Abstract
Background
The purpose of this study was to evaluate the efficacy and safety of two fixed combinations, ie, timolol 0.5% + brimonidine 0.2% + dorzolamide 2% (TBD) versus timolol 0.5% + brimonidine 0.2% (TB) in patients with primary open-angle glaucoma or ocular hypertension.
Methods
We performed a 3-month, randomized, double-blind study in patients with primary open-angle glaucoma or ocular hypertension and an intraocular pressure of 21–30 mmHg. Patients were randomly assigned to receive one drop of TBD or TB twice a day. The primary efficacy endpoint was change in intraocular pressure after 3 months of treatment. Safety measures were assessed by the presence of adverse events.
Results
Mean baseline intraocular pressure was similar at 8 am and 4 pm in the treatment groups (TBD 22.3 ± 0.9 mmHg, TB 22.4 ± 1.8 mmHg, P = 0.558; TBD 19.02 ± 1.3, TB 19.08 ± 1.2, P = 0.536, respectively). At the end of the study, the mean intraocular pressure was significantly lower in the TBD group at both 8 am (16.19 ± 2.0 mmHg versus 18.35 ± 1.4 mmHg, P = 0.000) and 4 pm (14.74 ± 2.4 mmHg versus 16.77 ± 1.4 mmHg, P = 0.000).
Conclusion
Fixed-combination TBD was more effective than fixed-combination TB for reducing IOP in patients with primary open-angle glaucoma.
Keywords: primary open-angle glaucoma, ocular hypertension, intraocular pressure, fixed combination
Introduction
Glaucoma is a common and potentially blinding ocular disease of multifactorial etiology. It is characterized by progressive acquired loss of retinal ganglion cells and their axons, leading to optic nerve atrophy and appearance of characteristic cupping of the optic disc associated with corresponding visual deficit. It is frequently associated with elevated intraocular pressure (IOP). This damage can lead to permanent vision loss if it is not treated.1,2
Population studies indicate that one in 40 adults older than 40 years has glaucoma, with loss of visual function. The disease affects approximately 60 million people worldwide, with 8.4 million being bilaterally blind. Even in developed countries, half of glaucoma cases are undiagnosed. Glaucoma is mostly asymptomatic until late in the disease when visual problems arise.3
Elevated IOP is an important and modifiable risk factor for the development and progression of glaucoma.1 It has been demonstrated that lowering IOP effectively delays development of glaucoma in patients with ocular hypertension, as well as progression of established glaucoma.4 Topical hypotensive medication is considered as first-line treatment in the initial management of increased IOP. Nevertheless, target IOP levels are not always achieved with a single medication, and patients frequently require multiple medications, which can lead to unsatisfactory adherence with treatment.3,5–7 According to the Ocular Hypertension Treatment Study, after 5 years of IOP-lowering treatment, 40% of patients need at least two drugs.8
Fixed combinations for glaucoma and ocular hypertension treatment are increasing. These fixed combinations contain two medications in a single bottle, and offers several advantages, including enhanced convenience, improved adherence, and reduced exposure to preservatives.6
The efficacy and safety of fixed combinations has been evaluated previously in different studies.7 The purpose of this study was to compare the efficacy and safety of fixed combinations of timolol 0.5% + brimonidine 0.2% + dorzolamide 2% (TBD) versus timolol 0.5% + brimonidine 0.2% (TB) for IOP reduction.
Materials and methods
We conducted a prospective 3-month, randomized, double-blind, multicenter clinical trial to compare the efficacy and safety of TBD versus TB on IOP reduction. The protocol was reviewed and approved by our ethics committee, and was conducted in compliance with the Declaration of Helsinki and in accordance with Good Clinical Practice standards. All patients who participated in the study provided their written informed consent. The study is registered on the International Standard Randomized Controlled Trial Number Register (ISRCTN48477221).
Adult patients with primary open-angle glaucoma, ocular hypertension, pseudoexfoliative glaucoma, or pigmentary glaucoma, and a mean IOP of 21–30 mmHg were enrolled. Primary exclusion criteria included blindness in one eye, visual field loss indicative of end-stage glaucoma, normal-tension glaucoma, optic nerve cupping ≥ 0.8, any active ocular disease other than glaucoma or ocular hypertension that would interfere with study interpretation, history of cataract surgery within 3 months prior to baseline, contraindication of any medication used in the protocol, and pregnancy, risk of pregnancy, or breastfeeding.
