Abstract
In many social species, competition between groups is a major factor proximately affecting group-level movement patterns and space use and ultimately shaping the evolution of group living and complex sociality. Here we evaluated the factors influencing group-level dominance among 5 social groups of wild baboons (Papio cynocephalus), in particular focusing on the spatial determinants of dominance and the consequences of defeat. When direct conflict occurred between conspecific baboon groups, the winning group was predicted by differences in the number of adult males in each group and/or groups that had used the areas surrounding the encounter location more intensively than their opponent in the preceding 9 or 12 months. Relative intensity of space use over shorter timescales examined (3 and 6 months) was a poor predictor of the interaction’s outcome. Losing groups but not winning groups experienced clear short-term costs. Losing groups used the area surrounding the interaction less following an agonistic encounter (relative to their intensity of use of the area prior to the interaction). These findings offer insight into the influences and consequences of intergroup competition on group-level patterns of space use.
Intergroup competition occurs in many social species and is considered a major factor shaping the evolution of group living and complex sociality. Comparable to factors influencing the outcome of individual-level contests, important determinants of dominance in group-level competition include asymmetries in both fighting abilities and perceived resource value. In intergroup conflicts, advantages in fighting ability (or “resource holding potential”) are most commonly associated with group size such that larger groups have a competitive advantage relative to outnumbered opponents (Maynard Smith & Parker 1976). However, asymmetries in fighting ability alone are insufficient to explain situations in which dominance roles reverse. For example, among territorial species, residents are thought to place a higher value on the area being contested than intruders, and thus have more to gain from winning and/or more to risk by defeat (Enquist & Leimar 1987). Ownership advantage may also reduce to arbitrary conventions, such as “residents always win”, in territorial species (Maynard Smith & Parker 1976; Kokko et al. 2006).
In situations characterized by overlapping home ranges rather than discrete territories, interpreting perceived resource value for each contestant is complicated when both groups in a pair-wise encounter utilize the area surrounding an interaction, i.e. when there is ambiguity in defining “resident” versus “intruder”. Previous studies typically have overcome this challenge by considering the relative distance between an interaction location and each contestant’s nest site or home range center: resources are considered more valuable when close to a central reference point [e.g. Steller’s jay (Cyanocitta stelleri): Brown 1963; ocellated antbird (Phaenostictus mcleannani): Willis 1973; chipmunk (Tamias striatus): Elliott 1978; capuchin monkey (Cebus capucinus): Crofoot et al. 2008]. For many species, however, areas used intensively may be highly valued regardless of their location in the home range. An alternative approach, therefore, is to directly measure asymmetric use of the area surrounding the interaction location with the prediction that intensity of use is a marker for the contestant’s value of a resource (Crofoot et al. 2008).
In this study, we investigated group-level power asymmetry, or dominance, in wild baboons (Papio cynocephalus). Baboons, like many other cercopithecine primates, live in discrete, stable multimale-multifemale social groups. Multiple social groups consisting of 20-100 individuals (Estes 1991) comprise a single population, and the home ranges of neighboring groups overlap extensively (e.g. Altmann & Altmann 1970; Shopland 1982; Markham et al. manuscript). Limited and concentrated resources essential to survival, such as waterholes and sleeping sites, occur within regions of overlap. Baboons are obligate users of both: drinking from waterholes is a near-daily necessity and sleeping groves of adequate size provide safety from nocturnal predators. In the Amboseli basin of Kenya, any single group utilizes multiple waterholes and groves in their home ranges, i.e. baboons are “multiple central place foragers” (sensu Chapman et al. 1989; McLaughlin & Montgomerie 1989). Typically, resource size and group intolerance is believed to limit simultaneous use to a single group, suggesting that groups rely upon temporal mechanisms to partition the landscape. How the outcomes of intergroup contests both influence and are influenced by group-level patterns of space use has not been investigated.
Our study addressed three specific objectives. First, we evaluated total group size and composition (number of adult males and number of adult females) as predictors of dominance in group-level interactions. If philopatry influences participation in intergroup aggression (e.g. Cheney 1987; Isbell 1991), the number of adult females (the non-dispersing sex in this species) would be a stronger predictor of dominance than total group size or number of adult males. However, adult male baboons are reported to exhibit more aggressive displays and be more actively involved in intergroup conflict relative to other sex-age classes (e.g. Maxim & Buettner-Janusch 1963; Stoltz & Saayman 1970; Paterson 1973; Cheney & Seyfarth 1977), suggesting asymmetries in the number of adult males may determine an interaction’s outcome. We therefore predicted that differences in the number of adult males would be a better predictor of dominance than either differences in total group size and number of adult females.
