Abstract
Purpose
To compare the diagnostic and prognostic value of FDG PET and bone scans (BS) in the assessment of osseous lesions in patients with progressing prostate cancer.
Experimental Design
In a prospective imaging trial, 43 patients underwent FDG PET and BS prior to experimental therapies. Bone scan index (BSI) and standardized uptake value (SUV) on FDG PET were recorded. Patients were followed until death (n=36) or at least 5 years (n=7). Imaging findings were correlated with survival.
Results
Osseous lesions were detected in 39 patients on BS and 32 on FDG PET (p=0.01). Follow-up was available for 105 FDG-positive lesions, and 84 (80%) became positive on subsequent BS. Prognosis correlated inversely with SUV (median survival 14.4 vs. 32.8 mos if SUVmax > 6.10 vs. ≤ 6.10, p=0.002) and BSI (14.7 vs. 28.2 mos if BSI >1.27 vs. < 1.27; p=0.004). Only SUV was an independent factor in multivariate analysis. In castrate resistant patients combining a nomogram for progressive prostate cancer with SUV dichotomized patients into a high vs. low risk group (median survival 14.4 vs. 34.6 mos, p=.015) more prognostic than either nomogram or SUV alone.
Conclusion
The current study of progressive prostate cancer confirms earlier work that BSI is a strong prognostic factor. Most FDG-only lesions at baseline become detectable on follow-up BS, suggesting their strong clinical relevance. FDG SUV is an independent prognostic factor and provides complementary prognostic information.
Keywords: prostate cancer, positron emission tomography, FDG, bone scan, prognosis
Introduction
The prognosis of patients with prostate cancer varies widely at all points in the disease. Even in the setting of progressive castration resistance, which represents the lethal variant of the disease, the survival of an individual patient can vary from months to years.1 To better define risk, several studies have focused on clinical or biologic parameters, patterns of spread, and/or disease extent. Few of these analyses include quantitative measures of disease burden. PSA doubling time has been proposed as a potential surrogate of disease activity. However, a method that provides a direct measure of disease activity is clearly needed. For prostate cancer, this requires developing more reliable methods to assess disease in bone, the most common site of metastasis.
Several prognostic models have been reported based on the number lesions on 99mTc-MDP bone scan or pattern of spread (axial or appendicular).2–3 In previous work we used standard man bone weights, and a semi-quantitative estimation technique to develop a new parameter, the bone scan index or BSI, which provides a quantitative measure of the percentage of the adult skeleton involved by tumor.4 Using BSI, survival times vary inversely with disease extent. We also showed that the early spread of prostate cancer to bone is highly confined to regions of the skeleton that normally contain active red marrow in the adult male.5
More recently we have explored the use of FDG-PET, which has the potential to provide a more direct measure of disease activity. In a pilot study of patients with progressive castrate resistant disease, we showed that at least one FDG positive lesions was present in 14 of 14 patients with positive bone scans, and that the individual FDG-positive lesion was clinically relevant.6 Here we expand on these findings in a larger group of patients studied under a prospective protocol, and investigate the utility of SUVmax, a measure of FDG accumulation in metastatic lesions, as a potential biomarker in progressive prostate cancer.
Materials and Methods
Patients
From an ongoing prospective study approved by our Institutional Review Board, we identified 51 patients (median age 68y; range: 47–86y) with progressive prostate cancer who were enrolled between June 1997 and December 2000 and who had follow-up for at least 5 years or until death. Study entry required histologically proven prostate adenocarcinoma and clinical evidence of disease progression as defined by a rising PSA and a detectable abnormality consistent with metastasis on a standard imaging study, such as a 99mTc MDP bone scan, CT or MRI. Written informed consent for this HIPPA compliant study was obtained from all patients at the time of enrollment.
All 51 patients had evidence of progressing metastatic prostate cancer: 39 patients had castrate resistant disease, and 12 had non-castrate disease. Progressing cancer was defined as a rising PSA on three observations taken at least 1 week apart (with a total rise of more than 50% from baseline) or an increase in preexisting lesions on bone scintigraphy or in measurable soft tissue disease by computerized tomography (CT) or magnetic resonance imaging (MRI) within 6 weeks of study entry or development of new lesions. Patients were considered castrate if testosterone levels were < 50 ng/dl in blood.
