Abstract
Objective
A human thymic epithelial cell (TEC) line expressing human leukocyte antigen (HLA)-ABC and HLA-DR was engineered to overexpress murine Delta-like 1 (TEC-Dl1) for the purpose of establishing a human culture system that supports T lymphopoiesis from hematopoietic progenitor cells (HPCs).
Materials and Methods
Cord blood (CB) or bone marrow (BM) HPCs were co-cultured with either the parental TEC line expressing low levels of the Notch ligands, Delta-like 1 and Delta-like 4 or with TEC-Dl1 to determine if these cell lines support human lymphopoiesis.
Results
In co-cultures with CB or BM HPCs, TEC-Dl1 cells promote de novo generation of CD7posCD1apos T-lineage committed cells. Most CD7posCD1ahi cells are CD4posCD8pos double positive (DP). We found that TEC-Dl1 cells are insufficient to generate mature CD3hi CD4pos or CD3hi CD8pos single positive (SP) T cells from the CD4posCD8pos DP T cells; however, we detected CD3lo cells within the DP and SP CD4 and CD8 populations. The CD3lo SP cells expressed lower levels of IL-2Rα and IL-7Rα compared to CD3lo DP cells. In contrast to the TEC-Dl1 line, the parental TEC-84 line expressing low levels of human Notch ligands permits HPC differentiation to the B-cell lineage.
Conclusions
We report for the first time a human TEC line that supports lymphopoiesis from CB and BM HPC. The TEC cell lines described herein provide a novel human thymic stroma model to study the contribution of HLA molecules and Notch ligands to T cell commitment and maturation and could be utilized to promote lymphopoiesis for immune cell therapy.
Keywords: lymphopoiesis, hematopoietic stem cell, stromal cells, umbilical cord blood
Introduction
The thymus is essential for the development of T cells from bone marrow-derived hematopoietic stem cells (HSCs) in animals and humans [1–3]. Genetic and functional studies show that thymic epithelial cells (TECs) play a critical role in T cell development [4–7]. Phenotype analyses of ex vivo human thymocytes have revealed distinct stages of T cell maturation. The stages of T cell development in the thymus have been defined as HSC (CD34posCD7negCD1aneg), preT/natural killer (CD34posCD7posCD1aneg), preT (CD34pos/loCD7posCD1apos), immature single positive (CD1aposCD7posCD4pos), early double positive (CD3negCD4posCD8pos), double positive (DP) (CD3posCD4posCD8pos), and single positive (SP) (CD3posCD4posCD8neg or CD3posCD8posCD4neg) [8].
Notch signaling is essential for T cell lineage commitment and differentiation [9, 10]; however, it is unclear which of the Notch ligands expressed by TEC trigger the physiological signal for T cell lineage commitment and/or maturation. Delta-like 4 (Dl4) and Delta-like 1 (Dl1) are both known to bind the receptor Notch-1 on HSC [11]. Murine BM stromal cell lines such as S17-DL1 and OP9-DL1 that overexpress the Notch ligand Dl1 and OP9-DL4 that overexpresses the Notch ligand Dl4 support T cell development from human cord blood (CB) HSCs [12–14] and bone marrow (BM) HSCs [15] as well as murine HSCs [16, 17].
Although BM stromal cells expressing Notch ligand support T lymphopoiesis in vitro, it is TECs that support T cell development in vivo. In this study, we describe human TEC lines that support human lymphopoiesis. Our data show that CB or BM hematopoietic progenitor cells (HPCs) co-cultured with a TEC line modified to overexpress the murine Dl1 (TEC-Dl1) give rise to T-lineage cells including CD1apos cells that express both CD4 and CD8. In contrast, the parental TEC-84 line that expresses low levels of human DL1 and DL4 permits HPC commitment to the B cell lineage. We propose that the human TEC lines described herein provide a new model that can be used to define the contributions of HLA molecules to T cell development and to delineate the role of Notch ligands in human T lymphopoiesis.
Materials and Methods
Human blood and tissue sample collection
Collection of human TECs, CB, and adult BM was approved by Loyola University Chicago’s Institutional Review Board and was done in accordance with the principles of the Helsinki Declaration.
Generation of the TEC-84 and TEC-Dl1 cell lines
TEC primary cultures were initiated by an explant technique as previously described [18]. TECs were immortalized by infection of the primary cultures with amphotropic retrovirus from a cell line, PA317 LXSN-16E6E7 [19] (American Type Culture Collection, Manassas, VA), containing the HPV E6E7 early genes and stable TEC lines were selected with G418 (800µg/ml). After two weeks in culture, the infected TECs were removed from selective media and expanded in TE media (3:1 DMEM:F12 medium with 5% FCS, 5.5µg/ml bovine insulin, 0.4µg/ml hydrocortisone, 9 ng/ml cholera toxin, 0.3% adenine hydrochloride, 1mM sodium pyruvate, 10ng/ml epidermal growth factor, 2.5µg/ml amphotericin B, and 55ng/ml gentamicin sulfate). The selected TECs (TEC-84) were subjected to four rounds of electronic sorting and expansion for CD104 (integrin β4), CD29 (integrin β1), and CD49f (VLA6). The MigR1-GFP retroviral vector was engineered to express the murine Delta-like 1 gene 5’ upstream of the internal-ribosomal entry site thereby allowing bicistronic expression of Dl1 and green fluorescence protein (GFP) [20]. Retrovirus containing Migr1-Dl1-GFP was generated using amphotropic Phoenix cells and supernatants were used to spinoculate TEC. Cells expressing high levels of Dl1 were sorted for GFP expression on a FACSARIA (Becton Dickinson (BD) Franklin Lakes, NJ, USA). Quantitative RT PCR was used to quantify the number of mouse and human Delta-like1 (DL1) and human Delta-like 4 (DL4) transcripts. RNA was extracted from TEC-84 or TEC-Dl1 cells using the RNeasy kit (Qiagen, Valencia, CA, USA). Primer sequences were as follows: Human DL1 (NM_005618): (5’) ACACCATAAGCCCTGCAAGAA, (3’) TCACAGATTTTGCCGTAGAAGC; Human DL4 (NM_019074): (5’) AGTGGTCATTGCGCTTCTTG, (3’) ACCTTCTCGCTCATCATCGAA; murine Dl1(NM_007865): (5’) CTTCTTTCGCGTATGCCTCAA; (3’) AGGCGGCTGATGAGTCTTTCT.
