Abstract
Noncommunicable diseases were estimated to claim more than 36 million lives worldwide in 2008. Major contributors to this burden were cardiovascular disease, cancer, chronic respiratory diseases, and diabetes. The United Nations General Assembly held a high-level meeting on noncommunicable diseases in September 2011 for heads of states and governments, conscious of the projected increases in disease incidence, particularly in low- and middle-income countries. This meeting followed the Special Session on HIV/AIDS in 2001, the only other high-level meeting to discuss a health topic and orient the global political agenda toward a growing threat to human development. Proposed strategies for control of noncommunicable diseases focused mainly on the shared risk factors of tobacco, harmful use of alcohol, physical inactivity, and unhealthy diet. However, for cancer, a broader response is required. Notably, the heterogeneity of cancer with respect to its geographical distribution, etiology, and pathology all demand a more nuanced, regional, or even local approach. Preparations for the meeting elicited enormous attention from governments and nongovernmental organizations, but the engagement of the research community was less evident. This commentary calls for the involvement of the cancer research community in response to the further action detailed in the United Nations Political Declaration emanating from the meeting, identifies a number of cancer-specific priorities, including vaccination against hepatitis B virus and human papillomavirus, cervical cancer screening, and early detection of breast cancer, and suggests areas where cancer research can provide the evidence base for cancer control, notably in improving the quality and coverage of cancer registration, elucidating cancer etiology, and evaluating interventions, including their implementation in low-resource health-care settings. Finally, the need for global cooperation in developing a research agenda for low- and middle-income countries is highlighted.
On September 19–20, 2011, the United Nations (UN) General Assembly held a high-level meeting for heads of states and governments on noncommunicable diseases that focused on four major contributors to global burden of disease: cardiovascular disease, cancer, chronic respiratory diseases, and diabetes. This was only the second high-level meeting to discuss a health topic—the first, in 2001, concerned human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome. The UN meeting followed the First Global Ministerial Meeting on Healthy Lifestyles and Noncommunicable Disease Control that was held in April 2011 in Moscow. For the first time, these two conferences turned the global political agenda toward noncommunicable diseases in a coordinated manner. These meetings followed more than a decade of leadership from the World Health Organization (WHO) and were supported by many partners, notably The NCD Alliance. The outcome of the UN General Assembly meeting was a Political Declaration (1) that recognizes that the burden of noncommunicable diseases will fall increasingly on the low- and middle-income countries and highlights the need for prevention of noncommunicable diseases to be a part of development initiatives to reduce poverty and associated social and health inequalities.
Noncommunicable Diseases: Increasing Political Awareness
From its inception, the WHO Global Strategy for the Prevention and Control of Noncommunicable Diseases (2), focused on the above-mentioned four major contributors to the global burden of noncommunicable diseases because they represent shared risk factors and have identifiable preventive interventions at both the population and individual levels (Figure 1). Specifically, WHO has provided global strategies to reduce the harmful use of alcohol and to encourage a better diet and more physical activity (3,4). The key achievement regarding tobacco was the Framework Convention for Tobacco Control in 2005, the first international treaty on health. The priorities for control of noncommunicable diseases were proposed based on this background (5,6).
Figure 1.
WHO global strategy for the prevention and control of noncommunicable diseases. Shown are the four major noncommunicable diseases (column 1) under consideration, together with both the shared population (column 2) and individual risk factors (column 3) highlighted in the WHO Global Status Report on noncommunicable diseases 2010 (7).
In its role as an autonomous agency within WHO, the International Agency for Research on Cancer (IARC) has cooperated closely with the WHO both in preparing for the meeting and in drafting the first Global Status Report on Noncommunicable Diseases (7). Two areas emerged that merit attention by the wider cancer research community. First, the visibility of the UN meeting appears to have been limited among cancer researchers. This is undesirable because the best possible evidence base derived from research is required to underpin priority setting and because the Political Declaration has the potential to shape the cancer research agenda in the coming years. Second, it is evident that a number of important features specific to cancer demand a broader, complementary approach to the one generally described for noncommunicable diseases. This commentary addresses the rationale for a cancer-specific agenda within noncommunicable disease control, explores how such an agenda can supplement the overall priorities for noncommunicable diseases, and suggests priorities for cancer research in response to the UN Political Declaration.
