Abstract
Our objective was to develop a surface modification strategy for a titanium alloy (TiAl6V4) to provide thromboresistance for surfaces in rigorous blood-contacting cardiovascular applications, such as that found in ventricular assist devices. We hypothesized that this could be accomplished by the covalent attachment of a phospholipid polymer, poly(2-methacryloyloxyethylphosphorylcholine (MPC)-co-methacryl acid) (PMA). TiAl6V4 was H2O plasma treated by radio frequency glow discharge, silanated with 3-aminopropyltriethoxysilane (APS), and ammonia plasma treated to increase surface reactivity. The TiAl6V4 surface was then modified with PMA via a condensation reaction between the amino groups on the TiAl6V4 surface and the carboxyl groups on PMA. The surface composition was verified by X-ray photoelectron spectroscopy, confirming successful modification of the TiAl6V4 surfaces with APS and PMA as evidenced by increased Si and P. Plasma treatments with H2O and ammonia were effective at further increasing the surface reactivity of TiAl6V4 as evidenced by increased surface PMA. The adsorption of ovine fibrinogen onto PMA-modified surfaces was reduced relative to unmodified surfaces, and in vitro ovine blood contact through a rocking test revealed marked reductions in platelet deposition and bulk phase platelet activation relative to unmodified TiAl6V4 and polystyrene controls. The results indicate that the PMA-modification scheme for TiAl6V4 surfaces offers a potential pathway to improve the thromboresistance of the blood-contacting surfaces of cardiovascular devices.
Keywords: titanium alloy, surface modification, phospholipid polymer, blood compatibility, cardiovascular devices
INTRODUCTION
The successful application of titanium and titanium alloys (e.g. TiAl6V4) in biomedical devices, such as hard tissue replacement and cardiovascular applications, has encouraged the development of surface modification techniques to improve application of specific properties such as topography, bioactivity, bone conductivity, wear resistance, corrosion resistance, and blood compatibility.1,2 One growing area of titanium alloy application in the area of cardiovascular disease is in the context of ventricular assist devices (VADs), which provide circulatory support to those in end-stage heart failure via pulsatile or rotary actuation of the blood. Unfortunately, VAD implantation is associated with a number of complications including infection, bleeding, and thromboembolism, and these biocompatibility concerns are a major reason why VADs are arguably underutilized in heart failure patients.3,4 VADs are of particular interest to those working in the biomaterial subspecialty of hemocompatible surfaces in that these devices represent a relatively large surface area of blood contact that remains in place for extended periods of time, ideally with minimal levels of anticoagulation and antiplatelet therapy.
Titanium and its alloys are employed as the blood-contacting surface and outer housing of many VADs due to machinability, corrosion resistance, and relative biocompatibility. However, platelet adhesion and thrombus formation still occur on these titanium surfaces, resulting in thromboembolism or increased bleeding risk due to the essential use of anticoagulation in these patients.1,4–6 Improvement of the surface chemistry of titanium to improve thromboresistance would represent a significant technological improvement that might contribute to more widespread adoption of these devices in latter stage heart failure patients.
Various surface mechanical, chemical, and physical surface modification methods have been applied to titanium alloys including machining or polishing, acidic or alkaline treatment, anodic oxidation, chemical vapor deposition, biochemical modification through silanization, physical vapor deposition, ion implantation, and glow discharge plasma treatment.1 For biological applications, plasma treatment using radio frequency glow discharge (RFGD) is especially attractive as it may be used to deposit active functional groups for covalent attachment of other polymers or biomolecules.1,7,8 Similarly, silane coupling agents with a terminal functional group have been used extensively for surface modification of inorganic silicas as well as metallic materials.9–13 Others have reported the modification of titanium surfaces by alkylsilanes to form organic films with good stability, furthermore, coupling agents such as organo-functional trialkoxysilanes have been applied to form durable chemical bonding between inorganic and organic molecules (or moieties).9,12,13
Biomembrane mimetic artificial surfaces prepared from phospholipid polymers [phosphorylcholine (PC)-containing polymers] have received considerable interest for chemical, biological, and medical application because they have previously been shown to possess excellent biological and blood compatibility.14–20 These surfaces have several defining characteristics, including strong affinity with natural phospholipids, high surface free water fraction due to the zwitterionic nature of PC, resistance to nonspecific protein adsorption, limited activation of plasma proteins, and lateral mobility of molecules without firm adhesion by proteins or cells.15–18 One of the most common monomers utilized in biological and biomedical applications is 2-methacryloyloxye-thylphosphorylcholine (MPC), which has biomimetic PC groups in its structure and can polymerize with other hydrophobic monomers.15,16 It has been extensively shown previously that surface modifications with MPC polymers are effective in improving hemocompatibility by suppressing protein adsorption, platelet adhesion, and platelet activation on blood-contacting materials such as dialysis membranes, vascular prosthesis, stents, and VADs.21–31 In the report on VAD surface modification, MPC was adsorbed as opposed to covalently attached, leading to erosion of the coating during chronic use.27
The objective of this study was to develop a surface modification strategy that could reduce platelet deposition onto the TiAl6V4 surface that we are currently utilizing in the construction of a pediatric VAD where blood biocompatibility concerns are of particular importance.32 Our approach focused on developing a method for covalently attaching a phospholipid polymer, poly(MPC-co-methacryl acid) (PMA), onto the TiAl6V4 surface and characterizing this surface in terms of composition and platelet adhesion and activation by the surface in vitro.
MATERIALS AND METHODS
Materials
Titanium alloy (TiAl6V4) was purchased (President Titanium, Hanson, MA, and California Metal & Supply, Gardena, CA) and polished with 3.0, 1.0, 0.25, and 0.1 μm diamond pastes (Electron Microscopy Sciences, Washington, PA). A phospholipid copolymer comprised of 70 mol % MPC and 30 mol % methacrylic acid (MA) (PMA, Mw = 5.5 × 105) was kindly provided by Dr. Kazuhiko Ishihara (University of Tokyo, Tokyo, Japan). 3-Aminopropyltrie-thoxysilane (APS; United Chemical Technologies, Horsham, PA) and 1-ethyl 3-(3-dimethylaminopropyl) carbodiimide-hydrochloride (EDC; Sigma-Aldrich, St. Louis, MO) was used for a silane coupling agent and a condensation agent, respectively. Heparin (Pharmacia & Upjohn, Ann Arbor, MI) was used for blood anticoagulation.
