Atrial fibrillation (AF) is the most common arrhythmia and is associated with increased risks of stroke, heart failure, dementia and death.1–8 Since the number of elderly individuals will increase over the years to come, the prevalence of AF is predicted to increase dramatically.9 Symptoms are a major reason patients with AF seek medical attention. Approximately two-thirds of all emergency department visits with a primary diagnosis of AF result in hospital admissions.10 AF and its related symptoms therefore represent a major therapeutic challenge and burden to healthcare systems. The major goals of AF therapy are to reduce of cardiovascular symptoms, morbidity and mortality. Since the outcome of rate versus rhythm control therapies is similar,11,12 the degree of symptoms related to the arrhythmia is a major consideration when selecting a treatment strategy. Given the cost and potential complications related to medications and ablation techniques utilized for rhythm-control, an accurate evaluation of the symptoms and functional status of patients with AF is crucial.
Despite the fact that AF was described in humans in 1906,13 no standardized assessment of symptoms or functional status has been accepted as the gold standard. The management of AF stands in marked contrast to heart failure for which there is a straightforward and widely used symptom scale. Although, AF and heart failure often coexist and both may cause similar symptoms, the New York Heart Association functional class was not developed for use in AF per se. The lack of a standardized approach may result in part from the complex clinical decision-making in patients with AF. Challenges arise because symptoms related to AF are highly variable, not only when comparing different patients, but also when assessing individual patients at different time points.
The most common symptoms include palpitations, chest pain and a reduction in exercise tolerance. Yet approximately 15% to 30% of patients with AF are asymptomatic.14–16 The relation between symptoms or impaired functional status and the onset or recurrences of the arrhythmia is not always obvious. Also, symptoms and impaired functional status may not be specific for AF since other cardiovascular conditions and risk factors for AF may cause similar symptoms. Furthermore, the magnitude of symptoms and functional status improvement attributable to specific AF therapies varies widely.
The aim of our review is to discuss symptomatology and functional status of individuals with AF. We will highlight current knowledge about the patient’s experience of AF, the pathophysiology and the prognostic implications of symptoms related to AF. In addition, we will provide an overview of the most frequently used tools to assess symptoms and functional status. Finally, we will summarize current gaps in the literature regarding AF symptomatology, and will underscore important potential research directions.
Review criteria
Our review was based on the authors’ knowledge of the literature and a structured search of the PubMed database (http://www.ncbi.nlm.nih.gov/pubmed/) using the terms “symptoms”, “atrial fibrillation”, “functional status”, “functional capacity”, “asymptomatic”, “quality of life”, “rate”, “ablation”, “rhythm”, “control”, alone or in combination. Further selection was based on abstracts and clinical relevance. When available we focused on randomized controlled trials, if unavailable we presented important observational studies. Our review is not intended to be all-inclusive; rather it represents the main studies published on this topic.
Symptoms related to atrial fibrillation
AF-symptomatology has mainly been studied in subjects referred for AF evaluation or treatment, and therefore may be overestimated. The French Etude en Activité Libérale de la Fibrillation Auriculaire (ALFA) study reported that patients with paroxysmal AF are more symptomatic than patients with persistent and permanent AF (Figure 1).14
Figure 1.
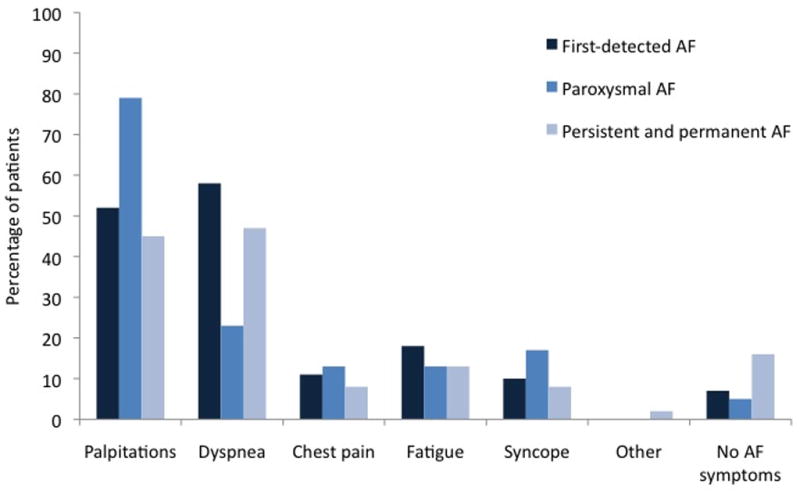
Symptoms according to type of AF in the ALFA study. (adapted and adjusted from Levy et al. Circulation 1999)14
Patients may experience palpitations, dyspnea, chest pain, dizziness and, less commonly, syncope or presyncope at some point during the life course of AF. Other, less specific symptoms reported in relation to AF include fatigue and anxiety. In the Euro Heart Survey on Atrial Fibrillation, 69% of AF patients had experienced symptoms related to the arrhythmia at some point since diagnosis. The majority of patients (54%) were asymptomatic at the time of the survey and the lowest symptom burden was reported in patients with permanent AF (Table 1).17
Table 1.
Presence of symptoms according to type of AF.
| AF-related symptoms* | First-detected AF (n=978) | Paroxysmal AF (n=1517) | Persistent AF (n=1167) | Permanent AF (n=1541) |
|---|---|---|---|---|
| Current | 77% | 77% | 73% | 55% |
| Previous | 7% | 16% | 15% | 20% |
| Never | 16% | 6% | 10% | 21% |
| Heart failure NYHA class III/IV | 17% | 8% | 15% | 25% |
Includes palpitations, syncope, dyspnea, chest pain, dizziness, fatigue, and non-specified symptoms. Adapted and adjusted from Nieuwlaat et al. Eur Heart J 200517
There is substantial intra- and inter-individual diversity in the type and severity of symptoms. Within an individual, symptoms may fluctuate widely over time. Also, there is a wide heterogeneity in patterns of AF, precipitants of AF, and therapeutic approaches. There are major gaps in our knowledge regarding the relations of race and ethnicity, advancing age, sex, and socioeconomic status, with AF-related symptoms and functional status.
Future research
To determine whether AF-therapies aimed at reducing symptoms are effective, it is imperative to know the natural history of AF-related symptoms. Research should focus on the incidence and prevalence of AF-related symptoms in different AF-subsets, different age ranges, other races and ethnicities, sexes, socioeconomic status, and hospital-based and community-based settings. The role of other cardiovascular diseases and different patterns of AF (paroxysmal, persistent and permanent AF) in generating symptoms needs to be determined. It is of great importance to know how AF-related symptoms change over time, and whether AF therapies reduce AF-related symptoms. New imaging modalities (e.g. magnetic resonance imaging, computer tomography and positron emission tomography) and novel biomarkers (e.g. natriuretic peptides) may facilitate the identification of specific AF endophenotypes related to particular AF symptoms. Emerging genomic, epigenomic, transcriptomic, proteomic and metabolomic data also may increase the discovery of novel tissue-specific biomarkers or genomic markers that may correlate with distinct symptoms.
Mechanisms of AF-related symptoms
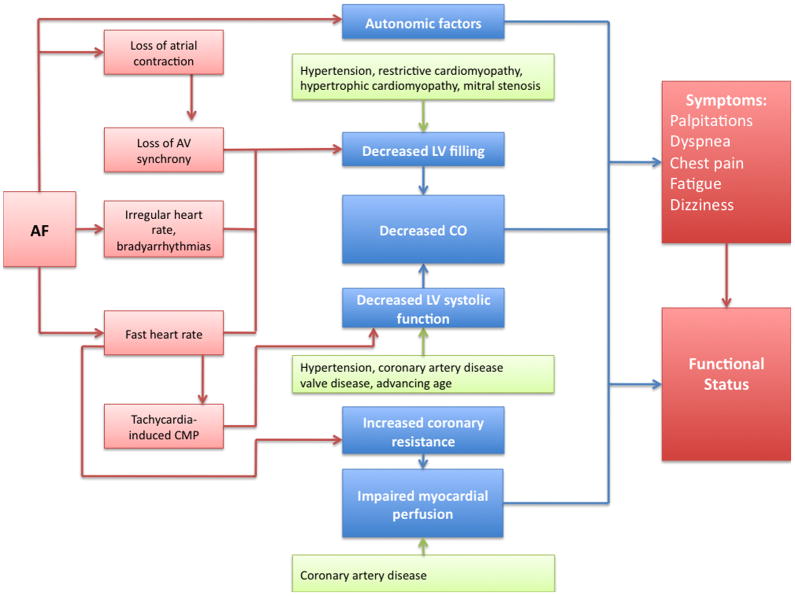
There is a striking paucity of data regarding the mechanisms by which AF causes symptoms.18 The investigation of the mechanistic link between symptoms and AF is complicated by the fact that AF often occurs in the presence of heart failure and valve disease, conditions that may present with similar symptoms. Heart failure is common in AF patients since both conditions share common risk factors, such as hypertension, diabetes, valve disease, myocardial infarction,19 and each disorder can predispose to the other condition. Furthermore, systolic and diastolic heart failure, and valve disease may not only mimic AF symptoms, but may also aggravate AF symptoms and functional status. Symptoms related to AF are likely to be multifactorial, due to both direct and indirect effects of the arrhythmia (Figure 2). At the current time, it is unclear whether symptom patterns are heritable.