Second, we evaluated relative space use in the area surrounding the interaction location as a predictor of dominance over 4 timescales (3, 6, 9, and 12 months) prior to the interaction. This approach offered novel, empirical insight into the theory that resource value is correlated with long-term use, specifically that the probability of winning is influenced by the duration of tenure (reviewed in Bradbury & Vehrencamp 1998).
Third, we analyzed the spatial consequences of agonistic interactions over the same 4 timescales for winners and losers by comparing space use in the area surrounding an interaction before and after the encounter. Theoretical and empirical research on individual-level agonisms suggests that losers should avoid areas of agonistic interaction if prior experience reliably predicts future conflict (reviewed in Stamps & Krishan 2001). Yet, to the best of our knowledge, spatial consequences of defeat have not been studied in group-level contests. We predicted that the losing group would exhibit avoidance of the area (relative to its former use) following the interaction whereas space-use patterns of the winning group would be unaltered.
METHODS
This study was part of ongoing research on baboons (Papio cynocephalus) living within the Amboseli ecosystem, a semi-arid short-grass savannah that straddles the Kenya-Tanzania border (Alberts & Altmann 2012). The data presented here represent a 9-year period from Aug 2000–Oct 2009, and focused on five social groups. All baboons within the study population were individually identifiable by ABRP field researchers, and each group was the focus of detailed observations several days each week. Consequently, demographic data were typically accurate to within a few days. Details on assessing maturational milestones relevant to calculating the number of adult males and females are provided by Alberts & Altmann (1995) and Gesquiere et al. (2007), respectively. Complete details on monitoring effort and data collection protocols can be accessed online (http://www.princeton.edu/~baboon/).
For this study, we used observer-recorded data on decided agonistic interactions between group pairs (N=222). Decided agonistic interactions were defined as contests in which only one group displayed clear dominance over another group and/or in which only one group displayed clear subordinance in response to another group. For each dyadic agonistic interaction, ABRP observers recorded group identities, date, time, and dominant/subordinate group-level behaviors. Specifically, the behaviors we considered in assessing interactions included direct aggression (N=3; 1.3%), chasing (N=11; 5.0%), spatial displacement (N=200; 90.1%), and blocking access to discrete ecological resources (N=8; 3.6%). To ensure independence of observations, we included only one agonistic interaction per dyad-day in our analyses, i.e. agonistic interactions have a daily resolution. Dyad-days were defined as days in which at least one of the two groups involved in the agonistic interaction was the focus of observation. For the 9-year period of this study (Aug 2000-Oct 2009), we had a total of 18,691 dyad-days, and the observation days had an average duration of 4.4 hours (± 0.03 SE; N=5,520).
Location of each agonistic interaction was determined by cross-referencing group identity, date, and time with observer-recorded GPS location data. Observers recorded half-hourly GPS locations of focal groups during each day of observation. Groups rarely traveled in areas that were inaccessible to observers; therefore GPS data are not spatially biased to times/locations for which observers were able to follow the animals. For a subset of interactions (N=25), GPS data were coincident with the time of the interaction. In other cases (N=182), we used Esri ArcGIS 9.2 (Redlands, CA) and the Hawth’s Tools extension (Beyer 2004) to calculate straight-line displacements from GPS readings taken ≤ 15 minutes before and after the interaction; interaction location was estimated along this line based on the time of the interaction and assuming constant travel speed. As a third and final source of locational information, in 7 cases we were able to use known coordinates of specified sleeping groves or waterholes referenced in observer-recorded notes about the interaction. We were not able to determine the interaction’s location in 8 cases.
Half-hourly GPS location data were also used to determine each group’s intensity of use in the area surrounding each interaction location. Observers recorded on average 115 (± 1.5 SE) GPS readings per group in each calendar month of this study (N=630 group-months). Intensity of prior use was assessed independently for each of the two groups participating in the interaction. For each group we calculated intensity of use as the proportion of that group’s total GPS locations that were with within 500 m of the interaction location over 4 timescales preceding and following the interaction date: 3, 6, 9, and12 months.