Cancer: A Heterogeneous Disease
Cancer needs a more nuanced policy approach than the other major diseases because of its heterogeneity. Cancer varies in its geographical distribution, etiology, and pathology. Therefore, the shared noncommunicable disease risk factors shown in Figure 1, although important, are insufficient for the prevention of many important types of cancer.
Geographical Heterogeneity
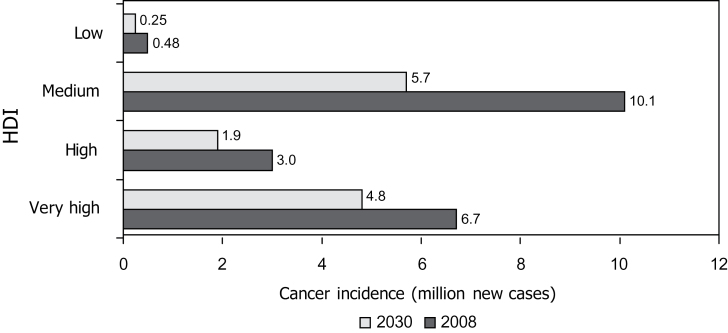
Of the 36 million deaths from noncommunicable diseases worldwide in 2008 (7), 7.6 million (~20%) were due to cancer. The global cancer incidence is projected to increase from 13.3 million to 21.4 million per year between 2010 and 2030 (8). This increase is driven by population growth and aging. It assumes no change in underlying cancer causes or incidence rates; however, if risk factors such as tobacco use or unhealthy diets and obesity are increasingly adopted by populations in low- and middle-income countries, the current projections will be underestimates. By 2030, approximately half of all cancers globally will occur in countries that are classified as medium on the Human Development Index (HDI) (Figure 2) because this category includes some of the world’s most populous nations (China, India, and Indonesia). By that time, these countries and the low-HDI countries will face almost a doubling in the number of new cancer cases per year. The health services and communities of many such countries are often least able to cope with this additional challenge.
Figure 2.
Cancer burden in relation to the Human Development Index (HDI). Data adapted from (28). The HDI is composed of three dimensions: health, as measured by life expectancy at birth; education, as measured by years of schooling; and living standards, as measured by gross national income per capita.
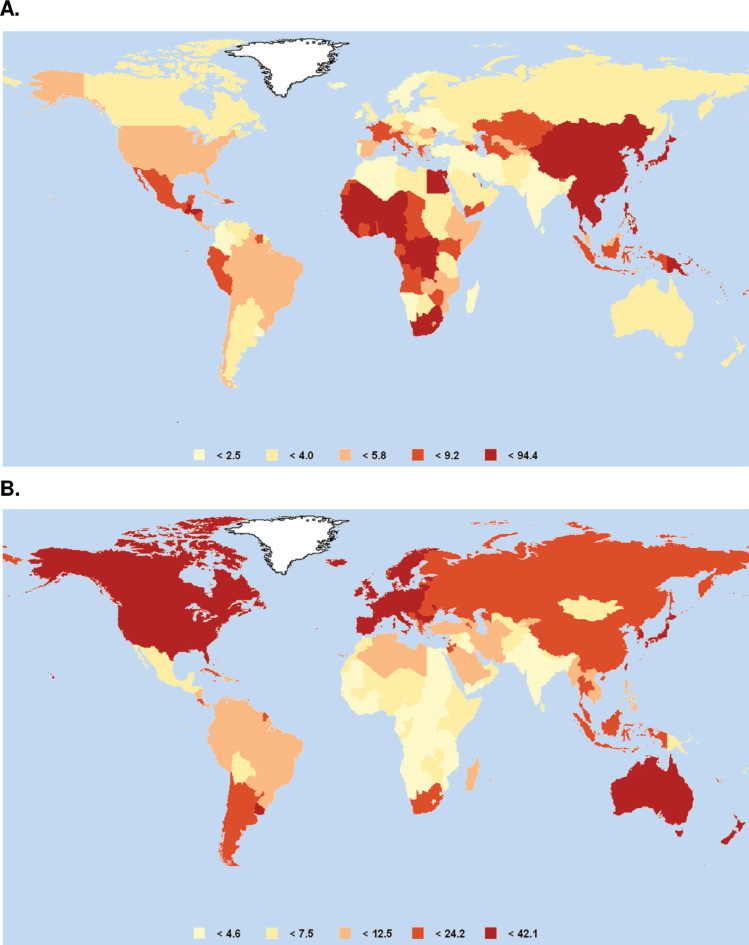
However, moving beyond total cancer burden, one sees markedly different patterns of cancer by region. The problem of simplifying such a diverse set of diseases under a single heading is immediately evident. For example, Figure 3 shows the worldwide occurrence of two common cancers—liver and colorectal—in 2008. Similar geographical diversity can be seen by comparing many other common cancers by continent (Table 1). Consequently, in light of this information, priorities for cancer control must be developed at a regional, national, or even local level. For example, early detection of colorectal cancer will have a different priority in a region with a high incidence than it does in a region with a low incidence. Similarly, the impact of human papillomavirus (HPV) vaccination on cervical cancer will vary geographically in relation to incidence and the availability of screening programs.