Figure 2.
Conceptual model of pathophysiological mechanisms relating AF and symptoms.
Palpitations
A significant proportion of AF patients experience palpitations, defined as an increased perception of the heartbeat. The sensory and mechanistic pathways underlying palpitations have yet to be defined.20,21 Interestingly, previous studies have reported that the neural stimuli for palpitations may not originate from the myocardium. Barsky et al. demonstrated that despite the absence of cardiac innervation, one-third of heart transplant recipients were accurately aware of their resting heartbeat.22 Further research is necessary to identify afferent neural pathways that transmit sensory information during palpitations.
Chest pain
Chest pain often occurs during episodes of AF, even in the absence of overt structural heart diseases.23 Although chest pain may be a nonspecific sensation of abnormal cardiac motion, it may also relate to impaired myocardial perfusion.24 It is unlikely that impaired myocardial perfusion is solely due to fast ventricular rates during AF, since chest pain also is observed in AF patients with slow ventricular rates.25 Previous studies have shown that coronary vascular resistance is elevated in patients with AF.26,27 Other factors, such as the irregularity of the ventricular response, as well as alteration in the sympathetic nervous system activation and the renin-angiotensin system also may contribute to the development of chest pain.24,26,27
Reduced exercise capacity, dyspnea and fatigue
Over half of all AF patients experience a reduction in exercise capacity measured by the New York Heart Association class.11 Reduced functional capacity may be a non-specific symptom or maybe due to symptoms such as dyspnea.28 Exercise performance depends on a number of factors including cardiac output and oxygen transport, which in turn depend on respiratory function. It has been proposed that during AF, cardiac output maybe compromised due to impaired diastolic filling secondary to rapid ventricular rates.29 Diastolic dysfunction in AF also may increase left-sided intracardiac pressures and predispose patients to episodes of subclinical pulmonary edema.30 Interestingly though, a hemodynamic study of patients in sinus rhythm or after the acute induction of AF, demonstrated that the arrhythmia was associated with normal or even low intracardiac pressures.31 Furthermore, structural heart diseases, accompanying AF, may be an important determinant of reduced exercise capacity. Lastly, AF-related stroke may lead to disability and subsequently reduce exercise capacity.
Dyspnea and reduced functional capacity also may be an indirect consequence of AF. Multiple studies have shown that long-term AF may induce left ventricular dysfunction or a tachycardiomyopathy. The loss of atrioventricular synchrony and the rapid ventricular rate are the primary mechanisms thought to adversely affect ventricular function and overall hemodynamic status.32,33 However, AF can also predispose to left ventricular dysfunction and tachycardiomyopathy at normal heart rates, presumably due to irregularity of RR intervals.32–35 Left ventricular dysfunction and heart failure may cause dyspnea and a reduction in exercise capacity. Conversion to sinus rhythm may improve left ventricular systolic function and reverse tachycardiomyopathy.36,37 Adequate rate control, however, may also improve left ventricular systolic dysfunction and AF-related symptoms.38,39
Dizziness
Dizziness, syncope and presyncope are relatively uncommon symptoms related to AF. Sympathovagal imbalance may play a role in patients with AF and dizziness.40 Increased sympathetic and parasympathetic impulses can both result in adverse hemodynamic effects, thus predicting the specific effects of these impulses during AF is challenging.18 Other potential mechanisms of dizziness or syncope include sinus node dysfunction with pauses upon conversion of AF to sinus rhythm or rapid ventricular rates in patients with underlying conditions such as hypertrophic cardiomyopathy, valvular stenosis, or an accessory pathway.41 In the absence of underlying cardiac conditions or a bypass tract, AF is unlikely to cause significant adverse hemodynamic effects.31
Future research
An in-depth understanding of pathophysiological mechanisms underlying AF symptomatology is likely to enhance our ability to more clearly define the relation between symptoms and arrhythmia. Although it is difficult to dissect whether symptoms are caused by AF itself or by other cardiac diseases, well-designed studies including both individuals with various types of AF, stratifying or adjusting for age, sex, race, socioeconomic status, pattern of AF, and medication use are warranted. The role of other cardiovascular diseases and different patterns of AF (paroxysmal, persistent and permanent AF) in generating symptoms needs to be determined. Emerging research in the fields of atrial imaging, biomarkers, and genomics of AF may elucidate pathophysiological mechanisms underlying AF-related symptoms.
Symptoms and documentation of AF
In patients with AF, it often remains unclear why some patients are asymptomatic whereas others are severely symptomatic. Both somatic and psychological factors20 are likely to contribute to the complex relation between symptoms and arrhythmia.42–44 A sub-analysis of the AFFIRM trial reported that patients with asymptomatic AF have less severe cardiac disease.15
Approximately 15–30% of patients with AF are asymptomatic.14–16 Asymptomatic AF is often discovered incidentally, for example during population surveys or when patients undergo routine physical examinations. In approximately 15–25% of patients with AF, stroke is the initial presenting sign of AF.45,46 Similarly, data from patients with implantable pacemakers or defibrillators revealed that high percentages (up to 70%) of paroxysms of AF are asymptomatic. 26,47–50 Thus, the simple awareness of symptoms is not a good discriminator of the presence or absence, nor the severity of the arrhythmia.
In patients with persistent AF, particularly elderly patients, symptoms decrease or may even disappear with longer durations of the arrhythmia, and AF may become permanent.14,15,41 Conversely, the presence of symptoms may prompt the clinician to interrupt the progression from persistent to permanent AF by more vigorous pursuit of rhythm control strategies. Symptoms also may abate when patients are commenced on pharmacological therapy, in some cases despite the persistence of arrhythmia.51,52 For instance, the SOFAT trial reported an inverse correlation between symptoms and use of antiarrhythmics, and a direct correlation between symptoms and high ventricular rates.53
The discordance between symptoms and the presence of AF is of particular relevance because as mentioned previously, recurrence of symptomatic AF is currently used to evaluate success of pulmonary vein ablation therapy.54–59 Not surprisingly, several reports demonstrated an underestimation of the recurrence rate of the arrhythmia, and an overestimation of the success rate of pulmonary vein ablation, because a high proportion of recurrences of AF are asymptomatic.60,61
Future research
Although it is known that there is a weak association between AF-related symptoms and the actual rhythm, little is known about influencing factors. Taking advantage of the implantable cardiac rhythm recorders such as implantable pacemakers, defibrillators, and loop recorders, it is possible to study the longitudinal history of symptoms in the same individual, and to determine why some episodes of AF are symptomatic and others asymptomatic. Information regarding the influence of AF-therapies or daily activities on specific symptoms on the transition from symptomatic to asymptomatic AF is within reach. Furthermore, current interest in the heritability and genetics of AF offer an opportunity to study whether presence or absence of AF-related symptoms are heritable.
AF-related symptoms systematic measures
In an attempt to provide a more objective assessment of symptoms and a more accurate measure of response to therapy, a number of questionnaires have been developed for patients with AF (summarized in Table 2). The majority of such questionnaires have been developed for research purposes. The most widely used symptom-scoring systems are the Symptom Checklist—Frequency and Severity Scale, (specifically developed for cardiac arrhythmias by Bubien and Kay, modified by Jenkins),65 and the University of Toronto Atrial Fibrillation Severity Scale.66,67 Published validation data for the AF specific scales are sparse. Whether the reproducibility of the symptoms measures is sufficient, and whether the symptom measures are generalizable to all AF subjects is unknown.
Table 2.
