Abstract
Three hundred and eighty-four pigs, mean initial live weight of 20.8 kg, were assigned randomly to groups of 24 (12 females, 12 castrated males). Each group was randomly assigned to 1 of 2 dietary treatments consisting of the same commercial barley-based diet, with or without the addition of tylosin phosphate. The barn where the animals were housed operates as an all-in all-out facility, and all pigs arrived on the same day as part of a group of 960 pigs. No new pigs were introduced into the facility during the period of this trial and pigs were sent to market over a 4-week period upon achieving a live weight of 110 kg. The pigs were weighed at the beginning of the trial and when they left the facility for slaughter. Feed consumption and incidence of disease, mortality, or both were recorded daily. At slaughter, carcass backfat depth over the last rib, 6.5 cm ventral to the dorsal midline (P-2 site); loin depth; carcass weight; predicted lean yield; and grade index were recorded. The sow herd supplying pigs to the unit was known to be free of the major swine diseases such as swine influenza, mycoplasma pneumonia, porcine reproductive and respiratory syndrome (PRRS), necroproliferative enteritis, and ascarids. A strict biosecurity protocol was employed to minimize the risk of introducing disease organisms into the unit. Prior to this study, no subtherapeutic antibiotics had been used in this facility. Tylosin phosphate supplementation had no significant effect on final weight, days on test, total gain, and daily gain. In both treatments, the pigs reached a mean market weight of 110.2 kg within 94.1 days, resulting in daily gains of the order of 950 grams per day. Due to the design of the trial, it was difficult to measure significant feed consumption effects. Feed consumption and conversion appeared to be similar for pigs in both treatment groups. At slaughter, tylosin phosphate supplementation appeared to significantly increase lean muscle content of the carcass as measured by loin muscle depth (P = 0.04). Mortality rates and the number of underweight pigs sent to market were low for this trial. Mortality was similar for both treatments; however, more of the control pigs than of the tylosin phosphate fed pigs were underweight when sent to market. From the results of this study, it appears that pigs of fast growing genotypes fed adequate diets and housed in a biosecure environment do not require dietary tylosin phosphate supplementation in order to maximize growth. There is some indication that tylosin phosphate supplementation may improve lean content of the carcass in pigs housed in such an environment.
Introduction
In pork production, antibiotics are commonly included in feed at subtherapeutic levels for 2 main economic reasons: disease prevention and growth promotion. According to Dewey et al (1,2), antibiotics are currently used in 90% of starter rations, 75% of grower rations, and 50% of finisher rations for pigs. In the past, it was recognized that subtherapeutic levels of antibiotics had a positive effect on growth performance and herd health (3). Feeding antibiotics to swine has been shown to increase weight gain by 3.3% to 8.8% and to improve feed utilization efficiency by as much as 7%. The mechanisms involved are not completely understood; however, there are several theories that have merit. Since subtherapeutic levels of antibiotics have been shown to reduce the incidence and severity of diseases, such as swine dysentery, and because farms with poor hygiene see relatively greater growth responses to antibiotics, it appears that antibiotics reduce levels of pathogens thus freeing up energy for growth, which might otherwise be used to fight off infection (4,5,6,7,8,9). Similarly, it has been suggested that antibiotics alter the normal pathogenic and nonpathogenic flora of the gut and that these changes have a beneficial effect on utilization of nutrients (10). Work with antibiotics, such as chlortetracycline and penicillin, has shown that treated pigs have high levels of insulin-like growth factor 1, suggesting that antibiotics play a role in metabolic functions in the pig (11,12). Other than having effects on the metabolism of the pig, it appears reasonable that antibiotics may have less of a growth promoting effect under conditions where pathogenic organisms are not present, or present at very low levels.
The positive growth effects of antibiotics have led the pork industry to the point where the greatest use of antibiotics in pigs is at subtherapeutic levels as growth promoters and disease prophylactics (13).
Following the publication of the Swann Report (14), attention has been focussed on the risk of bacterial resistance to specific antibiotics and the effects that these may have on animal and human health. For example, Threlfall et al (15) indicated that strong evidence existed that the prophylactic use of antibiotics in calf herds contributed to the multiresistant Salmonella Typhimurium DT204/193/20c outbreak in the United Kingdom in the early 1980s. Such strains were resistant to almost all of the commonly used antibiotics and were, therefore, untreatable (16). In recent years, the use of in-feed subtherapeutic antibiotics for growth promotion by the pork industry has come under increased scrutiny because of this potential risk (17,18). This has led to the banning of the use of some subtherapeutic antibiotics for swine in Sweden and Denmark, followed by the banning of 6 major subtherapeutic antibiotics (tylosin phosphate, virginianmycin, spiromycin, zinc bacitracin, carbodox, olaquinalox) by the European Union (EU) as a whole in 1999 (19). The active components of some of these antibiotics are similar to, or the same as, some antibiotics used in human medicine (tylosin phosphate used as a growth promoter in pigs and erythromycin used in human medicine) (19).