Image Acquisition
FDG PET and bone scans were performed at baseline (30 days before to 7 days after first treatment). All 51 patients had bone scan prior to treatment initiation; FDG PET was performed in 43 patients before and in 8 patients (1–20 days) after treatment initiation. Because of the possibility of confounding results of PET-FDG after treatment, these 8 patients were eliminated from further analysis, and the PET-FDG information in this manuscript was derived from this 43 patient sub-set. Follow-up bone scans were performed in 36 of these patients three to six weeks after the initiation of therapy.
For the PET scan, patients fasted for at least 6 hours before FDG injection, but liberal intake of water was allowed. Plasma glucose levels were measured before FDG injection and found to be in the acceptable range (< 200mg/dl) in all patients. Following intravenous injection of 555 MBq FDG (15 mCi) and an approximately 45–60 min uptake period, images were acquired from the level of the auditory meatus to the level of the mid thighs on an Advance® (GE Medical Systems, Waukesha, WI) whole-body PET scanner. Emission and transmission images were both obtained for 4 minutes per bed position. Transmission data were used for attenuation correction in all cases and an iterative reconstruction with segmented attenuation corrections (IRSAC) algorithm was employed.
Bone scans were obtained approximately 2–3 hours after the intravenous injection of 925 MBq (25 mCi) of 99mTc-MDP in the anterior and posterior projections (and spot views of selected body regions when indicated) using a dual head gamma camera (ADAC Laboratories, Milpitas, CA) equipped with a low-energy, high-resolution collimator at a scan speed of 20 cm/min.
Image Analysis
Attenuation-corrected PET images were reviewed by a radiologist and a nuclear medicine physician who were unaware of patients’ clinical data (except that all had progressive prostate cancer) and laboratory findings. Interpretation was performed on a dedicated workstation displaying three orthogonal planes and a maximum intensity projection image. SUVmax was calculated for the lesion with the most intense FDG uptake in a given PET scan.
Bone scans were reviewed on dedicated workstations by the same observers. The bone scan index (BSI) was calculated as described previously.4
For both image modalities, abnormal tracer accumulation in bones consistent with metastasis was defined as focal or linear in shape, and of intensity greater than that in adjacent normal skeleton. These bone lesions were recorded based on a consensus between the two readers. Discrepant cases were discussed with senior nuclear medicine physicians and then classified as either benign or malignant. When available, plain films, CT and MRI were used for a better understanding of findings on PET and bone scan. The behavior of lesions with discrepant findings on baseline FDG PET and bone scans was assessed on follow-up bone scans.
Statistical analyses
The McNemar test was employed to compare the number of osseous metastases on FDG PET and bone scan, adjusted for clustering.7 As the skull was not usually included in the PET field of view, skull lesion on the bone scan were not considered for this analysis. Recognizing that tissue diagnosis rather than imaging findings should represent the gold standard for detection of bone metastases, sensitivity and specificity of PET and bone scan were not estimated; instead the total number of lesions and their concordance on FDG and bone scans were compared. Survival probabilities were estimated by Kaplan-Meier method and compared with the log-rank test. Multivariate analyses were performed by proportional hazards regression. Prognostic values were dichotomized around the observed medians when necessary. This method of analysis permitted for reasonably sized groups in each category. All analyses were performed using SAS (version 9.1, Cary, NC). Two-tailed p values of less than 0.05 were considered significant.
Results
Imaging Findings
The median time between bone scan and PET scan was 11.6 days (maximum of 46 days). Overall, 37 of the 43 patients were thought to have osseous metastases; based on bone scan positivity, and FDG-PET was positive in 31 of these 43 patients. The bone scan indicated metastases in significantly more patients than did the FDG PET (p=0.01; see Table 1).
Table 1.