Surface pretreatment and silanization of titanium surfaces
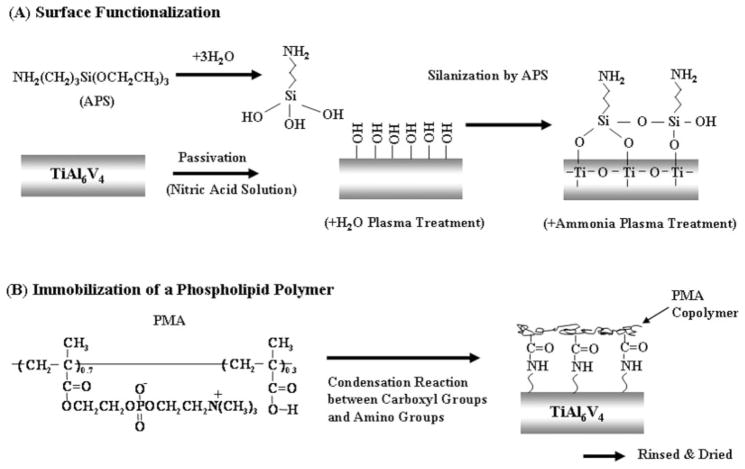
TiAl6V4 samples were cleaned ultrasonically three times for 5 min each with ethanol and acetone, and the surfaces were passivated with a 35% nitric acid solution for 1 h and rinsed with distilled water. Additionally, the TiAl6V4 surfaces were then pretreated by H2O plasma with RFGD (MARCH GCM250; March Instrument, CA). The plasma power applied was 25 W at a frequency of 13.65 MHz. The titanium surface was subjected to RFGD for 5 min at a water pressure of 0.4 Torr. Next, the pretreated TiAl6V4 surfaces were silanated with APS by immersion in an APS solution for 3 h in a 90°C oil bath. The APS solution consisted of 2% APS in ethanol that was hydrolyzed by adding water and stirring for 1 h. The silanated samples were rinsed with distilled water and dried at 110°C for 1 h. The silanated samples were then rinsed three times with ethanol and water and stirred in deionized water for 1 h to remove adsorbed APS. Additionally, a subset of silanated TiAl6V4 samples was treated with ammonia plasma by RFGD (25 W, 1 min) to further introduce amine groups on the surface [Fig. 1(A)].
Figure 1.
Scheme of surface functionalization and immobilization of a phospholipid polymer (PMA) on a titanium alloy surface.
Immobilization of a phospholipid polymer
Silanated and surface-aminated titanium samples were immersed in 0.1 wt % PMA copolymer solution in water and a condensation reagent was added (EDC, [EDC]/[MA] = 50). The PMA solution was stirred for 24 h in a 60°C oil bath. The PMA was chemically immobilized on the surface of the titanium sample through the reaction between the carboxyl groups in the PMA and the amine groups on the titanium surface [Fig. 1(B)]. After modification with the PMA, the titanium samples were washed three times with EtOH and water, rinsed with stirred, deionized water for 24 h, and then dried under vacuum.
Surface characterization
The surface composition of the titanium samples was analyzed by X-ray photoelectron spectroscopy (XPS) using a Surface Science Instruments S-probe spectrometer with a take-off angle of 55°. This take-off angle corresponds to a sampling depth of ~5 nm. Elemental composition spectra were acquired using a pass energy of 150 eV. High-resolution C1s spectra were acquired at an analyzer pass energy of 50 eV. The Service Physics ESCAVB Graphics Viewer program was used to determine peak area, calculate the elemental compositions from peak areas and peak fit the high resolution spectra. The surface composition on a given sample was averaged from two composition spots and one high resolution C1s analysis. The mean value for three different samples was determined.
Surface protein adsorption
Surface protein adsorption on modified and unmodified TiAl6V4 samples was assessed by a micro-bicinchoninic acid (BCA) assay.26 Ovine fibrinogen (Sigma-Aldrich) was prepared in phosphate-buffered solution (PBS; BD Biosciences, San Jose, CA) at a concentration of 0.03 g/dL. The samples were immersed in the fibrinogen solution at 37°C for 3 h. A protein analysis kit (Quantipro-Micro BCA kit; Sigma-Aldrich) based on the BCA method was used to quantify adsorbed fibrinogen. The mean value of fibrinogen adsorption from three independent samples, each measured in triplicate, was determined.
Blood collection and observation of acute thrombotic surface deposition
Whole ovine blood was collected by jugular venipuncture, directly into a syringe containing heparin (1.5 U/mL) using an 18-gauge 1 [1/2]″ needle, after discarding the first 3 mL. NIH guidelines for the care and use of laboratory animals were observed. The heparinized blood was treated with quinacrine dihydrochloride (Sigma) at a final concentration of 10 μM in PBS to fluorescently label the platelets prior to contact with the test surfaces. Modified titanium samples were placed into BD Vacutainer® blood collection tubes (BD Biosciences, Franklin Lakes, NJ) filled with 5 mL heparinized ovine blood and were rocked for 60 min at 37°C on a hematology mixer (Fisher Scientific, Pittsburgh, PA). Mural thrombus deposition and the distribution of adhered platelets were assessed macroscopically and microscopically using an epi-fluorescence microscope (Zeiss; Carl Zeiss, Thornwood, NY).
Scanning electron microscopy of platelet adhesion and morphology
Whole blood was again collected as above from healthy ovines, but without heparin, and 2.7 mL was then immediately added to monovette tubes containing 0.3 mL of 0.106M trisodium citrate (Sarstedt, Newton, NC). Modified and unmodified titanium samples were placed into BD Vacutainer tubes containing 5 mL citrated ovine blood and incubated for 3.5 h again with continuous rocking. The surface was then rinsed with PBS and immersed in a 2.5% glutaraldehyde solution for 2 h at 4°C to fix the surface adhered platelets, the samples were then washed three times in PBS (15 min each), treated for 1 h in 1% (w/v) OsO4 solution and washed three more times in PBS. Samples were then serially dehydrated with increasing ethanol solutions, critical point dried and sputter coated with gold/palladium. Each sample surface was observed by scanning electron microscopy (SEM; JSM-6330F; JEOL USA, Peabody, MA).
Quantification of surface adherent platelets
Modified and unmodified titanium samples were incubated with ovine blood (collected as earlier, but into a solution of 6 U/mL heparin) for 2 h at 37°C with continuous rocking as earlier. The surfaces were rinsed thoroughly after blood contact with 50 mL PBS and immersed in 1 mL of 2% Triton X-100 solution (Sigma) for 20 min to lyse surface adherent platelets. The number of platelets for each sample was then estimated by a lactate dehydrogenase (LDH) assay33 with an LDH Cytotoxicity Detection Kit (Takara Bio, Japan). Calibration of spectrophotometer absorbance results to platelet numbers was accomplished using a calibration curve generated from known dilutions of ovine platelet rich plasma in the lysing solution.
