Abstract
Gene-environment interactions are the indisputable cause of most respiratory diseases. However, we still have very limited understanding of the mechanisms that guide these interactions. Although the conceptual approaches to environmental genomics were established several decades ago, the tools are only now available to better define the mechanisms that underlie the interactions among these important etiological features of lung disease. In this article, we summarize recent insights in the environmental genomics (ecogenomics) of common nonmalignant respiratory diseases (asthma, COPD, pulmonary fibrosis, and respiratory infections), describe the framework of gene-environment interactions that inform the pathogenesis of respiratory diseases, and propose future research directions that will help translate scientific advances into public health gains.
Keywords: asthma, COPD, pulmonary fibrosis, respiratory infection, gene-environment interactions
INTRODUCTION
Respiratory disease is common throughout the world, is increasing in prevalence, and has significant public health impact. For instance, airway disease, including asthma and chronic obstructive pulmonary disease (COPD), affects up to 15% of the U.S. adult population and leads annually to a combined total of >15,000,000 lost work days, 1,100,000 hospitalizations, and 120,000 deaths, at an estimated cost burden of $23 billion in the United States (63, 76). Fibrotic lung disease, including idiopathic pulmonary fibrosis, affects 5–30 persons per 100,000, although misdiagnoses and underreporting likely lead to underestimates of true prevalence. Additionally, incident respiratory infections in the United States are reported at 80–100 episodes per 100 persons annually (1). Identifying effective treatment and preventive strategies for these diseases could lead to substantial public health gains in increased life expectancy, improved quality of life, decreased resource utilization, and health care cost savings.
The lung is continuously exposed to the environment, and environmental injury is very common throughout the life of an individual. Approximately 11,000 liters of air move through the respiratory system daily, containing dust, fumes, microbes, aerosolized toxins, and pollutants. Therefore, environmental triggers have been long implicated as important factors in the etiology and pathogenesis of respiratory diseases. For example, investigators determined in the 1700s that grain dust is a cause of asthma, in the early 1900s that asbestos is a cause of lung cancer, and in the 1960s that cigarette smoke is a cause of emphysema. However, significantly variable individual susceptibility to environmental injury was quite obvious. For example, ~10% of grain workers develop asthma, <1% of asbestos-exposed workers develop mesothelioma, and 20% of cigarette smokers develop COPD. A likely explanation for this differing susceptibility is that host susceptibility factors interact with environmental triggers in the pathogenesis of respiratory disease. Many host (genetic) factors, which may contribute to the differing susceptibility between individuals, have been recently identified using genomic tools from a new discipline called environmental genomics or ecogenomics.
Ecogenomics characterizes the interaction between genetic variants and environmental factors that leads to the development of disease in some individuals and maintains a healthy, homeostatic equilibrium in others. The ultimate goals of ecogenomics are to identify subjects at risk for environmentally induced diseases, to characterize which environmental exposures lead to disease in susceptible individuals, to develop biomarkers for disease development, and to reduce the burden of environmentally induced disease using preventive and therapeutic measures based on scientific knowledge. Although the conceptual approaches to environmental genomics were established several decades ago, we are only now beginning to make significant scientific gains. In this review, we summarize recent ecogenomic insights into common respiratory diseases including asthma, COPD, pulmonary fibrosis, and respiratory infections. These diseases are of significant public health importance in both developed and developing countries, and considerable research has been devoted to their pathogenesis. We demonstrate the complexity of gene-environment interactions in these respiratory diseases and describe how not only DNA polymorphisms, but also environmentally induced nongenetic DNA modifications (epigenetics), contribute to this field. Epigenetic mechanisms offer a new paradigm in the pathogenesis of disease and introduce a dynamic concept of gene-environment interactions that can be altered in an individual’s lifetime and can affect the phenotype of the individual and also their offspring. Finally, using our summary of available knowledge and identification of scientific gaps, we suggest directions for future research.
EPIGENETICS
Epigenetics is the study of changes in gene transcription that are dependent on mechanisms other than DNA sequence changes (31, 36), including both heritable changes in gene expression and stable, long-term alterations in the transcriptional potential that are not necessarily heritable. Because epigenetic processes are highly interdependent and regulate gene expression in an age-, state-, cell-, and tissue-dependent manner, these mechanisms constitute a complex system of molecular controls that can affect human diseases.
Epigenetic regulation of the genome results in a hierarchy of transcriptional switches that facilitate development, differentiation, and normal tissue function, and influence the host response to stress (31, 36). Three primary mechanisms are known to govern gene expression: DNA methylation, noncoding RNAs, and histone modification. These epigenetic changes may be inherited independently of the sequence of DNA (Table 1). Hyper-methylation of CpG motifs, particularly at promoter and enhancer sites, silences gene transcription. Alternatively, hypomethylation of these motifs enhances gene transcription. Noncoding RNAs bind to DNA and interfere with transcription and posttranscriptional regulation of gene expression. Histones undergo >100 types of conserved, covalent posttranslational modifications (methylation, acetylation, or phosphorylation) that affect chromatin structure and alter gene expression. These mechanisms are conserved in eukaryotic organisms, from yeast to humans, and regulate the transcriptional activity of specific genes, at specific stages of development, and in response to specific endogenous and exogenous stressors.
Table 1.
Known epigenetic mechanisms
| Mechanism | Description |
|---|---|
| DNA methylation | The methyl group is transferred from S-adenosylmethionine to the C-5 position of cytosine by a cytosine-methyltransferase. Hypermethylation of CpG motifs, particularly at promoter and enhancer sites, silences gene transcription. Alternatively, hypomethylation of these motifs enhances gene transcription. |
| Noncoding RNAs (also known as microRNAs) | MicroRNAs bind to DNA and interfere with transcription and posttranscriptional regulation of gene expression. |
| Histone modification | Histones, the building blocks of nucleosomes, undergo >100 conserved, covalent posttranslational modifications (methylation, acetylation, or phosphorylation) that regulate chromatin structure and gene expression. |
Although epigenetic marks (e.g., changes such as methylation and histone modification) can be inherited, they can also be modified throughout development (35) and by the environment (4). For instance, although monozygotic twins are genetically identical, twin pairs often differ in features as well as disease outcomes. We now know that early in life, monozygotic twins are epigenetically identical, whereas older twin pairs have divergent epigenetic marks that are associated with differences in gene expression (35). These findings suggest that life events such as environmental exposures can alter epigenetic marks that may account, in part, for phenotypic differences in twin pairs. In aggregate, these findings suggest that the epigenome can be reprogrammed, potentially affecting the risk, etiology, and treatment of various disease states.
ASTHMA
Asthma is a complex, heritable disease that is increasing in prevalence, incidence, and severity, particularly in developed countries (24, 27). It affects 11.2% of the U.S. population and accounts for $10 billion in direct health care costs (27), as well as 12.8 million missed days of school for children and 10 million missed days of work for adults annually (73). Gender and ethnic differences exist for women and black asthmatics, both having a significantly higher rate of outpatient asthma visits, emergency room evaluations, hospitalizations, and mortality than non-Hispanic Caucasian males (73). Moreover, asthma has a much higher prevalence and incidence in developed versus developing countries (24), which is increasing despite intensive research into its pathobiology, genetics, and treatment.