Flow cytometry
Cells were stained with fluorochrome labeled antibodies purchased from eBioscience, Biolegend, or BD Pharmingen (San Diego, CA, USA) or from Invitrogen (Carlsbad, CA, USA). Intracytoplasmic antibody staining was performed by fixing and permeabilizing cells with Cytofix/Cytoperm (BD Bioscience) prior to the addition of antibody. The percentage of positive cells for each marker was determined using a BD Canto or Canto II flow cytometer and FlowJo software (Tree Star, Ashland, OR, USA). Only cells within the lymphocyte gate were evaluated. Quadrants for positive and negative populations were based on cells stained with isotype control antibodies and fluorescence minus one (FMO) controls [21].
Co-culture of HPCs with TEC-84 or TEC-Dl1
CD34pos cells were enriched from CB or BM using the EasySep CD34 positive selection kit (StemCell Technologies, Vancouver, BC, Canada). For phenotyping experiments, CD34pos cells were stained with antibodies to lineage (Lin) specific markers: CD3, CD19, CD56, CD14, and CD15 (FITC); CD34 (PE); CD38 (PECy5 or APC); and CD45RA (PECy7 or APC). For co-culture experiments, LinnegCD34posCD38negCD45RApos HPCs were sorted on a FACSARIA flow cytometer (purity >99%) and 3000 HPCs/well were co-cultured with confluent TEC-Dl1 in α-MEM media containing 15% FCS, 5% human serum, and 5ng/ml each of human IL-7, SCF, and Flt-3L (Peprotech, Rocky Hill, NJ, USA). For B lymphopoiesis studies, sub-confluent TEC-84 were co-cultured with 2000 HPCs/well in media containing 10% FCS, and 10ng/ml each of human IL-7, SCF, and Flt-3L, 6.6ng/ml IL-3, 20ng/ml HGF (Peprotech), and 5% CB plasma. The total number of live cells harvested from TEC-Dl1 or TEC-84 co-cultures was counted on a hemocytometer.
Ex vivo human thymocytes
Cryogenically preserved human thymocytes were stained with the same antibodies used to analyze T lymphoid progenitors from co-cultures.
Results
Expression of HLA and adhesion molecules on TEC-Dl1
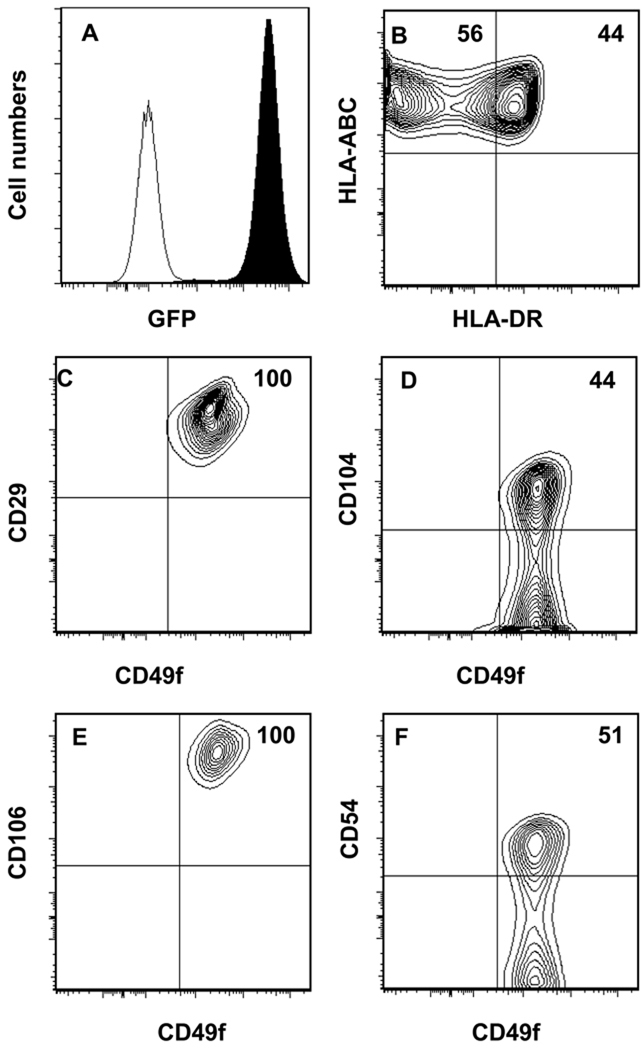
To establish a TEC line capable of supporting T lymphopoiesis from human HPCs, we developed a primary TEC culture from pediatric thymus tissue as described in “Materials and Methods”. By quantitative RT-PCR, the parental cell line, TEC-84 was found to express low levels of the human Notch ligands, DL1 (<1000 transcript copies/µg RNA) and DL4 (1300 transcript copies/µg RNA) (data not shown). Since mouse and human DL1 are highly homologous and since OP9 cells overexpressing mouse or human DL1 support T lymphopoiesis [16, 22], we overexpressed the murine Dl1 by infecting the TEC-84 cells with retrovirus containing Migr1-Dl1-GFP to enhance the potential of the TEC-84 cell line to support T lymphopoiesis. The GFPpos Dl1 expressing cells, TEC-Dl1, showed homogenous expression of GFP (Fig. 1A). By quantitative RT-PCR, we found that TEC-Dl1 express 1–1.3 ×106 Dl1 transcripts/µg of total RNA which is comparable to the expression of Dl1 in OP9-DL1 cells (data not shown). The TEC-Dl1 cells uniformly express HLA-ABC and nearly half of the cells also express HLA-DR (Fig. 1B). The TEC-Dl1 cells also express the epithelial cell markers, CD29 (integrin β1), CD104 (integrin β4), and CD49f (VLA6) (Fig. 1C–F). While the parental TEC-84 cells uniformly express CD104 (data not shown), expression of CD104 is reduced in the TEC-Dl1 cells (Fig. 1D). We found that all TEC-Dl1 cells express CD106 (Fig. 1E) and one-half of them also express CD54 (Fig. 1F). These data verify that the phenotype of the TEC-Dl1 line is similar to the phenotype of in vivo human TECs.
Figure 1. Cell surface marker characterization of the human TEC-Dl1 as determined by flow cytometric analysis.