Platelet activation quantification by flow cytometry
The percentage of activated ovine platelets in the bulk phase of the blood contacting the surface samples was quantified by a recently described flow cytometric assay using fluorescein conjugated Annexin V protein.34 Heparinized (6 U/mL) blood was incubated with titanium samples as described above for 2 h. Blood samples were prepared for flow cytometric analysis using 250 μL of Annexin V binding buffer and the level of platelet activation was determined as previously described.34 Activation levels from five independent samples were averaged for each surface type.
Statistical analyses
Data are presented as means with standard deviation. Statistical significance between sample groups was determined using ANOVA followed by post-hoc Newman–Keuls testing and accepted at p < 0.05.
RESULTS
Surface characterization of the modified TiAl6V4
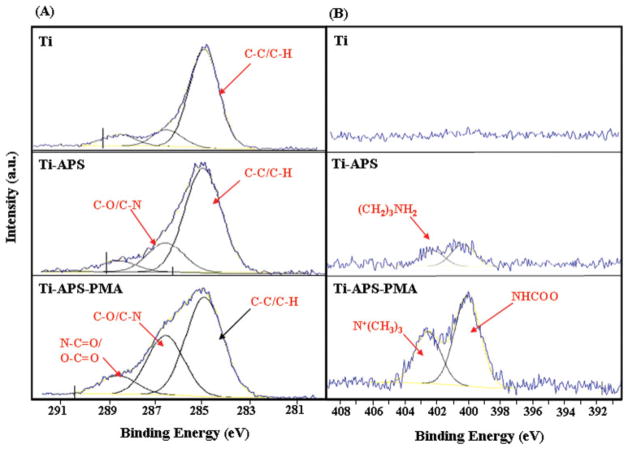
The high-resolution spectra from XPS are shown in Figure 2. The C1s data were calibrated to the hydrocarbon peak (C–C/C–H) at 285.0 eV. The peak fits for the APS-modified titanium surface (Ti-APS) show an increase attributable to a peak at 286.6 eV, which is likely due to C–O or C–N type species. For the PMA-modified surface a peak at 288.6 eV due to O–C =O and an amide type (N–C=O) species are present [Fig. 2(A)]. Furthermore, the amide bonding between the PMA and APS is confirmed from the N1s high resolution spectra on the Ti-APS-PMA surface. The PMA-modified surface clearly showed two peaks at 400.0 eV due to NHCOO species (amide bond between PMA and APS) and 402.5 eV due to N+(CH3)3 species present in MPC [Fig. 2(B)]. The surface atomic compositions of the TiAl6V4 samples are shown in Table I. The APS-modified surface (Ti-APS) also had an increase in Si composition which can be attributed to the presence of APS. All of the PMA-modified titanium surfaces [Ti-APS-PMA and Ti-APS-PMA (plasma)] showed an increase in atomic percentage of phosphorus (P), indicating successful modification with PMA. Furthermore, the Ti-APS-PMA (plasma) surfaces which were H2O and ammonia plasma treated by RFGD showed a statistically significant higher P composition than the Ti-APS-PMA surfaces which did not receive any plasma treatments.
Figure 2.
Spectra and peak fits for high resolution C1s (A) and N1s (B) XPS scans for unmodified and modified TiAl6V4 samples. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
TABLE I.
Atomic Percentage (Composition) as Determined by X-ray Photoelectron Spectroscopy (XPS)
| C | O | Ti | Al | Si | N | P | |
|---|---|---|---|---|---|---|---|
| Ti | 39.9 (±7.2) | 41.8 (±4.0) | 8.7 (±1.2) | 5.1 (±3.1) | 1.3 (±1.5) | 0.9 (±0.1) | 0.0 (±0.0) |
| Ti-APS | 42.4 (±4.2) | 37.4 (±3.4) | 4.3 (±0.5) | 4.1 (±1.8) | 10.2 (±2.8) | 1.3 (±0.3) | 0.1 (±0.1) |
| Ti-APS-PMA | 45.0 (±9.7) | 32.5 (±6.2) | 4.3 (±3.2) | 1.0 (±0.9) | 7.4 (±5.8) | 2.4 (±0.1) | 0.8 (±0.1) |
| Ti-APS-PMA (plasma) | 47.1 (±9.3) | 37.0 (±8.0) | 5.8 (±3.3) | 0.1 (±0.2) | 5.6 (±0.9) | 4.3 (±0.7) | 1.3 (±0.1) |
N = 3, Standard deviation (±SD).
The amount of adsorbed ovine fibrinogen on the unmodified and modified titanium surfaces as well as a tissue culture polystyrene control surface is shown in Figure 3. The Ti-APS-PMA and Ti-APS-PMA (plasma) showed a significant decrease in adsorbed fibrinogen relative to all other surfaces (p < 0.05).
Figure 3.
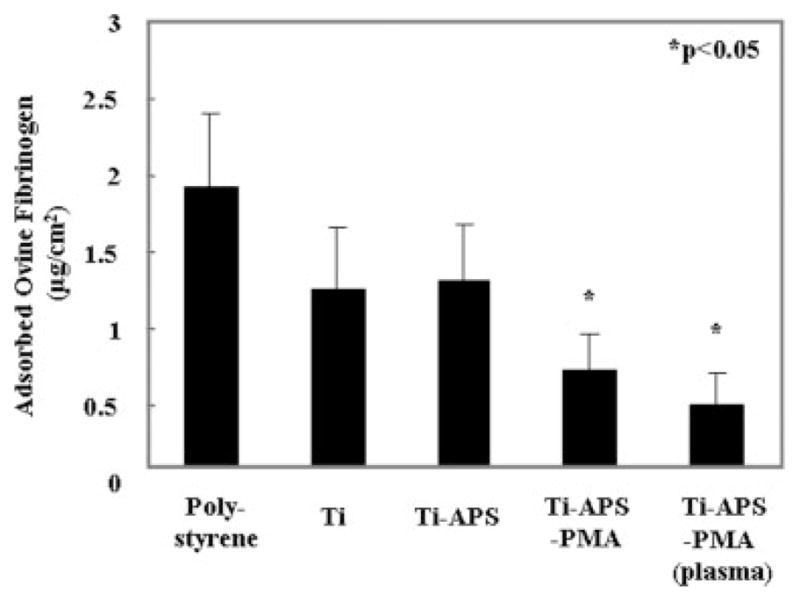
Ovine fibrinogen adsorption from buffer at 37°C for 3 h onto surfaces of control tissue culture polystyrene, unmodified, and modified TiAl6V4 samples as determined by micro-BCA assay.