Number of patients with bone lesions on FDG PET versus bone scan
| Negative FDG PET |
Positive FDG PET |
Total | |
|---|---|---|---|
|
Negative bone scan |
6 | 0 | 6 (13.9%) |
|
Positive bone scan |
6 (all BS remained positive on FU scans) |
31 | 37 (86.1%) |
| Total | 12 (27.9%) | 31 (72.1%) | 43 (100.0%) |
p=0.01, BS=bone scans; FU=follow-up
Among the 31 patients with osseous metastases on both bone scan and FDG PET, the PET also showed lymph node involvement in 8 patients and lymph node plus organ involvement (lung, pleura or liver) in another 4 patients. Of the 6 patients with positive bone scan but no osseous lesions on PET, two were found to have lymph node metastases by PET.
All of the 6 patients with negative bone scan (BS) also showed normal distribution of FDG throughout the skeleton on PET. However, one of these patients showed abnormal FDG uptake in lymph nodes and prostate bed. A patient example is shown in Figure 1.
Figure 1.


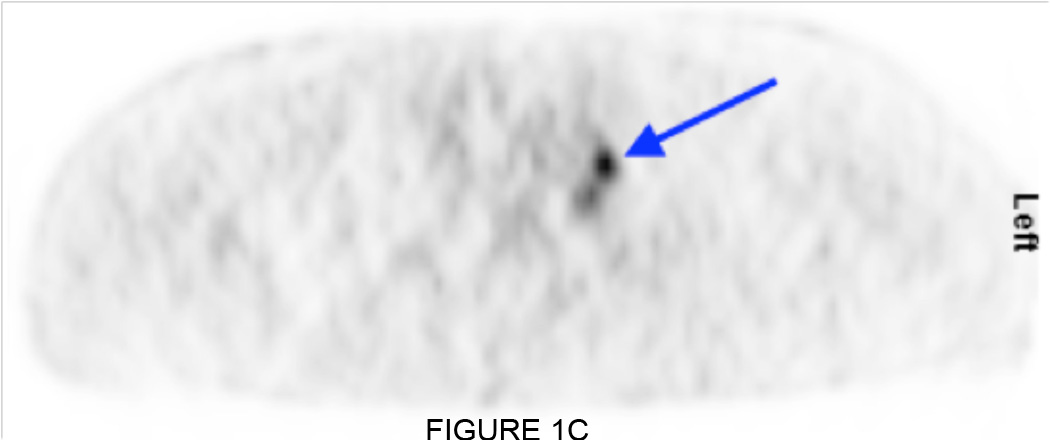
(A) Negative bone scan, except for foci of degenerative joint disease in the shoulders. (B, C) Coronal and sagittal FDG PET does not show skeletal lesions, but metastatic lymph node (arrows) is identified in the left supraclavicular region.
Twelve of the 43 patients (27.8%) had no evidence for osseous metastases on FDG PET; in six of these individuals the bone scan was also negative, but BS showed abnormal sites interpreted as metastases in the other 6 patients, including axial skeleton and/or thoracic cage (n=5) or widespread lesions throughout the skeleton (pelvis, spine, thoracic cage and skull; n=1).
Summarizing PET FDG vs BS in these 43 patients, 37 had a positive bone scan as an indicator of boney disease involvement; 31/43 had corresponding FDG + in bone ; 3/43 had FDG+ only in nodal or soft-tissue. Thus 8/43 (18.6%) of patients with progressing prostate cancer by our criteria had no site that was positive on PET FDG.
Table 2 shows the findings in individual bones. Discrepancies between bone scan and PET-FDG were noted in 241 bones. Overall, slightly fewer metastases were noted on bone scan than on the FDG PET. The median BSI was 1.3 (mean 6.44, range: 0.0–55). This amounts to about 3.4% of the red marrow containing skeleton in the average adult male. Fifteen patients showed focal abnormal radiotracer accumulation on the bone scan in sites not included in the PET field of view. In 14 cases, this involved the skull with or without simultaneous disease in the extremities, and in one case the tibia. However, none of these patients had lesions exclusively in those sites, and in each case the PET revealed osseous metastases in other locations. Eight additional patients showed solitary abnormalities in the mandible on bone scan, which were considered unlikely to represent metastatic prostate cancer. The median SUVmax was 6.1 (range: 2.2–16.8).