The primary efficacy endpoint was the IOP-lowering effect after 3 months of treatment. Patients were assessed for eligibility at a screening visit 6 weeks before baseline when a medical and ocular history was taken, IOP was measured in both eyes (using a calibrated Goldmann applanation tonometer), as well as Snellen visual acuity measurement and visual field test using automated perimetry (Humphrey’s 24-2 Sita Standard). Abnormal findings on slit-lamp examination (biomicroscopy) and fluorescein dye were graded as mild, moderate, or severe. Safety outcome measures included adverse events and ocular signs.
All IOP-lowering medications were suspended in eligible patients, who underwent a 6-week washout period prior to baseline and, if their IOPs were considered detrimental, they were excluded from the study. Patients were evaluated at five study visits, ie, at baseline, and at 15, 30, 60, and 90 days after enrollment. At each study visit, IOP was measured in each eye at 8 am and 4 pm, slit-lamp examination using biomicroscopy and Snellen visual acuity measurement tests were performed, and use of concomitant medications were recorded.
Patients were randomly assigned 1:1 to receive TBD or TB. To maintain masking, bottles of the study drugs were overlabeled so that they had an identical appearance when provided to patients. Patients were instructed to instill one drop of either TBD (Krytantek Ofteno®; Laboratorios Sophia, Guadalajara, Mexico) or TB (Combigan D®; Allergan Inc, Irvine, CA) in each eye twice a day, ie, in the morning at 8 am (±15 minutes) and in the evening at 8 pm (±15 minutes) for 3 months. The trial medication was discontinued if either the investigator or patient thought that it was not in the patient’s best interests to continue, or if the patient became pregnant.
Ocular findings and adverse events regardless of relationship to treatment were monitored throughout. Investigators recorded observed adverse events, as well as those reported by patients or elicited by questioning. Adverse events were classified as mild, moderate, or severe. Only randomized patients who concluded the study without major protocol violation and provided IOP measurements at all visits were included in the per-protocol analysis.
Statistical analysis
Baseline and final differences in IOP between the treatment groups were analyzed using the Mann–Whitney test. Before study initiation, it was determined that at least 42 patients were needed per group to detect a difference of at least 2 mmHg in mean IOP reduction between treatments using a significance level of 0.05, with a power of 0.80.
Results
Fifty-six patients per group were recruited for this study from 11 centers in Mexico. A total of 106 patients were included in the per-protocol analysis. None of the patients presented with pseudoexfoliative or pigmentary glaucoma. The mean age was 62.5 ± 10.2 years in the TBD group and 60.6 ± 12.7 years in the TB group. Demographic characteristics are shown in Table 1.
Table 1.