Ethical Note
All project protocols complied with regulations in Kenya (Republic of Kenya Research Permits NCST/5/002/R/776 to J.A. and NCST/5/002/R/777 to S.C.A.) and in the United States (Princeton University IACUC 1649), and adhered to the ASAB/ABS Guidelines for the Use of Animals in Research.
Statistical Analyses
To test for determinants of dominance between a pair of groups, we randomly selected one group from each intergroup interaction as the focal subject for analysis. Accounting for repeated observations of each group-pair, we used generalized estimating equations (GEE) to test whether difference in demographics and relative intensity of use (focal group’s intensity of use / opponent’s intensity of use) predicted the interaction’s outcome, i.e. whether the focal group won or lost, over 4 timescales (3, 6, 9, and 12 months) prior to the interaction. Three demographic predictors were evaluated: differences in total group size (focal group size – opponent’s group size), differences in the number of adult males (adult males in focal group – adult males in opponent group), and differences in the number of adult females (adult females in focal group – adult females in opponent group). Because these 3 predictors were highly correlated, we tested the effects of each in 3 separate models. We selected the single best model at each timescale using quasi-likelihood under the independence model criterion (QIC), which compares the adequacy of several models and identifies the model that best explains the variance of the dependent variable as that with the lowest QIC value (Pan 2001; Tsai et al. 2011). Additionally, we calculated QIC weights to determine the relative predictive ability of the models tested by normalizing each model based on its QIC value relative to the QIC value of the best model (Burnham & Anderson 2004).
For evaluating the effects of an encounter’s outcome over the same 4 timescales, we used a Wilcoxon rank test to determine whether each group’s intensity of use in the months following an interaction differed significantly from the intensity of use prior to the interaction. Intensity of use before and after the interaction was tested separately for winners and losers. All statistical tests were run individually for each timescale (3, 6, 9, and 12 months) and were performed in SPSS 17.0 (SPSS Inc., 2008). The alpha value for statistical significance was set to 0.05 for all analyses.
RESULTS
The average difference in total group size during N=222 interactions (where group size was measured on the day of the interaction) was 29.6 individuals (± 1.1 SE, range: 1-78). The average difference in the number of adult males was 5.2 males (± 0.2 SE, range: 0-13), and the average difference in the number of adult females was 9.1 females (± 0.35 SE, range: 0-26). Based on locational information and associated notes, the majority of intergroup agonistic interactions (143 of 222) occurred in apparent conflict over either waterholes (N=54; 24.3%) or sleeping groves (N=89; 40.1%). Field records for the remaining 79 interactions (35.6%) provided no explicit reference to waterholes and/or sleeping groves in the context of the interaction. Although intergroup agonisms varied in intensity of aggression and/or submission, no instances of wounding were observed in this 9-year period.
Group dominance was predicted by asymmetries in the number of adult males, particularly when relative differences in the number of adult males were large (Fig. 1). Although comparable results were found for asymmetries in total group size and number of adult females, differences in the number of adult males produced lower QIC values at all timescales examined (Table 1). Because the number of adult males predicted the observed outcomes better than either total group size or number of adult females did, we focus hereafter exclusively on the number of adult males.
Fig. 1.
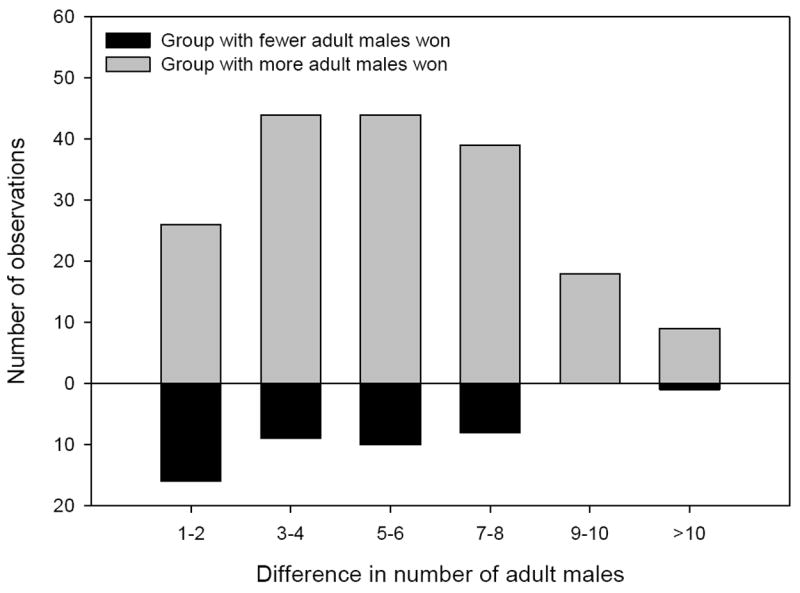
Group dominance was largely predicted by asymmetries between competing social groups in the number of adult males, particularly when relative differences in the number of adult males were large.
