Abstract
| Strategy, Management and Health Policy | ||||
|---|---|---|---|---|
| Enabling Technology, Genomics, Proteomics | Preclinical Research | Preclinical Development Toxicology, Formulation Drug Delivery, Pharmacokinetics | Clinical Development Phases I-III Regulatory, Quality, Manufacturing | Postmarketing Phase IV |
Replacement of the ribose moiety of adenosine 5′-triphosphate (ATP) with a carbocyclic ring constrained in either the Northern (N) or Southern (S) conformation produces agonists with widely differing activities at P2Y receptors (Kim et al. [2002] J Med Chem 45:208–218). We have used whole cell patch clamp recording to investigate the agonist activity of these two methanocarba analogs of ATP at four different P2X receptors (P2X1, P2X2, P2X3, and P2X2/3). On dorsal root ganglion neurons, (N) methanocarba-ATP ((1′S,2′R,3′S,4′R,5′S)-4-(6-amino-9H-purin-9-yl)-1-[triphosphoryloxymethyl] bicyclo[ 3.1.0]hexane-2,3-diol; MRS 2340) activated rapidly-desensitizing (P2X3) and slowly-desensitizing (P2X2/3) receptors with a similar potency to ATP. In contrast, (S) methanocarba-ATP ((±)-5-(6-amino-9H-purin-9-yl)-1-[triphosphoryloxymethyl] bicycle [3.1.0]hexane-2,3-diol MRS 2312) was devoid of agonist activity. On nodose ganglion neurones, that express mainly P2X2/3 receptors, ATP evoked a slowly desensitizing inward current with an EC50 value of 26 μM. MRS 2340 was an effective agonist, but less potent than ATP, while MRS 2312 at concentrations up to 100 μM produced a barely detectable response. On mammalian cell lines expressing recombinant hP2X1 and hP2X2 receptors, MRS 2340 evoked inward currents similar in amplitude to those produced by the same concentration of ATP or α,β-mATP. In contrast, MRS 2312 failed to give a detectable response. Although the conformation of the ribose affects agonist activity at P2Y receptors, there is a strong requirement for the (N) conformation for the activation of these P2X receptors. Furthermore, the region of the agonist binding site that accommodates the ribose moiety appears to be highly conserved among different P2X receptors. Drug Dev Res 61:227–232, 2004.
Keywords: ATP, P2X receptors, structure activity relationship
INTRODUCTION
Adenosine 5′ triphosphate is now well recognised as an important extracellular signalling molecule. It is used as a neurotransmitter in the central, peripheral and enteric nervous systems [Burnstock, 2003]. In addition, it can act as an autocrine/paracrine messenger molecule in a variety of tissues [Khakh et al., 2001; Jacobson et al., 2002]. The extracellular actions of ATP are mediated via two families of receptors, metabotropic G-protein coupled P2Y receptors and P2X receptors which are ligand-gated ion channels. Eight mammalian subtypes of P2Y receptor have been identified, while seven P2X receptor subunits have been cloned. These subunits can combine to form either homo- or hetero-multimeric receptors. So far, eleven functional P2X receptor multimers have been identified [North, 2002; Brown et al., 2002], but with each functional ion channel consisting of at least 3 subunits, there could be more.
The widespread distribution and large number of different receptor subtypes makes the P2 receptors potential therapeutic targets for many pathological conditions including cystic fibrosis, hypertension, and urinary incontinence [Burnstock, 2002]. However, the development of useful therapeutic agents will require the discovery of subtype selective agonists and antagonists. One approach to the identification of such agents is by the use of high throughput screening of large numbers of unrelated compounds. An alternative approach is by systematic investigation of the structural and conformational requirements of the natural ligand ATP. In solution, the ribose ring of nucleotides such as ATP may exist in a dynamic equilibrium between (N) (Northern; 2′-exo) or (S) (Southern; 2′-endo) conformations. To probe which of these two conformations is preferred at the P2 receptors, we have synthesized two analogues of ATP, MRS 2312 and MRS 2340, in which the ribose moiety has been replaced with a rigid carbocyclic ring system [Ravi et al., 2002]. These isomeric methanocarba analogs contain fused cyclopropane and cyclopentane rings, which are locked into either a (N) or (S) conformation depending on the position of the methanocarba bridge (Fig. 1). Constraining the pseudoribose has a profound effect on the agonist activity at P2Y receptors [Kim et al., 2002]. In the (N) conformation (MRS 2340), there is a dramatic increase in potency at the P2Y1 receptor, while at the hP2Y4 receptor this compound has weak agonist activity. In contrast, ATP is an antagonist at the P2Y4 receptor. Constraining the pseudoribose ring in the (S) conformation (MRS 2312) resulted in a selective decrease in potency at the P2Y2 receptor, and a loss of agonist potency at the P2Y11 receptor [Kim et al., 2002]. At the P2Y1, receptor the (S)-methanocarba analogue had similar potency to ATP.