Selected scoring systems for symptoms related to AF.
| Measure, Year Described | Description | Scores | Design/Validation Cohort | Comments/Limitations |
|---|---|---|---|---|
| Symptom Checklist - Frequency and Severity Scale,62–65 1989 | Score based on severity and frequency of symptoms (palpitations, dyspnea, dizziness, exercise intolerance, chest discomfort, and syncope) | 0–64 for frequency 0–48 for severity |
Validated in multiple cohorts (pacemaker, atrioventricular node ablation for AF, radiofrequency catheter ablation for supraventricular arrhythmias, and pulmonary vein ablation for AF) |
Advantages:
|
Limitations:
| ||||
|
| ||||
| University of Toronto Atrial Fibrillation Severity Scale,66–69 1998 | 14-item disease-specific scale - subjective and objective ratings of AF disease burden, including frequency, duration, and patient perceived severity of episodes, and health care utilization | 3–30 | Validated in patients with paroxysmal and persistent AF, included in the Canadian Trial of Atrial Fibrillation |
Advantages:
|
Limitations:
| ||||
|
| ||||
| Canadian Cardiovascular Society Severity of Atrial Fibrillation Scale,70,71 2009 | Score determined using three steps:
|
0–4 | Designed by members of the Primary Panel of the Canadian Cardiovascular Society Consensus Conference on Atrial Fibrillation |
Advantages:
|
| Validated in large cohort of paroxysmal, persistent and permanent AF |
Limitations:
|
|||
|
| ||||
| Atrial Fibrillation 6 Scale,72 2009 | 6 questions focusing on dyspnea at rest, exertion, limitations in daily life, feeling of discomfort, fatigue and worry/anxiety | 0–60 (1–10 on a Likert scale for each question) | Designed for patients at the AF clinic |
Advantages:
|
| Validated in AF patients peri-cardioversion |
Limitations:
|
|||
|
| ||||
| European Heart Rhythm Association (EHRA) classification,73 2007 | Classification based exclusively on patient reported symptoms and impact on normal daily activities | EHRA class I–IV | Proposed by panel of experts of European Heart Rhythm Association |
Proposed advantage:
|
| No validation |
Limitations:
|
|||
A panel of experts of European Heart Rhythm Association proposed a novel AF-related classification based exclusively on patient reported symptoms and impact on normal daily activities.73 Although not yet validated, it has been recommended in the recent European Society of Cardiology guidelines.74
The development and clinical use of scoring systems for AF-related symptoms is challenging for a number of reasons. First, there is a weak correlation between symptoms and arrhythmia. Second, systematic measures of both symptoms and functional capacity are strongly influenced by age, sex, race, socioeconomic situation, and concomitant cardiovascular conditions.75,76 Finally, the relations of symptoms to meaningful outcomes and prognosis have not been well-examined.76 A number of potential solutions have been suggested including combining existing AF-related symptoms measures with more generalized quality of life measures,76,77 or developing shorter, more specific scales that focus on symptoms related to AF.57,78–80
Recently, a novel questionnaire (Atrial Fibrillation Effect on QualiTy-of-life [AFEQT]) has been developed and validated by a broad range of researchers involved in the development of the previous symptom measures.81 The 20-item AFEQT measure consists of a 4-item Symptoms score, an 8-item Daily Activities score, a 6-item Treatment Concerns score, and a 2-item Treatment Satisfaction scale. The AFEQT score combines symptoms, functional status and quality of life in one measure. The score has been shown to be valid, reliable and responsive to clinical change. It represents an important step forward, although the tool will require further testing to assess whether it overcomes limitations of the other AF-related symptom measures.
Future research
As was suggested by Coyne et al. in 200580 for health outcome quality of life measures in AF, more research is warranted to increase the validity, reproducibility, test-retest reliability, accuracy of day-to-day variability, and generalizability of the AF-symptoms measures. Most measures were developed and tested in specific hospital-based cohorts or randomized trials; the validity in other settings is unknown and needs more study. Further, most measures have not been widely validated in different age groups, races and ethnicities, or socioeconomic backgrounds. Whether these symptom scores have specificity for AF-related symptoms versus symptoms caused by other cardiac diseases is largely unknown. To increase the comparability of trials using symptoms as outcome measures, a consensus will need to emerge among investigators and clinicians regarding which measures to use rather than developing new measures for each specific purpose. Finally, integrating these measures into management of AF will be critical.
Functional status systematic measures
As discussed previously, in a significant proportion of AF patients a reduction in exercise tolerance is the major presenting symptom. Previous studies have reported that in many AF patients, the reduction in exercise tolerance is on the order of 15–20%.28 It is therefore of clinical interest to measure functional status in patients with AF, both as a means of measuring symptom burden and as an outcome measure for therapeutic interventions.82
Similar to measures of AF-related symptoms, multiple tests are available for measuring functional status in AF, each of which has specific limitations (summarized in Supplementary Table 1).83 The most commonly used subjective measures are the New York Heart Association classification, the Canadian Cardiovascular Society classification, Duke Activity Scale Index, and the Goldman Specific Activity Scale. More objective measures include the 6-minute walk test or an exercise stress test.83 Importantly, the published measures have not been specifically designed or validated in patients with AF.
Review of the literature indicates that different studies have variably used terms such as functional performance, functional capacity, level of impairment, quality of life health status, physical functioning and activities of daily living to describe an individual’s functional status.83 Although many times these measures are used interchangeably, there are some subtle differences between the terminologies utilized to describe functional status. For instance, functional performance refers to the ability to perform day-to-day activities, specifically “to meet basic needs, fulfill usual roles, and maintain the health and well-being”.83 In contrast, functional capacity refers to an individual’s maximum potential to carry out activities of daily living or self-care. Whereas the New York Heart Association classification, the Canadian Cardiovascular Society Classification, and Goldman Specific Activity Scale are measures of functional performance, the Duke Activity Scale Index, the 6-minute walk, and exercise stress testing are measures of functional capacity.
The utilization of a variety of measures makes comparison of outcomes from different studies describing relations between functional status and AF difficult. Each of the functional status measures has specific characteristics in terms of sensitivity, discrimination, scaling, reliability, validity and applicability. The lack of consistency between studies has led to challenges in interpreting study results, and making comparisons between studies.
Although the functional status measures have been of value for research purposes to quantify the functional limitation in patients with AF, they are associated with some major drawbacks. There is no clearly defined relation between AF and functional capacity. Also, there is not much known regarding confounding by age, sex, race, socioeconomic background, other cardiac diseases, and medication use.83 As a result, the use of functional capacity as the sole outcome measure of AF therapies may be insufficient. In addition, some functional measures are associated with specific limitations e.g. exercise tests and walk tests may not be possible in elderly patients or patients with disabilities.
Future research
Considerations regarding validity, reproducibility and generalizability of the instruments also are applicable to the functional status assessments in AF. Since most measures are not specifically developed for AF, investigating the value of functional measures in AF patients will be essential.
Impact of therapies on AF-related symptoms
Trials that have investigated the relation of therapeutic interventions on symptom burden and functional status are summarized in Supplementary Table 2.
Pharmacological rate- and rhythm control therapies
Large randomized trials have demonstrated that a rate-control strategy was non-inferior to a rhythm-control strategy in terms of cardiovascular morbidity and mortality. In the majority of trials that directly compared the two treatment strategies, neither strategy was superior for improving AF-related symptoms.11,12,78,84–86 It should be noted that severely symptomatic patients were not included in the large rate versus rhythm control trials. The effect of the treatment strategy on functional status in these trials was less clear and in some cases depended on the specific tool used to measure functional capacity. For instance, whereas improvements in exercise tolerance were reported in the rhythm control arm using objective measures such as a 6 minute walk test and treadmill exercise test,78,85,87 the results were not consistent when using qualitative measures such as New York Heart Association and Canadian Cardiovascular Society classifications.78,84,85,87 Of note, the reported improvements in functional class were relatively modest. Interestingly, the presence of other cardiovascular conditions appeared to have a greater impact than AF per se in these trials.87,88 A number of sub-studies of rate versus rhythm control trials, and a recently published randomized trial also investigated the relations between the achieved heart rate and symptoms in the rate control arm.89–91 The rate-control studies reported no relation between heart rate and symptoms or functional status.
Multiple small, non-randomized studies have shown that rhythm control therapy with cardioversion results in an improvement in exercise capacity, typically within the range of 10% to 20%.28,37,92,93 Two trials compared different pharmacological rhythm control agents and found that restoration and maintenance of sinus rhythm was associated with improvements in both symptoms and functional status.94,95 However once again, the magnitude of difference in these studies was modest; 15–20% relative changes in Symptom Checklist scores were seen.76 Another trial compared different amiodarone regimes, found no improvement in symptoms with either treatment strategy.96 Not surprisingly, the greatest improvement in symptom reduction and functional status, if any, was seen in those with the most severe symptoms and worst functional status at baseline.