Asthma is a familial condition, with estimates of heritability ranging from 36% to 79%. To date, 10 genome-wide linkage and association studies have been completed in families with asthma or asthma-related disorders; all but 4 chromosomes (X, 10, 18, and 22) have been implicated in the development of the disease (86). Although there are some consistent results between different genome-wide screens, there are many more discrepancies. Genes for which association has been reported include ADBR2, HLA, TNFA, CD14, IL13, LTA, VDR, STAT6, NOS, ADAM33, and ORMDL3 (86). Major, large-effect susceptibility genes for asthma have not been definitively identified (86), and replications in separate study populations are lacking. We and others believe that these inconsistencies result from the complex clinical phenotype of asthma and consequent heterogeneity within the study populations, a polygenic pattern of inheritance, the substantial role of environmental exposures in the development and progression of asthma, and the possibility that epigenetic mechanisms play an important role (46).
Many studies have shown that the risk of transmission of atopic disease from an affected mother is approximately four times higher than from an affected father (66). Similar parent-of-origin effects have been noted in other immunological diseases, including type I diabetes (90), rheumatoid arthritis (55), and inflammatory bowel disease (2). These effects may result from immune interactions between the fetus and the mother, which take place through the placenta and through breast milk (47). Alternatively, the maternal effect may result from genomic imprinting (31). Several known genes show parent-of-origin effects on allergic disease, such as the FcεRI-βlocus on chromosome 11q13 (84) and the SPINK5 gene on chromosome 5q34 (88).
Environmental factors are important in the pathogenesis of asthma, both through direct effects and indirectly through complex interactions with gene variants (16). Demographic factors of age, race, and socioeconomic status are risk factors for development and progression of asthma (27). However, dramatic increases in the prevalence, incidence, and severity of asthma suggest that diet, aeroallergens, smoking behavior, agents in the workplace, indoor and outdoor air pollution, viruses, domestic and occupational exposure to endotoxins, and immunization against certain infectious diseases play particularly important roles in etiology and pathogenesis of this condition (87); these increases have occurred too rapidly to be accounted for by changes in primary DNA sequence alone. Importantly, epigenetic mechanisms are influenced by environmental exposures (4, 35), providing a vital interface between biology and environment.
Several investigators have addressed the interaction of genetic factors with cigarette smoke in development and exacerbation of asthma. A recent study identified an association among asthma symptoms, cigarette smoke exposure, and single nucleotide polymorphisms (SNPs) in several xenobiotic enzymes (EPHX1, CYP1B1, and CYP2D6) and demonstrated that specific patterns of allelic correlations among these genes were associated with an especially high risk for the development of asthma, suggesting that epistatic interactions can affect the metabolism of environmental toxicants and modulate exposure levels and asthmatic exacerbations (74). This study is a good example of gene-gene and gene-environment interactions as disease modifiers. Several other studies have reported positive associations between SNPs in asthma-related genes such as GSTP1, GSTM1, TGF-beta1, CD14, IL13, ADRB2, or IL1RN and spirometric or symptomatic decline in response to environmental cigarette smoke exposure (59). However, the effects were generally limited (OR 1.1–1.5), and the number of cases was low (a few hundred subjects per study). London & Romieu (59) concluded that no definitive associations can be inferred from these studies. As the number of studied individuals grows, such interactions may become more evident. More importantly, perhaps, improved analytical methodology will help recognize gene-gene and gene-environment interactions. For example, current genome-wide association study (GWAS) chips were estimated to cover only 60% of the genome (42). Additionally, better statistical and computational methods may identify genetic networks that lead to the development of asthma and thus uncover epistatic effects that are undetected in single-gene analyses, which are standard in published reports (93).
In utero exposures are important risk factors for the development of asthma (27). Although prenatal exposure to diesel exhaust particles and environmental tobacco smoke (ETS) are associated with increased risk of asthma, maternal ingestion of fruits, vegetables, and oily fish appears to be protective (16, 34). Gestational exposure to an environment rich in microbial compounds protects against the development of atopy and appears to downregulate expression of toll-like receptors (TLRs) (28). For ETS, the risk of developing asthma is further increased by 17q21 genetic variants (16). These associations suggest that fetal development and possibly preconception represent periods of vulnerability that affect T cell development and maturation, possibly via epigenetic marks (68). This possibility is supported by effects of maternal nutritional stress, tobacco smoke, endocrine disruptors (bisphenol A and diethstilbestrol), and diesel fumes on epigenetic mechanisms, including DNA methylation and chromatin modifications (25, 65). Interestingly, Li et al. (57) reported transgenerational association of a grandmother’s smoking with her grandchildren’s risk for asthma. Moreover, because epigenetic marks can change postnatally (35), they can be affected by environmental exposures, diet, comorbidities, or even stochastic events.
Epigenetic mechanisms, as a cause of asthma, build on our current knowledge about asthma (non-Mendelian and parent-of-origin patterns of inheritance, environment, and in utero exposures) and provide an entirely novel paradigm for this disease. Although the prevailing hygiene hypothesis suggests that early post-natal exposures to microbial pathogens shape the immune system toward a Th1/Treg, nonallergic phenotype (27), after more than a decade of research the basic mechanisms underlying this immune switch have not been identified. Moreover, the hygiene hypothesis falls short of explaining the increasing prevalence, incidence, and severity of asthma observed over the past two decades in the United States (72). The novel hypothesis, that epigenetic mechanisms play a fundamental role in the etiology of asthma, provides a provocative new direction for asthma research that is mechanistically based and may have important public health implications.
We have recently demonstrated that in utero supplementation with methyl donors alters locus-specific DNA methylation and predisposes mice to allergic airway disease by directing the differentiation of T lymphocytes, with a skewing toward a Th2 phenotype; 82 distinct loci out of several thousand were differentially methylated when compared with control mice, each representing a potential candidate gene for allergic airway disease (46). This epigenetically controlled phenotype could be reversed with demethylating agents, consistent with epigenetic plasticity. Thus, in a mouse model, in utero dietary factors can modify the heritable risk of allergic airway disease during a vulnerable period of fetal development through epigenetic regulation that may, in fact, be reversible. Our results suggest that the period of in utero vulnerability or the postnatal reversibility of these methylation marks may provide opportunities for intervention. Our findings are supported by a recently published study examining a birth cohort of 32,077 children in whom perinatal folic acid supplements were associated with an increased risk of wheezing at 18 months of age (41). Other environmental exposures resulting in epigenetic marks may contribute to the development of asthma, including tobacco smoke, another in utero exposure associated with childhood asthma that can modify gene expression through DNA hypermethylation (57).
Our research (46), and others’ (41), suggests that too much dietary supplementation, especially with methyl donors during pregnancy, may have unexpected biological and pathophysiological consequences, at least in the mouse. However, given the demonstrated benefit of folate (a methyl donor) supplementation in preventing neural tube congenital abnormalities and the potential differences in murine and human biology with regard to adverse consequences of dietary supplementation during pregnancy, we must be cautious in considering any modifications to current recommendations for folate supplementation. Understanding the complex interactions between in utero exposures and epigenetic vulnerability in both species will provide insight into future interventions for individuals at risk of developing allergic asthma.
Epigenetics has the potential to transform asthma therapy from palliative to preventive and may alter our recommendations for pregnant women worldwide. Currently, other than avoidance of triggers, we are simply unable to prevent asthma. Most patients with asthma rely on chronic medications to reduce the frequency and severity of their symptoms. Understanding the importance of epigenetic mechanisms in the development of asthma and the periods of vulnerability in establishing epigenetic marks has the potential to prevent the development of this disease, not only in our offspring but in their children as well.