Fig. 1.
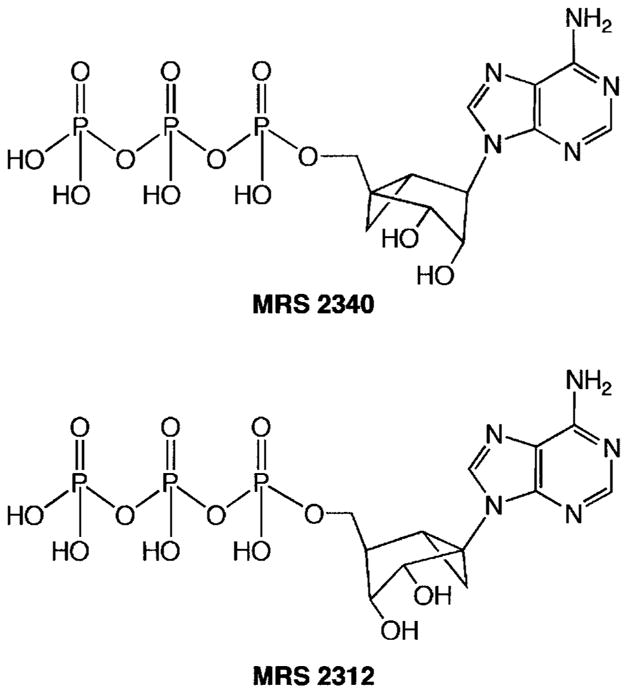
Chemical structures of the methanocarba-analogs of ATP tested. (1′S,2′R,3′S,4′R,5′S)-4-(6-amino-9H-purin-9-yl)-1-[triphosphoryloxymethyl] bicyclo[3.1.0]hexane-2,3-diol (MRS 2340) and (±)-5-(6-amino-9H-purin-9-yl)-1-[triphosphoryloxymethyl]bicyclo[3.1.0]hexane- 2,3-diol (MRS2312).
In this study, we have we have examined the ability of these two constrained analogs of ATP to activate four different P2X receptors (P2X1, P2X2, and P2X3 homomeric and the P2X2/3 heteromeric receptors), to see what effect these constraints have on the selectivity between P2X and P2Y receptors, or between different subtypes of P2X receptor.
MATERIALS AND METHODS
Cell Culture
Sensory ganglia from new-born (DRG) and 17-day-old (nodose) rats (Comparative Biology Unit, Royal Free and University College Medical School, London) were enzymatically dissociated and cultured as described previously [Dunn et al., 2000].
Human neuroblastoma 1321N1 cells stably expressing the hP2X2 receptor [Oglesby et al., 1999] were grown in DMEM with high glucose, supplemented with L-glutamine, pyridoxine, 10% foetal bovine serum, and geneticin. On reaching 80–90% confluency, cells were passaged (1:6). Following incubation in PBS + Versene, to release the cells, an appropriate volume of cell suspension was added to a new flask of growth medium. A small volume of this resultant cell suspension was placed in 35-mm culture dishes for electrophysiological recording.
Chinese hamster ovary (CHO-K1) cells stably transfected with a hP2X1/tetracycline sensitive repressor construct [Lachnitt et al., 2000] were grown in F-12 (HAM) medium with L-glutamine, supplemented with 10% foetal bovine serum 250 μg ml−1 G418, 100 μg ml−1 hygromycin, and 0.5 μg ml−1 tetracycline. Cells were passaged (1:6) on reaching 80–90% confluency. Cells were released by incubating in PBS + Versene. An appropriate volume of cell suspension was added to a new flask containing growth medium. Another aliquot of cells was diluted in medium without tetracycline, and placed in 35-mm culture dishes for electrophysiological recording. Cells were used 2–5 days after plating.