Demographic features of study groups
| n | Female n (%) | Male n (%) | Age* | |
|---|---|---|---|---|
| TBD | 56 | 42 (76) | 14 (24) | 62.5 ± 10.2 |
| TB | 56 | 56 44 (80) | 12 (20) | 60.6 ± 12.7 |
Note:
Data is presented in mean ± standard deviation.
Abbreivations: TBD, timolol-brimonidine-dorzolamide; TB, timololbrimonidine.
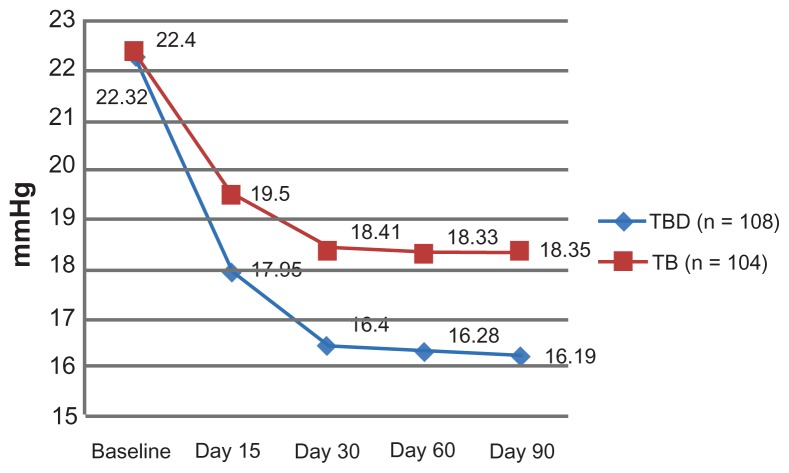
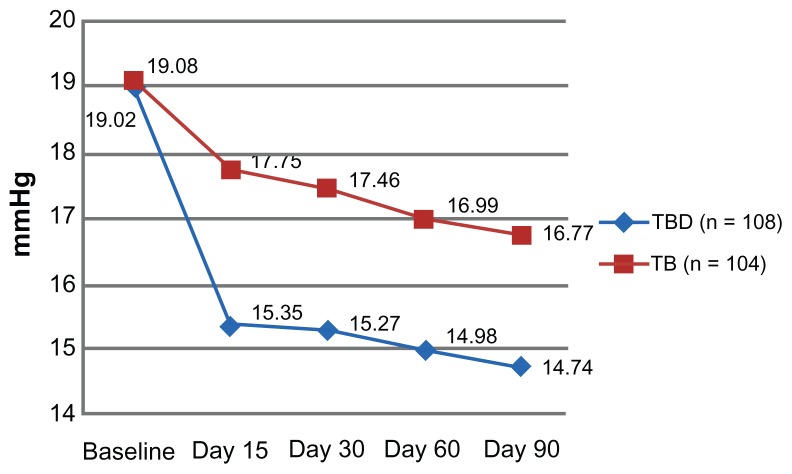
The primary efficacy endpoint was the IOP-lowering effect at both 8 am and 4 pm after 3 months of treatment. The mean baseline IOP was similar between the groups at 8 am (TBD 22.3 ± 0.9 mmHg, TB 22.4 ± 1.8 mmHg, P = 0.558) and at 4 pm (TBD 19.02 ± 1.3 mmHg, TB 19.08 ± 1.2 mmHg, P = 0.536, Table 2). After baseline, and at all study visits, the mean 8 am IOP at 15, 30, and 60 days after enrolment was significantly lower in the TBD group (Figure 1). The same pattern was observed when IOP was assessed at 4 pm (Figure 2). Furthermore, at the conclusion of the study, the mean IOP was significantly lower in the TBD group at 8 am (16.19 ± 2.0 mmHg versus 18.35 ± 1.4 mmHg, P = 0.000) and at 4 pm (14.74 ± 2.4 mmHg versus 16.77 ± 1.4 mmHg, P = 0.000, Table 3).
Table 2.
Difference in IOP between groups at 8 am
| TBD (n = 108) | TB (n = 104) | P | |
|---|---|---|---|
| Baseline | 22.3 ± 0.9 | 22.4 ± 1.8 | 0.558 |
| Day 15 | 18.0 ± 2.0 | 19.5 ± 3.4 | 0.000 |
| Day 30 | 16.4 ± 1.5 | 18.4 ± 1.3 | 0.000 |
| Day 60 | 16.3 ± 2.5 | 18.3 ± 2.0 | 0.000 |
| Day 90 | 16.2 ± 2.0 | 18.4 ± 1.4 | 0.000 |
Note: Data is presented in mean ± standard deviation.
Abbreviations: IOP, Intra Ocular Pressure; TBD, timolol-brimonidine-dorzolamide; TB, timololbrimonidine.
Figure 1.
Mean change from baseline IOP at each visit between groups at 8 am.
Abbreviations: IOP, Intra Ocular Pressure; TBD, timolol-brimonidine-dorzolamide; TB, timolol-brimonidine.
Figure 2.
Mean change from baseline IOP at each visit between treatment groups at 4 pm.
Abbreviations: IOP, Intra Ocular Pressure; TBD, timolol-brimonidine-dorzolamide; TB, timolol-brimonidine.
Table 3.
Difference in IOP between groups at 4 pm
| TBD (n = 108) | TB (n = 104) | P | |
|---|---|---|---|
| Baseline | 19.0 ± 1.3 | 19.1 ± 1.2 | 0.536 |
| Day 15 | 15.4 ± 1.2 | 17.8 ± 0.9 | 0.000 |
| Day 30 | 15.3 ± 1.3 | 17.5 ± 1.7 | 0.000 |
| Day 60 | 15.0 ± 2.3 | 17.0 ± 2.6 | 0.000 |
| Day 90 | 14.7 ± 2.4 | 16.8 ± 1.4 | 0.000 |
Note: Data is presented in mean ± standard deviation.
Abbreviations: IOP, Intra Ocular Pressure; TBD, timolol-brimonidine-dorzolamide; TB, timololbrimonidine.
Adverse events were documented in eight patients, of whom six were excluded from the study. These events included ocular burning (mild in one patient and moderate in another), itching (severe in one patient), foreign body sensation (mild in one patient), redness (severe in one patient), dizziness (severe in one patient), headache (mild in one patient), and bradycardia (mild in one patient), the latter not being related to the study drug. These data are summarized in Table 4.