COPD
COPD is a clinical syndrome comprised of chronic bronchitis and emphysema. Most, but not all, COPD cases in the developed world are attributed to cigarette smoke. In contrast, a significant proportion of COPD cases worldwide are thought to be caused by inhalational exposure to incomplete combustion products from biomass fuel in stoves and open fires used for heating and cooking.
As mentioned earlier, only a minority of individuals develop cigarette smoking–induced COPD, which suggests a gene-environment interaction. As in most environmentally induced diseases, there is likely a dose-response continuum that dictates the genetic or environmental contribution to the development of the phenotype. In the extremes, certain genetic characteristics, such as alpha-1-antitrypsin (AAT) deficiency, or extreme environmental exposure to pollution may be sufficient to induce COPD independent of other factors. In the overwhelming majority of cases, however, genetic susceptibility supplements a correspondingly appropriate degree of environmental exposure to induce COPD. Even in patients with extreme AAT deficiency, cigarette smoking is an independent risk factor for severe COPD (23). In fact, COPD is a disease in which environmental exposures may dominate the disease presentation. For example, in the Lung Health Study, sustained smoking cessation was the most significant determinant of lung function over a period of 14 years (3), suggesting that individual host factors may be less important. However, evidence of genetic susceptibility to COPD does exist. For example, some studies have shown an association of SNPs in the inflammatory genes TNF-α and TGF-β; the antioxidant genes GSTM1, GSTP1, and HMOX-1; and the xenobiotic metabolizing enzyme gene EPHX1 with COPD severity and the rate of lung function decline in smokers with COPD, although many of these findings could not be replicated (21).
Genes that predispose individuals to addictive behavior have also been studied in relation to COPD susceptibility. Indeed, smokers exhibit significant addictive behavior. Sustained smoking cessation is observed in only 15%–30% of all smokers (75). Recent research suggests there is substantial variability in the genetic predisposition to smoking addiction. Because smoking cessation is the single most powerful intervention affecting the rate of lung function decline in COPD, it is important to identify individuals who may more readily respond to cessation counseling as compared with those who would require more intensive treatment and follow-up. Indeed, polymorphisms in nicotine-metabolizing cytochrome P450 enzymes (mainly CYP2A6), in nicotine receptors, and in genes of the dopamine and serotonin pathways (involved in nicotine reward effects in the brain) may be associated with smoking addiction and the likelihood to stop smoking (75). More studies are needed to confirm these findings of notable gene-environment interactions, especially because they involve behavioral patterns that may affect environmental exposures.
Epigenetic modifications have been investigated in COPD. Decreased activity of the histone deacetylase HDAC2 in distal lung segments and alveolar macrophages of COPD patients may lead to a proinflammatory milieu, which promotes the progression of disease (8, 9, 51). Decreased HDAC2 activity may also lead to corticosteroid resistance, which is a hallmark of COPD. The decreased HDAC2 activity may be attributable to increased oxidative stress in COPD lungs caused by smoking or pollutant exposure. This line of research elegantly demonstrates the epigenetic pathways through which environmental exposures may modify the host response to injury by influencing gene expression. The research suggests treatment options. Theophylline, a drug that has been used in COPD patients for many years, apparently reverses the HDAC2 decrease. More potent interventions may be promising in this generally recalcitrant disease.
PULMONARY FIBROSIS
Pulmonary fibrosis, or interstitial lung disease (ILD), can be conceptualized as the pathological healing response to a spatially and temporally heterogeneous lung injury (37). Several lines of evidence suggest that development of ILD is at least partly determined by genetic factors. Inbred strains of mice demonstrate variable susceptibility to fibrogenic agents (70, 91). Considerable variability exists in the development of pulmonary fibrosis among workers exposed to similar concentrations of fibrogenic dusts or organic antigens. ILD has been observed in individuals with genetic disorders, including Hermansky-Pudlak syndrome, neurofibromatosis, Niemann-Pick disease (26), and dyskeratosis congenita (5). Furthermore, pulmonary fibrosis has been reported in closely related family members, including monozygotic twins raised in different environments, genetically related members of several families, and family members separated at an early age. Most pedigrees are compatible with autosomal dominant inheritance with reduced penetrance.
We recently reported 111 families with 2 or more cases of ILD. Familial interstitial pneumonia (FIP) appears to comprise several subtypes of ILD caused by an interaction between cigarette smoke and predisposing genes (82). The only published linkage study of FIP pointed to a putative candidate gene, ELMOD2, on chromosome 4 (45). Mutations in genes that maintain telomere length (TERT and TERC) are associated with development of FIP (5, 85) and sporadic idiopathic pulmonary fibrosis (IPF) (85). Families with ILD in multiple generations had mutations in surfactant protein-C (SFTPC) (69, 83). Missense mutations in surfactant protein A2 (SFTPA2) were detected in two of 59 FIP kindreds (89) who presented with early-onset pulmonary fibrosis and/or lung cancer.
All these above-mentioned mutations account for <10% of all FIP and sporadic IPF cases. Our preliminary linkage study and others (45) have identified additional regions on chromosomes 4 (4q31), 10 (10p13–14), 11 (11p15.4–15.5), and 12 (12p11.2–q14.1) that likely contain genes contributing to familial forms of ILD. Thus, FIP/ILD may be caused by multiple genetic changes (TERT, TERC, SFTPC, SFTPA2, and genes within loci on chromosomes 4, 10, 11, and 12), but we have identified only small proportions of potentially relevant genes. In our FIP cohort, 40% of families exhibited multiple types of ILD (82); likewise, in several genetic studies, a specific variant or locus may be associated with different ILD phenotypes within the same family (5, 69, 83, 85). In sum, different forms of ILD may be related by genetic predisposition, and environmental factors influence the distinct clinical phenotype in each patient through direct action or through epigenetic modifications of the host genome.
IPF is more frequent in cigarette smokers (11, 80). In FIP, smoking was the strongest risk factor for development of interstitial pneumonia with an odds ratio (OR) of 3.6 (82). Epidemiological studies have established a consistent dose-related association between metal and wood dust exposure and IPF in the United States (10), Britain (50), and Japan (52). Occupational exposure to asbestos can cause ILD that is indistinguishable from the histology of IPF (17); both cigarette smoking (79) and gene variants (22) increase the risk of asbestosis.
RESPIRATORY INFECTIONS
The lung is constantly exposed to infectious agents, yet the distal lung is, to the best of our knowledge, sterile. The ability to specifically and efficiently eradicate noxious microorganisms is therefore of paramount importance to the preservation of lung function. Susceptibility of the lung to respiratory infections may result from genetically induced alterations in lung anatomy or physiology, which make the lung vulnerable to infections (e.g., cystic fibrosis), or from genetic susceptibility to infections, either global (immunoglobulin deficiencies) or specific to particular pathogens. This section focuses on the latter pattern.
Genetic susceptibility to infectious diseases has garnered much scientific attention and has been reviewed extensively elsewhere (64). Here, we focus on three common respiratory infections with public health relevance: community-acquired pneumonia (CAP), viral infections causing significant respiratory disease [respiratory syncytial virus (RSV) and coronaviruses/severe acute respiratory syndrome (SARS)], and tuberculosis (TB). We discuss only strongly supported genetic susceptibility factors for these diseases.