Figure 3.
Global map of cancer incidence. A) Liver cancer. B) Colorectal cancer. Data are age-standardized incidence rates per 100 000 per year. Source: GLOBOCAN 2008 (8).
Table 1.
Six most common cancers (incidence—age standardized rates) in both sexes by continent*
| Africa | Asia | South America | North America | Europe | Oceania |
| Breast | Breast | Prostate | Prostate | Breast | Prostate |
| Cervix† | Lung | Breast | Breast | Prostate | Breast |
| Prostate | Stomach† | Cervix† | Lung | Colorectum | Colorectum |
| Liver† | Cervix† | Lung | Colorectum | Lung | Melanoma |
| Colorectum | Liver† | Colorectum | Uterus | Uterus | Lung |
| NHL‡ | Colorectum | Stomach† | NHL‡ | Cervix† | NHL‡ |
* Source: GLOBOCAN 2008 (8). Cervix = cervix uteri; uterus = corpus uteri; melanoma = skin melanoma; NHL = non-Hodgkin lymphoma.
† Cancers that have a predominantly infectious etiology.
‡ Cancers for which a component of their etiology is associated with an infection.
The shared environmental and behavioral risk factors for noncommunicable diseases—listed as priorities by the UN (Figure 1)— make an important contribution to the global cancer burden. In addition to the global impact of tobacco on cancer in multiple organs, alcohol is associated with cancers of the liver, larynx, esophagus, pharynx, breast, and colorectum (9), and insufficient physical activity has been linked to cancers of the breast, colorectum, and endometrium (10). Reducing the consumption of sugar should help control obesity and overweight, which are risk factors for cancers of the esophagus, breast, colorectum, endometrium, kidney, and pancreas (10).
Crucially, however, the magnitude of the effect of the above-mentioned risk factors on cancer control by region will depend on their prevalence and the direction of their time trends. In some cases, the goal will be to reduce prevalent exposures, whereas in other cases it will be to prevent the introduction of a risk factor; in the latter case, the action taken will preempt projected increases in disease burden rather than tackling the existing burden. For example, tobacco control through implementation of the Framework Convention for Tobacco Control is universally beneficial for controlling cardiovascular diseases and cancer, either by reducing tobacco use in countries where it is already common or by restricting its introduction where its use is currently uncommon. Similar efforts should be made to prevent the adoption of so-called Westernized lifestyles that have insufficient physical activity and a high prevalence of obesity and overweight. However, the actions will not adequately address the existing cancer burden in regions where these latter risk factors remain, for the moment, at lower prevalence.
Etiological Heterogeneity
Geographical patterns of cancer arise because the prevalences of risk factors differ in a given population. Studies of geographical differences and of migrant populations (ie, their adoption of the cancer patterns of the host country) provided a cornerstone that established the role of environmental and lifestyle factors in the causation of cancer. Nevertheless, cancer differs from other noncommunicable diseases in that specific risk factors for a number of major cancer sites remain poorly defined.