Table 1.
QIC, ΔQIC, and model weights for the 3 models tested at each timescale (3, 6, 9, and 12 months). At each timescale, ΔQIC is the difference between a model and the model with the lowest QIC value, and the model weight is the normalized value of the model based on its ΔQIC value. All models were tested with relative intensity of use as an additional predictor variable (see text).
| Demographic predictor | QIC | ΔQIC | Model Weight |
|---|---|---|---|
| Timescale: 3 months | |||
| Difference in number of adult males | 191.11 | 0.00 | 0.962 |
| Difference in total group size | 197.60 | 6.48 | 0.038 |
| Difference in number of adult females | 212.78 | 21.66 | 0.000 |
| Timescale: 6 months | |||
| Difference in number of adult males | 205.83 | 0.00 | 0.831 |
| Difference in total group size | 209.02 | 3.19 | 0.169 |
| Difference in number of adult females | 222.39 | 16.56 | 0.000 |
| Timescale: 9 months | |||
| Difference in number of adult males | 208.76 | 0.00 | 0.952 |
| Difference in total group size | 214.78 | 6.02 | 0.047 |
| Difference in number of adult females | 227.31 | 18.55 | 0.000 |
| Timescale: 12 months | |||
| Difference in number of adult males | 209.12 | 0.00 | 0.954 |
| Difference in total group size | 215.20 | 6.08 | 0.046 |
| Difference in number of adult females | 228.13 | 19.01 | 0.000 |
In analyses of the subset of interactions for which location could be determined (N=214), the number of adult males was a significant determinant of dominance at all timescales; groups with more males experienced competitive advantages. Further, space use was a significant determinant of dominance; groups that used the area surrounding the location more intensively than their opponent in the preceding 9 or 12 months were more likely to win the encounter. In contrast, more recent space use, i.e. space use over shorter timescales (3 and 6 months), did not predict dominance. Table 2 provides summary statistics of the GEE at all timescales examined.
Table 2.
Results from a generalized estimating equation (GEE) testing the effects of asymmetries in the number of adult males (number of adult males in the focal group minus number of adult males in the other group) and intensity of use in the area surrounding the interaction’s location (focal group intensity of use/other group’s intensity of use) on the probability of winning across 4 timescales (3, 6, 9, and 12 months).
| Estimate | Wald χ2 | df | P | |
|---|---|---|---|---|
| Timescale: 3 months | ||||
| Intercept | 0.123 | 0.245 | 1 | 0.620 |
| Difference in number of adult males | 0.297 | 94.849 | 1 | <0.001 |
| Relative intensity of use | 0.008 | 0.010 | 1 | 0.918 |
| Timescale: 6 months | ||||
| Intercept | 0.031 | 0.028 | 1 | 0.868 |
| Difference in number of adult males | 0.285 | 78.134 | 1 | <0.001 |
| Relative intensity of use | 0.025 | 2.627 | 1 | 0.105 |
| Timescale: 9 months | ||||
| Intercept | 0.051 | 0.093 | 1 | 0.761 |
| Difference in number of adult males | 0.277 | 61.107 | 1 | <0.001 |
| Relative intensity of use | 0.028 | 4.072 | 1 | 0.044 |
| Timescale: 12 months | ||||
| Intercept | 0.041 | 0.061 | 1 | 0.806 |
| Difference in number of adult males | 0.278 | 62.333 | 1 | <0.001 |
| Relative intensity of use | 0.026 | 4.108 | 1 | 0.043 |
Statistically significant results are bolded
Groups that won interactions did not significantly change their intensity of use following an interaction at any of the 4 timescales. In contrast, groups that lost interactions used the area surrounding the interaction less in the 3 months following an agonistic encounter (relative to their intensity of use of the area 3 months prior to the interaction); there were no changes in intensity of space use over longer timescales (6, 9, or 12 months) for groups that lost interactions (Fig. 2). Table 3 provides summary statistics of the Wilcoxon rank test at all timescales examined.
