Abstract
Honey bee societies (Apis mellifera), the ectoparasitic mite Varroa destructor, and honey bee viruses that are vectored by the mite, form a complex system of host–parasite interactions. Coevolution by natural selection in this system has been hindered for European honey bee hosts since apicultural practices remove the mite and consequently the selective pressures required for such a process. An increasing mite population means increasing transmission opportunities for viruses that can quickly develop into severe infections, killing a bee colony. Remarkably, a few subpopulations in Europe have survived mite infestation for extended periods of over 10 years without management by beekeepers and offer the possibility to study their natural host–parasite coevolution. Our study shows that two of these “natural” honey bee populations, in Avignon, France and Gotland, Sweden, have in fact evolved resistant traits that reduce the fitness of the mite (measured as the reproductive success), thereby reducing the parasitic load within the colony to evade the development of overt viral infections. Mite reproductive success was reduced by about 30% in both populations. Detailed examinations of mite reproductive parameters suggest these geographically and genetically distinct populations favor different mechanisms of resistance, even though they have experienced similar selection pressures of mite infestation. Compared to unrelated control colonies in the same location, mites in the Avignon population had high levels of infertility while in Gotland there was a higher proportions of mites that delayed initiation of egg-laying. Possible explanations for the observed rapid coevolution are discussed.
Keywords: Adaptations, Apis mellifera, coevolution, host–parasite interactions, resistance
Introduction
Coevolutionary theories in the study of host–parasite interactions indicate that antagonistic reciprocal selection pressures will lead to an “arms race” with a series of adaptations and counter-adaptations by the host and parasite (Thompson 1994). Such antagonistic interactions actually accelerate molecular evolution compared to selection pressures of environmental changes (Paterson et al. 2010). The evolutionary dynamics of a host–parasite coevolution can lead to a relatively stable relationship, that is, fitness optimality for the host and/or the parasite, by means of a natural selection process (Schmid-Hempel 2010).
The ectoparasitic mite, Varroa destructor (Fig. 1), causes relatively little harm to its original host the Asian honey bee, Apis cerana, as behavioral and physiological traits of the host limit the mite population growth (reviewed in Rath 1999). A long evolutionary process with natural selective pressures has shaped this stable host–parasite relationship. Varroa mites were introduced to the Western honey bee, Apis mellifera, over 30 years ago and has since become the largest threat to honey bees and apiculture around the world. The mite is in part responsible for the recent global honey bee colony losses that have caused ecological and economical pressures on plant biodiversity and crop production, respectively (Potts et al. 2010). The coevolutionary process required for establishing a coexisting relationship between this parasite and its new host is lacking, both in time and in selective pressures because the selective disadvantage of being virulent is removed by apicultural practices aiming to control this damaging new mite pest. Therefore, studying natural selection host–parasite coevolution in this new host–parasite system has not been possible.
Figure 1.

Varroa destructor mite on the thorax of a male European honey bee (Apismellifera).
The Varroa mite is a highly specific parasite that relies completely on its host biology for its own survival and propagation. The mite feeds on bee hemolymph and reproduces in the brood cells of pupating bees. Moreover, the mite is an important component in the virulence of certain honey bee viruses, additional microparasites in this complex system of host–parasite interactions. In the absence of Varroa mites, honey bee viruses can occur as symptomless covert infections within a colony but when Varroa mites are present, a new transmission route is provided by this biological vector (Martin 2001; Shen et al. 2005; Gisder et al. 2009; de Miranda and Genersch 2010). According to theories in evolutionary epidemiology, vector-borne transmission often results in more virulent infections (Ewald 1994). As the mite population grows within a colony, increasing opportunities for transmission will lead to the development of overt viral infections that ultimately result in colony mortality within one to three years if the mite population is not reduced by beekeepers (Martin 2001; Boecking and Genersch 2008). Therefore, the virulence of the mite is considered an indirect measure of its ability to vector these viruses. Consequently, the viruses with a newly acquired vectorial transmission route will become more virulent, as their virulence is in general a measure of mite abundance.