It is important to recognize that cancer in low- and medium-HDI countries is not simply due to their adoption of the social and behavioral conditions found in the high- and very high-HDI countries. Both the current cancer patterns and future projections given in Figure 1 reflect indigenous risk factors prevalent in the low- and medium-HDI countries; these may be joined by the future superimposition of risk factors from high- and very high-HDI countries, resulting in a “double burden” of exposures. Perhaps the best example for which cancer-specific actions are needed is chronic infections. Infections are estimated to explain approximately 16% of cancers globally; however, in developing countries, infections explain 22.9% of cancers (11). The major contributors to cancer are infections with hepatitis B and C viruses (HBV and HCV), HPVs, and Helicobacter pylori. Consequently, several of the most common cancers (ie, liver, stomach, and cervix) in Africa, Asia, and South America are related to an infection (Table 1). Ignoring the substantial cancer burden related to infection would be a failure to address preventable causes of cancer in many parts of the world. Consequently, the inclusion of vaccination against cancer-related infections in the UN Political Declaration was an important step. Although such interventions against specific cancers tackle a few hundred thousand cancer cases per year, they represent highly achievable and measurable interventions (ie, their benefits have narrower margins of uncertainty compared with broader multilayer interventions). Other infections that are of lesser global significance but which can have a serious impact on a local or regional level and can also be addressed by available interventions include the combined role of Kaposi sarcoma herpes virus and HIV in Kaposi sarcoma in sub-Saharan Africa and the role of liver flukes in cholangiocarcinoma in parts of Asia (11).
Other categories of risk factors can also be addressed. These include environmental and occupational agents, such as reduction in exposure to aflatoxins, indoor air pollution, radon, arsenic, and excess sunlight, as well as regulatory protection of workers in certain industries (9). Such preventive measures will be priorities in some regions even though the impact on global cancer incidence will be comparatively modest. For specific risk factors, the contribution to cancer burden can vary markedly by region (eg, the primary importance of HBV for liver cancer in sub-Saharan Africa compared with that of HCV and alcohol in Europe, North America, and Japan).
Pathological Heterogeneity
Cancers differ markedly in underlying molecular pathology, resulting in different rates of disease progression. Remarkable advances are being made in understanding carcinogenic mechanisms and in the availability of technologies to study genetic and epigenetic alterations in cancer and its precursor lesions. Translating this information not only to clinical practice but also to the study of etiology and prevention presents one of the great opportunities for cancer research in the 21st century (12). A new generation of biomarkers promises to refine the treatment of cancer, to permit the earlier detection and diagnosis of the disease, and to reveal the risk factors that drive these underlying molecular events.
The relatively long latency of the majority of cancers offers opportunities for the early detection of precancerous lesions or malignancy. Different cancers (by organ and cell type) have different disease trajectories, but for most, their early detection makes treatment more effective. The best current examples of common cancers for which early detection makes treatment more effective are cervical and breast cancers: even in low-income countries with limited therapeutic means, early detection is linked to improvements in survival (13). This is an important and hopeful message and aligns with the call for better access to affordable cancer therapies in the developing world (14).
Although not included in the list of highest priorities for noncommunicable diseases (Figure 1), increased access to cost- effective cancer screening programs is included in the UN Political Declaration (1). Initially, the focus should be on breast, cervical, colorectal, and, in some regions, oral cancers. Methods should be tailored to the countries concerned. For some countries, this will be screening programs (eg, mammography), whereas for other countries, early detection would be by other means (eg, breast awareness and clinical breast examination) (15). The evidence to date suggests that the best results are obtained with population-based, high-coverage approaches and rigorous quality-control procedures. However, high-risk groups of individuals can benefit from targeted surveillance (eg, those at high risk of familial cancers or precancerous conditions such as cirrhosis and gastrointestinal diseases, including Barrett’s esophagus).
Specific Priorities for Cancer Control
Controlling shared risk factors for noncommunicable diseases (ie, by reducing tobacco exposure and harmful alcohol use, by main taining or increasing physical activity levels, and controlling obesity) will benefit cancer control. These interventions will be reflected in the voluntary targets for noncommunicable disease control that countries have requested WHO to propose. However, I propose four additional high-priority interventions that would provide the basis for a number of cancer-specific targets highly relevant to low- and middle-income countries. These four priority areas were selected because of the potential global impact of the approaches proposed. All have feasible strategies for evaluating the effects of the intervention. These selections are not intended to imply that interventions against other regional priorities mentioned above (etiological heterogeneity) should be ignored. Their selection also presupposes the importance of interventions against tobacco and other shared noncommunicable disease risk factors. Finally, it is important to note that the emphasis for this commentary is on cancer prevention and its relation to cancer research. Other complementary priority areas for action on cancer in low- and middle-income countries have been highlighted by others (19–21), including the need for adequate access to palliative care, the need for access to affordable, effective treatments (eg, for childhood cancers such as Burkitt lymphoma and acute lymphobastic leukemia), survivorship issues, and the need to strengthen health infrastructures, including training and retention of qualified professionals.