Fig. 2.
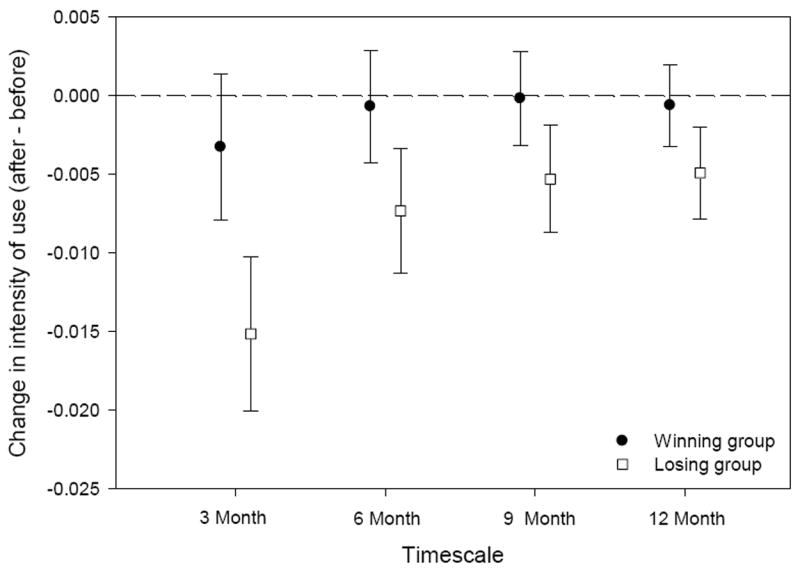
Following an agonistic interaction, losing groups tended to avoid the interaction location (relative to their intensity of use prior to the interaction); results were significant only for the losing group in the 3 months following the interaction. At all 4 timescales, winning groups did not change their intensity of use around the interaction location following an encounter.
Table 3.
Results from a Wilcoxon rank test to determine whether intensity of use in the months following an interaction differed significantly from the intensity of use prior to the interaction across 4 timescales (3, 6, 9, and 12 months).
| N | Z | P | |
|---|---|---|---|
| Timescale: 3 months | |||
| Winning group | 214 | -1.596 | 0.110 |
| Losing group | 214 | -3.186 | 0.001 |
| Timescale: 6 months | |||
| Winning group | 214 | -0.807 | 0.420 |
| Losing group | 214 | -1.841 | 0.066 |
| Timescale: 9 months | |||
| Winning group | 214 | -0.323 | 0.747 |
| Losing group | 214 | -1.721 | 0.087 |
| Timescale: 12 months | |||
| Winning group | 214 | -0.305 | 0.761 |
| Losing group | 214 | -1.776 | 0.076 |
Statistically significant results are bolded
DISCUSSION
This study demonstrates that success in contests between neighboring groups of wild baboons was determined by asymmetries in both the number of adult males and space-use patterns. Competitive advantages were experienced by groups with more adult males and/or groups that had used the areas surrounding the interaction location more intensively than their opponent in the preceding 9 or 12 months. Interestingly, shorter term intensity of use was not a significant predicator of dominance.
The influence of group size on contest outcome is well-established (e.g. Nagel 1973; McComb et al. 1994; Crofoot et al. 2008). However, few studies have evaluated whether differences in group composition provide a competitive advantage (but see Hamilton et al. 1975; Mosser & Packer 2009) despite recognition that aggressive involvement in intergroup encounters is not necessarily shared equally amongst group members (reviewed in Cheney 1987). In primates, female aggression during intergroup encounters is commonly reported in species characterized by female philopatry (Cheney 1987; Isbell 1991; but see Okamato & Matsumura 2002). However, in baboons, males are more likely than females to engage actively in intergroup interactions (e.g. Maxim & Buettner-Janusch 1963; Stoltz & Saayman 1970; Paterson 1973; Cheney & Seyfarth 1977). This male-biased participation in baboons may be explained by the high degree of sexual dimorphism in body size (Altmann et al. 1993) and canine size (Walker 1984) characterizing the species: individuals of the smaller sex and/or individuals lacking “weaponry” (e.g. large canines) may be unlikely to challenge rivals (Packer & Pusey 1979). Because aggressive involvement in intergroup encounters is not shared equitably, we predicted that differences in the number of adult males would be a stronger determinant of dominance than differences in total group size or number of adult females would be. Our results support this prediction, suggesting that a group’s fighting ability is best assessed by numerical differences in the age-sex class most likely to be actively involved in the encounter.