Acute thromboresistance assessment
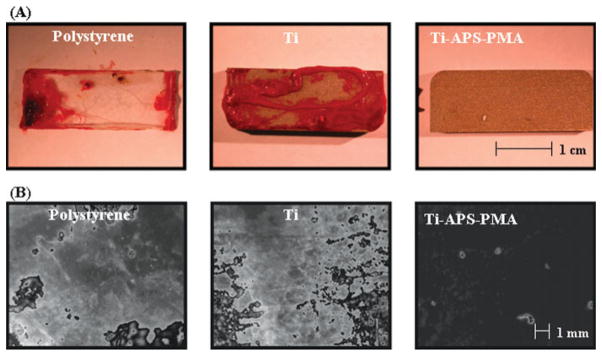
Figure 4(A) shows representative macroscopic images of Ti-APS-PMA and control surfaces after contact with minimally anticoagulated ovine blood. There was a marked difference between Ti-APS-PMA, which had relatively low thrombotic deposition, and the moderate or heavy deposition seen on polystyrene and unmodified TiAl6V4 surfaces, respectively. These results are supported by the fluorescent micrographs in Figure 4(B) where the polystyrene and unmodified titanium control surfaces exhibit heavy and nearly complete surface coverage with deposited platelets, while the Ti-APS-PMA surface contained only sparse, adherent platelets.
Figure 4.
(A) Macroscopic and (B) fluorescent micrograph images of unmodified and modified TiAl6V4 samples after contact with minimally anticoagulated (1.5 U/mL heparin) ovine blood for 50 min at 37°C. Scale bars = 1 cm for (A) and 1 mm for (B). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Evaluation of platelet deposition and activation
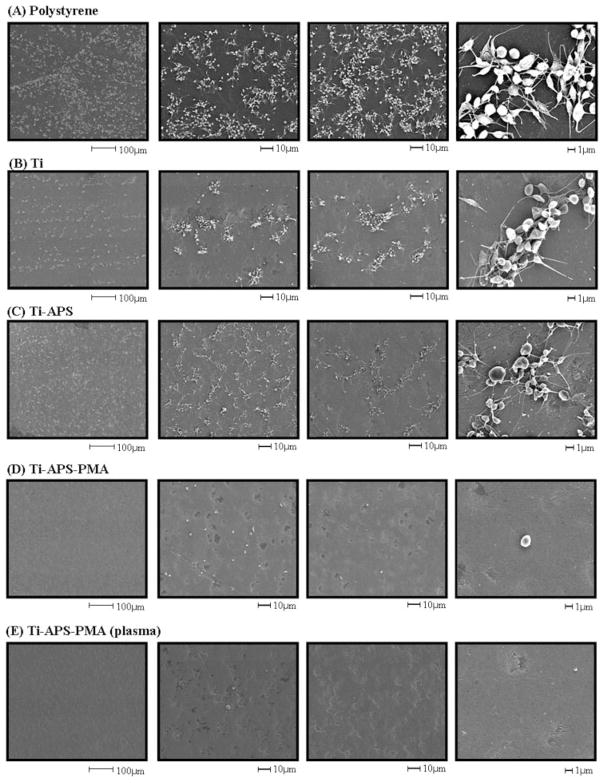
The SEM micrographs on the surfaces of polystyrene, unmodified, and modified titanium samples following 3.5 h contact with citrated ovine blood under rocking conditions are shown in Figure 5. There were many adhered and aggregated platelets on the polystyrene control surface [Fig. 5(A)] and most of these platelets exhibited extended pseudopodia. The unmodified titanium surface [Fig. 5(B)] also had many adherent platelets although the level of deposition appeared qualitatively lower than on the polystyrene surface. The silanated titanium surface [Fig. 5(C)] had a similar degree of platelet deposition to unmodified titanium. In contrast, platelet deposition and evidence of platelet activation were decreased dramatically on the PMA-modified titanium surfaces [Fig. 5(D,E)]. The Ti-APS-PMA surface had some adherent platelets, but most of these maintained their discoid morphology. Moreover, because of the sparse deposition, it was challenging to identify adherent platelets on the Ti-APS-PMA (plasma) surface [Fig. 5(E)].
Figure 5.
SEM micrographs of polystyrene, unmodified, and modified TiAl6V4 samples after contact with citrated ovine blood for 3.5 h at 37°C. (A) Polystyrene, (B) Ti, (C) Ti-APS, (D) Ti-APS-PMA, and (E) Ti-APS-PMA (plasma). Platelet activation as evidenced by pseudopodia extension is clearly present in images (A–C).
The number of deposited platelets as quantified by the LDH assay after blood contact is shown in Figure 6. Platelet deposition onto the PMA-modified surfaces [Ti-APS-PMA and Ti-APS-PMA (plasma)] was significantly less than deposition onto all of the other surfaces (p < 0.01). Finally, flow cytometric assessment of platelet activation in the bulk phase in terms of Annexin V binding is shown in Figure 7. Blood contacted with Ti-APS-PMA (plasma) samples showed a statistically significant reduction in the percentage of activated platelets in comparison to polystyrene, Ti, and Ti-APS samples (p < 0.05), and was not significantly different than the blood that was rocked in a tube without a test surface (no test surface).
Figure 6.
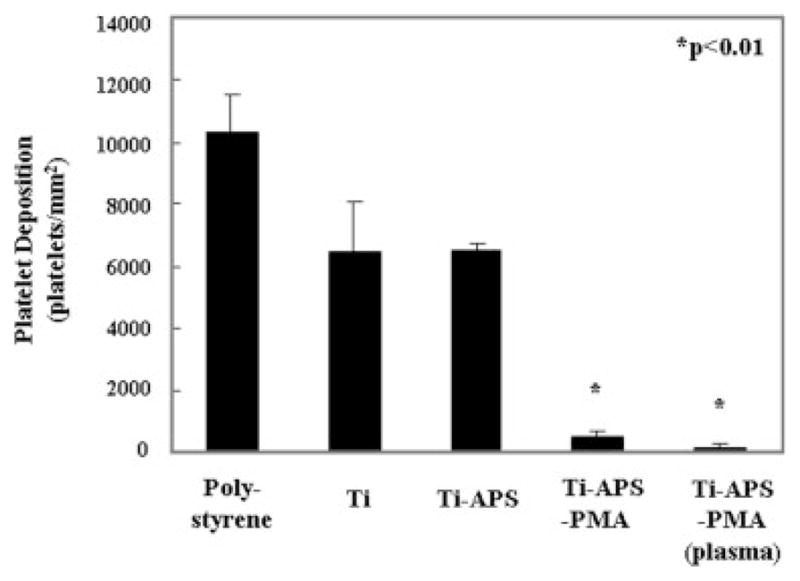
Platelet deposition onto surfaces after contact with ovine blood for 3.5 h as determined by a lactate dehydrogenase (LDH) assay.