The established TEC-Dl1 cell line was analyzed for : (A) Expression of GFP as a measure for murine Dl1 expression; filled histogram is TEC-Dl1, unfilled histogram is control parental TEC-84; (B) human MHC class I (HLA-ABC) APC and MHC class II (HLA-DR) PE; (C) CD29 (integrin β1) FITC and CD49f (VLA6) APC; (D) CD104 (integrin β4) PE and CD49f APC; (E) CD106 (VCAM) PE and CD49f APC; and (F) CD54 (ICAM) PE and CD49f APC. All axes except the y axis of (A) display log10 fluorescence. Numbers shown in quadrants are percentages.
TEC-Dl1 support of HPC differentiation to the T cell lineage
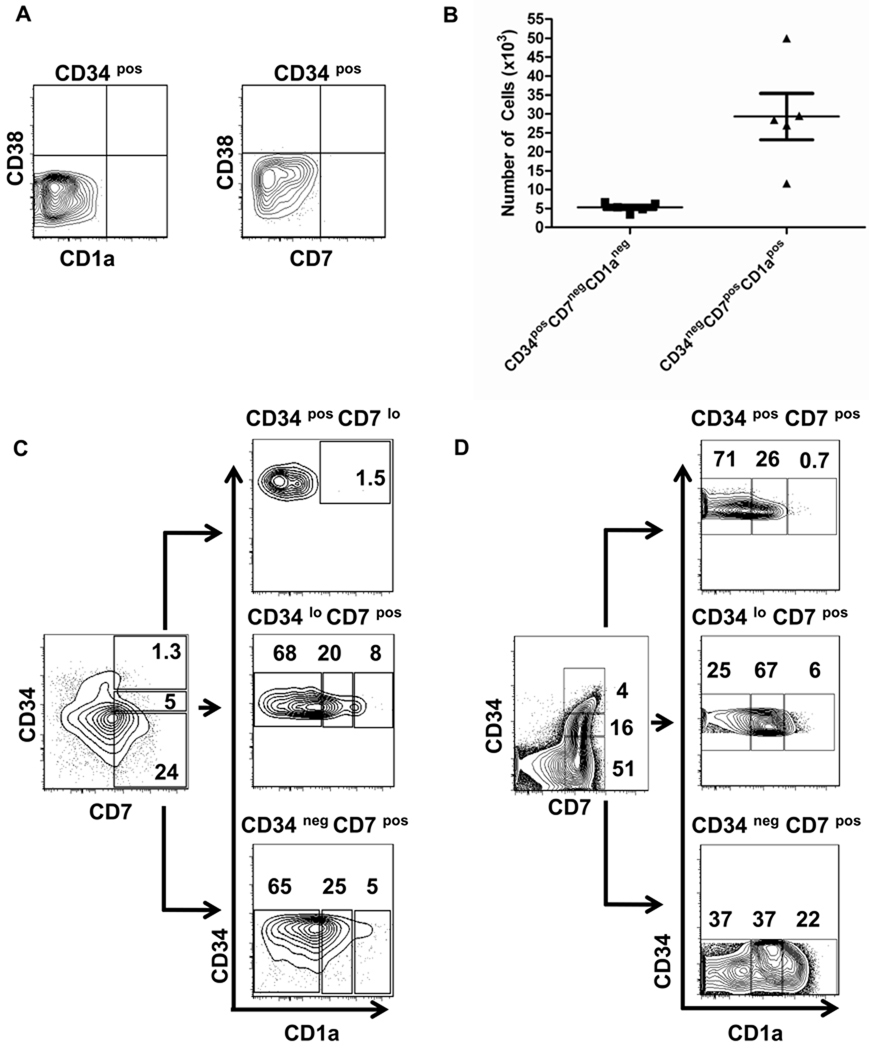
To determine if the TEC-Dl1 line could promote T cell lineage commitment and differentiation, LinnegCD34posCD38negCD45RApos cells isolated from CB were co-cultured with TEC-Dl1 in media supplemented with human IL-7, SCF, and Flt-3L. We chose to isolate CD45RApos cells because of their bias toward lymphopoiesis [13]. We first verified that HPCs were not already committed to the T cell lineage prior to culture. Enriched CB CD34pos cells were stained with antibodies to CD38, a marker of cells that have progressed beyond the HSC stage, the early T/NK cell marker CD7 and the T cell lineage commitment marker CD1a; flow cytometric analysis showed that the enriched CD34pos cells that were CD38neg displayed undetectable levels of CD1a or CD7 (Fig. 2A).
Figure 2. Expansion of CB HPCs and expression of T lineage surface markers on CB HPCs after co-culture with TEC-Dl1.
(A) Lack of CD38 (APC), CD7 (biotin + SA-APC-Cy7) and CD1a (biotin + SA-APC-Cy7) expression on enriched CD34pos CB cells before culture; (B) CB HPCs (3000 cells) were co-cultured with TEC-Dl1 for two weeks; the total number of CD34posCD7negCD1aneg cells uncommitted to the T cell lineage (n=5) and the total number of CD34negCD7posCD1apos cells committed to the T cell lineage (n=5) was determined. The bars indicate the means ± S.D.; C and D) CD1a expression within the CD34posCD7lo, CD34loCD7pos, and CD34negCD7pos subsets generated from CB HPC-TEC-Dl1 co-cultures (C) or cryopreserved ex vivo pediatric human thymocytes (D) as determined by flow cytometry. Numbers shown are percentages. These data are representative of five CB HPC cultures and thymocytes from three human thymi. The scale for each axis is log10 fluorescence.
CB HPCs (3×103 cells) were cultured with TEC-Dl1 and after two weeks, the total number of cells was counted and these cells were analyzed by flow cytometry. On average, the cell number increased 60-fold (range= 1–3 × 105, n=7; data not shown) demonstrating that cell proliferation was occurring in these co-cultures. By the end of the culture period, a modest expansion of CD34posCD7negCD1aneg cells had occurred suggesting that some CD34pos cells may undergo proliferation without differentiation (Fig. 2B). These data are similar to a previous study showing an initial expansion of CD34pos cells cultured on OP9-DL1 [14] and are consistent with the role of Notch signaling in the maintenance and survival of hematopoietic progenitors [23].