Table 2.
FDG PET versus bone scan for the detection of bone lesions as a fraction of all bones assessed
| Negative FDG PET |
Positive FDG PET |
Total | |
|---|---|---|---|
|
Negative bone scan |
1079 | 121 (BS FU for 105 lesions: 84 (80%) became positive) |
1200 (69.8%) |
|
Positive bone scan |
120 (BS FU for 83 lesions: all remained positive) |
400 | 520 (31.2%) |
| Total | 1199 (69.8%) | 521 (30.2%) | 1720(100.0%) |
p=0.75 (N.S.), BS=bone scans; FU=follow-up
Follow-up bone scans were available in 36 subjects with at least one discrepancy between number and distribution of osseous lesions on bone scan versus FDG PET, involving a total of 214 bones. Of note, of the 105 bones with lesions seen on PET, 84 (80. %) became positive on follow-up bone scans and 21 (18.4%) remained negative (Figure 2). All lesions identified only on bone scans remained positive on subsequent bone scans.
Figure 2.



53 year old male with castrate resistant metastatic prostate cancer. (A) The initial bone scan shows several osseous metastases in the right 5th rib, sternum, left iliac bone and acetabulum. (B) FDG PET scan 4 days later shows the same lesions and in addition 2 metastatic foci in the lumbar spine (arrows). (C) A bone scan 6 months later shows abnormal tracer uptake in these lumbar vertebrae (dotted arrows).
Prognostic Value of Imaging Findings
Median survival of the entire sample was 21.3 months (95% confidence interval: 14.7–32.8). The hazard ratio of FDG-SUVmax in bone, when used as a continuous variable, was estimated to be 1.11: that is, a 11% increase in risk of death is expected for each unit increase in SUV (95% CI for hazard ratio: 1.03–1.19, p=0.007) Kaplan-Maier curves for SUVmax ≤ 6.10 against SUVmax > 6.10 (observed median), are shown in Figure 3A. In the 22 patients whose metastases had a SUVmax ≤ 6.10 the median survival was 32.8 months (95% CI: 23.6–41.6). By comparison, in the 21 patients whose metastases had a SUVmax > 6.10, the median survival was only 14.4 months (95% CI: 13.9–21.7, p=0.002).The hazard ratio for BSI versus prognosis was estimated at 1.02 (95% CI: 1.00–1.04, p=0.120). Median survival was 27.0 months (95% CI: 23.6–50.5) for the 26 patients with a BSI ≤1.27 as compared to 14.4 months (95% CI: 11.1–24.9) for the 25 patients with a BSI > 1.27 (p=0.004; Figure 3B). When both SUVmax and BSI were used in a multivariate analysis, SUVmax retained its significance (p=0.010, hazard ratio=1.098, 95% CI: 1.024–1.177), and BSI showed a trend toward significance (p=0.215, hazard ratio=1.019, 95% CI: 0.981–1.040). We further noted that dichotomized versions of these two variables formed two distinct groups: Among the 26 patients with SUVmax ≤ 6.10 and BSI ≤ 1.27, the median survival was 34.6 months (95% CI: 23.6-NR). On the other hand, among the 19 patients who had metastases with either SUVmax >6.10 a BSI > 1.27, or both, the median survival was only 14.7 months (95% CI: 13.8–27.0). The corresponding survival curves are shown in Figure 3C, and the statistical summary is shown in Table 3.
Figure 3.



(A) Kaplan-Meier survival curves: FDG PET SUV: Survival curves for 22 patients with low (≤6.10) and 21 patients with high (>6.10) SUVmax, p=0.002. (B) Kaplan-Meier survival curves: Bone scan index: Survival curves for 22 patients with low (≤ 1.27) and 21 patients with high (>1.27) BSI, p=0.004. (C) Joint analysis of SUVmax and BSI showing two patient groups with distinct prognosis. In the low SUV and low BSI group, median survival is 32.6 months; in the “high” SUV and BSI group, survival was 14.4 months and there is a distinct difference between the curves (p=0.001).
