Abstract
The roles of adaptation, chance, and history on evolution of the toxic dinoflagellate Alexandrium minutum Halim, under selective conditions simulating global change, have been addressed. Two toxic strains (AL1V and AL2V), previously acclimated for two years at pH 8.0 and 20°C, were transferred to selective conditions: pH 7.5 to simulate acidification and 25°C. Cultures under selective conditions were propagated until growth rate and toxin cell quota achieved an invariant mean value at 720 days (ca. 250 and ca. 180 generations for strains AL1V and AL2V, respectively). Historical contingencies strongly constrained the evolution of growth rate and toxin cell quota, but the forces involved in the evolution were not the same for both traits. Growth rate was 1.5–1.6 times higher than the one measured in ancestral conditions. Genetic adaptation explained two-thirds of total adaptation while one-third was a consequence of physiological adaptation. On the other hand, the evolution of toxin cell quota showed a pattern attributable to neutral mutations because the final variances were significantly higher than those measured at the start of the experiment. It has been hypothesized that harmful algal blooms will increase under the future scenario of global change. Although this study might be considered an oversimplification of the reality, it can be hypothesized that toxic blooms will increase but no predictions can be advanced about toxicity.
Keywords: Adaptation, Alexandrium minutum, chance, historical contingency, toxic red tides
Introduction
Although genetic adaptation, as a consequence of natural selection, is considered the main force driving evolution, two other factors also contribute to evolutionary change: chance and historical contingency (Gould 2002). The effects of chance are usually due to genetic drift events and random mutations without value for the organisms (Crow and Kimura 1970; Kimura 1983; Spiess 1989); the final consequence is that alleles that neither improve nor decrease adaptation are maintained in populations. Historical contingency can become important if certain genetic changes of adaptive value in the past constrain or promote evolutionary outcomes (Gould and Lewontin 1979; Blount et al. 2008). To disentangle the effects of adaptation, chance, and history on evolutionary change, Gould (1989, pp. 320–321) proposed a theoretical experiment, which consisted of “replaying life's tape” to test the repeatability of evolution and thereby to evaluate their respective roles; the experiment was envisioned to demonstrate the processes involved in macroevolutionary events. Obviously, an experiment such as that envisioned by Gould (1989) cannot be performed, as Lenski and Travisano (1994) stated: “the limitations reflect our lack of access to better machines for time travel.” However, this theoretical experiment can be empirically addressed in microevolution by the robust experiment of Travisano et al. (1995) in which, instead of “replaying life's tape” sequentially (where each tape recording is a replication of the experiment, each of them separated in time), one can achieve the same objective by replicating independent isolates propagated simultaneously. At the starting point, identical isolates (replicates) from a single ancestral genotype are established and the initial mean value of a specific phenotypic trait (in our case, growth rate and toxin cell quota) is measured for each of them. This value is expected to be identical among strains, within statistical limits of measurement error, at the beginning of the experiment. After a period of time, the value of each trait is measured again for each isolate. Differences between the initial and final mean values are explained as a result of the effect of adaptation, chance, or history (Fig. 1). Thus, a significant change in the mean value in relation to that of the ancestral isolate means that the trait has been a target of natural selection or that it is correlated with some other trait that has been selected. On the other hand, a significant increase in its variance represents the occurrence of divergence among the evolved isolates (the specific trait has not been a target for adaptation but reflects the effects of random mutations or drift or their interactions with other evolutionary processes). Other alternatives are the occurrence of both adaptation and chance, or on the other hand, the occurrence of neither.
Figure 1.
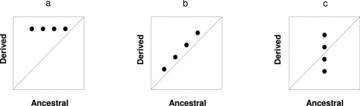
Schematic representation of evolutionary change determined by adaptation (a), history (b), and chance (c). The effects of chance are demonstrated for a set of clones of a single ancestral genotype, whereas adaptation and history are illustrated for several independent ancestors. Isoclines (dotted line) represent the score location if no changes take place. Adapted from Travisano et al. (1995) and Waagenar and Adami (2004).