Although intergroup agonistic interactions in our population occasionally escalated to physical aggression, most observed displays of dominance involved one group displacing another in the absence of physical conflict. The dominance of a group measured by its ability to displace others at discrete resources has been noted previously in several baboon populations (e.g. Altmann & Altmann 1970; Saayman 1971; Nagel 1973; Hamilton et al. 1975, Hamilton et al. 1976; Maples et al. 1976; Rasmussen 1979; Sugarwara 1979).
Among primates, contests between groups are most likely to intensify to extreme, even lethal, aggression in situations when groups temporarily fission into smaller subgroups such that imbalances of power vary across time (reviewed in Crofoot & Wrangham 2010). For example, “warfare” between male subgroups of neighboring communities has been studied extensively in the fission-fusion society of chimpanzees (Pan troglodytes). Although the number of members in each subgroup reliably predicts dominance in chimpanzees, variability in subgroup composition and size contributes to the likelihood that chimpanzee conflicts will escalate (Manson & Wrangham 1991). In contrast, the multimale-multifemale groups in baboon society are remarkably stable over time. As a result, each baboon group may be able to assess its fighting ability relative to its neighbor over long timescales. However, the effect of temporary subgroup formation (wherein stable groups occasionally fission and range separately for several hours) on the propensity for violence and/or dominance has not yet been evaluated. We do not know of any attempt to do so and the data available from our studies are not adequate to address this topic.
In addition to the effects of adult male numerical superiority, we demonstrated that intensity of space use surrounding the interaction location was a significant predictor of dominance: groups with fewer adult males than their opponent were occasionally able to win contests if they had used the interaction area more intensely than their opponent in the preceding 9 or 12 months. We are aware of only one study that similarly evaluated effects of both group size and location on the outcome of group-level agonistic interactions. Comparable to our findings, Crofoot et al. (2008) observed that the effects of numerical superiority on intergroup dominance patterns in capuchin monkeys varied across space. We agree with their interpretation that this tendency of relative “residents” to win contests, despite numerical disadvantages, may be an important mechanism by which a range of group sizes is maintained within a population.
Despite recognition that intensity of space use confers a competitive advantage in contests between animals, we know of no empirical studies that have explicitly addressed the timescale over which space use influences contest outcome. We found that intergroup dominance was predicted by long-term space use (space use over shorter timescales was a poor predictor of the interaction’s outcome), perhaps reflecting the periodicity of resource/space use, a possibility that is beyond our current ability to evaluate. In contrast, spatial consequences of defeat involved short-term (but not long-term) perturbations for the subordinate group. Together, these findings have important implications for the ways in which intergroup dominance and space use are interrelated. If intergroup encounter rates were higher in some species or under some conditions, we postulate that the repeated negative reinforcement may be more long-lasting and result in more spatial separation between groups. To the best of our knowledge, theoretical studies demonstrating that frequency of interaction influences spatial overlap among solitary animals (e.g. Jetz et al. 2004) have not yet been extended to empirical research on species obligated to group-living.
Acknowledgments
We are grateful to the government of the Republic of Kenya, to the Kenya Wildlife Services, the staff and wardens of Amboseli National Park, and the local community of the Amboseli region. Tremendous thanks go to ABRP researchers for their contributions to data collection and outstanding dedication in the field: R. Mututua, S. Sayialel, and J.K. Warutere. We also thank N. Learn and L. Maryott for their invaluable database assistance. Two anonymous reviewers provided helpful comments on a previous draft of this manuscript. We benefited from S. Mathews’ preliminary research on group-level dominance in the Amboseli baboon population; her contribution enhanced our understanding of intergroup interactions. Financial support was provided by American Society of Primatologists (to A.C.M.), Animal Behavior Society (to A.C.M.), International Primatological Society (to A.C.M.), NIA (R01AG034513-01 to J.A. and S.C.A.), NSF (IBN-0322613 to J.A. and S.C.A.; IOS-0919200 S.C.A.; BCS-0851750 to J.A. and A.C.M.), and Sigma Xi (to A.C.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberts SC, Altmann J. Preparation and activation: determinants of age at reproductive maturity in male baboons. Behavioral Ecology and Sociobiology. 1995;36:397–406. [Google Scholar]
- Alberts SC, Altmann J. The Amboseli Baboon Research Project: 40 Years of Continuity and Change. In: Kappeler P, Watts D, editors. Long-Term Field Studies of Primates. Berlin: Spring-Verlag; 2010. pp. 261–287. [Google Scholar]
- Altmann J, Schoeller D, Altmann SA, Muruthi P, Sapolsky RM. Body size and fatness of free-living baboons reflect food availability and activity levels. American Journal of Primatology. 1993;30:149–161. doi: 10.1002/ajp.1350300207. [DOI] [PubMed] [Google Scholar]
- Altmann SA, Altmann J. Baboon Ecology. Chicago: University of Chicago Press; 1970. [Google Scholar]
- Beyer H. Hawth’s Tools: An extension for ArcGIS. 2004 http://www.spatialecology.com.