Apis mellifera races in Africa, A. m. scutellata, and in South and Central America, of African origin (Africanized bees), are exceptions to the eventual mortality associated with mite infestation (Rosenkranz 1999; Allsopp 2006). Wild and feral colonies under natural selective pressures in these populations have evolved resistance and are able to pass traits to managed colonies through natural mating events (Rosenkranz 1999). Unfortunately, the exact mechanisms involved in their resistance remain unclear. Wild and feral colonies are rare in Europe and North America since the introduction of the mite; however, a few subset populations of European honey bee races have been exposed to natural selective pressures of long-term mite infestation, as opposed to apicultural pressures or selective breeding programs. Remarkably, these “natural” populations have been sustainably surviving mite infestation for extended periods, some over 10 years, without mite control treatments (De Jong and Soares 1997; Rinderer et al. 2001; Kefuss et al. 2004; Fries et al. 2006; Le Conte et al. 2007; Seeley 2007). These populations enable for the first time the possibility to study natural selection host–parasite coevolution between European honey bees and Varroa, though such studies have been lacking. Recently, the long-term survival despite mite infestation of one such population on the island of Gotland, Sweden, has been confirmed to be a host adaptation rather than reduced virulence of the mite (Fries and Bommarco 2007) and reduced mite reproductive success has been observed in this “natural” population (Locke and Fries 2011). The question remains however, if other “natural” European honey bee populations have developed resistance to Varroa mites and if so, has the same mechanisms of adaptive resistance occurred.
The ability of a host to suppress the reproductive success and ultimately the population growth rate of a parasite has a strong adaptive value that would be an effective strategy toward establishing host resistance. Successful mite reproduction requires the maturation of at least two eggs laid by a reproducing mother mite inside the brood cell: a male mite and a sister female mite, which must mate before bee eclosion (Rosenkranz et al. 2010). The male mite offspring will die when the bee hatches from the cell but any mated daughter mites will enter the colony's mite population along with their mother to find a new brood cell for reproduction.
The present study investigates mite resistance in a “natural” honey bee population in Avignon, France by examining mite reproductive parameters. This population has survived mite infestation without mite control treatment for an extended period of time (i.e., over 10 years; Le Conte et al. 2007). Varroa mite reproductive parameters are compared to findings of a previous study on the Gotland population in Sweden by Locke and Fries (2011) to determine whether adaptive strategies to resist Varroa were similar or differed between geographically and genetically separate populations that are experiencing similar selective pressures.
Materials and Methods
Mite reproductive success is defined here as the ability of the mother mite to produce at least one viable mated female offspring before the developing bee pupa hatches as an adult. Within a mite's reproductive phase, a mother mite that lays no eggs, lays only one egg, produces no male offspring, or begins egg-laying too late in relation to larval development, will not contribute any progeny to the mite population.
Data for the Avignon population that has survived since 1999 were collected in September 2009 and July 2010 in France. Data from the mite-surviving population in Gotland, Sweden, used for comparison with the French population, were collected in July and August in both 2008 and 2009 (Locke and Fries 2011). Both populations were compared first with mite-susceptible control colonies in the same location to determine any significant differences in the mite reproductive parameters. These control colonies were of different genetic background and receiving regular beekeeping management including Varroa population control treatment during the autumn before experimentation.
Detailed descriptions of the methods for investigating mite reproductive success are found in Locke and Fries (2011). In short, worker bee pupae older than approximately 190 h (brown eyes and yellow body stage), but before pupal eclosion at approximately 280 h (Martin 1994), were carefully removed in the laboratory. The developmental stage of each pupa was recorded based on the appearance description given by Martin (1994). Complete mite families from cells infested with a single mother mite were removed using a fine brush, examined with a stereo-microscope and recorded.