Non-pharmacological rate control therapies
In selected patients with severely symptomatic AF and uncontrollable heart rates despite antiarrhythmic drug therapy, atrioventricular node ablation with permanent pacemaker implantation maybe an effective alternative. Many, mostly non-randomized, studies have addressed the impact of this treatment strategy on symptoms related to AF and quality of life.97 In two studies, atrioventricular node ablation and pacemaker implantation was associated with a reduction in symptom scores between 18–30%.98,99 Functional capacity was measured in one of the trials and did not significantly improve in the treatment arm. In a meta-analysis including 21 studies with a total of 1,181 patients, both symptoms and functional status (measured with a variety of scales and scores) improved after ablation and pacing therapy.97
An alternative rate control approach, which only has been used in research settings, is to implant a permanent pacemaker with ventricular response pacing algorithms in patients with intact atrioventricular node conduction.100 The ventricular response-pacing algorithm is designed to promote regularity of the ventricular rate whilst ensuring that the mean ventricular rate does not increase. However, in a small crossover study including of 45 patients ventricular response pacing reduced the severity of AF-related symptoms but did not improve functional status (Duke Activity Status Index and 6-minute walk test).100
Non-pharmacological rhythm control therapies
Alternatives for pharmacological rhythm control for treatment of AF include Maze surgery and PVI for AF. The use of Maze surgery to treat isolated AF has decreased dramatically, mainly because of the emergence of less-invasive alternatives. There are several small series showing the efficacy of the Maze procedure; however, data regarding symptom reduction and functional status improvement are sparse.101,102 Multiple, mostly non-randomized, PVI studies have reported an improvement in symptoms (measured with the Symptom Checklist or a derived score based on the Symptom Checklist).76 A few small randomized controlled trails have compared PVI to other treatment strategies.103 In two randomized multicenter studies (n=112 and n=167) of patients with paroxysmal AF who failed on 1 antiarrhythmic drug,59,104 PVI was reported to be superior to antiarrhythmic drugs, both in terms of short and intermediate maintenance of sinus rhythm, and improvement in symptoms and functional status (as measured by Symptom Checklist and exercise test respectively). In a trial of heart failure patients comparing PVI to atrioventricular node ablation and biventricular pacemaker implantation, PVI was reported to be superior in terms of functional status measured by 6-minute-walk distance after 6 months.105
It is difficult to compare results of PVI studies to pharmacological rhythm control studies because of differences in patient characteristics. However, it is likely that in the subset of patients with paroxysmal AF with a high symptom burden, PVI is more effective than pharmacological rhythm control in terms of short- to intermediate-term symptom alleviation,59 even allowing for ascertainment bias from unblinded evaluation.106
Exercise training and impact on AF-related symptoms
Interestingly, in a few studies an exercise-training program improved the symptoms (Symptom Checklist) and functional status (exercise test) of AF patients.107–109 A specific exercise-training program might be an interesting treatment approach for AF, although more research is needed to support this therapy.
Future research
A more uniform and standardized approach to measuring AF-related symptoms and functional status is of vital importance to the field. Another important area of inquiry involves the relation of symptom and functional status scores to study endpoints and prognosis. Randomized clinical trials specifically aiming to reduce AF-related symptoms and functional status are needed. Also, prospective evaluation of AF-related symptoms and functional status and use of specific therapies to reduce symptom burden is essential. Lastly, the effects of AF-related symptoms and functional status on healthcare utilization and costs require further study.
Future research opportunities
In Table 3 we summarize potential future research topics detailed after each section to clarify the relation between AF and symptoms. To address these needs we advocate a multi-pronged cross-disciplinary and translational research program capitalizing on experimental, observational databases and randomized controlled trials. The main opportunities for future research are to (1) determine the variation in AF-related symptoms by patient demographics, comorbid conditions, therapies, and AF subtype; (2) determine pathophysiological mechanisms underlying AF-related symptoms; (3) describe the temporal relations between the presence versus absence of AF-related symptoms; (4) enhance the validity, reproducibility and generalizability of the AF-related symptoms and functional status measures, (5) standardize the methods of measuring AF-related symptoms in both clinical trials and clinical practice to enhance the interpretability and comparability of AF trials, (6) determine whether AF-related symptom and functional status measures are related to meaningful health outcomes, healthcare utilization and costs of care.
Table 3.
Future directions.
| Nr. | Field | Goals |
|---|---|---|
| 1 | Symptoms related to AF |
|
| 2 | Pathophysiology of AF- related symptoms |
|
| 3 | Temporal relation between AF and symptoms |
|
| 4 | Systematic AF- related symptom and functional status measures |
|
| 5 | AF-symptoms as outcome measure of AF-therapies |
|
In Bold are key recommendations.
Conclusions
Although there are still multiple unanswered questions on the mechanisms of symptoms and functional status in AF, and the impact of other cardiac diseases and AF therapies, the symptom burden associated with AF is a major consideration when deciding on the treatment strategy. Based on the available literature, AF therapies are only moderately effective in relieving symptoms and improving functional status. With the emergence of ablation techniques as a treatment for AF, more accurate assessment of symptom burden and functional status is increasingly important. More systematic research is urgently warranted to answer the many unresolved questions on the relation between symptoms and AF.
Supplementary Material
Acknowledgments
Sources of funding
Dr. Rienstra is supported by a grant from the Netherlands Organization for Scientific Research (Rubicon grant 825.09.020). This work was supported by grants from the NIH to Drs. Benjamin and Ellinor (1RO1HL092577), Dr. Benjamin (RO1AG028321, RC1-HL01056, 1R01HL102214); Dr. Magnani (R21) and Benjamin (Evans Center for Interdisciplinary Biomedical Research ARC on “Atrial Fibrillation at Boston University http://www.bumc.bu.edu/evanscenteribr/) and Dr. Ellinor (5R21DA027021, 1RO1HL104156, 1K24HL105780) and 6R01-NS 17950, N01-HC 25195. Dr. Magnani is supported by American Heart Association Award 09FTF219028. Dr. Sinner is supported by the German Heart Foundation. Dr. Van Gelder is supported by the Netherlands Heart Foundation, Interuniversity Cardiology Institute The Netherlands, Astra Zeneca, Biotronik, Medtronic, Sanofi-Aventis, Boehringer Ingelheim, St Jude Medical, Boston Scientific.
Footnotes
Disclosures
Dr. Van Gelder is receiving honoraria from Medtronic, MSD, Sanofi-Aventis, Boehringer Ingelheim, AGA Medical.