Figure 7.
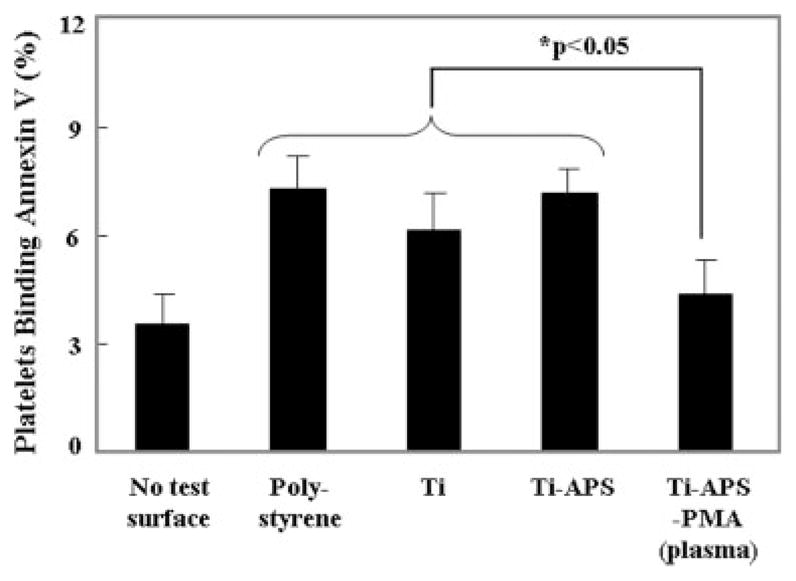
Quantification of activated platelets in the bulk phase of ovine blood after surface contact under continuous rocking. No test surface indicates blood from a rocked tube into which no test surface was placed. Platelet activation was quantified by flow cytometric measurement of Annexin V binding.
DISCUSSION
A common approach to improve hemocompatibility on metallic surfaces is the utilization of inorganic coatings such as titanium oxides, nitrides (oxynitride), and diamond-like carbon (DLC).1,35 These coatings exhibit high inertness, mechanical and chemical stability together with generally improved hemocompatibility relative to the corresponding untreated materials. The improved blood interaction has been attributed to high hydrophobicity and smoothness in the case of DLC, where protein adsorption levels are relatively high, but the predomination of the non–cell-adhesive albumin over fibrinogen on the surface could lead to the low thrombogenicity observed.36 For titanium oxides, a thicker oxide layer has been shown to be beneficial as have oxynitride coatings in terms of in vitro and in vivo performance relative to unmodified surfaces.37,38 However, the applicability of DLC via line-of-sight deposition techniques to complex geometries (e.g. a small rotary VAD) is limited and the relative hemocompatibility improvements with oxide and oxynitride layers may not be adequate.39
Metallic surface modification using organic materials has also been frequently pursued in an effort to improve hemocompatibility. Biologically inert hydrophilic polymer coatings such as polyethylene glycol have been shown to be effective in enhancing blood compatibility.40–42 Surface coatings with biomolecules or nitric oxide releasing materials are also applicable modification strategies for metallic surfaces.43,44 However, the effectiveness of surface modification using organic materials depends greatly on the specific modification methods and stability and efficacy must be considered for long-term implantable devices, as is the case with many cardiovascular applications. To immobilize organic materials onto metallic surfaces with good stability, functional groups or coupling agents are required, and an adequate number of functional groups must be introduced.
The introduction of biomimetic organic materials on metallic surfaces is also a promising approach to enhance hemocompatibility. In particular, self-assembled monolayer (SAM) studies have shown that PC assembled monolayers represent promising antifouling surfaces resistant to nonspecific protein adsorption and cell adhesion.45–48 A PC-containing polymer coating already has been successfully applied on metallic surfaces such as vascular stents.22,49,50 Also, there are a few reports where a PC-containing polymer was grafted onto a titanium oxide layer or metal alloys to obtain a relatively stable hemocompatible interface.51,52 However, despite widespread examination of grafting PC groups onto polymer or silica surfaces, the covalent immobilization of PC-containing polymers onto biomedically relevant metals has not been extensively studied, particularly with hemocompatibility evaluation of the resulting surfaces.
In this study, we chose an MPC copolymer (PMA) possessing carboxyl functional groups to immobilize by condensation onto a TiAl6V4 surface which had been functionalized with amino groups. This approach follows from a recent report where PMA was successfully attached using similar chemistry onto a cellulose acetate membrane surface.29 Further-more, plasma treatments using H2O vapor and ammonia gas by RFGD were also applied in this study as an additional surface pretreatment on pre- and post-silanated TiAl6V4 surfaces to enhance the surface activity for PMA immobilization. The PMA-modified TiAl6V4 (Ti-APS-PMA) surfaces that did not undergo any plasma treatments were shown to be successfully modified with PMA, and were associated with significant reductions in fibrinogen adsorption and platelet deposition. Also, the Ti-APS-PMA (plasma) surfaces which underwent both H2O and ammonia plasma treatments in the surface processing showed an increase in the amount of modified PMA on the surface relative to Ti-APS-PMA (p < 0.05). This results support the hypothesis that H2O and ammonia plasma contributed additional surface reactive hydroxyl and amino groups onto the surface, allowing additional APS and PMA molecules, respectively, to react onto the surface. However, the amount of modified PMA on the Ti-APS-PMA surfaces treated with either H2O or ammonia plasma did not differ significantly from the Ti-APS-PMA surfaces in this study, even though these plasma-treated TiAl6V4 surfaces exhibited an increase in either surface oxygen or nitrogen composition, relative to the untreated TiAl6V4 surfaces (data not shown). Further investigation of this phenomenon could be pursued under varying preparative conditions where amino groups are quantified after each plasma treatment, with a focus on the relationship between each plasma treatment and PMA immobilization.
There are several limitations to the experimental results that are worth discussing. Probably of most significance, only acute assessments of blood bio-compatibility were performed using an in vitro system with ovine blood and under flow conditions that are not well defined and not intended to replicate any specific application. Acute in vitro hemo-compatibility assessment represents a first hurdle to overcome prior to proceeding to more costly and burdensome in vivo studies for longer term blood contact evaluations. Subtle levels of thrombosis or coagulation activation may not be apparent under the levels of anticoagulation employed in vitro, and the potentially masked hemo-incompatibilities may be magnified in vivo. In addition, a lack of surface platelet deposition may not indicate a lack of bulk phase platelet activation. We sought to perform some of our thrombosis evaluations with minimally anticoagulated blood to examine a more rigorous setting and quantified bulk phase platelet activation in addition to surface deposition. Ovine blood varies from human blood in terms of thrombogenic potential; however, pre-clinical testing for the particular device we would be coating (a pediatric VAD) is performed in the ovine model.32,53,54 In terms of the flow conditions, particularly the high shear developed in rotary VADs, an appropriate next step would be to coat components of a VAD, for instance a rotor, and then to assess the hemocompatibility of this surface in a mock circulatory loop under device operating conditions.