In France, 430 cells infested by a single mother mite were examined in 16 Varroa-surviving colonies, and 211 such cells were examined in eight Varroa-susceptible control colonies. In Sweden, 614 cells were examined in 23 surviving colonies, and 592 cells were examined in 21 control colonies. Observations were most often of 30 cells per colony but varied between 10 and 35 cells with lower numbers for those colonies where not enough mite-infested cells were found. Within each pupal cell, the following parameters were recorded: the fertility (whether the mother mite laid eggs); the fecundity (number of eggs laid); the presence or absence of male offspring; the number of dead offspring; and the incidence of delayed egg-laying by mother mites (identified by relating the developmental stage of mite offspring to the developmental appearance, and thus the determined age, of the infested pupa). The yellow thorax stage of the pupae, that is, the earliest stage of pupal exocuticle sclerotization, is the longest stage ranging from approximately 190–230 h old. However, the male mite typically does not become an adult until the pupal age is around 210 h (Martin 1994). Therefore, to eliminate biased recordings, any yellow thorax stage infested pupa where no adult male mite was observed was recorded as “uncertain” since immature male mites are extremely difficult to distinguish from early stage immature female mite offspring. Individual mite reproductive parameters recorded from France were compared to the results obtained in Sweden (Locke and Fries 2011).
All statistical analyses were performed in SAS 9.1 for Windows. A mixed-effects model (SAS proc Mixed) was used to test the effects that surviving colonies compared to control colonies had on the proportion of successfully reproducing mites and individual mite reproductive parameters (α= 0.05). This model was also used to compare the mite reproductive parameters between the Avignon and Gotland populations. The random effects included in the model were date and location and a linear repeated-measures factor was used with the covariance structure selected according to Aikaike's information criteria (Littell et al. 1996). The assumption of normality and equal variance was verified by analysis of residuals (Littell et al. 1996).
Results
The average proportion of successfully reproducing Varroa mites was significantly lower in surviving colonies compared to control colonies in both Avignon, France (F1,21.9= 116.25, P < 0.0001; Fig. 2), and in Gotland, Sweden (F1,41.4= 75.78, P < 0.0001; Fig. 2). Interestingly, a similar between-colony difference in mite reproductive success was found in Avignon (surviving vs. control colonies (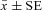 ) 0.59 ± 0.02 vs. 0.90 ± 0.02, respectively) and in Gotland (0.48 ± 0.02 vs. 0.78 ± 0.02 for surviving vs. control colonies, respectively; Fig. 2). The random effects included in the statistical model, that is, date and location did not render significant differences in any of the comparisons.
) 0.59 ± 0.02 vs. 0.90 ± 0.02, respectively) and in Gotland (0.48 ± 0.02 vs. 0.78 ± 0.02 for surviving vs. control colonies, respectively; Fig. 2). The random effects included in the statistical model, that is, date and location did not render significant differences in any of the comparisons.
Figure 2.
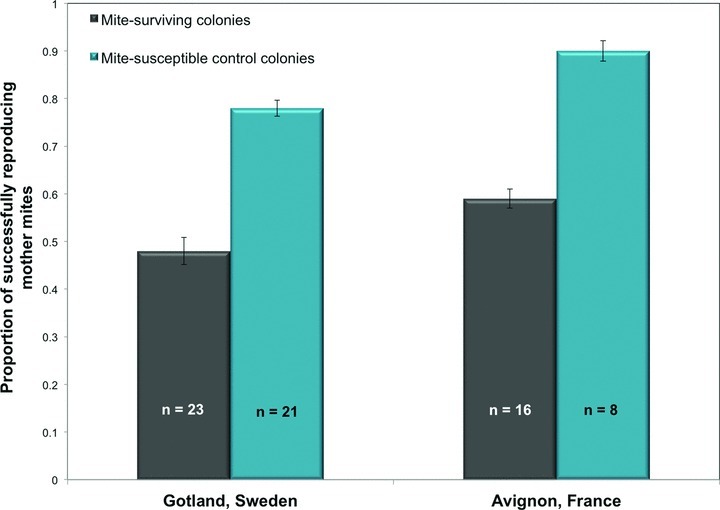
Mean proportion of successfully reproducing mother mites in the Varroa mite-surviving colonies and the mite-susceptible control colonies in Avignon, France, compared to Gotland, Sweden, with standard error bars.