CAP remains a major cause of morbidity and mortality throughout the world despite the advent of antibiotics (49), suggesting that host factors play significant roles in the susceptibility and course of this disease. Coronavirus infections that led to severe pneumonia caused the very disruptive SARS pandemic characterized by severe lung injury and high mortality. RSV infects virtually all infants by the age of 2 years, is the most common cause for hospitalization related to lower respiratory tract infections in infants younger than 1 year of age, and causes ~1 million deaths worldwide annually (48). Finally, TB is one of the most common causes of death worldwide (60). TB often affects individuals in developing countries with scant resources. Identification of resistance or susceptibility factors could help in the allocation of resources and efforts to fight this disease. Genetic susceptibility to TB has been demonstrated in several studies (12).
Infections occupy a unique position in the study of environmentally induced lung diseases because host-pathogen interactions are the defining factor. Pathogen genetics may affect disease development as much as host genetics does. Genetic susceptibility to infections may also be unusual because monogenic defects, rather than complex polygenic or multifactorial traits, may account for a significant portion of the genetic susceptibility to infections (18). For example, a recent GWAS investigating HIV-1 susceptibility identified two independent groups of polymorphisms, associated with HLA loci B and C, that explain ~15% of the total variation in HIV-1 susceptibility (32). Ultimately, several large GWAS analyses will be needed to begin to clarify the contributions of monogenic versus polygenic effects in infectious disease susceptibility (43).
Given the biology of infections, most identified susceptibility genes are part of either the innate immune or the adaptive immune system. Innate immunity is particularly important in the response to invading microorganisms. The innate immune system recognizes common and conserved microbial antigens, called pathogen-associated molecular patterns (PAMPs), through pattern-recognition receptors such as TLRs. TLRs have relatively broad yet specific recognition patterns; for example, TLR2 recognizes lipoteichoic acid and peptidoglycans on Gram-positive bacterial or fungal walls, and TLR4 recognizes the Gram-negative wall component endotoxin. After microbial antigens have been engaged by membrane-bound TLRs, cytoplasmic adaptor molecules such as myeloid differentiation factor 88 (MyD88) and toll-interleukin 1 receptor domain-containing adaptor protein (TIRAP) mediate downstream effects that result in cellular activation, cytokine release, and microbiocidal activity. Importantly, innate immune activation is now understood to modulate adaptive immunity as well, thus providing both the initial response to infection and the follow-up cues to further immune activity (71). Innate immune gene polymorphisms are frequently found as susceptibility factors in respiratory infections. The hyporesponsive TLR4 polymorphisms Asp299Gly and Thr399Ile, as well as CD14 SNPs leading to higher circulating levels of CD14, have been associated with increased mortality from sepsis (39, 62). The same TLR4 SNPs were associated with increased susceptibility to severe RSV infection in high-risk (premature and congenital heart disease) infants (6); this study established a risk synergy between a genetic factor (TLR4 SNPs) and nongenetic comorbidity (prematurity), highlighting the complex endogenous and exogenous factors that define susceptibility to infections.
A recent study of bacterial and host genetic influences in the dissemination of TB (20) demonstrated that East Asian isolates of Mycobacterium tuberculosis were more likely to cause disseminated disease than were European isolates and that carriers of a TLR2 polymorphism were more susceptible to the East Asian strain. TLR activation in macrophages leads to increased di-hydroxy-vitamin D production, which exhibits potent immuno-modulating actions (15). Indeed, investigators have associated SNPs in the vitamin D receptor gene VDR with increased susceptibility to severe RSV (53) and TB (14, 94) infections. Some innate immune genetic polymorphisms have been associated with resistance to TB disease. TIRAP is an innate immune protein that transduces TLR2 and TLR4 stimuli. The S180L TIRAP polymorphism construct leads to reduced response to TLR2 and TLR4 activation in vitro when compared with wild-type TIRAP. This polymorphism was associated with a reduced OR for developing TB and systemic lupus erythematosus in case-control and family-based association studies (19, 54).
Circulating innate immune factors are also important in disease susceptibility. Mannose-binding lectin (MBL) is a circulating innate immune protein whose serum levels are genetically determined. Several studies found an association of genetically induced MBL deficiency with invasive pneumococcal disease (78), severe or lethal pneumococcal pneumonia (38), or risk for bacterial/viral coinfections (30), whereas other studies found limited (40) or no effect (56). These negative results may be due to limited case numbers, however, because a meta-analysis indicates that MBL deficiency confers a >5-fold risk of death from invasive pneumococcal disease (29). Several other studies reported associations of HLA and MBL SNPs with susceptibility to SARS (58, 67, 99), although these studies were not supported by others (96). MBL effects are not necessarily unidirectional: For example, MBL-deficient polymorphisms appear to confer protection from TB (44, 81).
Innate immune genes are not alone in determining susceptibility to respiratory infections. Several immune modifying or effector genes have been associated with susceptibility. Interferons modify lymphocyte and macrophage activity; known associations exist between SNPs in interferon-alpha and RSV susceptibility (53) and interferon-gamma and TB susceptibility (61, 77).
The coagulation system is increasingly recognized for its importance in the response to infections. Plasminogen activator inhibitor (PAI-1) polymorphisms can affect susceptibility to CAP both positively and negatively. Genotypes associated with increased expression of PAI-1 were associated with increased susceptibility to CAP in elderly Caucasians (97). The coagulation system probably plays a role in nonbacterial infections as well; plasminogen alleles influenced susceptibility to invasive aspergillosis in immune-compromised patients (98).
Finally, a number of SNPs in other effector genes (e.g., heat-shock protein HSP70 and lymphotoxin alpha/LTA) or HLA genes have been associated with susceptibility to severe CAP (92) or TB (7). Further associations are certainly forthcoming. A large GWAS in West and South Africans using affected sib-pairs used linkage and microsatellite mapping to identify two regions of the genome on chromosomes 15q and Xq with suggestive TB susceptibility genes (13), but no specific genes were identified. Certain other genetic traits may not affect susceptibility to TB but may modify the course of disease. A polymorphism in the adenosine receptor P2X7 gene increased susceptibility to disseminated TB (33).
In conclusion, potential public health contributions of ecogenomics in respiratory infections cannot be overestimated. Identification of individuals who are at risk or who may be protected from certain infectious diseases could help focus preventive measures, such as administration of influenza vaccines during times of shortage, and it could allow us to stratify protective or isolation measures depending on susceptibility in cases of pandemics. Additionally, identification of at-risk individuals may help inform treatment decisions, and it could also help identify medical workers who should preferentially be deployed versus those who should be held back during epidemics such as SARS, which caused significant morbidity and mortality among medical personnel (95).
THE PATH FORWARD
When the Human Genome Project reported the completed human genome sequence in 2003, many predicted the dawn of a new era in medicine—an era when every disease would be explained by its genetic determinants and eventually cured by genetic manipulation. Unfortunately, this promise seems distant. In hindsight, our approach to genetic associations of disease was too simplistic. In fact, gene-environment interactions are infinitely more complex, as illustrated by the pathogenesis of respiratory disease.