The acquisition of CD1a expression and downregulation of CD34 expression by HSC has been used to assess T cell lineage commitment [22, 24]. CD1a is one of the earliest markers of T cell commitment with studies showing that CD1apos cells no longer have natural killer or dendritic cell potential [25]. In the HPC-TEC-Dl1 co-cultures, between 104 and 5 ×104 cells were CD34negCD7posCD1apos demonstrating the de novo generation of approximately ten T-lineage committed cells from each HPC (Fig. 2B). Only cells that had downregulated CD34 expression (CD34lo or CD34neg) expressed the T-lineage marker CD1a; the CD1apos cells were also CD7pos and within this population, a distinct subset of CD1ahi cells was identifiable (Fig. 2C). Analysis of ex vivo human thymocytes showed the presence of similar cell subsets (Fig. 2D). No T-lineage cells were observed in our cultures when HPCs were cultured alone or in media supplemented with cytokines (data not shown), demonstrating that TEC-Dl1 cells were required to promote T lymphopoiesis.
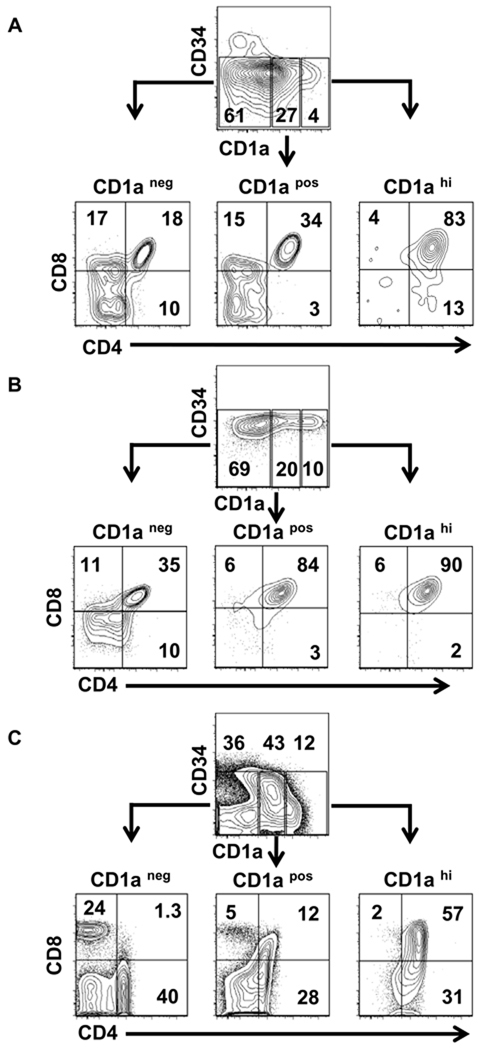
We further examined the phenotype of CD1a expressing cells (as shown in Fig. 2C–D) from CB HPCs (Fig. 3A) or BM HPCs (Fig. 3B) co-cultures for the expression of CD4 and CD8. Downregulation of CD1a expression correlates with maturation toward the SP stage, whereas CD1a expression is completely lost on mature SP cells and in recent thymic emigrants [26]. Similar to ex vivo human thymocytes (Fig. 3C), the majority of cells expressing CD1a in both CB and BM co-cultures were CD4posCD8pos DP. Some CD4posCD8neg immature single positive (ISP) cells that had not yet progressed to the DP stage were observed in the CD1ahi subset (Fig. 3A, lower right quadrant). We observed that BM cultures compared to CB cultures had higher percentages of DP cells within the CD1apos and CD1aneg subsets. It is unclear whether this difference is related to the age of the donating individuals or to the source of the HPCs; further study is required to explore these possibilities. Similar to ex vivo thymocytes, the CD1aneg subset from HPC co-cultures contained few DP cells. In contrast to the CD1aneg subset of ex vivo human thymocytes which contained a distinct population of CD4loCD8neg and CD8posCD4neg SP cells, only a small percentage of cells from the HPC co-cultures were CD4loCD8neg or CD8loCD4neg ; the expression of CD8 on SP cells was also lower in the co-cultures compared to ex vivo thymocytes (Fig. 3A–C). Taken together, these data demonstrate that de novo expression of CD1a and generation of DP T cells in co-cultures of HPCs with TEC-Dl1 parallels human thymocyte development; however SP T cell development in the co-cultures may be diminished.
Figure 3. Flow cytometric analysis of CD4 and CD8 expression in three populations identified by the expression of CD1a.
(A) Cells harvested from the CB HPC-TEC-Dl1 co-cultures after two weeks were analyzed for the expression of CD34 (PE), CD1a (APC), CD4 (AF750), and CD8 (PECy7). Expression of CD4 and CD8 was determined within the CD34neg CD1aneg (left), CD1apos (middle), and CD1ahi (right) populations. Similar analyses were performed for cells generated from BM HPCs co-cultured with TEC-Dl1 cells (B) and cryopreserved ex vivo human thymocytes (C). Numbers shown are percentages within each region or quadrant. Data shown are representative of five CB HPC co-cultures, two BM HPC co-cultures, and three human pediatric thymi. Log10 fluorescence is shown on the axes.