Investigations of the individual parameters involved in the mite's overall reproductive success also revealed differences between surviving and control colonies at each location, as well as differences between the two mite-surviving populations (Table 1). Although all the parameters rendered statistically significant differences between groups in each location, a few are highly significant and biologically interesting. In Avignon, the significantly high rates of infertility and secondly the high proportion of mites with delayed egg-laying seemed to be the most influential parameters in reducing the mite's reproductive success (Table 1). In Gotland however, the proportion of mites with delayed egg-laying was the parameter most significantly different from control colonies with a high proportion of mite offspring mortality a secondary significant factor. Fecundity was lower in the surviving colonies in both locations but this parameter does not necessarily contribute to the mite's ability to reproduce successfully. Instead, it represents only the number of eggs laid without accounting for the age of the offspring or the likelihood of them reaching maturity. Therefore, fecundity may not be independent from the incidence of delayed egg-laying since any mother mite that begins laying eggs late may consequently lay fewer eggs.
Table 1.
Mean values and standard errors (SE) of the different mite reproductive parameters investigated along with probability values of significant differences between the surviving colonies (SC) and control colonies (CC) within locations for each parameter investigated and probability values of significant differences between all surviving colonies and control colonies (between locations). Levels of significance are denoted with increasing number of asterisk.
| Within locations mean (SE), P | ||||||||
|---|---|---|---|---|---|---|---|---|
| Avignon, France | Gotland, Sweden | Between locations P | ||||||
| Mite reproductive parameter | SC | CC | P | SC | CC | P | SC | CC |
| Infertility | 0.15 (0.02) | 0.04 (0.01) | 0.0002*** | 0.08 (0.01) | 0.04 (0.01) | 0.0259* | 0.0002*** | 0.8679 |
| Dead progeny | 0.08 (0.01) | 0.02 (0.01) | 0.014* | 0.13 (0.01) | 0.06 (0.03) | 0.0050* | 0.0203* | 0.0554 |
| Absence of male | 0.04 (0.01) | 0.004 (0.01) | 0.0186* | 0.07 (0.01) | 0.03 (0.01) | 0.0104* | 0.0653 | 0.1590 |
| Delayed egg-laying | 0.13 (0.02) | 0.03 (0.02) | 0.0015** | 0.20 (0.02) | 0.08 (0.01) | <0.0001*** | 0.0131* | 0.0410* |
| Fecundity | 3.1 (0.09) | 4.1 (0.01) | <0.0001*** | 3.7 (0.09) | 4.3 (0.08) | <0.0001*** | 0.0006*** | 0.2810 |
Mite reproductive success was higher in the surviving population in Avignon than in the surviving population in Gotland (F1,17.8= 17.57, P= 0.0006; Fig. 2). However, comparing the two populations together shows significantly higher infertility rates and a lower mean fecundity in Avignon than in Gotland (Table 1). The mite reproductive success was also higher in the control colonies in Avignon than in the control colonies in Gotland (F1,26= 12.41, P= 0.0016; Fig. 2) and only delayed egg-laying was slightly significantly different in control colonies between locations. This result highlights that the surviving colonies in Avignon and in Gotland are unique from regular honey bee colonies and are distinctive from each other regarding the parameters involved in reduced mite reproductive success.
Discussion
In host–parasite interactions, host tolerance is defined as the ability to reduce the effect of the parasite, while host resistance is the ability to reduce the fitness of the parasite (Schmid-Hempel 2010). Until recently, the Avignon and Gotland populations have been considered tolerant to V. destructor since mites were still present but the damage of infestation was limited. In other words, colony mortality did not occur but the mechanisms behind the colonies’ survival were not understood. Our study presents two honey bee populations that have in fact evolved resistant traits enabling them to reduce the mite's fitness, measured as reproductive success. Nevertheless, in most cases, resistance and tolerance are correlated (Lipstich et al. 1996; Schmid-Hempel 2010). Varroa mites are still present in these colonies at rates near the normal colony mortality thresholds and likely both tolerance and resistance may operate simultaneously to enable the long-term survival of these honey bee populations.