- Bradbury WJ, Vehrencamp L. Principles of Animal Communication. 2. Sunderland: Sinauer Associates; 1998. [Google Scholar]
- Brown JL. Aggressiveness, dominance and social organization in the Steller Jay. The Condor. 1963;65:460–484. [Google Scholar]
- Burnham KP, Anderson DR. Model Selection and Inference. 2. New York: Springer-Verlag; 2002. [Google Scholar]
- Chapman CA, Chapman LJ, McLaughlin RL. Multiple central place foraging by spider monkeys: Travel consequences of using many sleeping sites. Oecologia. 1989;79:506–511. doi: 10.1007/BF00378668. [DOI] [PubMed] [Google Scholar]
- Cheney DL. Interactions and relationships between groups. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. Chicago: University of Chicago Press; 1987. pp. 267–281. [Google Scholar]
- Cheney DL, Seyfarth RM. Behavior of adult and immature male baboons during inter-group encounters. Nature. 1977;269:404–406. [Google Scholar]
- Crofoot MC, Gilby IC, Wikelski MC, Kays RW. Interaction location outweighs the competitive advantage of numerical superior in Cebus capucinus intergroup contests. Proceedings of the National Academy of Sciences. 2008;105:577–581. doi: 10.1073/pnas.0707749105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofoot MC, Wrangham RW. Intergroup aggression in primates and humans: The case for a unified theory. In: Kappeler P, Silk J, editors. Minding the Gap: Tracing the Origins of Human Universals. Berlin: Springer-Verlag; 2010. pp. 171–196. [Google Scholar]
- Elliott L. Social behavior and foraging ecology of the chipmunk (Tamias striatus) in the Adirondack Mountains. Smithsonian Contributions to Zoology. 1978;265 [Google Scholar]
- Enquist M, Leimar O. Evolution of fighting behaviour: The effect of variation in resource value. Journal of Theoretical Biology. 1987;127:187–205. [Google Scholar]
- Estes RD. The Behavior Guide to African Mammals. Berkeley: University of California Press; 1991. [Google Scholar]
- Gesquiere LR, Wango EO, Alberts SC, Altmann J. Mechanisms of sexual selection: Sexual swellings and estrogen concentrations as fertility indicators and cues for male consort decisions in wild baboons. Hormones and Behavior. 2007;51:114–125. doi: 10.1016/j.yhbeh.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Hamilton WJ, III, Buskirk RE, Buskirk WH. Chacma baboon tactics during intertroop encounters. Journal of Mammology. 1975;56:857–870. [PubMed] [Google Scholar]
- Hamilton WJ, III, Buskirk RE, Buskirk WH. Defense of space and resources by chacma (Papio ursinus) baboon troops in an African desert and swamp. Ecology. 1976;57:1264–1272. [Google Scholar]
- Isbell L. Contest and scramble competition: Patterns of female aggression and ranging behavior among primates. Behavioral Ecology. 1991;2:143–155. [Google Scholar]
- Jetz W, Carbone C, Fulford J, Brown JH. The scaling of animal space-use. Science. 2004;306:266–268. doi: 10.1126/science.1102138. [DOI] [PubMed] [Google Scholar]
- Kokko H, Lopez-Sepulcre A, Morrell LJ. From hawks and doves to self-consistent games of territorial behavior. American Naturalist. 2006;167:901–912. doi: 10.1086/504604. [DOI] [PubMed] [Google Scholar]
- Manson JH, Wrangham RW. Intergroup aggression in chimpanzees and humans. Current Anthropology. 1991;32:369–390. [Google Scholar]
- Maples WR, Maples MK, Greenwood WF, Walek ML. Adaptations of crop-raiding baboons in Kenya. American Journal of Physical Anthropology. 1976;45:309–315. [Google Scholar]