Maturation of the CD4posCD8pos DP cells in co-cultures of HPCs with TEC-Dl1
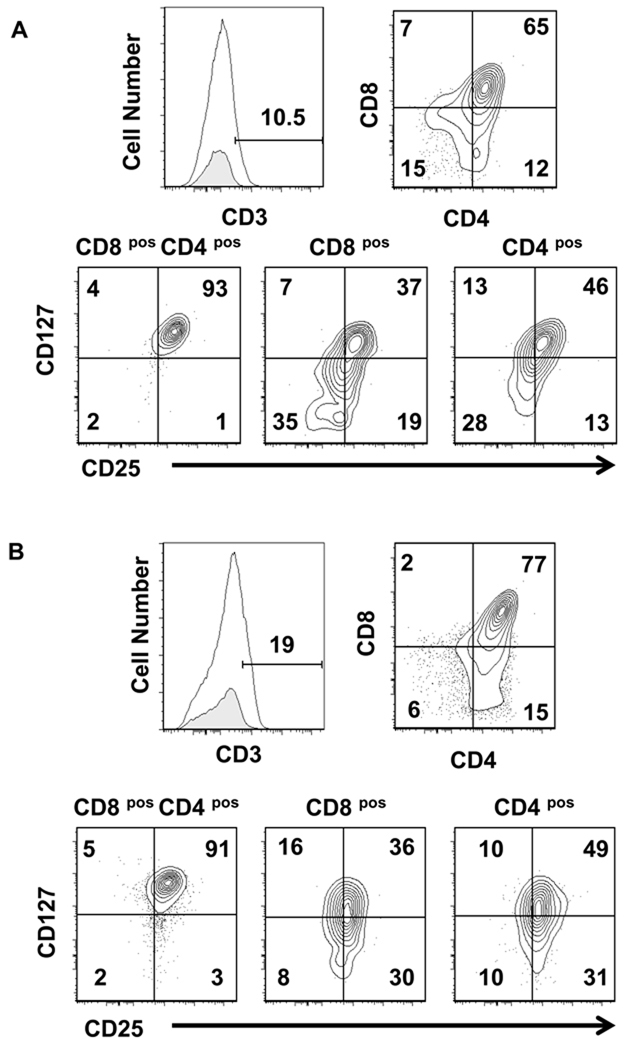
We hypothesized that the TEC-Dl1 should support T cell maturation of DP T cells to the SP stage because TEC-Dl1 express HLA Class I and Class II as well as the adhesion molecules CD106 (VCAM) and CD54 (ICAM) that are known to interact with developing thymocytes [27, 28] and function as co-stimulators of positive selection in vitro [29]. Positive selection results in the downregulation of either CD4 or CD8 co-receptors and increased expression of CD3 [30, 31]. To determine if T cells generated in the co-cultures of BM or CB HPCs with TEC-Dl1 were capable of maturation beyond the early DP stage, we analyzed these cells for expression of cell surface CD3, CD4, and CD8. In cultures of both CB and BM HPCs, a small percentage of CD3lo cells was detected (Fig. 4A–B). Co-cultures could not be continued beyond two to three weeks even when cells were transferred to fresh TEC-Dl1 cells due to a decrease in cell viability (data not shown). The majority of the CD3lo cells from TEC-Dl1 co-cultures were DP and a small fraction was either CD4lo or CD8lo SP cells. We found that >90% of the CD3lo DP cells express IL-7Rα(CD127) and the activation marker IL-2Rα (CD25). However, in comparison to the expression of these molecules on the CD4posCD8pos DP cells, the expression of CD127 and CD25 was downregulated on some CD3lo CD8lo and CD3lo CD4lo SP cells (Fig. 4A–B). We conclude that the majority of CD3lo cells are unable to progress through the DP stage to the SP stage since they retain expression of CD25, which is lost on DP T cells. Consistent with these data, TCRαβ or TCRγδcell surface expression was undetectable in the CD3lo population (data not shown).
Figure 4. Expression of CD25 and CD127 within CD3lo CD4posCD8pos double positive and CD4pos or CD8pos single positive populations.
(A) Cells were harvested from CB HPC-TEC-Dl1 co-cultures after two weeks and cell surface phenotype was analyzed by flow cytometry. The CD3lo cell population was determined by comparing cells stained with APC anti-CD3 (unshaded, top left panels) with an APC conjugated antibody that does not bind T cells (shaded, top left panels). The percentage of CD3lo cells is indicated. CD3lo cells were divided into subsets based on expression of CD4 (AF750) and CD8 (PECy7) (top right panels). These subsets were then analyzed for the expression of CD25 (PE) and CD127 (PECy5) (bottom panels). Numbers shown above markers or within quadrants are percentages. (B) Similar analyses were performed for BM HPC-TEC-Dl1 co-cultures. Representative data of five independent CB HPC co-culture and two independent BM HPC co-culture experiments are shown. Axes show log10 fluorescence.
TEC-84 cells that express low levels of DL1 and DL4 promote CB HPC differentiation to the B cell lineage
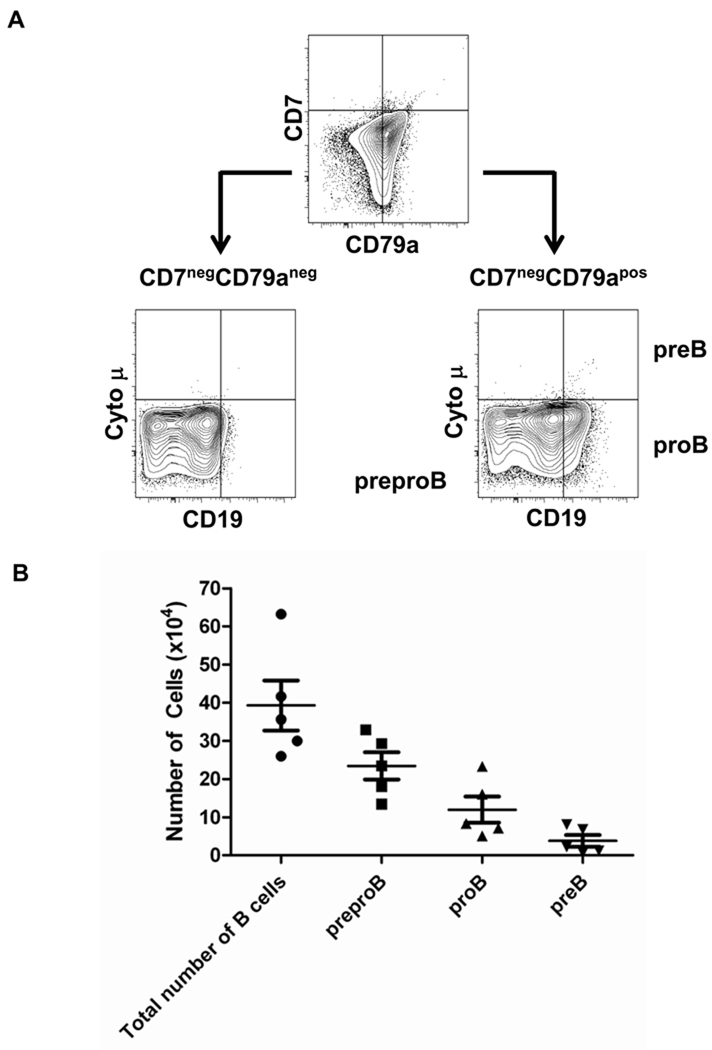
Several murine studies have demonstrated that thymic stroma cells can support B lymphopoiesis if Notch signaling is attenuated. CD4negCD8negTCRneg/CD3neg thymocytes can differentiate into B cells when cultured with a murine thymic stromal cell line [32] and murine thymic stroma cell monolayer cultures that express low levels of the Notch ligands, Dl1 and Dl4 support the differentiation of hematopoietic progenitor cells to the B-cell rather than the T-cell lineage [20]. It has also been demonstrated that CB HSCs cultured in a mouse fetal thymic organ culture develop into B cells when cultured in the presence of a high concentration of a Notch signaling inhibitor [33]. Based on these data, we hypothesized that the TEC-84 cell line would permit HPC differentiation to the B cell lineage. CB HPCs (4 × 103) were co-cultured with TEC-84 and the cells were analyzed after one to two weeks in culture. From a total of five independent experiments, we recovered on average, 92 × 104 live cells (range 50 × 104 – 12 × 105) from the cultures (data not shown). By flow cytometry, we detected myeloid lineage cells and some common lymphoid progenitors (data not shown); however nearly half of these cells were CD7neg CD79apos B-lineage cells (average of 39 × 104; range 26 × 104 – 63 × 104). B-lineage cells were not generated in the absence of TEC-84 cells (data not shown). The majority of the B-lineage progenitors were preproB cells (CD19negcytoplasmic µneg) (range 13 × 104 – 33 × 104 cells) or proB cells (CD19poscytoplasmic µneg) (range 5 × 104 – 23 × 104). On average, only 10% of the B cell progenitors were preB cells (CD19poscytoplasmic µpos) (range <1 × 104 – 8 × 104) (Fig. 5A–B). These data demonstrate that TECs expressing a low density of Notch ligand permit the development of B cells from HPC.
