CA) and HRP-linked secondary antibody and the color developed with tetramethyl benzidine (Substrate System for ELISA, Sigma Chem. Co., St. Louis, MO) and 1N H2SO4 was read at 450 nm (ELISA reader; BIO-TEK Instruments, Inc., Winooski, Vermont, USA) according to the manufacturer’s protocol. TNF-α measurement was done using ELISA kit as recommended (R&D Systems, Minneapolis, MN).
4.6. Data Analysis
Data are expressed as mean ± SEM. Significance was determined by one or two way ANOVA as appropriate by applying Tukey’s post-hoc test (GraphPad Software, Inc. San Diego, CA). A value of p< 0.05 was considered significant.
Research Highlights.
Acute versus sub-chronic oxidative stress differentially regulates Glyoxalase-1 and glutathione reductase-1 expression.
Sub-chronic oxidative stress activates calpain pathway and reduces Brain derived neurotrophic factor (BDNF) expression.
Sub-chronic oxidative stress activates transcription factor NFκB and increases angiotensin AT1 receptor expression
Sub-chronic oxidative stress also activates pro-inflammatory factors.
The study reveals potential molecular targets that collectively influence anxiety-like behavior and hypertension.
Acknowledgements
Funding: GEAR grant, UH (S.S), SURF award (CV), PURS award (AV), NIH/NIA AG29904 (M.A) and Academy of Finland NEURO research program and Academy Research Fellowship (I.H),
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
List of Abbreviations
X+XO (xanthine and xanthine oxidase), MDA (malondialdehyde), SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis), OF (open-field), LD (light-dark).
References
- Armando I, Seltzer A, Bregonzio C, Saavedra JM. Stress and angiotensin II: novel therapeutic opportunities. Curr Drug Targets CNS Neurol Disord. 2003;(6):413–9. doi: 10.2174/1568007033482661. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Bates TE, Stella AM. NO synthase and NO-dependent signal pathways in brain aging and neurodegenerative disorders: the role of oxidant/antioxidant balance. Neurochem. Res. 2000;(25):1315–41. doi: 10.1023/a:1007604414773. [DOI] [PubMed] [Google Scholar]
- Chan SH, Wu CW, Chang AY, Hsu KS, Chan JY. Transcriptional Upregulation of Brain-Derived Neurotrophic Factor in Rostral Ventrolateral Medulla by Angiotensin II. Significance in Superoxide Homeostasis and Neural Regulation of Arterial Pressure. Circ Res. 2010 Sep 2; doi: 10.1161/CIRCRESAHA.110.225573. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Charney DS, Drevets WC. The neurobiological basis of anxiety disorders. 2002 See Davis et al. 2002, pp. 901–30. [Google Scholar]
- Corcoran EE, Means AR. Defining Ca2+/calmodulin-dependent protein kinase cascades in transcriptional regulation. J Biol Chem. 2000;(5):2975–8. doi: 10.1074/jbc.R000027200. [DOI] [PubMed] [Google Scholar]
- Cowling RT, Zhang X, Reese VC, Dillmann WH, Greenberg BH. Effects of cytokine treatment on angiotensin II type 1A receptor transcription and splicing in rat cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2005;(3):1176–83. doi: 10.1152/ajpheart.00088.2005. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear potentiated startle. Am Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- de Oliveira MR, Silvestrin RB, Mello E, Souza T, Moreira JC. Oxidative stress in the hippocampus, anxiety-like behavior and decreased locomotory and exploratory activity of adult rats: effects of sub acute vitamin A supplementation at therapeutic doses. Neurotoxicology. 2007;(6):1191–9. doi: 10.1016/j.neuro.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Dell’Osso B, Buoli M, Baldwin DS, Altamura AC. Serotonin norepinephrine reuptake inhibitors (SNRIs) in anxiety disorders: a comprehensive review of their clinical efficacy. Hum Psychopharmacol. 2010 Jan;25(1):17–29. doi: 10.1002/hup.1074. [DOI] [PubMed] [Google Scholar]
- Di Loreto S, Zimmitti V, Sebastiani P, Cervelli C, Falone S, Amicarelli F. Methylglyoxal causes strong weakening of detoxifying capacity and apoptotic cell death in rat hippocampal neurons. Int. J. Biochem. Cell Biol. 2008;(40):245–257. doi: 10.1016/j.biocel.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Ditzen C, Jastorff AM, Kessler MS. Protein biomarkers in a mouse model of extremes in trait anxiety. Mol Cell Proteomics. 2006;(5):1914–20. doi: 10.1074/mcp.M600088-MCP200. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;(38):192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;(7):597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Einat H, Yuan P, Gould TD, Li J, Du J, Zhang L, Manji HK, Chen G. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J Neurosci. 2003;(19):7311–6. doi: 10.1523/JNEUROSCI.23-19-07311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elam M, Svensson TH, Thoren P. Differentiated cardiovascular afferent regulation of locus coeruleus neurons and sympathetic nerves. Brain Res. 1985 Dec 9;358(1-2):77–84. doi: 10.1016/0006-8993(85)90950-3. [DOI] [PubMed] [Google Scholar]