- Markham AC, Guttal V, Alberts SC, Altmann J. When good neighbors don’t need fences: Temporal landscape partitioning among baboon social groups. doi: 10.1007/s00265-013-1510-0. Unpublished manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxim PE, Buettner-Janusch J. A field study of the Kenya baboon. American Journal of Physical Anthropology. 1963;21:165–180. doi: 10.1002/ajpa.1330210209. [DOI] [PubMed] [Google Scholar]
- Maynard Smith J, Parker GA. The logic of asymmetric contests. Animal Behaviour. 1976;24:159–175. [Google Scholar]
- McComb K, Packer C, Pusey A. Roaring and numerical assessment in contests between groups of female lions, Panthera leo. Animal Behaviour. 1994;47:379–387. [Google Scholar]
- McLaughlin RL, Montgomerie RD. Brood dispersal and multiple central place foraging by Lapland longspur parents. Behavioral Ecology and Sociobiology. 1989;25:207–215. [Google Scholar]
- Mosser A, Packer C. Group territoriality and the benefits of sociality in the African lion, Panthera leo. Animal Behaviour. 2009;78:359–370. [Google Scholar]
- Nagel U. A comparison of anubis baboons, hamadryas baboons and their hybrids at a species border in Ethiopia. Folia Primatolgica. 1973;19:104–165. doi: 10.1159/000155536. [DOI] [PubMed] [Google Scholar]
- Okamato K, Matsumura S. Intergroup encounters in wild moor macaques (Macaca maurus) Primates. 2002;43:119–125. doi: 10.1007/BF02629671. [DOI] [PubMed] [Google Scholar]
- Packer C, Pusey AE. Female aggression and male membership in troops of Japanese macaques and olive baboons. Folia Primatologica. 1979;31:212–218. doi: 10.1159/000155884. [DOI] [PubMed] [Google Scholar]
- Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57:120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- Paterson JD. Ecologically differentiated patterns of aggressive and sexual behavior in two troops of Ugandan baboons, Papio anubis. American Journal of Physical Anthropology. 1973;38:641–648. doi: 10.1002/ajpa.1330380281. [DOI] [PubMed] [Google Scholar]
- Rasmussen DR. Correlates of patterns of range use of a troop of yellow baboons (Papio cynocephalus). I. Sleeping sites, impregnable females, births, and male emigrations and immigrations. Animal Behaviour. 1979;27:1098–1112. [Google Scholar]
- Saayman S. Behaviour of adult males in a troop of free ranging chacma baboons. Folia Primatologia. 1971;15:36–57. [PubMed] [Google Scholar]
- Shopland JM. An intergroup encounter with fatal consequences in yellow baboons (Papio cynocephalus) American Journal of Primatology. 1982;3:263–266. doi: 10.1002/ajp.1350030123. [DOI] [PubMed] [Google Scholar]
- Stamps JA, Krishan VV. How territorial animals compete for divisible space: A learning-based model with unequal competitors. The American Naturalist. 2001;157:154–169. doi: 10.1086/318634. [DOI] [PubMed] [Google Scholar]
- Stoltz LP, Saayman GS. Ecology and behaviour of baboons in the northern Transvaal. Annals of the Transvaal Museum. 1970;26:99–143. [Google Scholar]
- Sugarwara K. Socioecological study of a wild group of hybrid baboons between Papio anubis and Papio hamadryas in the Awash Valley, Ethiopia. Primates. 1979;20:21–56. [Google Scholar]
- Tsai M-Y, Wang J-F, Wu J-L. Generalized estimating equations with model selection for comparing dependent categorical agreement data. Computational Statistics & Data Analysis. 2011;55:2354–2362. [Google Scholar]
- Walker A. Mechanisms of honing in the male baboon canine. American Journal of Physical Anthropology. 1984;65:47–60. doi: 10.1002/ajpa.1330650108. [DOI] [PubMed] [Google Scholar]
- Willis EO. The behavior of ocellated antbirds. Smithsonian Contributions to Zoology. 1973:144. [Google Scholar]