Table 3.
Results of the uni- variate and multi-variate survival analysis
| Univariate Analysis | ||
|---|---|---|
| VARIABLE | HAZARD RATIO | p |
| SUV (continuous) | 1.11 (1.03–1.19) | 0.007 |
| SUV>6.10 | 2.90 (1.43–5.91) | 0.003 |
| BSI (continuous) | 1.02 (0.99–1.04) | 0.116 |
| BSI>1.27 | 2.71 (1.35–5.43) | 0.004 |
| Multivariate Analysis | |||
|---|---|---|---|
| MODEL | VARIABLE | HAZARD RATIO | p |
| Model 1 | SUV (continuous) | 1.10 (1.02–1.19) | 0.009 |
| BSI (continuous) | 1.02 (0.99–1.04) | 0.202 | |
| Model 2 | SUV>6.10 | 1.63 (0.67–3.97) | 0.278 |
| BSI>1.27 | 1.54 (0.63–3.74) | 0.345 | |
Discussion
Our study shows for the first time that FDG-PET SUVmax, a measure of the tumor glycolytic rate and Warburg effect, is strongly related to adverse prognosis in patients with progressive prostate cancer. Thus, progressing prostate cancer must now join the relatively long list of tumors for which FDG glycolytic rate, as captured by the baseline SUVmax, yields important prognostic information.8–20
This study also confirms several important observations that were made on a preliminary basis in earlier pilot trials. First, BSI was confirmed as a prognostic factor5, but in the current study even a relatively modest tumor involvement of 1.3% (the median in our data set) conferred a poor prognosis. Of note, in adult males bone marrow is confined to about 38% of the skeleton (axial skeleton, pelvis, variably the humeral and femoral heads, less commonly also the skull). Since osseous metastases initially localize to normal red marrow; a BSI of 1.3% corresponds to approximately 3.8% of the bones containing red marrow. Although a BSI of 1.3% then indicates a relatively modest involvement of marrow containing bone, in this group of patients with documented progressing prostate cancer, even this relatively low degree of involvement is an ominous sign. Second, we confirm our prior observation from a smaller group of patients6 that bone scan-positive but FDG-negative bone lesions are essentially static. On the other hand, the FDG-positive but bone scan-negative lesions at baseline eventually become detectable on follow-up bone scan. This supports the hypothesis that FDG-positive lesions are biologically active and aggressive, whereas lesions detectable on bone scan only (with negative FDG PET) have been treated successfully and tend to be stable, because active tumor has been removed. Thus, we believe that in patients with progressive disease, FDG-PET outperforms bone scanning for the detection of clinically active disease in the skeleton. Finally, we show that both FDG SUVmax and BSI were inversely correlated with survival, but only SUVmax was an independent predictor of survival in the multivariate analysis.
We recognize that our study plan also had limitations. For example, for purposes of accrual, 6/51 patients had FDG scans performed from 1–7 days after a change in therapy as permitted by protocol. Also, the SUV measurement itself can be quite variable, and the scans were performed, according to a protocol that permitted considerable variation in blood glucose, <200 mg/dl, and time after injection, (target of 60 minutes, but a broad range of 60 to 90 minutes. Also, the size of small lesions may have limited count recovery, and size of lesions is notoriously difficult to assess particularly in the bone. It is our view that all of these technical features of SUV response would be likely to increase random variation of the measure and reduce the predictive power of SUV, and in fact it is all the more remarkable that SUV was such a powerful predictor of survival given its technical limitations.
Based on our results, we therefore believe that FDG SUV, when used in proper clinical context, can serve as a biomarker of prognosis in progressing metastatic prostate cancer.