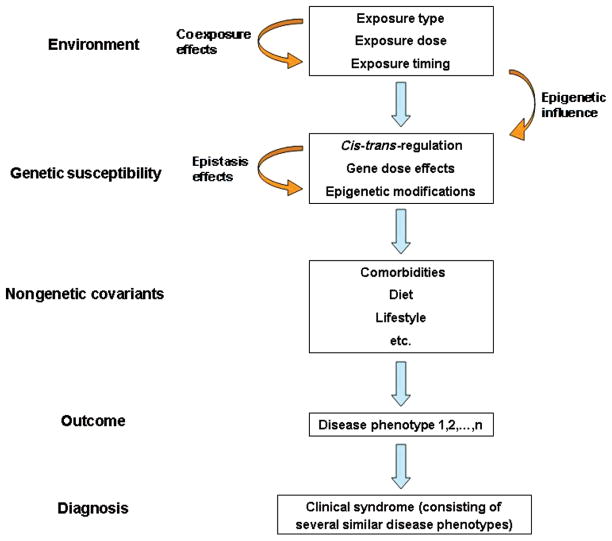
We now recognize several reasons for the lack of clear gene-disease associations in respiratory diseases (Figure 1). First, many of these diseases are phenotypic syndromes rather than distinct pathological entities. For example, asthma is defined by physiologic criteria and COPD by clinical and radiographic criteria. The diagnosis of these diseases surely encompasses several distinct pathophysiologic entities, which each contribute a minority/fraction of the total patient population. Second, these diseases likely result from complex traits; i.e., the simultaneous presence of multiple gene variants is necessary for the development of disease. These gene-gene interactions, called epistasis, may play a substantial role in the development of complex traits (93). Third, several types of genetic variations, such as copy number variation, cis-acting (intragene) versus trans-acting (extragene) regulatory variants, and noncoding DNA effects, may simultaneously affect genetic susceptibility, although this is currently incompletely understood. Fourth, environmental exposures in themselves can alter the genetic profiles of subjects and their offspring via epigenetic and mutational mechanisms. Fifth, nongenetic factors, such as comorbidities, lifestyle choices, diet, and exercise, may confound the gene-environment interaction. Finally, environmental exposures are never uniform or isolated. Duration, timing, and coexposures may alter the burden on a susceptible individual and thus ultimately affect disease presentation and severity. All these factors make it evident that our approach to ecogenomics must be complex and integrated.
Figure 1.
Gene-environment interaction complexity in the pathogenesis of respiratory diseases. Environmental exposures are the triggers that induce an individual response, based on genetic susceptibility factors. Coexposures and exposure characteristics modify the nature of the trigger, but environmental factors can also affect the genetic susceptibility through epigenetic mechanisms. Genetic susceptibility factors are the main determinants of the host response to an environmental trigger. Several genetic factors may be mutually interacting (epistasis), and gene expression can also be affected by environment–induced epigenetics. Nongenetic covariants may also alter the ultimate disease phenotype (outcome). Several clinically similar disease phenotypes may be included in a disease syndrome, which constitutes the final diagnosis.
Until now, our approach to gene-environment interactions has been focused predominantly on the identification of single gene polymorphisms. We need new tools for precise and thorough detection of environmental exposures, including development of portable exposure sensors and utilization of biological exposure indicators based on transcriptomics, proteomics, and metabolomics. The assembly of large patient cohorts will be necessary to identify relatively small genetic traits above the statistical background noise. We must expand the use of comparative genomics and utilize the study of gene-environment interactions in model organisms to focus our planning and design of human studies. Finally, new statistical, analytical, and computational tools will be needed to detect interactions among genes, epigenetics, and environmental exposures in their full complexity. Clearly this approach will require an integration of scientific disciplines: Collaboration among physicians, geneticists, engineers, epidemiologists, biologists, statisticians, and computer scientists will be necessary to establish the network needed for effective design, implementation, and interpretation of the studies that examine the combined environmental and genetic causes of complex lung diseases.
SUMMARY POINTS.
Gene-environmental interactions play a central role in all common respiratory diseases.
Environmental genomics or ecogenomics is the discipline investigating gene-environment interactions in disease pathogenesis.
Several modalities must be integrated for best utilization of ecogenomics: comprehensive genetic analysis, comprehensive longitudinal characterization of environmental stimuli, statistical and computational modeling of interactions, and use of animal models.
Public health gains from ecogenomic approaches to lung disease are substantial and include risk stratification, early recognition of disease, and disease prevention.
Glossary
- Asthma
airway disease characterized by inflammation, mucus production, smooth muscle hypertrophy, and variable obstruction
- Chronic obstructive pulmonary disease (COPD)
airway disease characterized by chronic bronchitis and/or emphysema and fixed obstruction
- Ecogenomics
a discipline that characterizes the interaction between genetic variants and environmental factors that lead to the development of disease
- Epigenetics
potentially heritable alterations in gene expression caused by mechanisms other than DNA sequence changes
- HLA
human leukocyte antigen
- SNP
single nucleotide polymorphism
- Genome-wide association study (GWAS)
gene-disease association studies, which use whole-genome single nucleotide polymorphism analysis and association of polymorphisms with disease in large subject cohorts
- TLR
toll-like receptor
- Interstitial lung disease (ILD)
parenchymal lung disease characterized by progressive scarring of alveoli and respiratory failure
- IPF
idiopathic pulmonary fibrosis
- CAP
community-acquired pneumonia
- SARS
severe acute respiratory syndrome
- TB
tuberculosis
- Innate immunity
part of the immune system that specifically recognizes conserved pathogen motifs and generates an immediate immune reaction without prior sensitization
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Stavros Garantziotis, Email: Garantziotis@niehs.nih.gov.
David A. Schwartz, Email: SchwartzD@NJHealth.org.