- Elks CM, Mariappan N, Haque M, Guggilam A, Majid DS, Francis J. Chronic NF-{kappa}B blockade reduces cytosolic and mitochondrial oxidative stress and attenuates renal injury and hypertension in SHR. Am. J. Physiol. Renal. Physiol. 2009;(296):298–305. doi: 10.1152/ajprenal.90628.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995;(5208):239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and neurodegeneration, where are we now? J. Neurochem. 2006;(97):1634–58. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- Hovatta I, Tennant RS, Helton R, et al. Glyoxalase1 and glutathione reductase 1 regulate anxiety in mice. Nature. 2005;(438):662–666. doi: 10.1038/nature04250. [DOI] [PubMed] [Google Scholar]
- Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000;(275):16023–29. doi: 10.1074/jbc.275.21.16023. J Pharmacol Exp Ther. (2):369-79. Epub May 2. [DOI] [PubMed] [Google Scholar]
- Kassed CA, Herkenham M. NF-kappaB p50-deficient mice show reduced anxiety-like behaviors in tests of exploratory drive and anxiety. Behav Brain Res. 2004;(2):577–84. doi: 10.1016/j.bbr.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Oh SJ, Park SH, Kang HJ, Park JB, Kim JI, Kim J, Lee JY. Neuronal loss in primary long-term cortical culture involves neurodegeneration-like cell death via calpain and p35 processing, but not developmental apoptosis or aging. Exp Mol Med. 2007;(1):14–26. doi: 10.1038/emm.2007.3. [DOI] [PubMed] [Google Scholar]
- Krömer SA, Kessler MS, Milfay D, Panhuysen M, Pütz B, Deussing JM, Holsboer F, Landgraf R, Turck CW. Identification of glyoxalase-I as a protein marker in a mouse model of extremes in trait anxiety. J Neurosci. 2005;(17):4375–84. doi: 10.1523/JNEUROSCI.0115-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Kessler MS, Spengler D, Zimbelmann M, Nussbaumer M, Singewald N, Rujescu D, Frank E. Candidate genes of anxiety-related behavior in HAB/LAB rats and mice: focus on vasopressin and glyoxalase-I. Neurosci Biobehav Rev. 2007;(1):89–102. doi: 10.1016/j.neubiorev.2006.07.003. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López JF, Akil H, Watson SJ. Neural circuits mediating stress. Biol Psychiatry. 1999 Dec 1;46(11):1461–71. doi: 10.1016/s0006-3223(99)00266-8. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;(9):1089–93. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Masood A, Nadeem A, Mustafa SJ, O’Donnell JM. Reversal of oxidative stress-induced anxiety by inhibition of phosphodiesterase-2 in mice. 2008 doi: 10.1124/jpet.108.137208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdale function: cautions and caveats. Eur J Neurosci. 2008;28:1661–1666. doi: 10.1111/j.1460-9568.2008.06485.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Camandola S. NF-kappaB in neuronal plasticity and neurodegenerative disorders. J. Clin. Invest. 2001;(107):247–54. doi: 10.1172/JCI11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard J, Treit D. Effects of centrally administered anxiolytic compounds in animal models of anxiety. Neurosci Biobehav Rev. 1999 Mar;23(4):591–613. doi: 10.1016/s0149-7634(98)00056-6. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Geissler EC, Wan GJ. Comorbidities and associated treatment charges in patients with anxiety disorders. Pharmacotherapy. 2003;(23):1251–6. doi: 10.1592/phco.23.12.1251.32700. [DOI] [PubMed] [Google Scholar]
- Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 2008;(11):851–76. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- O’Donovan A, Hughes BM, Slavich L, O’Farrelly C, Malone KM. Clinical anxiety, cortisol and interleukin-6: evidence for specificity in emotion-biology relationships. Brain Behav. Immun. 2010 doi: 10.1016/j.bbi.2010.03.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC. Anxiety and alcohol abuse disorders: a common role for CREB and its target, the neuropeptide Y gene. Trends Pharmacol Sci. 2003;(9):456–60. doi: 10.1016/S0165-6147(03)00226-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sixth edition. 1986. [DOI] [PubMed] [Google Scholar]