In prior studies in mixed or smaller groups of patients with metastatic prostate cancer, FDG PET showed detection rates of suspicious bone lesions between 18%21 and 65%.22 We consider it a serious problem that the patient populations in many of these earlier studies were not uniformly defined with regard to disease progression and distinct clinical states1, which carry a different prognosis and require different therapies and imaging strategies.23 This assessment also includes treated tumors. Of note, in the initial states of the disease, prostate cancer responds to antiandrogen therapies. These hormone-responsive tumors likely have a very low glycolytic rate, so that FDG-PET may not be a useful test in non-progressing patients with metastatic prostate cancer. In contrast, we have previously shown that in patients with progressing castrate resistant disease FDG PET can be highly useful for assessing treatment response, because a high positivity rate on baseline FDG can be evaluated as a useful indicator of response.24
Conclusion
In conclusion, in patients with progressing prostate cancer, osseous metastases are readily detected on FDG PET. While the extent of osseous disease, as quantified by BSI, is by itself a prognostic marker, SUV is a stronger prognostic indicator in progressing prostate cancer. In castrate-resistant patients, the subset with the most aggressive disease, SUV adds incremental prognostic information. FDG PET provides clinically relevant information that may improve the management of patients with progressing metastatic prostate cancer.
Translational Relevance.
FDG PET has incremental prognostic value in patients with progressive metastatic prostate cancer. There are currently only limited treatment options for this lethal form of the disease, and response assessment is difficult in particular for bone lesions. If confirmed in future studies, FDG PET might serve as a prognostic, and potentially also predictive, imaging biomarker in prostate cancer.
Acknowledgments
Grant Support: This study was supported by the ICMIC grant P50 CA086438-10, NCI/NIH.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest.
References
- 1.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 2.Soloway MS, Hardeman SW, Hickey D, et al. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer. 1988;61:195–202. doi: 10.1002/1097-0142(19880101)61:1<195::aid-cncr2820610133>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 3.Yamashita K, Denno K, Ueda T, et al. Prognostic significance of bone metastases in patients with metastatic prostate cancer. Cancer. 1993;71:1297–1302. doi: 10.1002/1097-0142(19930215)71:4<1297::aid-cncr2820710421>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Imbriaco M, Larson SM, Yeung HW, et al. A new parameter for measuring metastatic bone involvement by prostate cancer: the Bone Scan Index. Clin Cancer Res. 1998;4:1765–1772. [PubMed] [Google Scholar]
- 5.Sabbatini P, Larson SM, Kremer A, et al. Prognostic significance of extent of disease in bone in patients with androgen-independent prostate cancer. J Clin Oncol. 1999;17:948–957. doi: 10.1200/JCO.1999.17.3.948. [DOI] [PubMed] [Google Scholar]
- 6.Morris MJ, Akhurst T, Osman I, et al. Fluorinated deoxyglucose positron emission tomography imaging in progressive metastatic prostate cancer. Urology. 2002;59:913–918. doi: 10.1016/s0090-4295(02)01509-1. [DOI] [PubMed] [Google Scholar]
- 7.Obuchowski NA. On the comparison of correlated proportions for clustered data. Stat Med. 1998;17:1495–1507. doi: 10.1002/(sici)1097-0258(19980715)17:13<1495::aid-sim863>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 8.Costelloe CM, Macapinlac HA, Madewell JE, et al. 18F-FDG PET/CT as an indicator of progression-free and overall survival in osteosarcoma. J Nucl Med. 2009;50:340–347. doi: 10.2967/jnumed.108.058461. [DOI] [PubMed] [Google Scholar]