References
- 1.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: Population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The Framingham study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: The Framingham heart study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 4.Wolf PA, Benjamin EJ, Belanger AJ, Kannel WB, Levy D, D’Agostino RB. Secular trends in the prevalence of atrial fibrillation: The Framingham study. Am Heart J. 1996;131:790–795. doi: 10.1016/s0002-8703(96)90288-4. [DOI] [PubMed] [Google Scholar]
- 5.Hannon N, Sheehan O, Kelly L, Marnane M, Merwick A, Moore A, Kyne L, Duggan J, Moroney J, McCormack PM, Daly L, Fitz-Simon N, Harris D, Horgan G, Williams EB, Furie KL, Kelly PJ. Stroke associated with atrial fibrillation-incidence and early outcomes in the North Dublin population stroke study. Cerebrovasc Dis. 2010;29:43–49. doi: 10.1159/000255973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS, Anderson JL, Muhlestein JB, Horne BD, Lappe DL, Day JD. Atrial fibrillation is independently associated with senile, vascular, and alzheimer’s dementia. Heart Rhythm. 2010;7:433–437. doi: 10.1016/j.hrthm.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Dublin S, Anderson ML, Haneuse SJ, Heckbert SR, Crane PK, Breitner JC, McCormick W, Bowen JD, Teri L, McCurry SM, Larson EB. Atrial fibrillation and risk of dementia: A prospective cohort study. J Am Geriatr Soc. 2011;59:1369–1375. doi: 10.1111/j.1532-5415.2011.03508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyasaka Y, Barnes ME, Bailey KR, Cha SS, Gersh BJ, Seward JB, Tsang TS. Mortality trends in patients diagnosed with first atrial fibrillation: A 21-year community-based study. J Am Coll Cardiol. 2007;49:986–992. doi: 10.1016/j.jacc.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 9.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 10.Zimetbaum P, Reynolds MR, Ho KK, Gaziano T, McDonald MJ, McClennen S, Berezin R, Josephson ME, Cohen DJ. Impact of a practice guideline for patients with atrial fibrillation on medical resource utilization and costs. Am J Cardiol. 2003;92:677–681. doi: 10.1016/s0002-9149(03)00821-x. [DOI] [PubMed] [Google Scholar]
- 11.Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJ, Tijssen JG, Crijns HJ. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 12.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 13.Einthoven W. Le telecardiogramme. Arch Int Physiol. 1906;4:132–164. [Google Scholar]
- 14.Levy S, Maarek M, Coumel P, Guize L, Lekieffre J, Medvedowsky JL, Sebaoun A. Characterization of different subsets of atrial fibrillation in general practice in France: The ALFA study. The college of French cardiologists. Circulation. 1999;99:3028–3035. doi: 10.1161/01.cir.99.23.3028. [DOI] [PubMed] [Google Scholar]
- 15.Flaker GC, Belew K, Beckman K, Vidaillet H, Kron J, Safford R, Mickel M, Barrell P. Asymptomatic atrial fibrillation: Demographic features and prognostic information from the atrial fibrillation follow-up investigation of rhythm management (AFFIRM) study. Am Heart J. 2005;149:657–663. doi: 10.1016/j.ahj.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 16.Kerr C, Boone J, Connolly S, Greene M, Klein G, Sheldon R, Talajic M. Follow-up of atrial fibrillation: The initial experience of the Canadian registry of atrial fibrillation. Eur Heart J. 1996;17 (Suppl C):48–51. doi: 10.1093/eurheartj/17.suppl_c.48. [DOI] [PubMed] [Google Scholar]
- 17.Nieuwlaat R, Capucci A, Camm AJ, Olsson SB, Andresen D, Davies DW, Cobbe S, Breithardt G, Le Heuzey JY, Prins MH, Levy S, Crijns HJ. Atrial fibrillation management: A prospective survey in ESC member countries: The Euro heart survey on atrial fibrillation. Eur Heart J. 2005;26:2422–2434. doi: 10.1093/eurheartj/ehi505. [DOI] [PubMed] [Google Scholar]
- 18.MacRae CA. Symptoms in atrial fibrillation: Why keep score? Circ Arrhythm Electrophysiol. 2009;2:215–217. doi: 10.1161/CIRCEP.109.878355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: Epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91:2D–8D. doi: 10.1016/s0002-9149(02)03373-8. [DOI] [PubMed] [Google Scholar]
- 20.Sears SF, Serber ER, Alvarez LG, Schwartzman DS, Hoyt RH, Ujhelyi MR. Understanding atrial symptom reports: Objective versus subjective predictors. Pacing Clin Electrophysiol. 2005;28:801–807. doi: 10.1111/j.1540-8159.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- 21.Bhandari AK, Anderson JL, Gilbert EM, Alpert BL, Henthorn RW, Waldo AL, Cullen MT, Jr, Hawkinson RW, Pritchett EL. Correlation of symptoms with occurrence of paroxysmal supraventricular tachycardia or atrial fibrillation: A transtelephonic monitoring study. The flecainide supraventricular tachycardia study group. Am Heart J. 1992;124:381–386. doi: 10.1016/0002-8703(92)90601-q. [DOI] [PubMed] [Google Scholar]
- 22.Barsky AJ, Ahern DK, Brener J, Surman OS, Ring C, Dec GW. Palpitations and cardiac awareness after heart transplantation. Psychosom Med. 1998;60:557–562. doi: 10.1097/00006842-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Brown AM, Sease KL, Robey JL, Shofer FS, Hollander JE. The risk for acute coronary syndrome associated with atrial fibrillation among ed patients with chest pain syndromes. Am J Emerg Med. 2007;25:523–528. doi: 10.1016/j.ajem.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Goette A, Bukowska A, Dobrev D, Pfeiffenberger J, Morawietz H, Strugala D, Wiswedel I, Rohl FW, Wolke C, Bergmann S, Bramlage P, Ravens U, Lendeckel U. Acute atrial tachyarrhythmia induces angiotensin II type 1 receptor-mediated oxidative stress and microvascular flow abnormalities in the ventricles. Eur Heart J. 2009;30:1411–1420. doi: 10.1093/eurheartj/ehp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connolly SJ, Schnell DJ, Page RL, Wilkinson WE, Marcello SR, Pritchett EL. Symptoms at the time of arrhythmia recurrence in patients receiving azimilide for control of atrial fibrillation or flutter: Results from randomized trials. Am Heart J. 2003;146:489–493. doi: 10.1016/S0002-8703(03)00250-3. [DOI] [PubMed] [Google Scholar]
- 26.Kochiadakis GE, Skalidis EI, Kalebubas MD, Igoumenidis NE, Chrysostomakis SI, Kanoupakis EM, Simantirakis EN, Vardas PE. Effect of acute atrial fibrillation on phasic coronary blood flow pattern and flow reserve in humans. Eur Heart J. 2002;23:734–741. doi: 10.1053/euhj.2001.2894. [DOI] [PubMed] [Google Scholar]
- 27.Range FT, Schafers M, Acil T, Schafers KP, Kies P, Paul M, Hermann S, Brisse B, Breithardt G, Schober O, Wichter T. Impaired myocardial perfusion and perfusion reserve associated with increased coronary resistance in persistent idiopathic atrial fibrillation. Eur Heart J. 2007;28:2223–2230. doi: 10.1093/eurheartj/ehm246. [DOI] [PubMed] [Google Scholar]
- 28.Ueshima K, Myers J, Graettinger WF, Atwood JE, Morris CK, Kawaguchi T, Froelicher VF. Exercise and morphologic comparison of chronic atrial fibrillation and normal sinus rhythm. Am Heart J. 1993;126:260–261. doi: 10.1016/s0002-8703(07)80049-4. [DOI] [PubMed] [Google Scholar]
- 29.Skinner NS, Jr, Mitchell JH, Wallace AG, Sarnoff SJ. Hemodynamic consequences of atrial fibrillation at constant ventricular rates. Am J Med. 1964;36:342–350. doi: 10.1016/0002-9343(64)90160-3. [DOI] [PubMed] [Google Scholar]
- 30.Lau CP, Leung WH, Wong CK, Cheng CH. Haemodynamics of induced atrial fibrillation: A comparative assessment with sinus rhythm, atrial and ventricular pacing. Eur Heart J. 1990;11:219–224. doi: 10.1093/oxfordjournals.eurheartj.a059687. [DOI] [PubMed] [Google Scholar]
- 31.Alboni P, Scarfo S, Fuca G, Paparella N, Yannacopulu P. Hemodynamics of idiopathic paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 1995;18:980–985. doi: 10.1111/j.1540-8159.1995.tb04738.x. [DOI] [PubMed] [Google Scholar]