Despite the current interest in the reduction or elimination of subtherapeutic antibiotic use in livestock production, there may be a risk that such a reduction or elimination would have negative effects on animal welfare, nutrient utilization, manure production, and economic sustainability. Cromwell (3), for example, calculated that the subtherapeutic use of antibiotics resulted in a saving of $2.92 per pig in feed and housing costs. Close (13) suggested that in-feed antibiotics for pigs provide a 5- to 10-fold payback in terms of increased production efficiency. Hardy (19) summarized 7 earlier reports showing that dietary antimicrobial agents improved digestibility of energy, nitrogen, and phosphorus in pigs by 5.1%, 1.8%, and 3.4%, respectively.
Much of the evidence in favor of the use of subtherapeutic antibiotics was derived a number of years ago. Cromwell (3) summarized the results of a number of experiments conducted from 1950 to 1985 that showed positive effects from the use of antibiotics at subtherapeutic levels. Since 1985, genetic selection and improved understanding of the nutrient requirements of pigs has resulted in large improvements in growth rate and carcass composition (20). The development of all-in all-out pig flow has altered the way that many pigs are managed in modern facilities. It has also been suggested that the best alternative to the use of subtherapeutic antibiotics may be to manage pork production in such a way as to minimize exposure to disease-causing infectious agents (21). According to Hardy (19), biosecurity and hygiene practices are critical to minimizing disease challenges.
A trial was conducted in an all-in all-out, minimal disease, growing-finishing unit. A strict biosecurity protocol was used to evaluate the performance of pigs fed high quality commercial diets, with and without the addition of tylosin phosphate as a subtherapeutic growth promoting antibiotic.
Material and methods
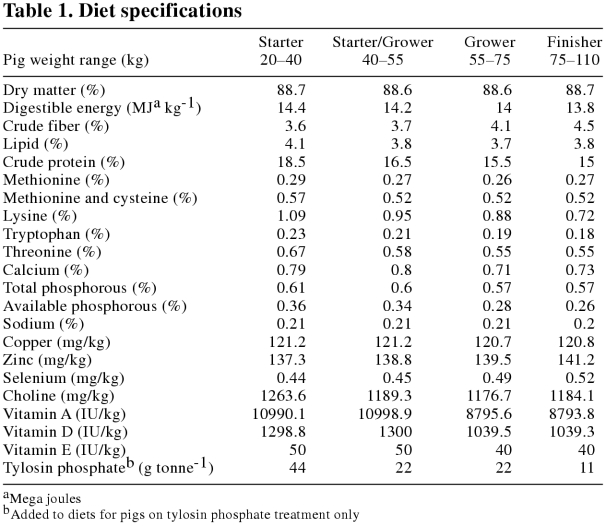
Three hundred and eighty-four pigs (192 females, 192 castrated males), mean initial live weight 20.8 kg, were obtained from a minimal disease sow herd in which batch farrowing was used as a means of producing large numbers of pigs of a similar age and weight. The pigs were assigned randomly to 2 dietary treatments consisting of the same commercial, barley-based diet, with or without the addition of tylosin phosphate. Table 1 lists the minimum nutrient content of the commercial diets used.
Table 1.
All pigs were housed in groups of 24 (12 females, 12 castrated males), and feed and water were provided ad libitum via wet/dry feeders that supplied 2 adjacent pens each. Each pen was 2.7 × 6.7 m in size and had a solid concrete floor bedded with 60 cm of mixed straw and sawdust. The barn where the animals were housed operates as an all-in all-out facility, and all pigs arrived on the same day as part of a group of 960 pigs. No new pigs were introduced into the facility during the period of this trial. As the pigs approached market weight, they were weighed individually and sent to market over a 4-week period upon achieving individual target weights of 110 kg. Any pig not reaching 110 kg by the end of the 4-week period was shipped anyway and designated as being shipped “under weight.” In this study, all-in all-out is defined as all pigs arriving on the same day and all pigs being removed from the facility by a given date before new pigs arrive. The barn has been described previously in detail (22).