Electrophysiological Recording
Culture dishes containing cells were mounted on an inverted microscope and cells viewed at 400 × magnification. The dish was perfused with an external solution containing (mM): NaCl 154, KCl 4.7, MgCl2 1.2, CaCl2 2.5, HEPES 10, and glucose 5.6; the pH was adjusted to 7.4 using NaOH. Whole cell patch clamp recordings were made using electrodes filled with internal solution that contained (mM): citric acid 56, MgCl2 3, CsCl 10, HEPES 40 and 10, EGTA 0.1; the pH was adjusted to 7.2 using CsOH (total Cs+ concentration 170 mM). For perforated patch recording, this solution was supplemented by the addition of 250 μg ml−1 amphotericin B. Data were acquired using pCLAMP software (Axon Instruments, Union City, CA). Signals were filtered at 2 kHz (−3 dB frequency, Bessel filter, 80 dB per decade).
MRS 2312 and MRS 2340 were synthesized as described [Ravi et al., 2002]. Drugs were applied rapidly through a four- or six-barrel manifold [see Dunn et al., 2000]. One barrel was used to apply drug-free solution to enable rapid termination of drug applications. Agonists were applied for 2 sec at 2-min intervals for P2X2 and P2X2/3 receptors, and for 1 sec at 4 min intervals for P2X1 and P2X3 receptors. These times were found to be sufficient for responses to be reproducible.
Tissue culture media were obtained from Invitrogen Ltd. (Paisley, UK) and other reagents were from Sigma-Aldrich (Poole, Dorset, UK).
Data Analysis
All values are expressed as mean ± S.E.M. Data traces and graphs were plotted using Origin v7 (Microcal). For concentration-response data, pooled data (11 cells from 4 experiments) were fitted with the Hill equation using Origin v7, and the results are presented as the fitted value ± SE, determined by the fitting routine.
RESULTS
DRG Neurones
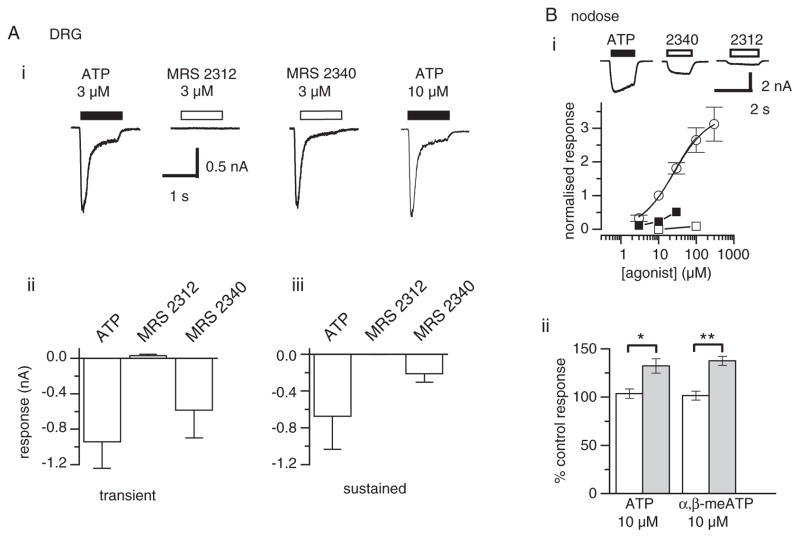
In the majority of voltage-clamped DRG neurones, fast application of ATP (3 μM) evoked a rapidly activating inward current, which desensitized during the 1-sec agonist application (Fig. 2Ai). This response has been attributed to the activation of homomeric P2X3 receptors [Grubb and Evans, 1999; Dunn et al., 2000]. Application of the same concentration of MRS 2312 to these neurones failed to evoke a detectable response. In contrast, MRS 2340 evoked an inward current with the same time course, though slightly smaller than the response to ATP (Fig. 2Ai, ii).
Fig. 2.