- Pietranera L, Saravia FE, Roig P, Lima A, De Nicola AF. Protective effects of estradiol in the brain of rats with genetic or mineralocorticoid-induced hypertension. Psychoneuroendocrinology. 2008 Apr;33(3):270–81. doi: 10.1016/j.psyneuen.2007.11.009. Epub 2007 Dec 31. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Byrne PP, Davidson KW, Kessler RC, Asmundson GJ, Goodwin RD, Kubzansky L, Lydiard RB, Massie MJ, Katon W, Laden SK, Stein MB. Anxiety disorders and comorbid medical illness. Gen Hosp Psychiatry. 2008;(30):208–25. doi: 10.1016/j.genhosppsych.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Sánchez-Lemus E, Benicky J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: Therapeutic implications. Psychoneuroendocrinology. 2011;(1):1–18. doi: 10.1016/j.psyneuen.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra JM. Brain angiotensin II: new developments, unanswered questions and therapeutic opportunities. Cell Mol Neurobiol. 2005;(253):485–512. doi: 10.1007/s10571-005-4011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S. Role of the central nucleus of the amygdala in the control of blood pressure: descending pathways to medullary cardiovascular nuclei. Clin Exp Pharmacol Physiol. 2005 May-Jun;32(5-6):450–6. doi: 10.1111/j.1440-1681.2005.04210.x. [DOI] [PubMed] [Google Scholar]
- Salim S, Sarraj N, Taneja M, Saha K, Tejada-Simon MV, Chugh C. Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behav Brain Res. 2010a;(208):545–52. doi: 10.1016/j.bbr.2009.12.039. [DOI] [PubMed] [Google Scholar]
- Salim S, Asghar M, Chugh G, Taneja M, Xia Z, Saha K. Oxidative stress: a potential recipe for anxiety, hypertension and insulin resistance. Brain Res. 2010b;(1359):178–85. doi: 10.1016/j.brainres.2010.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim S, Asghar M, Taneja M, Hovatta I, Wu YL, Saha K, Sarraj N, Hite B. Novel role of RGS2 in regulation of antioxidant homeostasis in neuronal cells. FEBS Lett. 2011 Apr 16; doi: 10.1016/j.febslet.2011.04.023. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayre LM, Perry G, Smith MA. Oxidative stress and neurotoxicity. Chem. Res. Toxicol. 2008;(2):172–88. doi: 10.1021/tx700210j. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, Workel JO, Vreugdenhil E, Oitzl MS, de Kloet ER. Correlation between hippocampal BDNF mRNA expression and memory performance in senescent rats. Brain Res. 2001;(915):227–33. doi: 10.1016/s0006-8993(01)02855-4. [DOI] [PubMed] [Google Scholar]
- Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;(119):9–17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Shumway SD, Maki M, Miyamoto S. The PEST domain of IkB is necessary and sufficient for in vitro degradation by m-calpain. J Biol Chem. 1999;(274):30874–81. doi: 10.1074/jbc.274.43.30874. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ. Unease on the role of glyoxalase 1 in high-anxiety-related behaviour. Trends Mol Med. 2006;(5):195–9. doi: 10.1016/j.molmed.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ. Dicarbonyl intermediates in the Maillard reaction. Annals of the New York Academy of Sciences. 2005;(1043):111–117. doi: 10.1196/annals.1333.014. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ. Glyoxalase I structure, function and a critical role in the enzymatic defence against glycation. Biochem Soc Trans. 2003;(6):1343–8. doi: 10.1042/bst0311343. [DOI] [PubMed] [Google Scholar]
- Tremper-Wells B, Vallano ML. Nuclear calpain regulates Ca2+-dependent signaling via proteolysis of nuclear Ca2+/calmodulin-dependent protein kinase type IV in cultured neurons. J Biol Chem. 2005;(3):2165–75. doi: 10.1074/jbc.M410591200. [DOI] [PubMed] [Google Scholar]
- Vollert C, Zagaar M, Hovatta I, Taneja M, Vu A, Dao A, Levine A, Alkadhi K, Salim S. Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: Potential role of oxidative stress mechanisms. Behav Brain Res. 2011 May 19; doi: 10.1016/j.bbr.2011.05.010. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Williams R, 4th, Lim JE, Harr B, Wing C, Walters R, Distler MG, Teschke M, Wu C, Tarantino LM, Borevitz JO, Palmer AA. A common and unstable copy number variant is associated with differences in Glo1 expression and anxiety-like behavior. PLoS ONE. 2009;(3):46–49. doi: 10.1371/journal.pone.0004649. [DOI] [PMC free article] [PubMed] [Google Scholar]