- 32.Clark DM, Plumb VJ, Epstein AE, Kay GN. Hemodynamic effects of an irregular sequence of ventricular cycle lengths during atrial fibrillation. J Am Coll Cardiol. 1997;30:1039–1045. doi: 10.1016/s0735-1097(97)00254-4. [DOI] [PubMed] [Google Scholar]
- 33.Daoud EG, Weiss R, Bahu M, Knight BP, Bogun F, Goyal R, Harvey M, Strickberger SA, Man KC, Morady F. Effect of an irregular ventricular rhythm on cardiac output. Am J Cardiol. 1996;78:1433–1436. doi: 10.1016/s0002-9149(97)89297-1. [DOI] [PubMed] [Google Scholar]
- 34.Natale A, Zimerman L, Tomassoni G, Kearney M, Kent V, Brandon MJ, Newby K. Impact on ventricular function and quality of life of transcatheter ablation of the atrioventricular junction in chronic atrial fibrillation with a normal ventricular response. Am J Cardiol. 1996;78:1431–1433. doi: 10.1016/s0002-9149(97)89296-x. [DOI] [PubMed] [Google Scholar]
- 35.Farshi R, Kistner D, Sarma JS, Longmate JA, Singh BN. Ventricular rate control in chronic atrial fibrillation during daily activity and programmed exercise: A crossover open-label study of five drug regimens. J Am Coll Cardiol. 1999;33:304–310. doi: 10.1016/s0735-1097(98)00561-0. [DOI] [PubMed] [Google Scholar]
- 36.Peters KG, Kienzle MG. Severe cardiomyopathy due to chronic rapidly conducted atrial fibrillation: Complete recovery after restoration of sinus rhythm. Am J Med. 1988;85:242–244. doi: 10.1016/s0002-9343(88)80352-8. [DOI] [PubMed] [Google Scholar]
- 37.Van Gelder IC, Crijns HJ, Blanksma PK, Landsman ML, Posma JL, Van Den Berg MP, Meijler FL, Lie KI. Time course of hemodynamic changes and improvement of exercise tolerance after cardioversion of chronic atrial fibrillation unassociated with cardiac valve disease. Am J Cardiol. 1993;72:560–566. doi: 10.1016/0002-9149(93)90352-d. [DOI] [PubMed] [Google Scholar]
- 38.Grogan M, Smith HC, Gersh BJ, Wood DL. Left ventricular dysfunction due to atrial fibrillation in patients initially believed to have idiopathic dilated cardiomyopathy. Am J Cardiol. 1992;69:1570–1573. doi: 10.1016/0002-9149(92)90705-4. [DOI] [PubMed] [Google Scholar]
- 39.Lazzari JO, Gonzalez J. Reversible high rate atrial fibrillation dilated cardiomyopathy. Heart. 1997;77:486. doi: 10.1136/hrt.77.5.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van den Berg MP, Hassink RJ, Tuinenburg AE, Lefrandt JD, de Kam PJ, Crijns HJ. Impaired autonomic function predicts dizziness at onset of paroxysmal atrial fibrillation. Int J Cardiol. 2001;81:175–180. doi: 10.1016/s0167-5273(01)00564-2. [DOI] [PubMed] [Google Scholar]
- 41.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Zamorano JL. Acc/aha/esc 2006 guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association task force on practice guidelines and the European Society of Cardiology committee for practice guidelines (writing committee to revise the 2001 guidelines for the management of patients with atrial fibrillation): Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 42.Barsky AJ, Cleary PD, Barnett MC, Christiansen CL, Ruskin JN. The accuracy of symptom reporting by patients complaining of palpitations. Am J Med. 1994;97:214–221. doi: 10.1016/0002-9343(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 43.Zeldis SM, Levine BJ, Michelson EL, Morganroth J. Cardiovascular complaints. Correlation with cardiac arrhythmias on 24-hour electrocardiographic monitoring. Chest. 1980;78:456–461. doi: 10.1378/chest.78.3.456. [DOI] [PubMed] [Google Scholar]
- 44.Clark PI, Glasser SP, Spoto E., Jr Arrhythmias detected by ambulatory monitoring. Lack of correlation with symptoms of dizziness and syncope. Chest. 1980;77:722–725. doi: 10.1378/chest.77.6.722. [DOI] [PubMed] [Google Scholar]
- 45.Lin HJ, Wolf PA, Benjamin EJ, Belanger AJ, D’Agostino RB. Newly diagnosed atrial fibrillation and acute stroke. The Framingham study. Stroke. 1995;26:1527–1530. doi: 10.1161/01.str.26.9.1527. [DOI] [PubMed] [Google Scholar]
- 46.Asberg S, Henriksson KM, Farahmand B, Asplund K, Norrving B, Appelros P, Stegmayr B, Asberg KH, Terent A. Ischemic stroke and secondary prevention in clinical practice: A cohort study of 14,529 patients in the Swedish stroke register. Stroke. 2010;41:1338–1342. doi: 10.1161/STROKEAHA.110.580209. [DOI] [PubMed] [Google Scholar]
- 47.Fetsch T, Bauer P, Engberding R, Koch HP, Lukl J, Meinertz T, Oeff M, Seipel L, Trappe HJ, Treese N, Breithardt G. Prevention of atrial fibrillation after cardioversion: Results of the PAFAC trial. Eur Heart J. 2004;25:1385–1394. doi: 10.1016/j.ehj.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 48.Page RL, Wilkinson WE, Clair WK, McCarthy EA, Pritchett EL. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation. 1994;89:224–227. doi: 10.1161/01.cir.89.1.224. [DOI] [PubMed] [Google Scholar]
- 49.Page RL, Tilsch TW, Connolly SJ, Schnell DJ, Marcello SR, Wilkinson WE, Pritchett EL. Asymptomatic or “silent” atrial fibrillation: Frequency in untreated patients and patients receiving azimilide. Circulation. 2003;107:1141–1145. doi: 10.1161/01.cir.0000051455.44919.73. [DOI] [PubMed] [Google Scholar]
- 50.Glotzer TV, Hellkamp AS, Zimmerman J, Sweeney MO, Yee R, Marinchak R, Cook J, Paraschos A, Love J, Radoslovich G, Lee KL, Lamas GA. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: Report of the atrial diagnostics ancillary study of the mode selection trial (MOST) Circulation. 2003;107:1614–1619. doi: 10.1161/01.CIR.0000057981.70380.45. [DOI] [PubMed] [Google Scholar]
- 51.Wolk R, Kulakowski P, Karczmarewicz S, Karpinski G, Makowska E, Czepiel A, Ceremuzynski L. The incidence of asymptomatic paroxysmal atrial fibrillation in patients treated with propranolol or propafenone. Int J Cardiol. 1996;54:207–211. doi: 10.1016/0167-5273(96)02631-9. [DOI] [PubMed] [Google Scholar]
- 52.Savelieva I, Camm AJ. Clinical relevance of silent atrial fibrillation: Prevalence, prognosis, quality of life, and management. J Interv Card Electrophysiol. 2000;4:369–382. doi: 10.1023/a:1009823001707. [DOI] [PubMed] [Google Scholar]
- 53.Patten M, Maas R, Karim A, Muller HW, Simonovsky R, Meinertz T. Event-recorder monitoring in the diagnosis of atrial fibrillation in symptomatic patients: Subanalysis of the SOPAT trial. J Cardiovasc Electrophysiol. 2006;17:1216–1220. doi: 10.1111/j.1540-8167.2006.00609.x. [DOI] [PubMed] [Google Scholar]
- 54.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 55.Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, Salvati A, Dicandia C, Mazzone P, Santinelli V, Gulletta S, Chierchia S. Circumferential radiofrequency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation. 2000;102:2619–2628. doi: 10.1161/01.cir.102.21.2619. [DOI] [PubMed] [Google Scholar]
- 56.Pappone C, Rosanio S, Augello G, Gallus G, Vicedomini G, Mazzone P, Gulletta S, Gugliotta F, Pappone A, Santinelli V, Tortoriello V, Sala S, Zangrillo A, Crescenzi G, Benussi S, Alfieri O. Mortality, morbidity, and quality of life after circumferential pulmonary vein ablation for atrial fibrillation: Outcomes from a controlled nonrandomized long-term study. J Am Coll Cardiol. 2003;42:185–197. doi: 10.1016/s0735-1097(03)00577-1. [DOI] [PubMed] [Google Scholar]
- 57.Oral H, Pappone C, Chugh A, Good E, Bogun F, Pelosi F, Jr, Bates ER, Lehmann MH, Vicedomini G, Augello G, Agricola E, Sala S, Santinelli V, Morady F. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006;354:934–941. doi: 10.1056/NEJMoa050955. [DOI] [PubMed] [Google Scholar]
- 58.Hsu LF, Jais P, Sanders P, Garrigue S, Hocini M, Sacher F, Takahashi Y, Rotter M, Pasquie JL, Scavee C, Bordachar P, Clementy J, Haissaguerre M. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med. 2004;351:2373–2383. doi: 10.1056/NEJMoa041018. [DOI] [PubMed] [Google Scholar]