HBV vaccination. Infant HBV vaccination, including a birth dose, is recommended by the WHO, given the global importance of liver cancer and chronic liver disease. A target prevalence of less than 1% hepatitis B surface antigen positive among children younger than 5 years is achievable; additional information on vaccine coverage is available from national immunization programs.
HPV vaccination. HPV vaccination using the quadrivalent or bivalent vaccine is recommended by the WHO, targeting adolescent girls before the onset of sexual activity. The cost of the vaccine is falling, and evidence of the efficacy of fewer than the standard three doses of vaccine may lead to easier administration (16). The decision to vaccinate or not depends on country-specific factors, including the prevalence of HPV, the incidence of cervical cancer, vaccine affordability, and the national immunization program infrastructure. The target for HPV vaccination should be to increase the number of high-risk, low-resource countries that can achieve good vaccine coverage (>70%), and an impact that is measured through a gradual decrease in infections and, ultimately, cervical cancer.
Cervical cancer screening. Even where HPV vaccination is introduced, cervical cancer screening will remain a priority to prevent deaths in women who are too old to benefit from vaccination. At least 70% of women aged 30–49 years should be screened at least once for cervical cancer. Affordable and feasible HPV testing methods offer an effective alternative to cytology. Randomized screening trials and cohort studies have indicated very low rates of detection of high-grade cervical lesions and cervical cancer for many years after a negative HPV DNA test (17). Screening intervals between 5 and 7 years seem advisable at present when resources are limited; shorter screening intervals are unnecessary and costly. Low-cost visual inspection with acetic acid is another alternative cervical cancer screening approach in low-resource countries and can pave the way for building the infrastructure that may eventually facilitate the introduction of affordable HPV-based screening. The availability of effective treatment for early cervical lesions is, of course, a prerequisite in these programs. Although a screen-and-treat approach without triage may be currently considered in some low-resource countries, triaging women with positive HPV tests is recommended before referral for colposcopy and further management.
Breast cancer early detection. Breast cancer is now the leading cancer among women in many low- and middle-income countries. Early detection and appropriate treatment are the most effective approaches to control the burden of this disease. The specific approach will depend on the incidence of the disease as well as the logistics and financial implications of implementing a quality-assured national screening program. In many low- and middle-income countries, mammography screening is neither affordable nor feasible. In such countries, an alternative approach may be to increase breast awareness among women and improve opportunities in health services for clinical breast examination (15,18). If these approaches succeed in downstaging breast cancers, improvements in survival would be achieved even in countries where cancer services are currently limited (13).
Priorities for Cancer Research in Relation to the High-Level Meeting
A number of research areas would provide vital evidence in establishing the most reliable evidence base for cancer control. Some that are particularly relevant to low- and middle-income countries are described below.
Improved surveillance of cancer morbidity and mortality and prevalence of major risk factors. Coverage of cause-of-death statistics is essential and is still lacking or incomplete in more than half the world’s countries. To plan cancer services, governments need to know not only how many people are dying but how many will develop the disease and how many will live with it as cancer survivors. An understanding of temporal trends in the population prevalence of major cancer risk factors complements cancer surveillance to improve long-term cancer projections and planning. Cancer registries provide a platform for research, in addition to cancer statistics. They reveal geographical heterogeneity and temporal trends that generate etiological hypotheses. Registries are also vital in assessing the outcome of prevention-associated interventions, including community-randomized trials and planned public health interventions (eg, cervical cancer screening, early detection of breast cancer and HPV, or HBV vaccination). Population-based cancer registries are integral to both cancer control and research and as such should be a core component of any noncommunicable disease surveillance plan. Sadly, such registries are lacking or inadequate in many countries, especially in developing regions of the world. Sufficient knowledge and experience exist to provide support to countries to develop cancer registries that, with a modest level of sustained investment, would cover a defined region of a country, comprise a minimal dataset, and use open-source software (eg, CanReg5) to allow standard analyses and international comparisons. The IARC-led Global Initiative for Cancer Registry Development in Low- and Middle-Income Countries (22) is an international cooperation designed to create regional centers of expertise in cancer registration, which in turn will provide the infrastructure to transform the coverage and quality of registration.