Table 4.
Adverse events presented in study groups
| TBD (n = 56) | TB (n = 56) | |
|---|---|---|
| Ocular burning | 1b | 1a |
| Itching | 1c | |
| Foreign body sensation | 1a | |
| Redness | 1c | |
| Dizziness | 1c | |
| Cephalea | 1a | |
| Bradycardia | 1a |
Note: Classified as:
Mild;
moderate;
severe.
Abbreivations: TBD, timolol-brimonidine-dorzolamide; TB, timolol-brimonidine.
Discussion
The aim of treating glaucoma and ocular hypertension is to reduce IOP.5 Elevated IOP is an important risk factor for progression of glaucoma, so achieving and maintaining a low IOP minimizes the risk of glaucomatous progression and vision loss.9,10
Different classes of drugs are available for IOP reduction, the most commonly used being the prostaglandin analogs, beta-blockers, alpha agonists, and carbonic anhydrase inhibitors. Single medication is sometimes insufficient to reduce IOP, and patients may require different combinations to achieve their target IOP.6,10
Fixed combinations, ie, two drugs contained in a single bottle, have emerged as a treatment option, offering several advantages and fewer adverse events.6 A number of studies have evaluated the efficacy and safety of these combinations, and it has been demonstrated that combinations are superior to monotherapy with their constituent parts.1,4–7,10,11
García-Sánchez et al5 compared administration of a fixed combination of latanoprost + timolol with an unfixed combination of brimonidine and timolol in patients with elevated IOP for 6 months. They observed that the fixed combination reduced IOP more effectively at all assessment times and was better tolerated than the unfixed combination. In our study, we compared a triple fixed combination of TBD and a double fixed combination of TB. Although our study is different because we used fixed combinations and did not use a prostaglandin analog, we can compare our results for IOP reduction taking into account the use of fixed combinations, and we observed that the TBD combination was more effective than TB.
In a previous study, Baiza-Durán et al12 compared two fixed combinations, ie, timolol + dorzolamide + brimonidine versus timolol + dorzolamide. The conclusion of their study was that the triple combination appeared to be safer and more effective for reducing IOP than the double fixed combination. Similarly, our investigation demonstrated that the TBD combination was more effective than TB in reducing IOP in the population studied.
Finally, a study by Fechtner et al7 compared triple therapy (a fixed combination of brimonidine-timolol plus latanoprost) with double therapy (timolol plus latanoprost); in this study, patients were on latanoprost treatment and required additional IOP-lowering. Adjunctive therapy comprised a fixed combination of brimonidine + timolol or timolol only plus latanoprost; thus, a triple combination was used. This study concluded that triple therapy (fixed combination of brimonidine + timolol plus latanoprost) reduced IOP significantly more effectively than double therapy (timolol plus latanoprost). Our study results are similar in that we also demonstrated that a triple combination is more effective than a double combination when reducing IOP. In the study by Fechtner et al7, triple therapy was used, but was not a fixed combination and involved two different bottles of medication.
The ultimate goal of treating glaucoma is to preserve the remaining visual field. Only treatment to reduce IOP has shown evidence of being effective in preserving the visual field. Monotherapy remains the preferred initial choice of treatment in glaucoma, using prostaglandin analogs and β-blockers as initial treatment for lowering IOP. Prostaglandin analog eye drops are preferred due to their strong IOP-reducing action and the convenience of requiring only once-daily administration, except in the event of side effects, intolerance, or patient refusal. Nevertheless, target IOP levels are not always achieved with a single medication, and patients frequently require multiple medications, which can lead to unsatisfactory adherence with treatment.3,5–7,13 Adherence with treatment for glaucoma is better when regimens are simple rather than complex.14 Use of two or more bottles of IOP-lowering medication may be associated with an increase in noncompliance, and the advantage of fixed combinations is that a single bottle can contain up to three medications, thus minimizing the number of bottles and drops that need to be used by the patients, and facilitating adherence to treatment. Fixed combinations are important adjuncts for the treatment of glaucoma but should generally be used only when monotherapy has not provided adequate IOP reduction. TBD is a good treatment option when a combination is required. One possible disadvantage is that TBD must be administered twice a day as opposed to prostaglandins, which only need to be administered once a day. Another possible disadvantage is that the incidence of treatment-related adverse events could be higher on TBD than on any other combination. Another drawback could be that it is not possible to change the drug concentration or dosing schedule for one component medication independently of the other when using a fixed combination like TBD. Further studies will be needed to determine the long-term safety and efficacy of a fixed combination of TBD, as well as its effectiveness in providing additional IOP-lowering over 24 hours.