In Avignon, France and in Gotland, Sweden, Varroa mite-resistant honey bee colonies reduce the average reproductive success of their infesting mites by about 30% compared to local control colonies. Although these resistant populations are genetically unrelated and separated by over 2000 km, natural selection has in both cases resulted in the reduce reproductive success of this parasitic mite. The main cause of colony mortality related to high mite infestation is the virus infections vectored by the mite, particularly deformed wing virus for its close association with Varroa (Martin 2001; Boecking and Genersch 2008). The ability to suppress the mite's reproductive success would delay or limit the mite's population growth within the colony. This would consequently also limit the virus infections to less effective transmission pathways that rarely lead to colony mortality, such as vertical transmission through infected honey bee eggs and/or horizontal transmission to larvae by nurse bees through infected food (Chen et al. 2006; de Miranda and Genersch 2010). It is unknown what the observations from this study might mean for the evolution of the viruses.
From an evolutionary perspective, the Varroa mite's strict dependence on its host's biology causing a reduction in host fitness from parasitic infestation has imposed strong selective pressures leading to a coevolutionary arms race. In most cases of coevolution, parasites will have an evolutionary advantage above their host due to their faster evolution caused by a shorter generation time (Hafner et al. 1994; Schmid-Hempel 2010). However, in this particular system, V. destructor is of clonal origin in Europe with low genetic variation (Solignac et al. 2005). In addition, the honey bee has 10 times higher genetic recombination levels than any higher order eu-karyote analyzed thus far (Beye et al. 2006). These aspects may have provided the honey bee with an evolutionary advantage in the arms race with V. destructor, an arms race that possibly is in the hosts favor, with mite adaptations limited. A counter-adaptation could be expected according to co-evolution theory (Thompson 1994; Schmid-Hempel 2010) but with the lack of genetic diversity among mites this may take a long time. On the other hand, the adapted resistance in these two honey bee populations has evolved incredible fast by natural selection.
Mechanistic explanations of the bees’ ability to suppress mite reproductive success remain unknown. Both the Avignon and Gotland populations have experienced similar selection pressures of natural mite infestation that is unique compared to most other European honey bee populations due to apicultural management and both have evolved a similar colony-level mite-resistant trait. However, these populations have different life-history traits and different environmental factors that would also be involved in their adaptive responses to the mite pressure. The evolved mechanisms behind the ability to suppress reproductive success of mites may differ between these two distinct populations. In general, one may expect different traits to be favored in different populations living in distinct environments even with similar natural selection pressures, especially in traits involved in coevolutionary relationships (Thompson 1999). Although the two populations have clearly both evolved the ability to reduce mite reproductive success, the between-population differences are less clear. Therefore, more detailed investigations are necessary to identify and tease apart the possible mechanistic differences.
A suggested mechanism involved in reducing the mite's reproductive success could be for example, the adult bee behavior known as Varroa-sensitive hygiene (VSH), which involves the uncapping or removal of mite-infested brood (Harbo and Harris 2005; Ibrahim and Spivak 2006). It has been shown that bee colonies expressing this behavioral trait may selectively remove pupae with reproducing mites resulting in the remaining infested cells having a misrepresented higher proportion of infertile mites (Harbo and Harris 2005; Ibrahim and Spivak 2006). This could potentially be a mechanism of the Avignon population, in light of the observed high mite infertility rates. Since the Gotland population does not demonstrate hygienic behavior (Locke and Fries 2011) nor had significantly high proportions of infertile mites, there is no reason to suspect that they are expressing VSH. Instead, the suppression of mite reproductive success in Gotland may be due to another mechanism, such as pupal volatile compounds that can inhibit the initiation of egg-laying of mites (Garrido and Rosenkranz 2003; Milani et al. 2004).
Besides suppressing mite reproduction, both Varroa-resistant European honey bee populations in this study also share the fact that they have been unmanaged, enabling natural selection (as opposed to artificial) to shape the evolution of their mite resistance. This is an important consideration since it highlights the impact that apicultural practices otherwise have on these host–parasite interactions (Fries and Camazine 2001), suggesting a human interference in co-evolution between species.
This tri-layered complex host–parasite system between honey bees (a multilevel organism with high genetic recombination rates), the Varroa mite (with a fast generation time but low genetic variation), and the viruses (vectored by Varroa) that infect both the bee and the mite (de Miranda and Genersch 2010), challenges basic coevolutionary theories and has not been fully exploited by evolutionary biologists as a model for host–parasite interaction theories. Our hope is to stimulate interdisciplinary research between apicultural studies and evolutionary biology to provide new insight into parasitic interactions of this system. A deeper understanding of how honey bee colonies naturally coevolve with parasites, and understanding the mechanisms and traits behind such coevolution, is necessary for establishing new optimal and long-term sustainable honey bee health management strategies in apiculture.