Activity of methanocarba-analogs of ATP at native P2X receptors on sensory neurones and smooth muscle cells. (A) i: Comparison of the transient response evoked in voltage clamped DRG neurones by ATP, MRS 2312, and MRS 2340. ii: Mean data from 4 neurones. iii: Comparison of the amplitude of the sustained response to these agonists observed in a further 5 DRG neurons. (B) Comparison of the action of MRS 2312 and ATP on rat nodose ganglion neurones. i: Representative traces, showing the sustained response to ATP (10 μM) in these cells. While MRS 2340 (30 μM) produces a similar response, MRS 23112 at 100 μM produces a barely detectable response. The concentration-response curve for ATP yields an EC50 of 27 ± 2 μM with a Hill coefficient of 0.97 ± 0.06. In contrast, MRS 2312 at 100 μM barely evokes any response, while MRS 2340 produces responses at 10 and 30 μM but is considerably less active than ATP. Points represent the mean ± s.e.m. from 4 to 8 cells (a total of 11 cells from 4 separate experiments). Where not shown, error bars lie within the symbol. ii: MRS 2312 potentiates responses to ATP and α,β-mATP. After recording a control response, a response to agonist plus MRS 2312 was recorded following a 2-min incubation with (shaded columns) or without (open columns) MRS 2312. Responses were then normalized with respect to the preceding control response. While co-application of MRS 2312 produced no significant effect, following a 2-min pre-incubation with MRS 2312, there was a significant increase in the response to both agonists. Columns represent the mean response from 5 cells. Significantly different *P < 0.05; **P < 0.01 by an unpaired Student’s t-test.
In some DRG neurones, ATP evoked a slowly desensitizing response attributed to activation of heteromeric P2X2/3 receptors [Zhong et al., 2003]. In these neurones, MRS 2340 (3 μM) again evoked an inward current smaller than, but kinetically similar to, that produced by the same concentration of ATP. However, the same (3 μM) concentration of MRS 2312 failed to produce a measurable response (Fig. 2Aiii).
Nodose Ganglion Neurones
Neurones from the nodose ganglion express almost exclusively heteromeric P2X2/3 receptors [Lewis et al., 1995]. In these neurones, ATP evoked a concentration-dependent sustained inward current with an EC50 value of 26 ± 2 μM (pooled data from 11 neurons; Fig. 2Bi). In contrast, MRS 2312 in concentrations up to 100 μM produced a barely detectable response, while MRS 2340 produced clear responses at 10 and 30 μM, although this compound was between three and ten times less potent than ATP (Fig. 2Bi). Insufficient quantities of MRS 2340 were available to construct a full concentration-response curve.
The lack of agonist activity of MRS 2312 might arise from a lack of affinity for the receptor, or a lack of intrinsic activity. In an attempt to distinguish between these possibilities, we investigated the ability of this compound to act as an antagonist at the P2X2/3 receptor. In these experiments, a control response to either 10 μM ATP or 10 μM α,β-meATP was recorded. Control solution, or 30 μM MRS 2312 was applied for 2 min, and then a response to agonist plus MRS 2312 (30 μM) was recorded. Although co-application of MRS 2312 and agonist had no significant effect on the response, application of MRS 2312 for 2 min significantly increased the response to both agonists (Fig. 2Bii).
Recombinant hP2X1 Receptors
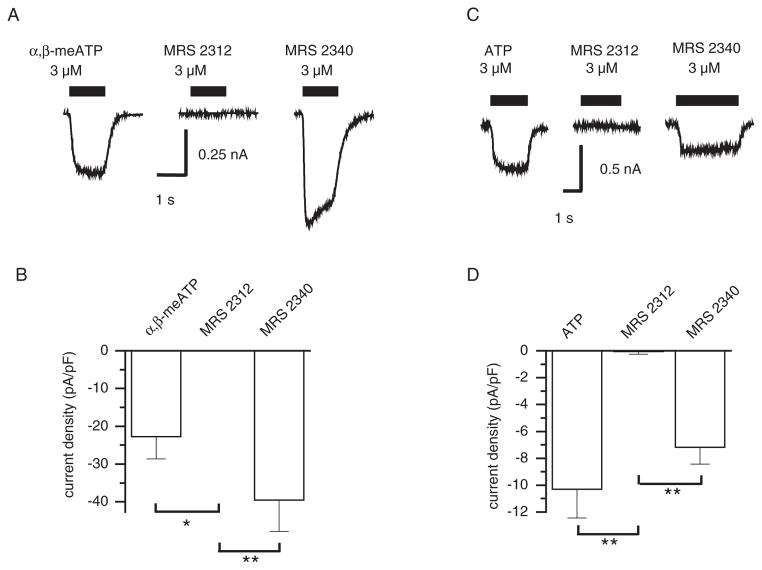
To investigate further whether MRS 2312 and MRS 2340 exhibit any selectivity between different P2X receptor sub-types, we examined their activity at recombinant P2X1 and P2X2 receptors. In CHO-K1 cells expressing the P2X1 receptor, α,β-meATP (3 μM) evoked a rapidly activating inward current, which desensitized to a variable extent during the agonist application. Again, MRS 2340 was an effective agonist, giving responses approximately twice the size of those to the same concentration of α,β-mATP. MRS 2312 at concentrations up to 3 μM again failed to evoke a response (Fig. 3A). Pooled data from 6 cells is shown in Figure 3B.