The composition of Si on the unmodified and modified TiAl6V4 samples varied greatly as seen in Table I. Some of the unmodified TiAl6V4 samples already had Si on the surfaces and the Si composition on the silanated samples (Ti-APS) was much higher than for the N composition although in theory the increase in their surface compositions should have been similar following the silanization step. This high Si composition indicates some potential contamination of the source materials or some additional contamination in the surface modification process. More meticulous polishing and additional cleaning steps could decrease the Si contamination.
The PMA surface modification approach described in this study also has some limitations worth noting, even though the modification effect was enough to decrease platelet deposition by the immobilized PMA copolymer on the TiAl6V4 surface. Based on the surface composition data from the XPS analysis, the coverage of modified PMA could be considered to be low and nonuniform in comparison to physical adsorption and self assembled monolayer coverage. We believe this is in part a result of the steric hindrance caused by immobilization of the long-chain PMA copolymer on the titanium surface. Despite this concern about the amount of PMA coverage on the titanium surface, the modification effect was enough to almost complete abrogate any platelet deposition on the modified TiAl6V4 surface. Also, the surface coverage and uniformity of the modified PMA will depend upon the density and distribution of reactive amine groups on the TiAl6V4 surface. Functionalization of TiAl6V4 surfaces through APS reaction with hydroxyl groups likely does not provide the optimal density and distribution of surface amines. Preparation of a complete APS SAM on the TiAl6V4 surface could be considered. Also, even if PMA were to cover all of a TiAl6V4 surface, the PC moiety would not cover the surface uniformly because the PMA is a random copolymer of MPC and MA. The consequence of such uneven PC coverage is not clear. A similar surface modification strategy for introducing the PC moiety uniformly that has fewer steps may be advantageous in order to enhance incorporation of this technology in the manufacture of the blood-contacting device surfaces.
Finally, we point out the uniqueness of our approach in terms of covalent attachment of the MPC copolymer on a titanium alloy surface. Previous studies involving in vivo biocompatibility assessments with physisorbed MPC polymer on the blood-contacting surfaces of a VAD were shown to be superior in terms of decreased thrombotic deposition and platelet activation when compared with devices with DLC-coated surfaces.27,31 Despite the improved hemocompatibility in the above study, they showed the elution of the MPC polymer with long-term operation; the primary concern was due to the fact the MPC polymer was physically adsorbed onto the titanium surfaces. As the method reported here involves covalent modification of the titanium alloy, the durability of the PMA surface modification would be expected to be improved over adsorption for longer implant periods, but such evaluations remain to be performed.
CONCLUSIONS
TiAl6V4 surfaces were modified with a phospholipid polymer, PMA, through a condensation reaction following TiAl6V4 surface functionalization with a silane coupling agent (APS). XPS data demonstrated successful modification of the TiAl6V4 surfaces with APS and PMA. The PMA-modified TiAl6V4 surface exhibited markedly improved blood compatibility with significant decreases in fibrinogen adsorption, acute platelet deposition, and bulk phase platelet activation relative to unmodified Ti samples. This approach may prove valuable in the covalent surface modification of titanium alloy blood-contacting surfaces associated with chronically implanted cardiovascular devices.
Acknowledgments
The surface analysis data from NESAC/BIO were provided with support from NIH. We are indebted to Prof. Kazuhiko Ishihara who kindly provided the PMA copolymer.
Contract grant sponsor: NIH; contract grant numbers: HHSN268200448192C, EB-002027
References
- 1.Liu X, Chu PK, Ding C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater Sci Eng R. 2004;47:49–121. [Google Scholar]
- 2.Rack HJ, Qazi JI. Titanium alloys for biomedical application. Mater Sci Eng C. 2006;26:1269–1277. [Google Scholar]
- 3.Wagner WR, Borovetz HS, Griffith BP. Implantable cardiac assist devices. In: Ratner B, Hoffman A, Schoen F, Lemons J, editors. Biomaterials Science: An Introduction to Materials in Medicine. San Diego, CA: Elsevier Academic Press; 2004. pp. 494–506. [Google Scholar]
- 4.Deng MC, Edwards LB, Hertz MI, Rowe AW, Keck BM, Kormos RL, Naftel DC, Kirklin JK, Taylor DO. Mechanical circulatory support device database of the International Society for Heart and Lung Transplantation: Third annual report 2005. J Heart Lung Transplant. 2005;24:1182–1187. doi: 10.1016/j.healun.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 5.McBride LR, Naunheim KS, Fiore AC, Moroney DA, Swartz MT. Clinical experience with 111 Thoratec ventricular assist devices. Ann Thorac Surg. 1999;67:1233–1239. doi: 10.1016/s0003-4975(99)00246-5. [DOI] [PubMed] [Google Scholar]
- 6.Schaub RD, Kameneva MK, Borovetz HS, Wagner WR. Assessing acute platelet adhesion on opaque metallic and polymeric biomaterials with fiber optic microscopy. J Biomed Mater Res. 2000;49:460–468. doi: 10.1002/(sici)1097-4636(20000315)49:4<460::aid-jbm4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Xiao SJ, Textor M, Spencer ND. Covalent attachment of cell-adhesive, (Arg-Gly-Asp)-containing peptides to titanium surfaces. Langmuir. 1998;14:5507–5516. [Google Scholar]
- 8.Chevallier P, Castonguay M, Turgeon S, Dubrulle N, Mantovani D, McBreen PH, Wittmann JC, Laroche G. Ammonia RF-plasma on PTFE surfaces: Chemical characterization of the species created on the surface by vapor-phase chemical derivatization. J Phys Chem B. 2001;105:12490–12497. [Google Scholar]