To test the effect of history, it is necessary to carry out a similar experiment using different ancestral genotypes (Travisano et al. 1995; Fig. 1). The experiment of Travisano et al. (1995) was designed for bacterial populations, and with the appropriate modifications it has been recently addressed with digital organisms (Wagenaar and Adami 2004) in an apicomplexan parasite (Pérez-Zaballos et al. 2005), a marine dinoflagellate (Flores-Moya et al. 2008), and cyanobacterium (Rouco et al. 2011). Certainly, this experimental evolutionary study can be carried out in any microorganism that can be asexually grown and easily manipulated during many generations.
Blooms of the toxic dinoflagellate Alexandrium minutum Halim were previously known only from the waters of the Mediterranean Sea and South Australia (Hallegraeff et al. 1988) but toxic populations have been later detected in northern France (Belin 1993), northwestern Spain (Franco et al. 1994), the North Sea (Nehring 1998; Elbrachter 1999; Persson et al. 2000; Hansen et al. 2003), India (Godhe et al. 2001), Malaysia (Usup et al. 2002), Vietnam (Yoshida et al. 2000), and South Africa (Pitcher et al. 2007). The increase in reports of harmful algal blooms has been related to increased numbers of scientific studies (Anderson 1989), eutrophication or unusual climatic conditions in coastal waters (Hallegraeff 1993), and to the spread of toxic algae by natural (Vila et al. 2001; Persich et al. 2003) or human-assisted vectors (Lilly et al. 2002; Fahnenstiel et al. 2009). More recently, the factors linked to anthropogenic global change have also been implicated in the expansion of harmful algal blooms (Hallegraeff 2009). In relation to global change, it must be take into account that the increasing CO2 concentration in the last two centuries (and the concomitant acidification and increased temperature at the sea surface) means that A. minutum (and all marine organisms) are being exposed to changes that are taking place faster than those that they underwent in their evolutionary past (Raven et al. 2005; Rost et al. 2008). However, few phytoplankton experiments have gone on long enough for genotype selection to be significant (Reboud and Bell 1996; Collins and Bell 2004; Collins et al. 2006; Flores–Moya et al. 2008; Rouco et al. 2011). For this reason, it has been suggested that long-term selection experiments must be performed in order to understand the potential adaptation and evolution resulting from global change in the oceans (Raven et al. 2005).
The aim of this work was to evaluate the contribution of adaptation (separating acclimation from selection of favored mutants), chance, and history to the phenotypic change in growth rate and toxin cell quota in two toxic strains of A. minutum. For this purpose, long-term cultures (previously acclimated to pH 8.0 and 20°C) were performed at a temperature increased by 5°C and a pH drop of 0.5 units; then, the ancestral and derived scores of growth rate and toxin cell quota were compared. Although the experimental approach followed here is an oversimplification of the reality, it constitutes a novel way to explore the evolutionary response of toxic dinoflagellates to anthropogenic-induced changes in environmental conditions.
Materials and Methods
Experimental organism and growth conditions
Two strains of A. minutum Halim (both isolated from the Ría de Vigo, NW Spain), one of which (Al1V) is more toxic than the other (Al2V; Flynn et al. 1994), were used. The strains are available from the Algal Culture Collection of the Facultad de Veterinaria (Universidad Complutense, Madrid, Spain). Cultures of both strains were maintained in 50-mL Greiner tissue-culture flasks in 20-mL f/2 medium supplied by Sigma Aldrich Chemie (Taufkirchen, Germany) at 20°C, pH 8.0 under 60 µmol photons m–2 sec–1 over the waveband 400–700 nm, 16:8 h light:dark photoperiod. Exponential growth of cells was achieved by serial transfer of an inoculum to fresh medium every 21 days, for two years (Fig. 2). More data on isolation, culture, and genetics of these strains are compiled in Costas and López-Rodas (1994, 1996), Costas et al. (1995), and Mendoza et al. (1995). The absence of viable bacteria was tested periodically by epifluorescence microscopy after staining with acridine orange.