Fig. 3.
Activity of methanocarba-analogs of ATP at recombinant P2X receptors. A: Comparison of the activity of α,β-mATP, MRS 2312, and MRS 2340 on rP2X1 receptors expressed in CHO cells. B: The histogram shows the mean response from 6 cells, which have been normalised with respect to cell membrane capacitance to compensate for the large variation in cell size. C: Comparison of the responses produced by ATP, MRS 2312, and MRS 2340 at hP2X2 expressed in 1321N1 cells. D: The histogram shows the mean data from 5 cells. Significantly different by unpaired Student’s t-test *P < 0.05; **P < 0.01.
Recombinant hP2X2 Receptors
Subtypes of P2X receptors can be divided into two broad groups, those activated by α,β-meATP, and those that are not. We, therefore, investigated the action of MRS 2312 and MRS 2340 on recombinant hP2X2 receptors expressed in 1321N1 cells, as an example of a receptor that is insensitive to α,β-meATP. In these cells, ATP evoked a sustained inward current, which was mimicked by MRS 2340. In contrast, MRS 2312 failed to evoke a response (Fig. 3.C,D).
DISCUSSION
In this study, we have compared the activity of two analogs of ATP as agonists at four different P2X receptors (P2X1, P2X2, P2X3, and P2X2/3) expressed in native cells, or in cell lines expressing recombinant receptors. Although the small quantity of these compounds available prevented the construction of detailed concentration-response curves, the main finding of this study is that constraining the pseudoribose moiety in the (N) conformation (MRS 2340) results in an effective agonist, with a potency similar to, or slightly less than, that of ATP at all four P2X receptor subtypes studied. In contrast, constraining the ribose in the (S) conformation (MRS 2312) yields a compound lacking significant agonist activity at these P2X receptors.
The inability of MRS 2312 to evoke a response might be due to a lack of affinity for the agonist-binding site, or a lack of intrinsic activity. Since this compound failed to show any antagonist activity at a concentration of 30 μM, the former appears most likely. Interestingly, MRS 2312 actually enhanced the response to ATP at the P2X2/3 receptor. The P2X2 subunit is known to possess a number of allosteric sites, including one at which diadenosine pentaphosphate (Ap5A) exerts a positive modulatory effect [Pintor et al., 1996]. It is conceivable that the potentiation by MRS 2312 is due to an interaction at this site on the P2X2 subunit.
Our results on P2X receptors are in marked contrast with previous studies on the action of these compounds at P2Y receptors, where the effect of constraining the pseudoribose differs from one receptor subtype to another. At P2Y2 receptors, MRS 2340 is equipotent with ATP, while MRS 2312 is approximately 50 times less potent. However, at P2Y1 receptors, MRS 2312 is equipotent with ATP, while MRS 2340 is 30 times more potent [Kim et al., 2002]. The ability to synthesize agents that can differentiate between P2X and P2Y receptors may be important for investigating the physiological roles of these receptors in vivo and for the development of P2 receptors as therapeutic targets. While α,β-meATP is a selective agonist for P2X receptors, it only activates some subtypes of this class of receptors. Conversely, some P2Y receptors can be activated by uracil nucleotides, which lack significant activity at P2X receptors. We have now demonstrated that the constrained methanocarba-analogs of ATP provide alternative ways of generating P2Y selective agents. Constraining the pseudoribose in the (S) conformation (MRS 2312) yields a compound that lacks activity at P2X receptors, but is still an agonist at P2Y receptors. More significantly, although constraint in the (N) conformation (MRS 2340) generates an agonist which is 30 times more potent than ATP at P2Y1, there is no increase in potency at a range of P2X receptors.