- 59.Jais P, Cauchemez B, Macle L, Daoud E, Khairy P, Subbiah R, Hocini M, Extramiana F, Sacher F, Bordachar P, Klein G, Weerasooriya R, Clementy J, Haissaguerre M. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: The A4 study. Circulation. 2008;118:2498–2505. doi: 10.1161/CIRCULATIONAHA.108.772582. [DOI] [PubMed] [Google Scholar]
- 60.Hindricks G, Piorkowski C, Tanner H, Kobza R, Gerds-Li JH, Carbucicchio C, Kottkamp H. Perception of atrial fibrillation before and after radiofrequency catheter ablation: Relevance of asymptomatic arrhythmia recurrence. Circulation. 2005;112:307–313. doi: 10.1161/CIRCULATIONAHA.104.518837. [DOI] [PubMed] [Google Scholar]
- 61.Senatore G, Stabile G, Bertaglia E, Donnici G, De Simone A, Zoppo F, Turco P, Pascotto P, Fazzari M. Role of transtelephonic electrocardiographic monitoring in detecting short-term arrhythmia recurrences after radiofrequency ablation in patients with atrial fibrillation. J Am Coll Cardiol. 2005;45:873–876. doi: 10.1016/j.jacc.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 62.Bubien R, Packa D, Karst G, Kay G. Does activity sensing rate adaptive pacing improve quality of life? Pacing Clin Electrophysiol. 1989;12:688. [Google Scholar]
- 63.Kay G, Bubien R, Karst G. Metabolic versus activity sensors for rate-adaptive pacing: A prospective comparison utilizing quality of life measurements. Pacing Clin Electrophysiol. 1989;12:641. [Google Scholar]
- 64.Bubien R, Kay G. A randomized comparison of quality of life and exercise capacity with DDD and VVIR pacing modes. Pacing Clin Electrophysiol. 1990;13:524. [Google Scholar]
- 65.Bubien RS, Knotts-Dolson SM, Plumb VJ, Kay GN. Effect of radiofrequency catheter ablation on health-related quality of life and activities of daily living in patients with recurrent arrhythmias. Circulation. 1996;94:1585–1591. doi: 10.1161/01.cir.94.7.1585. [DOI] [PubMed] [Google Scholar]
- 66.Maglio C, Sra J, Paquette M, Dorian P, Bygrave A, Wood K, Ayers G. Measuring quality of life and symptom severity in patients with atrial fibrillation. Pacing Clin Electrophysiol. 1998:21. [Google Scholar]
- 67.Dorian P, Jung W, Newman D, Paquette M, Wood K, Ayers GM, Camm J, Akhtar M, Luderitz B. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: Implications for the assessment of investigational therapy. J Am Coll Cardiol. 2000;36:1303–1309. doi: 10.1016/s0735-1097(00)00886-x. [DOI] [PubMed] [Google Scholar]
- 68.Paquette M, Roy D, Talajic M, Newman D, Couturier A, Yang C, Dorian P. Role of gender and personality on quality-of-life impairment in intermittent atrial fibrillation. Am J Cardiol. 2000;86:764–768. doi: 10.1016/s0002-9149(00)01077-8. [DOI] [PubMed] [Google Scholar]
- 69.Dorian P, Paquette M, Newman D, Green M, Connolly SJ, Talajic M, Roy D. Quality of life improves with treatment in the Canadian trial of atrial fibrillation. Am Heart J. 2002;143:984–990. doi: 10.1067/mhj.2002.122518. [DOI] [PubMed] [Google Scholar]
- 70.Dorian P, Cvitkovic SS, Kerr CR, Crystal E, Gillis AM, Guerra PG, Mitchell LB, Roy D, Skanes AC, Wyse DG. A novel, simple scale for assessing the symptom severity of atrial fibrillation at the bedside: The CCS-SAF scale. Can J Cardiol. 2006;22:383–386. doi: 10.1016/s0828-282x(06)70922-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dorian P, Guerra PG, Kerr CR, O’Donnell SS, Crystal E, Gillis AM, Mitchell LB, Roy D, Skanes AC, Rose MS, Wyse DG. Validation of a new simple scale to measure symptoms in atrial fibrillation: The Canadian cardiovascular society severity in atrial fibrillation scale. Circ Arrhythm Electrophysiol. 2009;2:218–224. doi: 10.1161/CIRCEP.108.812347. [DOI] [PubMed] [Google Scholar]
- 72.Harden M, Nystrom B, Kulich K, Carlsson J, Bengtson A, Edvardsson N. Validity and reliability of a new, short symptom rating scale in patients with persistent atrial fibrillation. Health Qual Life Outcomes. 2009;7:65. doi: 10.1186/1477-7525-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kirchhof P, Auricchio A, Bax J, Crijns H, Camm J, Diener HC, Goette A, Hindricks G, Hohnloser S, Kappenberger L, Kuck KH, Lip GY, Olsson B, Meinertz T, Priori S, Ravens U, Steinbeck G, Svernhage E, Tijssen J, Vincent A, Breithardt G. Outcome parameters for trials in atrial fibrillation: Recommendations from a consensus conference organized by the German atrial fibrillation competence network and the European Heart Rhythm Association. Europace. 2007;9:1006–1023. doi: 10.1093/europace/eum191. [DOI] [PubMed] [Google Scholar]
- 74.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation: The task force for the management of atrial fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 75.Reynolds MR, Lavelle T, Essebag V, Cohen DJ, Zimetbaum P. Influence of age, sex, and atrial fibrillation recurrence on quality of life outcomes in a population of patients with new-onset atrial fibrillation: The fibrillation registry assessing costs, therapies, adverse events and lifestyle (FRACTAL) study. Am Heart J. 2006;152:1097–1103. doi: 10.1016/j.ahj.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reynolds MR, Ellis E, Zimetbaum P. Quality of life in atrial fibrillation: Measurement tools and impact of interventions. J Cardiovasc Electrophysiol. 2008;19:762–768. doi: 10.1111/j.1540-8167.2007.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thrall G, Lane D, Carroll D, Lip GY. Quality of life in patients with atrial fibrillation: A systematic review. Am J Med. 2006;119:448 e441–419. doi: 10.1016/j.amjmed.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 78.Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation--pharmacological intervention in atrial fibrillation (PIAF): A randomised trial. Lancet. 2000;356:1789–1794. doi: 10.1016/s0140-6736(00)03230-x. [DOI] [PubMed] [Google Scholar]
- 79.Brignole M, Gianfranchi L, Menozzi C, Alboni P, Musso G, Bongiorni MG, Gasparini M, Raviele A, Lolli G, Paparella N, Acquarone S. Assessment of atrioventricular junction ablation and DDDR mode- switching pacemaker versus pharmacological treatment in patients with severely symptomatic paroxysmal atrial fibrillation: A randomized controlled study. Circulation. 1997;96:2617–2624. doi: 10.1161/01.cir.96.8.2617. [DOI] [PubMed] [Google Scholar]
- 80.Coyne K, Margolis MK, Grandy S, Zimetbaum P. The state of patient-reported outcomes in atrial fibrillation: A review of current measures. Pharmacoeconomics. 2005;23:687–708. doi: 10.2165/00019053-200523070-00004. [DOI] [PubMed] [Google Scholar]
- 81.Spertus J, Dorian P, Bubien R, Lewis S, Godejohn D, Reynolds MR, Lakkireddy DR, Wimmer AP, Bhandari A, Burk C. Development and validation of the atrial fibrillation effect on quality-of-life (AFEQT) questionnaire in patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2010 doi: 10.1161/CIRCEP.110.958033. [DOI] [PubMed] [Google Scholar]
- 82.Atwood JE, Myers JN, Tang XC, Reda DJ, Singh SN, Singh BN. Exercise capacity in atrial fibrillation: A substudy of the sotalol-amiodarone atrial fibrillation efficacy trial (SAFE-T) Am Heart J. 2007;153:566–572. doi: 10.1016/j.ahj.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 83.Coyne KS, Allen JK. Assessment of functional status in patients with cardiac disease. Heart Lung. 1998;27:263–273. doi: 10.1016/s0147-9563(98)90038-3. [DOI] [PubMed] [Google Scholar]
- 84.Carlsson J, Miketic S, Windeler J, Cuneo A, Haun S, Micus S, Walter S, Tebbe U. Randomized trail of rate-control versus rhythm-control in persistent atrial fibrillation: The strategies of treatment of atrial fibrillation (STAF) study. J Am Coll Cardiol. 2003;41:1690–1696. doi: 10.1016/s0735-1097(03)00332-2. [DOI] [PubMed] [Google Scholar]
- 85.Opolski G, Torbicki A, Kosior DA, Szulc M, Wozakowska-Kaplon B, Kolodziej P, Achremczyk P. Rate control vs rhythm control in patients with nonvalvular persistent atrial fibrillation: The results of the Polish how to treat chronic atrial fibrillation (HOT CAFE) study. Chest. 2004;126:476–486. doi: 10.1378/chest.126.2.476. [DOI] [PubMed] [Google Scholar]