- 9.Nanci A, Wuest JD, Peru L, Brunet P, Sharma V, Zalzal S, Mckee MD. Chemical modification of titanium surfaces for covalent attachment of biological molecules. J Biomed Mater Res. 1998;40:324–335. doi: 10.1002/(sici)1097-4636(199805)40:2<324::aid-jbm18>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 10.Daniel MW, Sefcik J, Francis LF, McCormick AV. Reactions of trifunctional silane coupling agent in the presence of colloidal silica sols in polar media. J Colloid Interface Sci. 1999;219:351–356. doi: 10.1006/jcis.1999.6497. [DOI] [PubMed] [Google Scholar]
- 11.Jesionowski T, Krysztafkiewicz A. Influence of silane coupling agents on surface properties of precipitated silicas. Appl Surf Sci. 2001;172:18–32. [Google Scholar]
- 12.Matinlinna JP, Laajalehto K, Laiho T, Kangasniemi I, Lassila LVJ, Vallittu PK. Surface analysis of Co-Cr-Mo alloy and Ti substrates silanizated with trialkoxysilanes and silane mixtures. Surf Interface Anal. 2004;36:246–253. [Google Scholar]
- 13.Matinlinna JP, Areva S, Lassila LVJ, Vallittu PK. Characterization of siloxane films on titanium substrate derived from three aminosilane. Surf Interface Anal. 2004;36:1314–1322. [Google Scholar]
- 14.Hayward JA, Chapman D. Biomembrane surface as model for polymer design: The potential for heamocompatibility. Biomaterials. 1984;5:135–142. doi: 10.1016/0142-9612(84)90047-4. [DOI] [PubMed] [Google Scholar]
- 15.Ishihara K, Aragaki R, Ueda T, Watanabe A, Nakabayashi N. Reduced thrombogenicity of polymers having phospholipids polar group. J Biomed Mater Res. 1990;24:1069–1077. doi: 10.1002/jbm.820240809. [DOI] [PubMed] [Google Scholar]
- 16.Iwasaki Y, Mikami A, Kurita K, Yui N, Ishihara K, Nakabayashi N. Reduction of surface-induced platelet activation on phospholipid polymer. J Biomed Mater Res. 1997;36:508–515. doi: 10.1002/(sici)1097-4636(19970915)36:4<508::aid-jbm8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 17.Ishihara K, Nomura H, Mihara T, Kurita K, Iwasaki Y, Nakabayashi N. Why do phospholipids polymers reduce protein adsorption? J Biomed Mater Res. 1998;39:323–330. doi: 10.1002/(sici)1097-4636(199802)39:2<323::aid-jbm21>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 18.Nakaya, Li YJ. Phospholipid polymers. Prog Polym Sci. 1999;24:143–181. [Google Scholar]
- 19.Lewis AL. Phosphorylcholine-based polymers and their use in the prevention of biofouling. Colloids Surf B: Biointerfaces. 2000;18:261–275. doi: 10.1016/s0927-7765(99)00152-6. [DOI] [PubMed] [Google Scholar]
- 20.Lewis AL, Cumming ZL, Goreshi HH, Kirkwood LC, Tolhurst LA, Stratford PW. Crosslinkable coatings from phosphorylcholine-based polymer. Biomaterials. 2001;22:99–111. doi: 10.1016/s0142-9612(00)00083-1. [DOI] [PubMed] [Google Scholar]
- 21.Ishihara K. Bioinspired phospholipid polymer biomaterials for making high performance artificial organs. Sci Technol Adv Mater. 2000;1:131–138. [Google Scholar]
- 22.Whelan DM, Giessen WJ, Krabbendam SC, Vliet EA, Verdouw PD, Serruys PW, Beusekom HMM. Biocompatibility of phosphorylcholine coated stents in normal porcine coronary arteries. Heart. 2000;83:338–345. doi: 10.1136/heart.83.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasegawa T, Iwasaki Y, Ishihara K. Preparation and performance of protein-adsorption-resistant asymmetric porous membrane composed of polysulfone/phospholipid polymer blend. Biomaterials. 2001;22:243–251. doi: 10.1016/s0142-9612(00)00180-0. [DOI] [PubMed] [Google Scholar]
- 24.Ye SH, Watanabe J, Iwasaki Y, Ishihara K. Novel cellulose acetate membrane blended with phospholipid polymer for hemo-compatible filtration system. J Membr Sci. 2002;210:411–421. [Google Scholar]
- 25.Yoneyama T, Sugihara K, Ishihara K, Iwasaki Y, Nakabayashi N. The vascular prosthesis without pseudointima prepared by antithrombogenic phospholipid polymer. Biomaterials. 2002;23:1455–1459. doi: 10.1016/s0142-9612(01)00268-x. [DOI] [PubMed] [Google Scholar]
- 26.Ye SH, Watanabe J, Iwasaki Y, Ishihara K. Antifouling blood purification membrane composed of cellulose acetate and phospholipid polymer. Biomaterials. 2003;24:4143–4152. doi: 10.1016/s0142-9612(03)00296-5. [DOI] [PubMed] [Google Scholar]
- 27.Kihara S, Yamazaki K, Litwak P, Kameneva MV, Ushiyama H, Tokuno T, Borzelleca DC, Umezu M, Tomioka J, Tagusari O, Akimoto T, Koyanagi H, Kurosawa H, Kormos RL, Griffith BP. In vivo evaluation of MPC polymer coated continuous flow left ventricular assist system. Artif Organs. 2003;27:188–192. doi: 10.1046/j.1525-1594.2003.t01-2-06993.x. [DOI] [PubMed] [Google Scholar]
- 28.Ye SH, Watanabe J, Iwasaki Y, Ishihara K. In situ modification on cellulose acetate hollow fiber membrane modified phospholipid polymer for biomedical application. J Membr Sci. 2005;249:133–141. [Google Scholar]
- 29.Ye SH, Watanabe J, Iwasaki Y, Takai M, Ishihara K. High functional hollow fiber membrane modified with phospholipid polymers for a liver assist bioreactor. Biomaterials. 2006;27:1955–1962. doi: 10.1016/j.biomaterials.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 30.Nakabayashi N. Preparation of biocompatible materials and their evaluation. Macromol Symp. 2006;245–246:591–598. [Google Scholar]
- 31.Snyder TA, Tsukui H, Kihara S, Akimoto T, Litwak KN, Kameneva MV, Yamazaki K, Wagner WR. Preclinical biocompatibility assessment of the EVAHEART ventricular assist device: Coating comparison and platelet activation. J Biomed Mater Res A. 2007;81:85–92. doi: 10.1002/jbm.a.31006. [DOI] [PubMed] [Google Scholar]