LITERATURE CITED
- 1.Adams PF, Hendershot GE, Marano MA. Current estimates from the National Health Interview Survey, 1996. Vital Health Stat. 1999;10:1–203. [PubMed] [Google Scholar]
- 2.Akolkar PN, Gulwani-Akolkar B, Heresbach D, Lin XY, Fisher S, et al. Differences in risk of Crohn’s disease in offspring of mothers and fathers with inflammatory bowel disease. Am J Gastroenterol. 1997;92:2241–44. [PubMed] [Google Scholar]
- 3.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Int Med. 2005;142:233–39. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- 4.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–69. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–26. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 6.Awomoyi AA, Rallabhandi P, Pollin TI, Lorenz E, Sztein MB, et al. Association of TLR4 polymorphisms with symptomatic respiratory syncytial virus infection in high-risk infants and young children. J Immunol. 2007;179:3171–77. doi: 10.4049/jimmunol.179.5.3171. [DOI] [PubMed] [Google Scholar]
- 7.Balamurugan A, Sharma SK, Mehra NK. Human leukocyte antigen class I supertypes influence susceptibility and severity of tuberculosis. J Infect Dis. 2004;189:805–11. doi: 10.1086/381689. [DOI] [PubMed] [Google Scholar]
- 8.Barnes PJ. Role of HDAC2 in the pathophysiology of COPD. Annu Rev Physiol. 2009;71:451–64. doi: 10.1146/annurev.physiol.010908.163257. [DOI] [PubMed] [Google Scholar]
- 9.Barnes PJ, Ito K, Adcock IM. Corticosteroid resistance in chronic obstructive pulmonary disease: inactivation of histone deacetylase. Lancet. 2004;363:731–33. doi: 10.1016/S0140-6736(04)15650-X. [DOI] [PubMed] [Google Scholar]
- 10.Baumgartner KB, Samet JM, Coultas DB, Stidley CA, Hunt WC, et al. Occupational and environmental risk factors for idiopathic pulmonary fibrosis: a multicenter case-control study. Collaborating Centers. Am J Epidemiol. 2000;152:307–15. doi: 10.1093/aje/152.4.307. [DOI] [PubMed] [Google Scholar]
- 11.Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1997;155:242–48. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- 12.Bellamy R. Genetic susceptibility to tuberculosis. Clin Chest Med. 2005;26:233–46. vi. doi: 10.1016/j.ccm.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Bellamy R, Beyers N, McAdam KP, Ruwende C, Gie R, et al. Genetic susceptibility to tuberculosis in Africans: a genome-wide scan. Proc Natl Acad Sci USA. 2000;97:8005–9. doi: 10.1073/pnas.140201897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellamy R, Ruwende C, Corrah T, McAdam KP, Thursz M, et al. Tuberculosis and chronic hepatitis B virus infection in Africans and variation in the vitamin D receptor gene. J Infect Dis. 1999;179:721–24. doi: 10.1086/314614. [DOI] [PubMed] [Google Scholar]
- 15.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94:26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouzigon E, Corda E, Aschard H, Dizier MH, Boland A, et al. Effect of 17q21 variants and smoking exposure in early-onset asthma. N Engl J Med. 2008;359:1985–94. doi: 10.1056/NEJMoa0806604. [DOI] [PubMed] [Google Scholar]
- 17.Brody A. Asbestos-induced lung disease. Environ Health Perspect. 1993;100:21–30. doi: 10.1289/ehp.9310021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casanova JL, Abel L. Inborn errors of immunity to infection: the rule rather than the exception. J Exp Med. 2005;202:197–201. doi: 10.1084/jem.20050854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castiblanco J, Varela DC, Castano-Rodriguez N, Rojas-Villarraga A, Hincapie ME, Anaya JM. TIRAP (MAL) S180L polymorphism is a common protective factor against developing tuberculosis and systemic lupus erythematosus. Infect Genet Evol. 2008;8:541–44. doi: 10.1016/j.meegid.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Caws M, Thwaites G, Dunstan S, Hawn TR, Lan NT, et al. The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog. 2008;4:e1000034. doi: 10.1371/journal.ppat.1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cookson WO. State of the art. Genetics and genomics of chronic obstructive pulmonary disease. Proc Am Thoracic Soc. 2006;3:473–75. doi: 10.1513/pats.200603-036MS. [DOI] [PubMed] [Google Scholar]
- 22.Corsini E, Luster MI, Mahler J, Craig WA, Blazka ME, Rosenthal GJ. A protective role for T lymphocytes in asbestos-induced pulmonary inflammation and collagen deposition. Am J Respir Cell Mol Biol. 1994;11:531–39. doi: 10.1165/ajrcmb.11.5.7946383. [DOI] [PubMed] [Google Scholar]
- 23.Demeo DL, Sandhaus RA, Barker AF, Brantly ML, Eden E, et al. Determinants of airflow obstruction in severe alpha-1-antitrypsin deficiency. Thorax. 2007;62:806–13. doi: 10.1136/thx.2006.075846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devereux G. The increase in the prevalence of asthma and allergy: food for thought. Nat Rev. 2006;6:869–74. doi: 10.1038/nri1958. [DOI] [PubMed] [Google Scholar]
- 25.Dolinoy DC, Weidman JR, Jirtle RL. Epigenetic gene regulation: linking early developmental environment to adult disease. Reprod Toxicol. 2007;23:297–307. doi: 10.1016/j.reprotox.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 26.du Bois RM. Genetic factors in pulmonary fibrotic disorders. Semin Respir Crit Care Med. 2006;27:581–88. doi: 10.1055/s-2006-957330. [DOI] [PubMed] [Google Scholar]
- 27.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–35. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 28.Ege MJ, Bieli C, Frei R, van Strien RT, Riedler J, et al. Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. J Allergy Clin Immunol. 2006;117:817–23. doi: 10.1016/j.jaci.2005.12.1307. [DOI] [PubMed] [Google Scholar]
- 29.Eisen DP, Dean MM, Boermeester MA, Fidler KJ, Gordon AC, et al. Low serum mannose-binding lectin level increases the risk of death due to pneumococcal infection. Clin Infect Dis. 2008;47:510–16. doi: 10.1086/590006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Endeman H, Herpers BL, de Jong BA, Voorn GP, Grutters JC, et al. Mannose-binding lectin genotypes in susceptibility to community-acquired pneumonia. Chest. 2008;134:1135–40. doi: 10.1378/chest.08-0642. [DOI] [PubMed] [Google Scholar]
- 31.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4:143–53. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 32.Fellay J, Shianna KV, Ge D, Colombo S, Ledergerber B, et al. A whole-genome association study of major determinants for host control of HIV-1. Science. 2007;317:944–47. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernando SL, Saunders BM, Sluyter R, Skarratt KK, Goldberg H, et al. A polymorphism in the P2X7 gene increases susceptibility to extrapulmonary tuberculosis. Am J Respir Crit Care Med. 2007;175:360–66. doi: 10.1164/rccm.200607-970OC. [DOI] [PubMed] [Google Scholar]
- 34.Fitzsimon N, Fallon U, O’Mahony D, Loftus BG, Bury G, et al. Mothers’ dietary patterns during pregnancy and risk of asthma symptoms in children at 3 years. Irish Med J. 2007;100(Suppl):27–32. [PubMed] [Google Scholar]
- 35.Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci USA. 2005;102:10604–9. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gal-Yam EN, Saito Y, Egger G, Jones PA. Cancer epigenetics: modifications, screening, and therapy. Annu Rev Med. 2007;59:267–80. doi: 10.1146/annurev.med.59.061606.095816. [DOI] [PubMed] [Google Scholar]