- 86.Jenkins LS, Brodsky M, Schron E, Chung M, Rocco T, Jr, Lader E, Constantine M, Sheppard R, Holmes D, Mateski D, Floden L, Prasun M, Greene HL, Shemanski L. Quality of life in atrial fibrillation: The atrial fibrillation follow-up investigation of rhythm management (AFFIRM) study. Am Heart J. 2005;149:112–120. doi: 10.1016/j.ahj.2004.03.065. [DOI] [PubMed] [Google Scholar]
- 87.Chung MK, Shemanski L, Sherman DG, Greene HL, Hogan DB, Kellen JC, Kim SG, Martin LW, Rosenberg Y, Wyse DG. Functional status in rate- versus rhythm-control strategies for atrial fibrillation: Results of the atrial fibrillation follow-up investigation of rhythm management (AFFIRM) functional status substudy. J Am Coll Cardiol. 2005;46:1891–1899. doi: 10.1016/j.jacc.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 88.Rienstra M, van Gelder IC, Hagens VE, Veeger NJ, van Veldhuisen DJ, Crijns HJ. Mending the rhythm does not improve prognosis in patients with persistent atrial fibrillation: A subanalysis of the RACE study. Eur Heart J. 2006;27:357–364. doi: 10.1093/eurheartj/ehi637. [DOI] [PubMed] [Google Scholar]
- 89.Cooper HA, Bloomfield DA, Bush DE, Katcher MS, Rawlins M, Sacco JD, Chandler M. Relation between achieved heart rate and outcomes in patients with atrial fibrillation (from the atrial fibrillation follow-up investigation of rhythm management [AFFIRM] study) Am J Cardiol. 2004;93:1247–1253. doi: 10.1016/j.amjcard.2004.01.069. [DOI] [PubMed] [Google Scholar]
- 90.Groenveld HF, Crijns HJ, Rienstra M, Van den Berg MP, Van Veldhuisen DJ, Van Gelder IC. Does intensity of rate control influence outcome in persistent atrial fibrillation? Data of the RACE study. Am Heart J. 2009;158:785–791. doi: 10.1016/j.ahj.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 91.Van Gelder IC, Groenveld HF, Crijns HJ, Tuininga YS, Tijssen JG, Alings AM, Hillege HL, Bergsma-Kadijk JA, Cornel JH, Kamp O, Tukkie R, Bosker HA, Van Veldhuisen DJ, Van den Berg MP. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–1373. doi: 10.1056/NEJMoa1001337. [DOI] [PubMed] [Google Scholar]
- 92.Ueshima K, Myers J, Morris CK, Atwood JE, Kawaguchi T, Froelicher VF. The effect of cardioversion on exercise capacity in patients with atrial fibrillation. Am Heart J. 1993;126:1021–1024. doi: 10.1016/0002-8703(93)90732-o. [DOI] [PubMed] [Google Scholar]
- 93.Atwood JE, Myers J, Sullivan M, Forbes S, Sandhu S, Callaham P, Froelicher V. The effect of cardioversion on maximal exercise capacity in patients with chronic atrial fibrillation. Am Heart J. 1989;118:913–918. doi: 10.1016/0002-8703(89)90223-8. [DOI] [PubMed] [Google Scholar]
- 94.Roy D, Talajic M, Dorian P, Connolly S, Eisenberg MJ, Green M, Kus T, Lambert J, Dubuc M, Gagne P, Nattel S, Thibault B. Amiodarone to prevent recurrence of atrial fibrillation. Canadian trial of atrial fibrillation investigators. N Engl J Med. 2000;342:913–920. doi: 10.1056/NEJM200003303421302. [DOI] [PubMed] [Google Scholar]
- 95.Singh SN, Tang XC, Singh BN, Dorian P, Reda DJ, Harris CL, Fletcher RD, Sharma SC, Atwood JE, Jacobson AK, Lewis HD, Jr, Lopez B, Raisch DW, Ezekowitz MD. Quality of life and exercise performance in patients in sinus rhythm versus persistent atrial fibrillation: A veterans affairs cooperative studies program substudy. J Am Coll Cardiol. 2006;48:721–730. doi: 10.1016/j.jacc.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 96.Ahmed S, Ranchor AV, Crijns HJ, Van Veldhuisen DJ, Van Gelder IC. Effect of continuous versus episodic amiodarone treatment on quality of life in persistent atrial fibrillation. Europace. 2010;12:785–791. doi: 10.1093/europace/euq049. [DOI] [PubMed] [Google Scholar]
- 97.Wood MA, Brown-Mahoney C, Kay GN, Ellenbogen KA. Clinical outcomes after ablation and pacing therapy for atrial fibrillation: A meta-analysis. Circulation. 2000;101:1138–1144. doi: 10.1161/01.cir.101.10.1138. [DOI] [PubMed] [Google Scholar]
- 98.Kay GN, Ellenbogen KA, Giudici M, Redfield MM, Jenkins LS, Mianulli M, Wilkoff B. The ablate and pace trial: A prospective study of catheter ablation of the av conduction system and permanent pacemaker implantation for treatment of atrial fibrillation. APT investigators. J Interv Card Electrophysiol. 1998;2:121–135. doi: 10.1023/a:1009795330454. [DOI] [PubMed] [Google Scholar]
- 99.Weerasooriya R, Davis M, Powell A, Szili-Torok T, Shah C, Whalley D, Kanagaratnam L, Heddle W, Leitch J, Perks A, Ferguson L, Bulsara M. The Australian intervention randomized control of rate in atrial fibrillation trial (aircraft) J Am Coll Cardiol. 2003;41:1697–1702. doi: 10.1016/s0735-1097(03)00338-3. [DOI] [PubMed] [Google Scholar]
- 100.Tse HF, Newman D, Ellenbogen KA, Buhr T, Markowitz T, Lau CP. Effects of ventricular rate regularization pacing on quality of life and symptoms in patients with atrial fibrillation (atrial fibrillation symptoms mediated by pacing to mean rates [AF symptoms study]) Am J Cardiol. 2004;94:938–941. doi: 10.1016/j.amjcard.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 101.Jessurun ER, van Hemel NM, Defauw JA, Stofmeel MA, Kelder JC, de la Riviere AB, Ernst JM. Results of maze surgery for lone paroxysmal atrial fibrillation. Circulation. 2000;101:1559–1567. doi: 10.1161/01.cir.101.13.1559. [DOI] [PubMed] [Google Scholar]
- 102.Hemels ME, Gu YL, Tuinenburg AE, Boonstra PW, Wiesfeld AC, Van Den Berg MP, van Veldhuisen DJ, van Gelder IC. Favorable long-term outcome of maze surgery in patients with lone atrial fibrillation. Ann Thorac Surg. 2006;81:1773–1779. doi: 10.1016/j.athoracsur.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 103.Bonanno C, Paccanaro M, La Vecchia L, Ometto R, Fontanelli A. Efficacy and safety of catheter ablation versus antiarrhythmic drugs for atrial fibrillation: A meta-analysis of randomized trials. J Cardiovasc Med. 2010;11:408–418. doi: 10.2459/JCM.0b013e328332e926. [DOI] [PubMed] [Google Scholar]
- 104.Reynolds MR, Walczak J, White SA, Cohen DJ, Wilber DJ. Improvements in symptoms and quality of life in patients with paroxysmal atrial fibrillation treated with radiofrequency catheter ablation versus antiarrhythmic drugs. Circ Cardiovasc Qual Outcomes. 2010;3:615–623. doi: 10.1161/CIRCOUTCOMES.110.957563. [DOI] [PubMed] [Google Scholar]
- 105.Khan MN, Jais P, Cummings J, Di Biase L, Sanders P, Martin DO, Kautzner J, Hao S, Themistoclakis S, Fanelli R, Potenza D, Massaro R, Wazni O, Schweikert R, Saliba W, Wang P, Al-Ahmad A, Beheiry S, Santarelli P, Starling RC, Dello Russo A, Pelargonio G, Brachmann J, Schibgilla V, Bonso A, Casella M, Raviele A, Haissaguerre M, Natale A. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med. 2008;359:1778–1785. doi: 10.1056/NEJMoa0708234. [DOI] [PubMed] [Google Scholar]
- 106.Wyse DG, Anter E, Callans DJ. Cardioversion of atrial fibrillation for maintenance of sinus rhythm: A road to nowhere. Circulation. 2009;120:1444–1452. doi: 10.1161/CIRCULATIONAHA.109.884387. [DOI] [PubMed] [Google Scholar]
- 107.Mertens DJ, Kavanagh T. Exercise training for patients with chronic atrial fibrillation. J Cardiopulm Rehabil. 1996;16:193–196. doi: 10.1097/00008483-199605000-00007. [DOI] [PubMed] [Google Scholar]
- 108.Vanhees L, Schepers D, Defoor J, Brusselle S, Tchursh N, Fagard R. Exercise performance and training in cardiac patients with atrial fibrillation. J Cardiopulm Rehabil. 2000;20:346–352. doi: 10.1097/00008483-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 109.Hegbom F, Stavem K, Sire S, Heldal M, Orning OM, Gjesdal K. Effects of short-term exercise training on symptoms and quality of life in patients with chronic atrial fibrillation. Int J Cardiol. 2007;116:86–92. doi: 10.1016/j.ijcard.2006.03.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.