The pigs were weighed at the beginning of the trial; their mean weight when they left the facility for slaughter was 110.2 kg. Feed was allocated daily and consumption was determined at the end of the study by weighing the feed remaining in the feeder. Occurrence of disease, mortality, or both was recorded daily. At slaughter, carcass backfat depth, loin depth, carcass weight, predicted lean yield, and grade index (1999 PEI Hog Settlement Table) were recorded.
The facility used in this trial has been in operation since 1998. From veterinary inspection, necropsy, and slaughter checks via the Animal Productivity and Health Information Network (APHIN), the sow herd supplying pigs to the unit was known to be free of major swine diseases such as swine influenza, mycoplasma pneumonia, porcine reproductive and respiratory syndrome (PRRS), necroproliferative enteritis, and ascarids. The strict biosecurity protocol employed to minimize the risk of introducing disease organisms into the unit was as follows: Prior to the arrival of the pigs, all pens (including floors, partitions, feeders, walls, and ceilings) and alleyways were pressure washed, disinfected, and allowed to sit empty for 7 d. No cleaning of pens occurred during the course of the trial. Any equipment, such as bedding carts and weigh scales, used in the facility was either purchased new and used only in the facility or washed and disinfected before being brought into the facility.
Prior to this study, no subtherapeutic antibiotics had been used in the facility. Any pigs remaining in the facility on day 110 after arrival were sent to market regardless of live weight to permit cleaning and disinfection of the facility prior to the scheduled arrival of a new batch of pigs.
The facility was located 800 m away from a public road and 1.2 km away from the nearest hog operation. This facility, since construction, has been supplied exclusively with pigs from a single sow herd. From previous trials in this facility, the genotype of the supplying herd has been shown to be fast growing with growth rates of the order of 900 to 1000 g/d and feed conversion efficiencies of the order of 2.7 kg feed per kg gain, without the use of subtherapeutic antibiotics. Entrance from the public road was limited to employees of the facility. Entry into the building was via an air-locked entry way. Footwear was removed at that point, after which anyone exposed to pigs in the past 48 h was required to shower and wear clothing and footwear provided by the facility, while those not exposed to pigs in the past 48 h were required to wear clothing and footwear provided by the facility. Those who had been exposed to pigs in the past 24 h were refused entry. Human traffic in the facility was limited to that required in the building for maintenance, animal husbandry, or supervision of experiments. Rodents were controlled inside and along the perimeter of the building by using traps and bait. All external vents and other openings were covered with 1-cm nylon mesh to eliminate entry by birds and other wildlife.
Bedding was added to the pens at the beginning of the trial and further amounts to control dampness in the pens were added as required. No bedding was removed during the course of the study.
An automatic curtain natural ventilation system was employed to maintain good air quality (low levels of dust and ammonia as observed by the barn manager) and an ambient temperature of 16°C.
Growth data (weight at start and end of the trial) was collected from individual animals resulting in 24 observations per pen and 8 pens per treatment. Because each feeder supplied feed to 2 pens, there were 4 feed consumption observations per treatment.
The data was analyzed by ANOVA or, where appropriate, by analysis of covariance with initial pig weight as the covariate, according to a completely randomized design for 2 treatments randomly assigned to 8 pens each. These analyses compared the effect of tylosin phosphate on the basis of pen means, and were computed by using the ANOVA directive in Genstat 5 (23). The variability of the pigs in the tylosin phosphate pens was visually compared with that of the pigs in the without tylosin phosphate pens with box plots.
This trial was conducted in accordance with the guidelines set out by the Canadian Council on Animal Care (24). Local approval for this study was given by the Atlantic Veterinary College Animal Care Committee.
Results
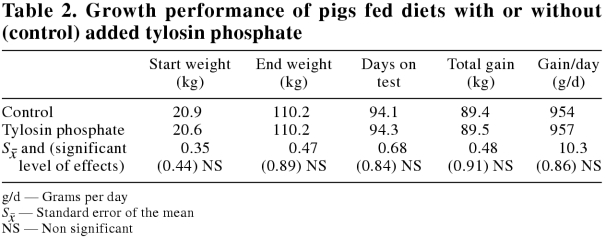
Final weight, days on test, total gain, and daily gain were not affected by tylosin phosphate supplementation (Table 2). In both treatments, the pigs reached a mean market weight of 110.2 kg within 94.1 d, resulting in daily gains of the order of 950 g per day. This is considered to be a high growth rate by commercial standards. Figure 1 shows the distribution of pigs reaching market weight on a weekly basis.