Figure 5. Characterization of B cells generated from CB HPCs co-cultured with TEC-84.
CB HPCs (2 wells of 2×103) were cultured with TEC-84 for two weeks and analyzed by flow cytometry for the immature B cell subset markers CD79a (PE), CD7 (PECy5), CD19 (PECy7) and intracellular IgM (APC). (A) CD79anegCD7neg cells were not considered to be committed to the B-cell lineage and were negative for two other B cell markers, CD19 and cytoplasmic IgM (cyto µ) (bottom left). CD7negCD79apos cells were considered committed to the B-cell lineage and were classified as preproB, proB, or preB cells based on the expression of CD19 and cyto µ (bottom right). The axes show log10 fluorescence. (B) The total number of B cells represents all cells that were positive for the B cell lineage marker, CD79a. The number of each B cell progenitor was calculated by multiplying the percentage of cells with each B cell progenitor phenotype by the total number of CD79apos cells. The bars indicate the mean ±S.D. of five independent CB HPC cultures.
Discussion
In these studies, we describe human TEC lines that support human lymphopoiesis. Although the TEC-Dl1 cells were homogenous for expression of Dl1, HLA-ABC, CD29, CD106, and CD49f, bimodal distribution of HLA-DR, CD104, and CD54 was observed. The appearance of the bimodal expression of HLA-DR, CD104 and CD54 was not due to heterogeneity of the TEC-Dl1 cell line because analysis of several TEC-Dl1 clones obtained by limiting dilution showed similar surface phenotype characteristics (data not shown). While the parental TEC-84 line was established based on homogenous expression of CD104, expression of CD104 was reduced in all subclones of TEC-Dl1 (data not shown). We have previously demonstrated that TEC express various isoforms of TGF-β in vivo and in vitro [18]. Expression of CD104 in epithelial cells is down modulated by TGF-β via epigenetic regulation [34]. We speculate that the interaction of murine Dl1 with human Notch on the TEC-Dl1 may lead to activation of TGF-β signaling resulting in a down modulation of CD104 expression.
In our co-culture system, TEC-Dl1 promoted commitment of CB or BM HPC to the T cell lineage as evidenced by the emergence of CD34lo/negCD7pos cells that are either CD1apos or CD1ahi. In agreement with a previous report, we found that the CB CD34posCD45RApos cells that were CD38neg displayed undetectable levels of CD7 expression [13] indicating that the CD7posCD1apos cells generated in co-cultures of HPCs with TEC-Dl1 were generated de novo. Importantly, the de novo CD1a cell subsets were phenotypically similar to those found in ex vivo human thymocytes, demonstrating that the TEC-Dl1 co-culture system mimics in vivo thymocyte development. Similar to our findings, a recent kinetic study showed that the emergence of CD1a expression correlates with the downregulation of CD34; however, CD7 expression in our TEC-Dl1 co-cultures (Fig. 2C) was less prominent than that seen in co-cultures of CB HSCs with OP9-DL1 [35]. CD7 expression may not be directly mediated by Notch signaling but rather by an unknown mediator(s) present in the murine OP9 cells because both the TEC-Dl1 and OP9-DL1 cell lines express similar levels of Notch (data not shown).
Currently, the exact role of Notch signaling in the maturation of DP cells to CD4 or CD8 SP cells remains controversial (reviewed in [36]). In mice, Notch expression is progressively downregulated at the CD4pos and CD8pos SP stage and is expressed at low levels in the peripheral T cells [37]. Although we observed CD8loCD4neg and CD4loCD8neg SP cells in co-cultures of HPC with TEC-Dl1, these cells have low expression of CD3, suggesting that Dl1 signaling is not sufficient and that additional developmental signals are required for upregulation of CD3 expression. It is possible that Notch signaling via DL4 is necessary for this developmental stage. Although the differential effect of DL1 and DL4 is not known in humans, mouse Dl4 is required for T cell development in vivo; while inactivation of Dl1 does not inhibit T cell development, the absence of Dl4 signaling completely blocks T cell development [38, 39]. To further investigate the roles of Dl1 and DL4 in T cell development, we will generate TEC lines with varying Dl1 and human DL4 expression and determine the ability of these cell lines to support HPC differentiation to the SP T cell stage.
Constitutive Notch signaling early in T cell development may lead to an irreversible block in maturation. It has been demonstrated that expression of DELTEX1 (DTX1) and NRARP, genes that are normally downregulated during TCRαβ lineage differentiation [40], is induced in HPCs co-cultured with OP9-DL1 cells [17]. Although we did not assess the expression of these genes in lymphoid progenitors from our co-cultures, we did observe that >90% of the CD3lo DP T cells from our co-cultures express CD127/IL-7Rα. Notch signaling has been shown to induce constitutive expression of CD127/IL-7Rα on thymocyte precursors [41]. We attempted to address this by disaggregating the developing lymphocytes from the TEC-Dl1 stroma and transferring them to the parental TEC line that did not overexpress Dl1, however this resulted in reduced cell yield without further development (data not shown). It is possible that T cell progenitors may require a gradient of Notch ligand expression during development; we are currently in the process of generating multiple TEC lines varying in DL1 expression to test this hypothesis. We are also in the process of generating TEC lines expressing mouse and human DL4. Perhaps, signaling through DL4 is needed for maturation of DP cells to SP cells. The low level of DL4 expression in our TEC-Dl1 cells may not support DP T cell maturation toward the SP stage. Once the TEC-DL4 cell lines are developed, we will be able to determine the contribution of DL1 and DL4 to the development of human SP T cells.