Conclusion
New trends in glaucoma treatment suggest more aggressive strategies are needed for IOP reduction. This can be achieved using different drugs with different pharmacodynamics. Using this fixed combination, low doses of different drugs can be administered, and in this way, the onset of adverse effects could be delayed or diminished. The use of fixed combinations is preferred over separate use of their components to improve adherence and persistence with treatment. A fixed combination of TBD administered twice a day is more effective than a fixed combination of TB twice a day for reducing IOP.
Acknowledgments
The Krytantek study group comprised Drs Alfonso García López, Marco Antonio Cortés Gastélum, Félix Gil Carrasco, Curt Hartleben Matkin, Jésus Jiménez Román, Miguel Luis Moreno Marín, José Antonio Paczka Zapata, Gustavo Velasco Gallegos, Marco Antonio Beltrán Loustaunau, and Carlos Naranjo Ahumada.
Footnotes
Disclosure
This study was sponsored by Laboratorios Sophia, SA de CV. The authors are employees of Laboratorios Sophia, SA de CV.
References
- 1.Lee A, McCluskey P. Clinical utility and differential effects of prostaglandin analogs in the management of raised intraocular pressure and ocular hypertension. Clin Ophthalmol. 2010;4:741–764. doi: 10.2147/opth.s10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duker JS, Yanoff M. Ophthalmology. 3rd ed. Edinburgh, UK: Mosby; 2009. [Google Scholar]
- 3.Quigley H. Glaucoma. Lancet. 2011;377:1367–1377. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- 4.Papaconstantinou D, Georgalas I, Kourtis N, et al. Preliminary results following the use of a fixed combination of timolol-brimonidine in patients with ocular hypertension and primary open-angle glaucoma. Clin Ophthalmol. 2009;3:227–230. doi: 10.2147/opth.s5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García-Sánchez J, Rouland JF, Spiegel D, et al. A comparison of the fixed combination of latanoprost and timolol with the unfixed combination of brimonidine and timolol with the unfixed combination of brimonidine and timolol in patients with elevated intraocular pressure. A six month, evaluator masked, multicentre study in Europe. Br J Ophthalmol. 2004;88:877–833. doi: 10.1136/bjo.2003.029330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higginbotham E. Consideration in glaucoma therapy: fixed combinations versus their component medications. Clin Ophthalmol. 2010;4:1–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Fechtner R, Harasymowycz P, Nixon D, et al. Twelve-week, randomized, multicenter study comparing a fixed combination of brimonidine-timolol with timolol as therapy adjunctive to latanoprost. Clin Ophthalmol. 2011;5:945–953. doi: 10.2147/OPTH.S19999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 9.Leske M, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: The Early Manifest Glaucoma Trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 10.Tanna A, Rademaker A, Stewart W, et al. Meta-analysis of the efficacy and safety of α2-adrenergic agonists, β-adrenergic antagonists, and topical carbonic anhydrase inhibitors with prostaglandin analogs. Arch Ophthalmol. 2010;128:825–833. doi: 10.1001/archophthalmol.2010.131. [DOI] [PubMed] [Google Scholar]
- 11.Lanzl I, Raber T. Efficacy and tolerability of the fixed combination of brinzolamide 1% and timolol 0.5% in daily practice. Clin Ophthalmol. 2011;5:291–298. doi: 10.2147/OPTH.S16355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baiza-Durán L, Álvarez-Delgado J, Contreras-Rubio A, et al. The efficacy and safety of two fixed combinations: timolol-dorzolamide-brimonidine versus timolol-dorzolamide. A prospective, randomized, double masked, multi-center, 6-month clinical trial. Ann Ophthalmol (Skokie) 2009;41:174–178. [PubMed] [Google Scholar]
- 13.McKinnon SJ, Goldberg LD, Peeples P, et al. Current management of glaucoma and the need for complete therapy. Am J Manag Care. 2008;14:S20–S27. [PubMed] [Google Scholar]
- 14.Olthoff C, Schouten J, Van de Borne B, et al. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension an evidence-based review. Ophthalmology. 2005;112:953–961. doi: 10.1016/j.ophtha.2004.12.035. [DOI] [PubMed] [Google Scholar]