Table 2.
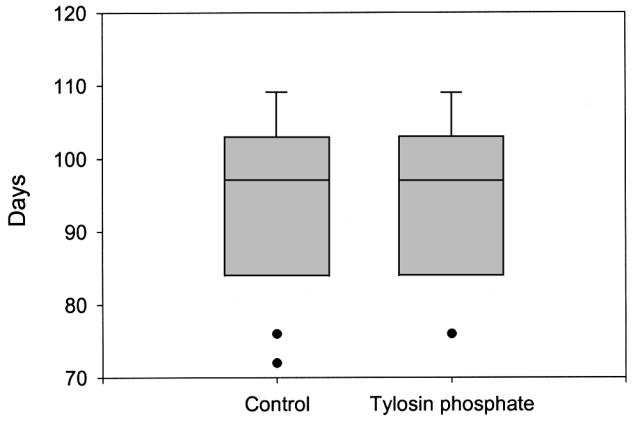
Figure 1. Days to market of pigs fed diets with or without tylosin phosphate added.
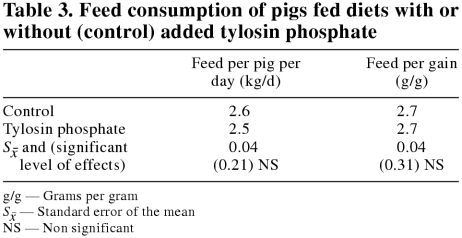
The design of the research facility limited the study to 4 replications per treatment for feed consumption parameters. Mean daily per-pig feed consumption and feed conversion efficiency values for each treatment are shown in Table 3. Feed consumption appeared to be similar for the pigs on either dietary treatment.
Table 3.
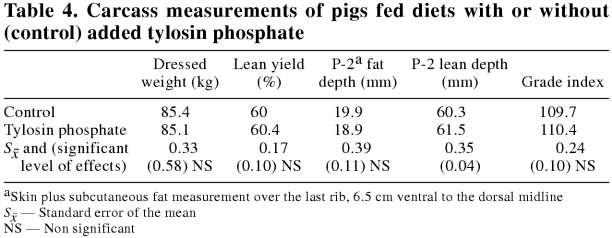
Carcass measurements for the pigs on trial are presented in Table 4. Tylosin phosphate supplementation appeared to increase lean muscle content of the carcass significantly as measured by loin muscle depth (P = 0.04) over the last rib, 6.5 cm ventral to the dorsal midline (P-2 site). The difference in lean measurement was not sufficient to significantly affect carcass value, as determined by grade index.
Table 4.
Mortality rates and the number of underweight pigs sent to market are given in Table 5. Mortality rates were similar for both treatments; however, more control pigs were underweight when sent to market than were tylosin phosphate fed pigs.
Table 5.
Discussion
The inclusion of tylosin phosphate at the level employed in this study did not have a statistically significant effect on economically important growth and carcass quality parameters. This is contrary to some reports in the literature: The Joint Expert Technical Advisory Committee on Antibiotic Resistance (25) reported that the average weight gain and the average feed conversion efficiency were improved in young pigs by 6.8% and 4.6%, respectively, and in grower pigs, by 1.9% and 1.7%, respectively, when subtherapeutic in-feed antibiotics were provided. Cromwell (3) summarized data from over 1000 experiments conducted in the United States from 1950 to 1985. This summary clearly indicated that growth rate and feed conversion efficiency were improved in young pigs by 16.4% and 6.9%, respectively, and in growing/finishing pigs by 4.2% and 2.2%, respectively. It is interesting to note, however, that even with these performance improvements, the mean growth rate and feed conversions of the pigs provided with subtherapeutic levels of antibiotics in Cromwell's summary were substantially lower than those obtained from the pigs in this study. From 20 kg to 110 kg live weight, the growth rate and feed conversion efficiency of the pigs without antibiotics was 956 g d-1 and 2.7 g g-1, respectively, in our study, while the values from 24 kg to 89 kg live weight for pigs fed antibiotics in Cromwell's study were 720 g d-1 and 3.23 g g-1, respectively. Cromwell's data illustrate how growth performance of pigs has been improved with the development of fast growing genotypes and with increased understanding of the nutrient and environmental requirements of pigs. Fast growing genotypes, when fed diets containing an adequate balance of amino acids and energy, have been shown to have growth rates of 1000 g d-1 or more (20).