- 37.Garantziotis S, Steele MP, Schwartz DA. Pulmonary fibrosis: thinking outside of the lung. J Clin Invest. 2004;114:319–21. doi: 10.1172/JCI22497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-Laorden MI, Sole-Violan J, Rodriguez de Castro F, Aspa J, Briones ML, et al. Mannose-binding lectin and mannose-binding lectin-associated serine protease 2 in susceptibility, severity, and outcome of pneumonia in adults. J Allergy Clin Immunol. 2008;122:368–74. 374, e1–2. doi: 10.1016/j.jaci.2008.05.037. [DOI] [PubMed] [Google Scholar]
- 39.Gibot S, Cariou A, Drouet L, Rossignol M, Ripoll L. Association between a genomic polymorphism within the CD14 locus and septic shock susceptibility and mortality rate. Crit Care Med. 2002;30:969–73. doi: 10.1097/00003246-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Gomi K, Tokue Y, Kobayashi T, Takahashi H, Watanabe A, et al. Mannose-binding lectin gene polymorphism is a modulating factor in repeated respiratory infections. Chest. 2004;126:95–99. doi: 10.1378/chest.126.1.95. [DOI] [PubMed] [Google Scholar]
- 41.Haberg SE, London SJ, Stigum H, Nafstad P, Nystad W. Folic acid supplements in pregnancy and early childhood respiratory health. Arch Dis Child. 2009;94:180–84. doi: 10.1136/adc.2008.142448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hao K, Schadt EE, Storey JD. Calibrating the performance of SNP arrays for whole-genome association studies. PLoS Genet. 2008;4:e1000109. doi: 10.1371/journal.pgen.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill AV. Aspects of genetic susceptibility to human infectious diseases. Annu Rev Genet. 2006;40:469–86. doi: 10.1146/annurev.genet.40.110405.090546. [DOI] [PubMed] [Google Scholar]
- 44.Hoal-Van Helden EG, Epstein J, Victor TC, Hon D, Lewis LA, et al. Mannose-binding protein B allele confers protection against tuberculous meningitis. Pediatr Res. 1999;45:459–64. doi: 10.1203/00006450-199904010-00002. [DOI] [PubMed] [Google Scholar]
- 45.Hodgson U, Pulkkinen V, Dixon M, Peyrard-Janvid M, Rehn M, et al. ELMOD2 is a candidate gene for familial idiopathic pulmonary fibrosis. Am J Hum Genet. 2006;79:149–54. doi: 10.1086/504639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest. 2008;118:3462–69. doi: 10.1172/JCI34378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Holt PG, Macaubas C, Stumbles PA, Sly PD. The role of allergy in the development of asthma. Nature. 1999;402:B12–17. doi: 10.1038/35037009. [DOI] [PubMed] [Google Scholar]
- 48.Howard TS, Hoffman LH, Stang PE, Simoes EA. Respiratory syncytial virus pneumonia in the hospital setting: length of stay, charges, and mortality. J Pediatr. 2000;137:227–32. doi: 10.1067/mpd.2000.107525. [DOI] [PubMed] [Google Scholar]
- 49.Hoyert DL, Kung HC, Smith BL. Deaths: preliminary data for 2003. Natl Vital Stat Rep. 2005;53:1–48. [PubMed] [Google Scholar]
- 50.Hubbard R, Lewis S, Richards K, Johnston I, Britton J. Occupational exposure to metal or wood dust and etiology of cryptogenic fibrosing alveolitis. Lancet. 1996;347:284–89. doi: 10.1016/s0140-6736(96)90465-1. [DOI] [PubMed] [Google Scholar]
- 51.Ito K, Ito M, Elliott WM, Cosio B, Caramori G, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med. 2005;352:1967–76. doi: 10.1056/NEJMoa041892. [DOI] [PubMed] [Google Scholar]
- 52.Iwai K, Mori T, Yamada N, Yamaguchi M, Hosoda Y. Idiopathic pulmonary fibrosis. Epidemiologic approaches to occupational exposure. Am J Respir Crit Care Med. 1994;150:670–75. doi: 10.1164/ajrccm.150.3.8087336. [DOI] [PubMed] [Google Scholar]
- 53.Janssen R, Bont L, Siezen CL, Hodemaekers HM, Ermers MJ, et al. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. J Infect Dis. 2007;196:826–34. doi: 10.1086/520886. [DOI] [PubMed] [Google Scholar]
- 54.Khor CC, Chapman SJ, Vannberg FO, Dunne A, Murphy C, et al. A Mal functional variant is associated with protection against invasive pneumococcal disease, bacteremia, malaria and tuberculosis. Nat Genet. 2007;39:523–28. doi: 10.1038/ng1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koumantaki Y, Giziaki E, Linos A, Kontomerkos A, Kaklamanis P, et al. Family history as a risk factor for rheumatoid arthritis: a case-control study. J Rheumatol. 1997;24:1522–26. [PubMed] [Google Scholar]
- 56.Kronborg G, Weis N, Madsen HO, Pedersen SS, Wejse C, et al. Variant mannose-binding lectin alleles are not associated with susceptibility to or outcome of invasive pneumococcal infection in randomly included patients. J Infect Dis. 2002;185:1517–20. doi: 10.1086/340216. [DOI] [PubMed] [Google Scholar]
- 57.Li YF, Langholz B, Salam MT, Gilliland FD. Maternal and grandmaternal smoking patterns are associated with early childhood asthma. Chest. 2005;127:1232–41. doi: 10.1378/chest.127.4.1232. [DOI] [PubMed] [Google Scholar]
- 58.Lin M, Tseng HK, Trejaut JA, Lee HL, Loo JH, et al. Association of HLA class I with severe acute respiratory syndrome coronavirus infection. BMC Med Genet. 2003;4:9. doi: 10.1186/1471-2350-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.London SJ, Romieu I. Gene by environment interaction in asthma. Annu Rev Public Health. 2009;30:55–80. doi: 10.1146/annurev.publhealth.031308.100151. [DOI] [PubMed] [Google Scholar]
- 60.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL, editors. Global Burden of Disease and Risk Factors. New York: Oxford Univ. Press; 2006. [PubMed] [Google Scholar]
- 61.Lopez-Maderuelo D, Arnalich F, Serantes R, Gonzalez A, Codoceo R, et al. Interferon-gamma and interleukin-10 gene polymorphisms in pulmonary tuberculosis. Am J Respir Crit Care Med. 2003;167:970–75. doi: 10.1164/rccm.200205-438BC. [DOI] [PubMed] [Google Scholar]
- 62.Lorenz E, Mira JP, Frees KL, Schwartz DA. Relevance of mutations in the TLR4 receptor in patients with gram-negative septic shock. Arch Intern Med. 2002;162:1028–32. doi: 10.1001/archinte.162.9.1028. [DOI] [PubMed] [Google Scholar]
- 63.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma—United States, 1980–1999. MMWR Surveill Summ. 2002;51:1–13. [PubMed] [Google Scholar]
- 64.McNicholl JM, Downer MV, Udhayakumar V, Alper CA, Swerdlow DL. Host-pathogen interactions in emerging and re-emerging infectious diseases: a genomic perspective of tuberculosis, malaria, human immunodeficiency virus infection, hepatitis B, and cholera. Annu Rev Public Health. 2000;21:15–46. doi: 10.1146/annurev.publhealth.21.1.15. [DOI] [PubMed] [Google Scholar]
- 65.Miller RL. Prenatal maternal diet affects asthma risk in offspring. J Clin Invest. 2008;118:3265–68. doi: 10.1172/JCI37171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moffatt MF, Cookson WO. The genetics of asthma. Maternal effects in atopic disease. Clin Exp Allergy. 1998;28(Suppl 1):56–61. doi: 10.1046/j.1365-2222.1998.0280s1056.x. discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 67.Ng MH, Lau KM, Li L, Cheng SH, Chan WY, et al. Association of human-leukocyte-antigen class I (B*0703) and class II (DRB1*0301) genotypes with susceptibility and resistance to the development of severe acute respiratory syndrome. J Infect Dis. 2004;190:515–18. doi: 10.1086/421523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nicolae D, Cox NJ, Lester LA, Schneider D, Tan Z, et al. Fine mapping and positional candidate studies identify HLA-G as an asthma susceptibility gene on chromosome 6p21. Am J Hum Genet. 2005;76:349–57. doi: 10.1086/427763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nogee LM, Dunbar AE, 3rd, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344:573–79. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 70.Ortiz LA, Lasky J, Hamilton RF, Jr, Holian A, Hoyle GW, et al. Expression of TNF and the necessity of TNF receptors in bleomycin-induced lung injury in mice. Exp Lung Res. 1998;24:721–43. doi: 10.3109/01902149809099592. [DOI] [PubMed] [Google Scholar]