While overexpression of Dl1 in TEC promotes T lineage commitment, our data demonstrating that HPCs co-cultured with TEC-84 that express low levels of DL1 and DL4 become B cell progenitors indicate that B cell development is permitted in the human thymic microenvironment in the absence of strong Notch signaling. However, maturation of B cell progenitors to surface IgMpos B cells was not supported by the TEC-84 line, even when the culture period was extended to three weeks (data not shown). The lack of IgMpos B cells may be due to the absence of a B cell maturation factor in the cultures. More likely, B cell maturation was inhibited by Notch signaling because even temporary activation of Notch signaling prevents the development of HSC beyond the proB stage [42] and leads to degradation of transcription factors and kinases essential for B cell development [43].
Taken together, these data demonstrate that TEC-Dl1 promote HPC differentiation to various T cell subsets with phenotypes similar to those observed with ex vivo thymocytes, whereas TEC-84 promote HPCs to develop into B-lineage cells. These human TEC lines represent a novel tool for studying human lymphopoiesis and have the potential to generate lymphocytes for in vivo therapy in patients with hematologic malignancies and immunodeficiency disorders.
Acknowledgements
We are grateful to the staff of the Women’s Health Department at Loyola University Medical Center for collecting cord blood samples. We thank Dr. William Hopkinson of the Loyola University Medical Center Orthopedics Department for collection of bone marrow samples. We are indebted to Patricia Simms of the flow cytometry core facility for assistance with data acquisition and analysis and the retroviral core for generating viral stocks used for the infection (CA105049). This work was supported by Illinois Regenerative Medicine Institute 63080019 (PJS), NIH AG023809 (PTL), NIH AI068390 (KLK), and NHLBI F32HL096278 (BCBZ). JCZP is supported by a Canada Research Chair in Developmental Immunology and by the Krembil Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure
The authors have no conflicting financial interests.
References
- 1.Miller JF. Immunological function of the thymus. Lancet. 1961;2:748–749. doi: 10.1016/s0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- 2.Miller JF. Effect of thymectomy in adult mice on immunological responsiveness. Nature. 1965;208:1337–1338. doi: 10.1038/2081337a0. [DOI] [PubMed] [Google Scholar]
- 3.Markert ML, Boeck A, Hale LP, et al. Transplantation of thymus tissue in complete DiGeorge syndrome. N Engl J Med. 1999;341:1180–1189. doi: 10.1056/NEJM199910143411603. [DOI] [PubMed] [Google Scholar]
- 4.Denning SM, Kurtzberg J, Le PT, Tuck DT, Singer KH, Haynes BF. Human thymic epithelial cells directly induce activation of autologous immature thymocytes. Proc Natl Acad Sci U S A. 1988;85:3125–3129. doi: 10.1073/pnas.85.9.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haynes BF, Denning SM, Le PT, Singer KH. Human intrathymic T cell differentiation. Semin Immunol. 1990;2:67–77. [PubMed] [Google Scholar]
- 6.Nehls M, Pfeifer D, Schorpp M, Hedrich H, Boehm T. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature. 1994;372:103–107. doi: 10.1038/372103a0. [DOI] [PubMed] [Google Scholar]
- 7.Pignata C, Gaetaniello L, Masci AM, et al. Human equivalent of the mouse Nude/SCID phenotype: long-term evaluation of immunologic reconstitution after bone marrow transplantation. Blood. 2001;97:880–885. doi: 10.1182/blood.v97.4.880. [DOI] [PubMed] [Google Scholar]
- 8.Spits H. Development of alphabeta T cells in the human thymus. Nature Rev Immunol. 2002;2:760–772. doi: 10.1038/nri913. [DOI] [PubMed] [Google Scholar]
- 9.Radtke F, Wilson A, Stark G, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 10.Pui JC, Allman D, Xu L, et al. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 11.Karanu FN, Murdoch B, Miyabayashi T, et al. Human homologues of Delta-1 and Delta-4 function as mitogenic regulators of primitive human hematopoietic cells. Blood. 2001;97:1960–1967. doi: 10.1182/blood.v97.7.1960. [DOI] [PubMed] [Google Scholar]
- 12.Jaleco AC, Neves H, Hooijberg E, et al. Differential effects of Notch ligands Delta-1 and Jagged-1 in human lymphoid differentiation. J Exp Med. 2001;194:991–1002. doi: 10.1084/jem.194.7.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haddad R, Guardiola P, Izac B, et al. Molecular characterization of early human T/NK and B-lymphoid progenitor cells in umbilical cord blood. Blood. 2004;104:3918–3926. doi: 10.1182/blood-2004-05-1845. [DOI] [PubMed] [Google Scholar]
- 14.La Motte-Mohs RN, Herer E, Zuniga-Pflucker JC. Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood. 2005;105:1431–1439. doi: 10.1182/blood-2004-04-1293. [DOI] [PubMed] [Google Scholar]
- 15.De Smedt M, Hoebeke I, Plum J. Human bone marrow CD34+ progenitor cells mature to T cells on OP9-DL1 stromal cell line without thymus microenvironment. Blood Cells Mol Dis. 2004;33:227–232. doi: 10.1016/j.bcmd.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 17.Mohtashami M, Shah DK, Nakase H, Kianizad K, Petrie HT, Zuniga-Pflucker JC. Direct comparison of Dll1- and Dll4-mediated Notch activation levels shows differential lymphomyeloid lineage commitment outcomes. J Immunol. 2010;185:867–876. doi: 10.4049/jimmunol.1000782. [DOI] [PubMed] [Google Scholar]
- 18.Schluns KS, Grutkoski PS, Cook JE, Engelmann GL, Le PT. Human thymic epithelial cells produce TGF-beta 3 and express TGF-beta receptors. Int Immunol. 1995;7:1681–1690. doi: 10.1093/intimm/7.10.1681. [DOI] [PubMed] [Google Scholar]