In conclusion, the conformation of the ribose moiety is important for agonist activity at P2 receptors, with the (N) conformation required for activation of P2X receptors. Furthermore, the subset of the agonist-binding site, which accommodates the ribose moiety, would appear to be conserved among different P2X receptors.
References
- Brown SG, Townsend-Nicholson A, Jacobson KA, Burnstock G, King BF. Heteromultimeric P2X(1/2) receptors show a novel sensitivity to extracellular pH. J Pharmacol Exp Ther. 2002;300:673–680. doi: 10.1124/jpet.300.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Potential therapeutic targets in the rapidly expanding field of purinergic signalling. Clin Med. 2002;2:45–53. doi: 10.7861/clinmedicine.2-1-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic receptors in the nervous system. In: Schwiebert EM, editor. Current topics in membranes, Vol. 54. Purinergic receptors and signalling. San Diego: Academic Press; 2003. pp. 307–368. [Google Scholar]
- Dunn PM, Liu M, Zhong Y, King BF, Burnstock G. Diinosine pentaphosphate: an antagonist which discriminates between recombinant P2X(3) and P2X(2/3) receptors and between two P2X receptors in rat sensory neurones. Br J Pharmacol. 2000;130:1378–1384. doi: 10.1038/sj.bjp.0703404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb BD, Evans RJ. Characterization of cultured dorsal root ganglion neuron P2X receptors. Eur J Neurosci. 1999;11:149–154. doi: 10.1046/j.1460-9568.1999.00426.x. [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Jarvis MF, Williams M. Purine and pyrimidine (P2) receptors as drug targets. J Med Chem. 2002;45:4057–4093. doi: 10.1021/jm020046y. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PP. International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev. 2001;5:107–118. [PubMed] [Google Scholar]
- Kim HS, Ravi RG, Marquez VE, Maddileti S, Wihlborg A-K, Erlinge D, Malmsjö M, Boyer JL, Harden TK, Jacobson KA. Methanocarba modification of uracil and adenine nucleotides: High potency of Northern ring conformation at P2Y1, P2Y2, or P2Y4 and P2Y11, but not P2Y6 receptors. J Med Chem. 2002;45:208–218. doi: 10.1021/jm010369e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachnit WG, Oglesby IB, Gever JR, Gever M, Huang C, Li XC, Jin H, McGivern JG, Ford AP. Regulated expression of the rat recombinant P2X(3) receptor in stably transfected CHO-K1 tTA cells. J Auton Nerv Syst. 2000;81:75–81. doi: 10.1016/s0165-1838(00)00120-x. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- Oglesby IB, Lachnit WG, Burnstock G, Ford APDW. Subunit specificity of polyclonal antisera to the carboxy terminal region of P2X receptors P2X1 through P2X7. Drug Dev Res. 1999;47:189–195. [Google Scholar]
- Pintor J, King BF, Miras-Portugal MT, Burnstock G. Selectivity and activity of adenine dinucleotides at recombinant P2X2 and P2Y1 purinoceptors. Br J Pharmacol. 1996;119:1006–1012. doi: 10.1111/j.1476-5381.1996.tb15771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi RG, Kim HS, Servos J, Zimmermann H, Lee K, Maddileti S, Boyer JL, Harden TK, Jacobson K. Adenine nucleotides analogues locked in a Northern methanocarba conformation: Enhanced stability and potency as P2Y1 receptor agonists. J Med Chem. 2002;45:2090–2100. doi: 10.1021/jm010538v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Banning AS, Cockayne DA, Ford AP, Burnstock G, McMahon SB. Bladder and cutaneous sensory neurons of the rat express different functional P2X receptors. Neuroscience. 2003;120:667–675. doi: 10.1016/s0306-4522(03)00243-4. [DOI] [PubMed] [Google Scholar]