Acknowledgments
We thank J. de Miranda, T. Pärt, and R. Bommarco for their comments on the manuscript. This work was financially supported by the EU-funded 7th Framework project BEE DOC, Grant Agreement 244956, and the Montagu Foundation Switzerland SAVE project. Financial support for maintaining the Gotland population was obtained from the EC-funded national program for production and sale of honey through the Swedish Board of Agriculture to I. F. Financial support for maintaining the Avignon populations was funded by a European Grant for beekeeping, FEOGA-convention 07-17/2004-2007 to Y. L. C.
References
- Allsopp M. M.S. Thesis. Pretoria, South Africa: University of Pretoria; 2006. Analysis of Varroa destructor infestation of southern African honey bee populations. [Google Scholar]
- Beye M, Gattermeier I, Hasselmann M, Gempe T, Schioett M, Baines JF, Schlipalius D, Mougel F, Emore C, Rueppell O. Exceptionally high levels of recombination across the honey bee genome. Genome Res. 2006;16:1339–1344. doi: 10.1101/gr.5680406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boecking O, Genersch E. Varroosis—the ongoing crisis in beekeeping. J. Consum. Protect. Food Safety. 2008;3:221–228. [Google Scholar]
- Chen YP, Evans JD, Feldlaufer MF. Horizontal and vertical transmission of viruses in the honey bee, Apis mellifera. J. Invertebr. Pathol. 2006;92:152–159. doi: 10.1016/j.jip.2006.03.010. [DOI] [PubMed] [Google Scholar]
- De Jong D, Soares AEE. An isolated population of Italian bees that has survived Varroa jacobsoni infestation without treatment for over 13 years. Am. Bee J. 1997;137:742–745. [Google Scholar]
- de Miranda JR, Genersch E. Deformed wing virus. J. Invertebr. Pathol. 2010;103:48–61. doi: 10.1016/j.jip.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Ewald PW. Evolution of infectious disease. Oxford, U.K: Oxford Univ. Press; 1994. [Google Scholar]
- Fries I, Bommarco R. Possible host-parasite adaptations in honey bees infested by Varroa destructor mites. Apidologie. 2007;38:525–533. [Google Scholar]
- Fries I, Camazine S. Implications of horizontal and vertical pathogen transmission for honey bee epidemiology. Apidologie. 2001;32:199–214. [Google Scholar]
- Fries I, Imdorf A, Rosenkranz P. Survival of mite infested (Varroa destructor) honey bee (Apis mellifera) colonies in a Nordic climate. Apidologie. 2006;37:1–7. [Google Scholar]
- Garrido C, Rosenkranz P. The reproductive program of female Varroa destructor mites is triggered by its host, Apis mellifera. Apidologie. 2003;35:419–430. doi: 10.1023/b:appa.0000010386.10686.9f. [DOI] [PubMed] [Google Scholar]
- Gisder S, Aumeier P, Genersch E. Deformed wing virus: replication and viral load in mites (Varroa destructor. J. Gen. Virol. 2009;90:463–467. doi: 10.1099/vir.0.005579-0. [DOI] [PubMed] [Google Scholar]
- Hafner MS, Sudman PD, Villablanca FX, Sradling TA, Demastes JW, Nadler SA. Disparate rates of molecular evolution in conspeciated hosts and parasites. Science. 1994;19:1087–1090. doi: 10.1126/science.8066445. [DOI] [PubMed] [Google Scholar]
- Harbo JR, Harris JW. Suppressed mite reproduction explained by the behaviour of adult bees. J. Apic. Res. 2005;44:21–23. [Google Scholar]
- Ibrahim A, Spivak M. The relationship between hygienic behaviour and suppression of mite reproduction as honey bee (Apis mellifera) mechanisms of resistance to Varroa destructor. Apidologie. 2006;37:31–40. [Google Scholar]