- 32.Wearden PD, Morell VO, Keller BB, Webber SA, Borovetz HS, Badylak SF, Boston JR, Kormos RL, Kameneva MV, Simaan M, Snyder TA, Tsukui H, Wagner WR, Antaki JF, Diao C, Vandenberghe S, Gardiner J, Li CM, Noh D, Paden D, Paden B, Wu J, Bearnson GB, Jacobs G, Kirk J, Khanwilkar P, Long JW, Miles S, Hawkins JA, Kouretas PC, Shaddy RE. The PediaFlow™ pediatric ventricular assist device. Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006;9:92–98. doi: 10.1053/j.pcsu.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Tamada Y, Kulik EA, Ikada Y. Simple method for platelet counting. Biomaterials. 1995;16:259–261. doi: 10.1016/0142-9612(95)92126-q. [DOI] [PubMed] [Google Scholar]
- 34.Johnson CA, Jr, Snyder TA, Woolley JR, Wagner WR. Flow cytometric assays for quantifying activated ovine platelets. Artif Organs. 2008;32:136–145. doi: 10.1111/j.1525-1594.2007.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Werner C, Maitz MF, Sperling C. Current strategies towards hemocompatible coatings. J Mater Chem. 2007;17:3376–3384. [Google Scholar]
- 36.Jones MI, McColl IR, Grant DM, Parker KG, Parker TL. Protein adsorption and platelet attachment and activation, on TiN, TiC, and DLC coatings on titanium for cardiovascular applications. J Biomed Mater Res. 2000;52:413–421. doi: 10.1002/1097-4636(200011)52:2<413::aid-jbm23>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 37.Huang N, Chen YR, Luo JM, Yi J, Lu R, Xiao J, Xue ZN, Liu XH. In vitro investigation of blood compatibility of Ti with oxide layers of rutile structure. J Biomater Appl. 1994;8:404–412. doi: 10.1177/088532829400800406. [DOI] [PubMed] [Google Scholar]
- 38.Windecker S, Mayer I, De Pasquale G, Maier W, Dirsch O, De Groot P, Wu YP, Noll G, Leskosek B, Meier B, Hess OM. Stent coating with titanium-nitride-oxide for reduction of neointimal hyperplasia. Circulation. 2001;104:928–933. doi: 10.1161/hc3401.093146. [DOI] [PubMed] [Google Scholar]
- 39.Alanazi A, Nojiri C, Kido T, Noguchi T, Ohgoe Y, Matsuda T, Hirakuri K, Funakubo A, Sakai K, Fukui Y. Engineering analysis of diamond-like carbon coated polymeric materials for biomedical applications. Artif Organs. 2000;24:624–627. doi: 10.1046/j.1525-1594.2000.06576.x. [DOI] [PubMed] [Google Scholar]
- 40.Lee JH, Lee HB, Andrade JD. Blood compatibility of polyethylene oxide surfaces. Prog Polym Sci. 1995;20:1043–1079. [Google Scholar]
- 41.Zhang F, Kang ET, Neoh KG, Wang P, Tan KL. Surface modification of stainless steel by grafting of poly(ethylene glycol) for reduction in protein adsorption. Biomaterials. 2001;22:1541–1548. doi: 10.1016/s0142-9612(00)00310-0. [DOI] [PubMed] [Google Scholar]
- 42.Tosatti S, Paul SM, Askendal A, VandeVondele S, Hubbell JA, Tengvall P, Textor M. Peptide functionalized poly (L-lysine)-g-poly(ethylene glycol) on titanium: resistance to protein adsorption in full heparinized human blood plasma. Biomaterials. 2003;24:4949–4958. doi: 10.1016/s0142-9612(03)00420-4. [DOI] [PubMed] [Google Scholar]
- 43.Haude M, Konorza TFM, Kalnins U, Erglis A, Saunamäki K, Glogar H, Grube E, Gil R, Serra A, Richardt HG, Sick P, Erbel R. Heparin-coated stent placement for the treatment of stenoses in small coronary arteries of symptomatic patients. Circulation. 2003;107:1265–1270. doi: 10.1161/01.cir.0000053442.64637.34. [DOI] [PubMed] [Google Scholar]
- 44.Frost MC, Reynolds MM, Meyerhoff ME. Polymers incorporating nitric oxide releasing/generating substances for improved biocompatibility of blood-contacting medical devices. Biomaterials. 2005;26:1685–1693. doi: 10.1016/j.biomaterials.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 45.Holmlin RE, Chen X, Chapman RG, Takayama S, Whitesides GM. Zwitterionic SAMs that resist nonspecific adsorption of protein from aqueous buffer. Langmuir. 2001;17:2841–2850. doi: 10.1021/la0015258. [DOI] [PubMed] [Google Scholar]
- 46.Tegoulia VA, Rao W, Kalambur AT, Rabolt JF, Cooper SL. Surface properties, fibrinogen adsorption, and cellular interactions of a novel phosphorylcholine-containing self-assembled monolayer on gold. Langmuir. 2001;17:4396–4404. [Google Scholar]
- 47.Chung YC, Chiu YH, Wu YW, Tao YT. Self-assembled biomimetic monolayers using phospholipid containing disulfides. Biomaterials. 2005;26:2313–2324. doi: 10.1016/j.biomaterials.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 48.Kim HK, Kim KM, Byun YR. Preparation of chemically anchored phospholipid monolayer on acrylated polymer substrate. Biomaterials. 2005;26:3435–3444. doi: 10.1016/j.biomaterials.2004.09.066. [DOI] [PubMed] [Google Scholar]
- 49.Galli M, Sommariva L, Prati F, Zerboni S, Politi A, Bonattie R, Mameli S, Butti E, Pagano A, Ferrari G. Acute and mid-term results of phosphorylcholine-coated stents in primary coronary stenting for acute myocardial infaction. Catheter Cardiovasc Interv. 2001;53:182–187. doi: 10.1002/ccd.1145. [DOI] [PubMed] [Google Scholar]
- 50.Morimoto N, Sato I, Watanabe A, Nakabayashi N, Iwasaki Y, Ishihara K. Coating stability and blood compatibility of stainless steel surfaces modified with phospholipid polymer. Kobunshi Ronbunshu. 2002;59:432–437. [Google Scholar]
- 51.Iwasaki Y, Saito N. Immobilization of phosphorylcholine polymers to Ti supported vinyldimethylsilyl monolayers and reduction of albumin adsorption. Colloids Surf B: Biointerfaces. 2003;32:77–84. [Google Scholar]
- 52.Kyomoto M, Iwasaki Y, Moro T, Konno T, Miyaji F, Kawaguchi H, Takatori Y, Nakamura K, Ishihara K. High lubricious surface of cobalt-chromium-molybdenum alloy prepared by grafting poly(2-methacryloyloxyethyl phosphorylcholine) Biomaterials. 2007;28:3121–3130. doi: 10.1016/j.biomaterials.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 53.Goodman SL. Sheep, pig, and human platelet-material interactions with model cardiovascular biomaterials. J Biomed Mater Res. 1999;45:240–250. doi: 10.1002/(sici)1097-4636(19990605)45:3<240::aid-jbm12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 54.Pelagalli A, Belisario MA, Tafuri S, Lombardi P, d’Angelo D, Avallone L, Staiano N. Adhesive properties of platelets from different animal species. J Comp Pathol. 2003;128:127–131. doi: 10.1053/jcpa.2002.0615. [DOI] [PubMed] [Google Scholar]