- 19.Halbert CL, Demers GW, Galloway DA. The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J Virol. 1991;65:473–478. doi: 10.1128/jvi.65.1.473-478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohtashami M, Zuniga-Pflucker JC. Three-dimensional architecture of the thymus is required to maintain delta-like expression necessary for inducing T cell development. J Immunol. 2006;176:730–734. doi: 10.4049/jimmunol.176.2.730. [DOI] [PubMed] [Google Scholar]
- 21.Tung JW, Parks DR, Moore WA, Herzenberg LA, Herzenberg LA. New approaches to fluorescence compensation and visualization of FACS data. Clin Immunol. 2004;110:277–283. doi: 10.1016/j.clim.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Weerkamp F, Baert MR, Brugman MH, et al. Human thymus contains multipotent progenitors with T/B lymphoid, myeloid, and erythroid lineage potential. Blood. 2006;107:3131–3137. doi: 10.1182/blood-2005-08-3412. [DOI] [PubMed] [Google Scholar]
- 23.Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16:232–236. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terstappen LW, Huang S, Picker LJ. Flow cytometric assessment of human T-cell differentiation in thymus and bone marrow. Blood. 1992;79:666–677. [PubMed] [Google Scholar]
- 25.Spits H, Blom B, Jaleco AC, et al. Early stages in the development of human T, natural killer and thymic dendritic cells. Immunol Rev. 1998;165:75–86. doi: 10.1111/j.1600-065x.1998.tb01231.x. [DOI] [PubMed] [Google Scholar]
- 26.Res P, Blom B, Hori T, Weijer K, Spits H. Downregulation of CD1 marks acquisition of functional maturation of human thymocytes and defines a control point in late stages of human T cell development. J Exp Med. 1997;185:141–151. doi: 10.1084/jem.185.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le PT, Singer KH. Human thymic epithelial cells: adhesion molecules and cytokine production. Int J Clin Lab Res. 1993;23:56–60. doi: 10.1007/BF02592284. [DOI] [PubMed] [Google Scholar]
- 28.Salomon DR, Crisa L, Mojcik CF, Ishii JK, Klier G, Shevach EM. Vascular cell adhesion molecule-1 is expressed by cortical thymic epithelial cells and mediates thymocyte adhesion. Implications for the function of alpha4beta1 (VLA4) integrin in T-cell development. Blood. 1997;89:2461–2471. [PubMed] [Google Scholar]
- 29.Paessens LC, Singh SK, Fernandes RJ, van Kooyk Y. Vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) provide co-stimulation in positive selection along with survival of selected thymocytes. Mol Immunol. 2008;45:42–48. doi: 10.1016/j.molimm.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Scott B, Bluthmann H, Teh HS, von Boehmer H. The generation of mature T cells requires interaction of the alpha beta T-cell receptor with major histocompatibility antigens. Nature. 1989;338:591–593. doi: 10.1038/338591a0. [DOI] [PubMed] [Google Scholar]
- 31.Kisielow P, Teh HS, Bluthmann H, von Boehmer H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature. 1988;335:730–733. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- 32.Montecino-Rodriguez E, Dorshkind K. Long-term culture of triple-negative thymocytes. J Immunol. 1996;156:957–962. [PubMed] [Google Scholar]
- 33.De Smedt M, Hoebeke I, Reynvoet K, Leclercq G, Plum J. Different thresholds of Notch signaling bias human precursor cells toward B-, NK-, monocytic/dendritic-, or T-cell lineage in thymus microenvironment. Blood. 2005;106:3498–3506. doi: 10.1182/blood-2005-02-0496. [DOI] [PubMed] [Google Scholar]
- 34.Yang X, Pursell B, Lu S, Chang TK, Mercurio AM. Regulation of beta 4-integrin expression by epigenetic modifications in the mammary gland and during the epithelial-to-mesenchymal transition. J Cell Sci. 2009;122:2473–2480. doi: 10.1242/jcs.049148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Awong G, Herer E, Surh CD, Dick JE, La Motte-Mohs RN, Zuniga-Pflucker JC. Characterization in vitro and engraftment potential in vivo of human progenitor T cells generated from hematopoietic stem cells. Blood. 2009;114:972–982. doi: 10.1182/blood-2008-10-187013. [DOI] [PubMed] [Google Scholar]
- 36.Laky K, Fowlkes BJ. Notch signaling in CD4 and CD8 T cell development. Curr Opin Immunol. 2008;20:197–202. doi: 10.1016/j.coi.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fiorini E, Merck E, Wilson A, et al. Dynamic regulation of notch 1 and notch 2 surface expression during T cell development and activation revealed by novel monoclonal antibodies. J Immunol. 2009;183:7212–7222. doi: 10.4049/jimmunol.0902432. [DOI] [PubMed] [Google Scholar]
- 38.Hozumi K, Negishi N, Suzuki D, et al. Delta-like 1 is necessary for the generation of marginal zone B cells but not T cells in vivo. Nat Immunol. 2004;5:638–644. doi: 10.1038/ni1075. [DOI] [PubMed] [Google Scholar]
- 39.Koch U, Fiorini E, Benedito R, et al. Delta-like 4 is the essential, nonredundant ligand for Notch1 during thymic T cell lineage commitment. J Exp Med. 2008;205:2515–2523. doi: 10.1084/jem.20080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van de Walle I, De Smet G, De Smedt M, et al. An early decrease in Notch activation is required for human TCR-alphabeta lineage differentiation at the expense of TCR-gammadelta T cells. Blood. 2009;113:2988–2998. doi: 10.1182/blood-2008-06-164871. [DOI] [PubMed] [Google Scholar]
- 41.Magri M, Yatim A, Benne C, et al. Notch ligands potentiate IL-7-driven proliferation and survival of human thymocyte precursors. Eur J Immunol. 2009;39:1231–1240. doi: 10.1002/eji.200838765. [DOI] [PubMed] [Google Scholar]
- 42.Benne C, Lelievre JD, Balbo M, Henry A, Sakano S, Levy Y. Notch increases T/NK potential of human hematopoietic progenitors and inhibits B cell differentiation at a pro-B stage. Stem Cells. 2009;27:1676–1685. doi: 10.1002/stem.94. [DOI] [PubMed] [Google Scholar]
- 43.Nie L, Perry SS, Zhao Y, et al. Regulation of lymphocyte development by cell-type-specific interpretation of Notch signals. Mol Cell Biol. 2008;28:2078–2090. doi: 10.1128/MCB.00844-07. [DOI] [PMC free article] [PubMed] [Google Scholar]