- Kefuss J, Vanpouke J, Ducos De Lahitte J, Ritter W. Varroa tolerance in France of Intermissa Bees from Tunisia and their naturally mated descendants: 1993–2004. Am. Bee J. 2004;144:563–568. [Google Scholar]
- Le Conte Y, de Vaublanc G, Crauser D, Jeanne F, Rousselle J-C, Bécard J-M. Honey bee colonies that have survived Varroa destructor. Apidologie. 2007;38:566–572. [Google Scholar]
- Lipstich M, Siller S, Norwak MA. The evolution of virulence in pathogens with vertical and horizontal transmission. Evolution. 1996;50:1729–1741. doi: 10.1111/j.1558-5646.1996.tb03560.x. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS system for mixed models. 2nd ed. Cary, NC: SAS Press; 1996. [Google Scholar]
- Locke B, Fries I. Characteristics of honey bee colonies (Apis mellifera) in Sweden surviving Varroa destructor infestation. Apidologie. 2011;42:533–542. [Google Scholar]
- Martin SJ. Ontogenesis of the mite Varroa jacobsoni Oud. in worker brood of the honeybee Apis mellifera L. under natural conditions. Exp. Appl. Acarol. 1994;18:87–100. [Google Scholar]
- Martin SJ. The role of Varroa and viral pathogens in the collapse of honeybee colonies: a modelling approach. J. Appl. Ecol. 2001;38:1082–1093. [Google Scholar]
- Milani N, Della VG, Nazzi F. (Z)-8-Heptadecene reduces the reproduction of Varroa destructor in brood cells. Apidologie. 2004;35:265–274. [Google Scholar]
- Paterson S, Vogwill T, Buckling A, Benmayor R, Spiers AJ, Thomson NR, Quail M, Smith F, Walker D, Libberton B. Antagonistic coevolution accelerates molecular evolution. Nature. 2010;464:275–279. doi: 10.1038/nature08798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 2010;1227:1–9. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Rath W. Co-adaptation of Apis cerana Fabr. and Varroa jacobsoni Oud. Apidologie. 1999;20:339–343. [Google Scholar]
- Rinderer TE, de Guzman LI, Delatte GT, Stelzer JA, Lancaster VA, Kuznetsov V, Beaman L, Watts R, Harris JW. Resistance to the parasitic mite Varroa destructor in honey bees from far eastern Russia. Apidologie. 2001;32:381–394. [Google Scholar]
- Rosenkranz P. Honey bee (Apis mellifera L.) tolerance to Varroa jacobsoni Oud. In South America. Apidologie. 1999;30:159–172. [Google Scholar]
- Rosenkranz P, Aumeier P, Ziegelmann B. Biology and control of Varroa destructor. J. Invert. Pathol. 2010;103:96–119. doi: 10.1016/j.jip.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P. Evolutionary parasitology. The integrated study of infections, immunology, ecology, and genetics. New York, NY: Oxford Univ. Press; 2010. [Google Scholar]
- Seeley TD. Honey bees of the Arnot Forest: a population of feral colonies persisting with Varroa destructor in the northeastern United States. Apidologie. 2007;38:19–29. [Google Scholar]
- Shen MQ, Yang XL, Cox-Foster D, Cui LW. The role of Varroa mites in infections of Kashmir bee virus (KBV) and deformed wing virus (DWV) in honey bees. Virology. 2005;342:141–149. doi: 10.1016/j.virol.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Solignac M, Cornuet JM, Vautrin D, Le Conte Y, Anderson D, Evan J, Cros-Arteil S, Navajas M. The invasive Korean and Japan types of Varroa destructor, ectoparasitic mite of the Western honey bee (Apis mellifera), are two partly isolated clones. Proc. R. Soc. Lond. B. 2005;272:411–419. doi: 10.1098/rspb.2004.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JN. The coevolutionary process. Chicago, IL: Univ. of Chicago Press; 1994. [Google Scholar]
- Thompson JN. The evolution of species interactions. Science. 1999;284:2116–2118. doi: 10.1126/science.284.5423.2116. [DOI] [PubMed] [Google Scholar]