Cancer etiology. The four shared noncommunicable disease risk factors (1) require further research in relation to cancer. Examples of the research required are the need for more precision about the type and amount of physical exercise required to reduce cancer risk as well as a better understanding of the risks associated with specific nutritional and metabolic factors. Nevertheless, cancer also differs from other noncommunicable diseases in that risk factors for a number of major cancer sites remain poorly defined. Examples of common cancers where information on etiology is lacking include prostate cancer, colorectal cancer, leukemia, lymphoma, kidney cancer, and a substantial proportion of breast cancer. For other cancers, such as esophageal cancer, carcinogenic agents are known in developed countries but are less well understood in developing countries. Consequently, it is vital that research into the causes of cancer proceeds in parallel with research into prevention and implementation. Priorities for low- and middle-income countries should be focused on cancers that are common in the region and for which the risks cannot be easily inferred from previous research in high-income countries, either because investigations are lacking or because risk factors differ. In addition, exposure over the life course, including early in life, merits further study (23).
New knowledge about carcinogenic mechanisms and technologies to investigate biological changes subsequent to specific exposures offers important new opportunities to investigate cancer etiology (24,25). Progress in molecular cancer epidemiology is fundamental to moving forward in areas that have been refractory to yielding conclusive public health advice in recent years.
Prevention research. Low-income countries have different cancer incidence patterns compared with high-income countries, notably in relation to cervical, liver, stomach, and HIV-associated cancers (Table 1). As mentioned above, for some cancers, the challenge is to implement established interventions; for other cancers, the research priority should be to identify prevention strategies. For example, H. pylori infection is a well-established risk factor for stomach cancer, the second leading cause of cancer death worldwide, and yet optimal H. pylori eradication and its impact on stomach cancer incidence remain to be defined. Although HBV is associated with a majority of liver cancer cases worldwide, there are 350 million chronic HBV carriers whom HBV vaccination cannot help and in many HBV-endemic areas, dietary staples are contaminated with aflatoxins, a potent human liver carcinogen (26). There is currently no vaccine against HCV. Therefore, research into reducing exposure to aflatoxins and effective low-cost treatments for chronic HBV and HCV carriers should be priorities. Similarly, research into cancer risk in individuals living with HIV is needed, particularly in relation to their susceptibility to other cancer-associated chronic infections. As breast cancer becomes the most common cancer in women and prostate cancer incidence likewise continues to increase in men, research into the most effective early detection approaches is vital, even in many low-income countries.
Implementation research. Community-based or clinical cancer prevention trials are a valuable component in cancer control planning. However, the subsequent implementation and evaluation of prevention measures at the population level is often neglected. There may be many facilitators of and barriers to (eg, behavioral, cultural, system-based) successful integration of known prevention measures into health services. These factors need to be understood. In addition, the scale of effect of the intervention in practice may differ from that in a trial setting. These different aspects fall under the domain of implementation research, an area that merits a far greater priority globally. A key component of this area of research is an understanding of inequalities and disparities in relation to the effectiveness of cancer-control strategies (27). There are several relevant areas directly related to the priority interventions proposed above. For example, in some health service settings, it will be challenging to develop a delivery platform for HPV vaccination of adolescent girls (as opposed to young children or infants), and there may be cultural and social sensitivities with regard to preventing a cancer caused by a sexually transmitted infection. Similarly, important questions remain about barriers to participation in early detection of breast cancer and cervical cancer screening programs, including what factors influence whether women attend treatment after having an anomaly detected. It is vital that prevention is not only evidence based but also subsequently evaluated during implementation; there are opportunities in this regard for observational studies of public health interventions and health services research to pinpoint areas where improvements would have most impact. This type of research requires an interdisciplinary approach that involves social scientists, behavioral epidemiologists, and health service researchers to elucidate the personal, programmatic, and structural challenges to implementing cancer prevention.