The lack of effect from dietary tylosin phosphate supplementation in this study may have been due to the minimal disease status of the pigs on test and the biosecurity protocol employed in the facility where they were housed. In some studies, the adverse effects of challenge with infective organisms on growth performance have been restored by feeding antibiotics at growth promoting levels (26). This suggests that the benefits of subtherapeutic antibiotics are greatest under these conditions. Conversely, the benefits appear to be reduced or eliminated in animals raised in pathogen-free environments (27), suggesting that growth promoting antibiotics may work by controlling or reducing the proliferation of pathogens, thus freeing up nutrients for growth that might otherwise be made unavailable by an infection.
As in all animals, the immune system of the pig functions to contain or eliminate pathogens that may harm it. Upon exposure to an antigen, the immune system is activated. Cytokines are released and dietary nutrients are diverted away from fuelling the growth process in order to support the immune system (28). These events result in lower feed intake and reduced rates of protein accretion (29). In the presence of pathogenic bacteria, antibiotic growth promoters result in faster lean growth and improved feed utilization efficiency by reducing or eliminating the influence of antigens on the immune system (30).
It must also be recognized that commercially available diets were employed in this study, with the only modification being the removal of tylosin phosphate from the formulation. As can be seen in Table 1, the copper levels in the diet were 120 mg/kg, well above the minimum requirement of 4.51 mg/kg recommended by the US National Research Council in 1998 (31), and could be considered to be at growth promoting levels. According to Cromwell (32), copper fed at levels from 100 to 250 mg/kg stimulates growth in pigs and is independent of growth promotion from antimicrobials. It is possible that the copper content of the diets had a growth promoting effect in this study. As the copper levels were similar in both diets, and as such copper levels are common in commercial diets where subtherapeutic antibiotics are also supplemented, the dietary comparisons made here remain valid.
Other explanations for the lack of treatment effect may be associated with nutrition and genetics. The pigs on this study were from a local genotype known for fast lean growth and they were fed commercial diets designed to meet the nutrient requirements of such genotypes. As described earlier, recent reports suggest that the benefits of growth promotants have become less marked with improving feed formulation, hygiene, and genetics (26,27).
Despite the lack of statistical difference in economically important parameters, the pigs fed the tylosin phosphate supplemented diet appeared to have laid down more lean, as indicated by the P-2 loin measurement. This partitioning effect is similar to that reported by Stahly et al (30), where growth promoting antibiotics (carbadox) improved lean accretion rate in growing pigs.
From the results of this study, it appears that pigs of fast growing genotypes fed adequate diets and housed in a biosecure environment do not require dietary tylosin phosphate supplementation in order to maximize growth. There is some indication that tylosin phosphate supplementation may improve lean gain in pigs housed in such an environment; however, further work is required to verify this hypothesis. CVJ
Footnotes
Address all correspondence and reprint requests to Dr. Van Lunen.
References
- 1.Dewey CE, Cox BD, Straw BE, Bush EJ, Hurd HS. Use of antimicrobials in swine feeds in the United States. Swine Health Prod 1999;7:19–25.
- 2.Dewey CE, Cox BD, Straw BE, Bush EJ, Hurd HS. Associations between off-label feed additives and farm size, veterinary consult use, and animal age. Prev Vet Med 1997;31:133–146. [DOI] [PubMed]
- 3.Cromwell GL. Subtherapeutic use of antibiotics for swine: performance, reproductive efficiency and safety issues. Proc George A. Young Swine Conf 1999:69–87.
- 4.Committee on Drug Use in Animals, Institute of Medicine. The Use of Drugs in Food Animals. Washington, D.C.: Natl Acad Pr, 1999.
- 5.Corpet DE. Mechanisms of antimicrobial growth promoters used in animal feed (French). Rev Med Vet 2000;151:99–104.
- 6.Hays VW. The Hays Report: Effectiveness of Feed Additive Usage of Antimicrobial Agents in Swine and Poultry Production. Long Beach, California: Rachelle Laboratories; Report 12476-01, 5/81. 1981:91 pp.
- 7.Antimicrobial Feed Additives. Swedish Government Official Report. Stockholm. 1997.
- 8.Thomke S, Elwinger K. Growth promotants in feeding pigs and poultry. I. Growth and feed efficiency responses to antibiotic growth promotants (reprinted from J Royal Swed Acad Agric Forest. 1997;136:9–21. Ann Zootech 1998:85–97.
- 9.Thomke S, Elwinger K. Growth promotants in feeding pigs and poultry. II. Mode of action of antibiotic growth promotants. Ann Zootech 1998:153–167.