- 71.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–68. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 72.Platts-Mills TA, Erwin E, Heymann P, Woodfolk J. Is the hygiene hypothesis still a viable explanation for the increased prevalence of asthma? Allergy. 2005;60(Suppl 79):25–31. doi: 10.1111/j.1398-9995.2005.00854.x. [DOI] [PubMed] [Google Scholar]
- 73.Pleis JR, Lucas JW. Data From the National Health Interview Survey, No 240. Hyattsville, MD: US Dep. Health Hum. Serv., Cent. Dis. Control Prev; 2008. Summary health statistics for U.S. adults: National Health Interview Survey, 2007. [PubMed] [Google Scholar]
- 74.Polonikov AV, Ivanov VP, Solodilova MA. Genetic variation of genes for xenobiotic-metabolizing enzymes and risk of bronchial asthma: the importance of gene-gene and gene-environment interactions for disease susceptibility. J Hum Genet. 2009;54(8):440–49. doi: 10.1038/jhg.2009.58. [DOI] [PubMed] [Google Scholar]
- 75.Quaak M, van Schayck CP, Knaapen AM, van Schooten FJ. Genetic variation as a predictor of smoking cessation success. A promising preventive and intervention tool for chronic respiratory diseases? Eur Respir J. 2009;33:468–80. doi: 10.1183/09031936.00056908. [DOI] [PubMed] [Google Scholar]
- 76.Redd SC. Asthma in the United States: burden and current theories. Environ Health Perspect. 2002;110(Suppl 4):557–60. doi: 10.1289/ehp.02110s4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rossouw M, Nel HJ, Cooke GS, van Helden PD, Hoal EG. Association between tuberculosis and a polymorphic NFκB binding site in the interferon gamma gene. Lancet. 2003;361:1871–72. doi: 10.1016/S0140-6736(03)13491-5. [DOI] [PubMed] [Google Scholar]
- 78.Roy S, Knox K, Segal S, Griffiths D, Moore CE, et al. MBL genotype and risk of invasive pneumococcal disease: a case-control study. Lancet. 2002;359:1569–73. doi: 10.1016/S0140-6736(02)08516-1. [DOI] [PubMed] [Google Scholar]
- 79.Schwartz DA, Davis CS, Merchant JA, Bunn WB, Galvin JR, et al. Longitudinal changes in lung function among asbestos-exposed workers. Am J Respir Crit Care Med. 1994;150:1243–49. doi: 10.1164/ajrccm.150.5.7952547. [DOI] [PubMed] [Google Scholar]
- 80.Schwartz DA, Van Fossen DS, Davis CS, Helmers RA, Dayton CS, et al. Determinants of progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1994;149:444–49. doi: 10.1164/ajrccm.149.2.8306043. [DOI] [PubMed] [Google Scholar]
- 81.Soborg C, Madsen HO, Andersen AB, Lillebaek T, Kok-Jensen A, Garred P. Mannose-binding lectin polymorphisms in clinical tuberculosis. J Infect Dis. 2003;188:777–82. doi: 10.1086/377183. [DOI] [PubMed] [Google Scholar]
- 82.Steele MP, Speer MC, Loyd JE, Brown KK, Herron A, et al. Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med. 2005;172:1146–52. doi: 10.1164/rccm.200408-1104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thomas AQ, Lane K, Phillips J, 3rd, Prince M, Markin C, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165:1322–28. doi: 10.1164/rccm.200112-123OC. [DOI] [PubMed] [Google Scholar]
- 84.Traherne JA, Hill MR, Hysi P, D’Amato M, Broxholme J, et al. LD mapping of maternally and nonmaternally derived alleles and atopy in FcεRI-β. Hum Mol Genet. 2003;12:2577–85. doi: 10.1093/hmg/ddg290. [DOI] [PubMed] [Google Scholar]
- 85.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci USA. 2007;104:7552–57. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vercelli D. Discovering susceptibility genes for asthma and allergy. Nat Rev. 2008;8:169–82. doi: 10.1038/nri2257. [DOI] [PubMed] [Google Scholar]
- 87.von Mutius E. Gene-environment interactions in asthma. J Allergy Clin Immunol. 2009;123:3–11. doi: 10.1016/j.jaci.2008.10.046. quiz 2–3. [DOI] [PubMed] [Google Scholar]
- 88.Walley AJ, Chavanas S, Moffatt MF, Esnouf RM, Ubhi B, et al. Gene polymorphism in Netherton and common atopic disease. Nat Genet. 2001;29:175–78. doi: 10.1038/ng728. [DOI] [PubMed] [Google Scholar]
- 89.Wang Y, Kuan PJ, Xing C, Cronkhite JT, Torres F, et al. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet. 2009;84:52–59. doi: 10.1016/j.ajhg.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Warram JH, Krolewski AS, Gottlieb MS, Kahn CR. Differences in risk of insulin-dependent diabetes in offspring of diabetic mothers and diabetic fathers. N Engl J Med. 1984;311:149–52. doi: 10.1056/NEJM198407193110304. [DOI] [PubMed] [Google Scholar]
- 91.Warshamana GS, Pociask DA, Sime P, Schwartz DA, Brody AR. Susceptibility to asbestos-induced and transforming growth factor-β1-induced fibroproliferative lung disease in two strains of mice. Am J Respir Cell Mol Biol. 2002;27:705–13. doi: 10.1165/rcmb.2002-0096OC. [DOI] [PubMed] [Google Scholar]
- 92.Waterer GW, ElBahlawan L, Quasney MW, Zhang Q, Kessler LA, Wunderink RG. Heat shock protein 70–2+1267AA homozygotes have an increased risk of septic shock in adults with community-acquired pneumonia. Crit Care Med. 2003;31:1367–72. doi: 10.1097/01.CCM.0000063088.86079.03. [DOI] [PubMed] [Google Scholar]
- 93.Weiss ST, Raby BA, Rogers A. Asthma genetics and genomics 2009. Curr Opin Genet Dev. 2009;19:279–82. doi: 10.1016/j.gde.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 94.Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000;355:618–21. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 95.World Health Organ. Consensus document on the epidemiology of severe acute respiratory syndrome (SARS) 2003 http://www.who.int/csr/sars/en/WHOconsensus.pdf.
- 96.Xiong P, Zeng X, Song MS, Jia SW, Zhong MH, et al. Lack of association between HLA-A, -B and -DRB1 alleles and the development of SARS: a cohort of 95 SARS-recovered individuals in a population of Guangdong, southern China. Int J Immunogenet. 2008;35:69–74. doi: 10.1111/j.1744-313X.2007.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yende S, Angus DC, Ding J, Newman AB, Kellum JA, et al. 4G/5G plasminogen activator inhibitor-1 polymorphisms and haplotypes are associated with pneumonia. Am J Respir Crit Care Med. 2007;176:1129–37. doi: 10.1164/rccm.200605-644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zaas AK, Liao G, Chien JW, Weinberg C, Shore D, et al. Plasminogen alleles influence susceptibility to invasive aspergillosis. PLoS Genet. 2008;4:e1000101. doi: 10.1371/journal.pgen.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang H, Zhou G, Zhi L, Yang H, Zhai Y, et al. Association between mannose-binding lectin gene polymorphisms and susceptibility to severe acute respiratory syndrome coronavirus infection. J Infect Dis. 2005;192:1355–61. doi: 10.1086/491479. [DOI] [PMC free article] [PubMed] [Google Scholar]