Conclusions
The cancer research community needs to be fully engaged with developments following the UN meeting on noncommunicable diseases. The WHO has been called upon by member states to 1) develop a comprehensive global monitoring framework, including a set of indicators, to monitor trends and to assess progress in the implementation of national strategies and plans on noncommunicable diseases and 2) to prepare recommendations for a set of voluntary global targets for the prevention and control of noncommunicable diseases. As priorities for interventions to control noncommunicable diseases are considered and the targets are debated, cancer researchers nationally and internationally must ensure that the evidence base in formulating those decisions is of the highest relevance and quality and should advocate for this evidence to be taken fully into account when drawing priorities and policy recommendations. There must also be a concerted effort to evaluate the impact of specified interventions as they are implemented.
Research and development in relation to prevention and control of noncommunicable diseases is also specified in the UN Political Declaration. This political agenda should be developed and used to identify gaps in the evidence base for cancer control, including improvements in data collection (eg, cancer registration, surveillance of risk factor prevalence), and to define priorities for research, including the continued search for the causes of the disease. Greater international cooperation in research and capacity building in low- and middle-income countries is required. The National Cancer Institute’s new Center for Global Health can play an important leadership role in this respect. This collaborative effort may see financial and other valuable resources being drawn into cancer research as part of a revised development aid agenda emerging from a new political awareness of the priority of noncommunicable diseases globally and their acknowledged link to achieving development goals and reducing inequalities. Indeed, consideration is called for among funding agencies as to how development aid is used to address the control of noncommunicable diseases including cancer.
Although this is a time of great opportunity for cancer research, the opportunities will not be realized automatically. Focusing attention on cancer in low- and middle-income countries is vital because the pain of this disease is increasingly felt in those regions currently most ill-equipped to respond. Cooperation between the countries of the southern hemisphere—so-called south–south cooperation—is critical. It is a time for partners, not pupils. The response, therefore, to the open door provided by the UN political declaration needs to be a global one, with an agenda defined, shaped, and shared by a concerted partnership of governments, civil society, and national and international organizations. In this way, one can envisage the most effective translation of cancer research into cancer control to alleviate the burden of this disease that is projected to fall on many of the most vulnerable populations of the world in the coming two decades.
Funding
The author received no external funding.
Notes
The author would like to thank his colleagues, Drs Silvia Franceschi, David Forman, Joachim Schüz, and Rengwaswamy Sankaranarayanan, for their helpful comments on earlier drafts of the manuscript.
References
- 1.(2011). http://www.un.org/ga/search/view_doc.asp?symbol=A/66/L.1. http://www.un.org/ga/search/view_doc.asp?symbol=A/66/L.1. Political declaration of the High-level Meeting of the General Assembly on the Prevention and Control of Non-communicable Diseases, United Nations. Accessed October 20, 2011.
- 2.World Health Organization (WHO); WHO Mar, 2000. http://apps.who.int/gb/archive/pdf_files/WHA53/ea14.pdf. http://apps.who.int/gb/archive/pdf_files/WHA53/ea14.pdf Global Strategy for the Prevention and Control of Noncommunicable Diseases: Report by the Director-General. Geneva, Switzerland: ; 1999. Endorsed by Resolution WHA 53/14 ( ) Accessed October 20, 2011.
- 3. World Health Organization (WHO) Global Strategy to Reduce the Harmful Use of Alcohol. Geneva, Switzerland: WHO; 2010. Endorsed by Resolution WHA 63/13 (May 2010). http://www.who.int/substance_abuse/alcstratenglishfinal.pdf Accessed October 20, 2011
- 4. World Health Organization (WHO) Global Strategy on Diet, Physical Activity and Health. Geneva, Switzerland: WHO ; 2004. Endorsed by Resolution WHA 57/17 (May 2004). http://www.who.int/dietphysicalactivity/strategy/eb11344/strategy_english_web.pdf Accessed October 20, 2011
- 5. Beaglehole R, Bonita R, Horton R, et al. Priority actions for the non-communicable disease crisis Lancet 2011;377(9775):1438–1447 [DOI] [PubMed] [Google Scholar]
- 6. Beaglehole R, Bonita R, Alleyne G, et al. UN High-Level Meeting on Non-Communicable Diseases: addressing four questions Lancet 2011;378(9789):449–455 [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization (WHO) 2011. http://whqlibdoc.who.int/publications/2011/9789240686458_eng.pdf. http://whqlibdoc.who.int/publications/2011/9789240686458_eng.pdf Global Status Report on Noncommunicable Diseases 2010—Description of the Global Burden of NCDs, Their Risk Factors and Determinants. Geneva, Switzerland: WHO. Accessed October 20, 2011.