- 10.Jukes TH. The history of the “antibiotic growth effect”. Fed Proc 1977;37:2514–2518. [PubMed]
- 11.Hatheway MR, Dayton WR, White ME, Henderson TL, Henningson TB. Serum insulin-like growth factor 1 (IGF-1) concentrations are increased in pigs fed antimicrobials. J Anim Sci 1996;74:1541–1547. [DOI] [PubMed]
- 12.Hatheway MR, Dayton WR, White ME, Henderson TL, Young DA, Doan TN. Effect of feed intake on antimicrobially induced increases in porcine serum insulin-like growth factor 1. J Anim Sci 1999;77:3208–3214. [DOI] [PubMed]
- 13.Close WH. Producing pigs without antibiotic growth promoters. Adv Pork Prod 2000;11:47–56.
- 14.United Kingdom Joint Committee of Houses of Parliament. Report on the use of antibiotics in animal husbandry and veterinary medicine (‘Swann Report’), London: Her Majesty's Stationary Office, November 1969.
- 15.Threlfall EJ, Frost JA, Rowe B. Public health problems associated with the use of antibiotics. In: Garnsworthy PC, Wiseman J, Haresign W, eds. Recent Advances in Animal Nutrition. Nottingham: Nottingham Univ Pr, 1996:47–53.
- 16.Threlfall EJ, Frost JA, Ward LR, Rowe B. Increasing spectrum of resistance in multiresistant Salmonella typhimurium. Lancet 1996;347:1053–1054. [DOI] [PubMed]
- 17.Prescott JF. Antimicrobial drugs: Miracle drugs or pig feed? Adv Pork Prod 2000;11:37–45.
- 18.Choraine P. Antibiotic resistance and prudent use of antibiotics in veterinary medicine. Equine Vet Educ 2000;12:108–112.
- 19.Hardy B. A world without growth promoters. Concepts in pig science 1999. 1st Annu Turtle Lake Science Conf Nottingham: Nottingham Univ Pr 1999:53–70.
- 20.Van Lunen TA, Cole DJA. The effect of lysine/digestible energy ratio on growth performance and nitrogen deposition of hybrid boars, gilts and castrates male pigs. Anim Sci 1996;63:465–475.
- 21.Taylor DJ. The responsible use of antibiotics in pig medicine. The Pig J 1999;43:107–187.
- 22.Campbell AJ, Van Lunen TA, MacLeod JA, Hurnik D, Linkletter G. Design and performance of a 1000 head swine finishing research barn. Can J Agric Eng 2003. In Press.
- 23.Genstat 5 Committee, Payne RW, chair. Genstat 5 reference manual. Oxford: Claredon Pr, 1993.
- 24.Canadian Council on Animal Care. Guide to the care of experimental animals. Vol 1. Ottawa: Canadian Council on Animal Care, 1993.
- 25.JETACAR (Joint Expert Advisory Committee on Antibiotic Resistance). The use of antibiotics in food producing animals. Commonwealth of Australia 1999:107–116.
- 26.CAFA (Commission on Antimicrobial Feed Additives). Antimicrobial feed additives. Stockholm: Sweden Ministry of Agriculture, 1997.
- 27.Gropp JM, Schuhmacher A. Antimicrobial growth promoters in animal husbandry. In: WHO 1997: Report of a Meeting Organized by the WHO in Berlin, Germany, 13–17 October, 1997. Volume 1: Report and background papers 1997. Obtained from World Health Organization: http://www.who.int/en/
- 28.Kuby J. Immune response to infectious diseases. In: Goldsby R, Kindt T, Osborne B, eds. Immunology. New York: Freeman WH. 1997:459–484.
- 29.Williams NH, Stahly TS, Zimmerman DS. Impact of immune system activation on the rate, efficiency and composition of growth and amino acid needs of pigs fed from 130 to 250 pounds body weight. Iowa State University Swine Research Report. Ames, Iowa: Iowa State Univ Pr 1994:13–17.
- 30.Stahly TS, Williams NH, Zimmerman DS. Impact of carbadox on rate, efficiency and composition of growth in pigs with a low and high level of immune system activation. J Anim Sci 1994; 72:165. [DOI] [PubMed]
- 31.National Research Council. Washington, D.C. Nutrient Requirements of Swine. Nat Acad Pr, 1998.
- 32.Cromwell GL. Copper as a nutrient for animals. In: Richardson HW, ed. Handbook of Copper Compounds and Applications, New York: Marcel Dekker. 1997:177–202.