- 9.de Geus-Oei LF, Wiering B, Krabbe PF, Ruers TJ, Punt CJ, Oyen WJ. FDG-PET for prediction of survival of patients with metastatic colorectal carcinoma. Ann Oncol. 2006;17:1650–1655. doi: 10.1093/annonc/mdl180. [DOI] [PubMed] [Google Scholar]
- 10.Downey RJ, Akhurst T, Gonen M, et al. Preoperative F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value predicts survival after lung cancer resection. J Clin Oncol. 2004;22:3255–3260. doi: 10.1200/JCO.2004.11.109. [DOI] [PubMed] [Google Scholar]
- 11.Eary JF, O'Sullivan F, Powitan Y, et al. Sarcoma tumor FDG uptake measured by PET and patient outcome: a retrospective analysis. Eur J Nucl Med Mol Imaging. 2002;29:1149–1154. doi: 10.1007/s00259-002-0859-5. [DOI] [PubMed] [Google Scholar]
- 12.Pryma DA, Schoder H, Gonen M, Robbins RJ, Larson SM, Yeung HW. Diagnostic accuracy and prognostic value of 18F-FDG PET in Hurthle cell thyroid cancer patients. J Nucl Med. 2006;47:1260–1266. [PubMed] [Google Scholar]
- 13.Robbins RJ, Wan Q, Grewal RK, et al. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab. 2006;91:498–505. doi: 10.1210/jc.2005-1534. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki R, Komaki R, Macapinlac H, et al. [18F]fluorodeoxyglucose uptake by positron emission tomography predicts outcome of non-small-cell lung cancer. J Clin Oncol. 2005;23:1136–1143. doi: 10.1200/JCO.2005.06.129. [DOI] [PubMed] [Google Scholar]
- 15.Schoder H, Noy A, Gonen M, et al. Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:4643–4651. doi: 10.1200/JCO.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 16.Swisher SG, Maish M, Erasmus JJ, et al. Utility of PET, CT, EUS to identify pathologic responders in esophageal cancer. Ann Thorac Surg. 2004;78:1152–1160. doi: 10.1016/j.athoracsur.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 17.Vansteenkiste JF, Stroobants SG, Dupont PJ, et al. Prognostic importance of the standardized uptake value on (18)F-fluoro-2-deoxy-glucose-positron emission tomography scan in non-small-cell lung cancer: An analysis of 125 cases. Leuven Lung Cancer Group. J Clin Oncol. 1999;17:3201–3206. doi: 10.1200/JCO.1999.17.10.3201. [DOI] [PubMed] [Google Scholar]
- 18.Wong RJ, Lin DT, Schoder H, et al. Diagnostic and prognostic value of [(18)F]fluorodeoxyglucose positron emission tomography for recurrent head and neck squamous cell carcinoma. J Clin Oncol. 2002;20:4199–4208. doi: 10.1200/JCO.2002.02.590. [DOI] [PubMed] [Google Scholar]
- 19.Tateishi U, Gamez C, Dawood S, Yeung HW, Cristofanilli M, Macapinlac HA. Bone metastases in patients with metastatic breast cancer: morphologic and metabolic monitoring of response to systemic therapy with integrated PET/CT. Radiology. 2008;247:189–196. doi: 10.1148/radiol.2471070567. [DOI] [PubMed] [Google Scholar]
- 20.Specht JM, Tam SL, Kurland BF, et al. Serial 2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) to monitor treatment of bone-dominant metastatic breast cancer predicts time to progression (TTP) Breast Cancer Res Treat. 2007;105:87–94. doi: 10.1007/s10549-006-9435-1. [DOI] [PubMed] [Google Scholar]
- 21.Yeh SD, Imbriaco M, Larson SM, et al. Detection of bony metastases of androgen-independent prostate cancer by PET-FDG. Nucl Med Biol. 1996;23:693–697. doi: 10.1016/0969-8051(96)00044-3. [DOI] [PubMed] [Google Scholar]
- 22.Shreve PD, Grossman HB, Gross MD, Wahl RL. Metastatic prostate cancer: initial findings of PET with 2-deoxy-2-[F-18]fluoro-D-glucose. Radiology. 1996;199:751–756. doi: 10.1148/radiology.199.3.8638000. [DOI] [PubMed] [Google Scholar]
- 23.Schoder H, Larson SM. Positron emission tomography for prostate, bladder, and renal cancer. Semin Nucl Med. 2004;34:274–292. doi: 10.1053/j.semnuclmed.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Morris MJ, Akhurst T, Larson SM, et al. Fluorodeoxyglucose positron emission tomography as an outcome measure for castrate metastatic prostate cancer treated with antimicrotubule chemotherapy. Clin Cancer Res. 2005;11:3210–3216. doi: 10.1158/1078-0432.CCR-04-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]