- 8.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. http://globocan.iarc.fr. http://globocan.iarc.fr GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide. Lyon, France: International Agency for Research on Cancer; 2010. IARC Cancer Base No. 10. Accessed October 20, 2011.
- 9. Cogliano VJ, Baan R, Straif K, et al. Preventable exposures associated with human cancers J Natl Cancer Inst 2011;103:1827– 1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Cancer Research Fund/American Institute for Cancer Research (AICR). Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR;; 2007. [Google Scholar]
- 11.de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. The Lancet Oncology. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 12. Wild CP Future research perspectives on environment and health: the requirement for a more expansive concept of translational cancer research Environ Health 2011;10(suppl 1):S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sankaranarayanan R, Swaminathan R, Brenner H, et al. Cancer survival in Africa, Asia, and Central America: a population-based study Lancet Oncol 2010;11(2):165–173 [DOI] [PubMed] [Google Scholar]
- 14. Farmer P, Frenk J, Knaul FM, et al. Expansion of cancer care and control in countries of low and middle income: a call to action Lancet 2010;376(9747):1186–1193 [DOI] [PubMed] [Google Scholar]
- 15. Sankaranarayanan R, Ramadas K, Thara S, et al. Clinical breast examination: preliminary results from a cluster randomized controlled trial in India J Natl Cancer Inst 2011;103(19):1476–1480 [DOI] [PubMed] [Google Scholar]
- 16. Kreimer AR, Rodriguez AC, Hildesheim A, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine J Natl Cancer Inst 2011;103(19):1444–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Franceschi S, Denny L, Irwin KL, et al. Eurogin 2010 roadmap on cervical cancer prevention Int J Cancer 2011;128(12):2765–2774 [DOI] [PubMed] [Google Scholar]
- 18. Harford JB. Breast-cancer early detection in low-income and middle-income countries: do what you can versus one size fits all Lancet Oncol 2011;12(3):306–312 [DOI] [PubMed] [Google Scholar]
- 19.Institute of Medicine of the National Academies Committee on Cancer Control in Low- and Middle-Income Countries. Cancer Control Opportunities in Low- and Middle-Income Countries. Washington, DC: The National Academies Press; 2007. [PubMed] [Google Scholar]
- 20.Knaul FM, Frenk J,, Shulman L. L for the Global Task Force on Expanded Access to Cancer Care and Control in Developing Countries. Boston, MA: : Harvard Global Equity Initiative;; 2011. Closing the Cancer Divide: A Blueprint to Expand Access in Low and Middle Income Countries. [Google Scholar]
- 21. Union for International Cancer Control World Cancer Declaration http://www.uicc.org/declaration. Accessed June 19, 2012.
- 22. International Agency for Research on Cancer Global Initiative for Cancer Registry Development in Low- and Middle-Income Countries http://gicr.iarc.fr/. Accessed June 19, 2012.
- 23. Wild CP. How much of a contribution do exposures experienced between conception and adolescence make to the burden of cancer in adults? Cancer Epidemiol Biomarkers Prev 2011;20(4):580–581 [DOI] [PubMed] [Google Scholar]
- 24. Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology Cancer Epidemiol Biomarkers Prev 2005;14(8):1847–1850 [DOI] [PubMed] [Google Scholar]
- 25. Rappaport SM, Smith MT. Epidemiology. Environment and disease risks Science 2010;330(6003):460–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wild CP, Gong YY. Mycotoxins and human disease: a largely ignored global health issue Carcinogenesis 2010;31(1):71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goldie SJ, Daniels N. Model-based analyses to compare health and economic outcomes of cancer control: inclusion of disparities J Natl Cancer Inst 2011;103(18):1373–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. The Lancet Oncology. Forthcoming. [DOI] [PubMed]