Figure 2.
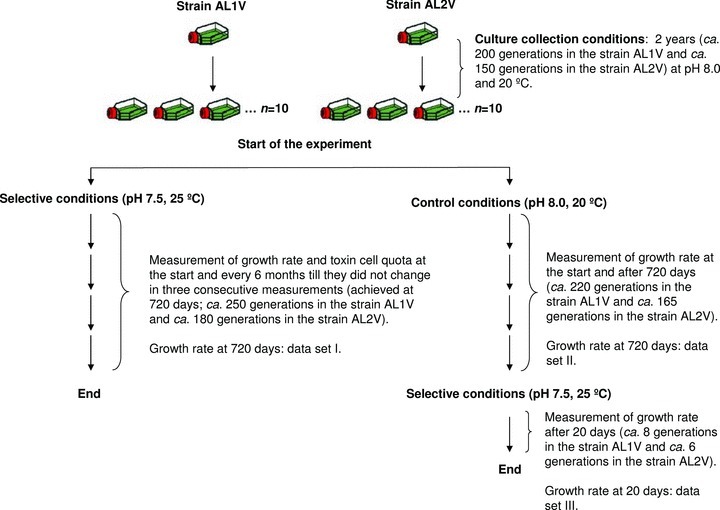
Schematic representation of the experimental design. Ten isolates for control (pH 8.0, 20°C) and 10 for selective conditions (pH 7.5, 25°C) of each Alexandrium minutum strain (AL1V and AL2V) were maintained through serial transfer into fresh medium. Under selective conditions, growth rate and toxin cell quota were determined every six months till these traits did not change in three consecutive measurements (720 days, corresponding to ca. 250 generations in strain AL1V and ca. 180 generations in strain AL2V). In order to disentangle the effects of acclimation from genetic adaptation, additional growth rate measurements were made in the controls after ca. 220 generations in strain AL1V and ca. 165 generations in strain AL2V. For this purpose, the control cultures were transferred to selective conditions (pH 7.5, 25°C) for 20 days (corresponding to ca. eight generations in strain AL1V and ca. six generations in strain AL2V).
Experimental design
Just before the experiment, both strains were recloned from a single vegetative cell, thus assuring their genetic homogeneity at the starting point. These newly established cultures were grown to mass populations in environmental conditions similar to those used to maintain cultures started from stocks from the Algal Culture Collection two years before (f/2 medium, 20°C, pH 8.0 under 60 µmol photons m–2 sec–1 over the waveband 400–700 nm, 16:8 h light:dark photoperiod). Cultures were then used to found 10 independent populations of each strain, and their growth rates and toxin cell quota were measured (Fig. 2). These populations were transferred to new culture conditions that were chosen in order to mimic the proposed environmental conditions in the middle latitude oceans for the next few decades. For this purpose, only two of the principal environmental factors involved in global changes in the oceans were chosen: temperature and pH. Studies on global change predict a warming of 2–6°C (Hansen et al. 2000; IPCC 2007) and a pH drop in the sea surface of 0.2–0.5 units (Zeebe and Wolf–Gladrow 2001; Caldeira and Wickett 2003) below today's mean, by 2100–2200. Consequently, the cultures were transferred to 25°C and pH 7.5 but the other culture conditions (f/2 medium, 60 µmol photons m–2 sec–1 over the waveband 400–700 nm, 16:8 h light:dark photoperiod) were maintained as previously described. To reduce the pH to 7.5, 1N HCl was added to the culture medium. Because the pH increased to 8.0 in 8–10 days due to the photosynthetic activity of cells, the pH was adjusted every three days (at this moment, pH ranged from 7.5 to 7.7).
Experimental cultures were serially propagated by 32-fold dilution into fresh medium every 18–12 days for strain AL1V and 25–17 days for strain AL2V (this interval was shortened as the experiment progressed), in order to achieve ca. 5 generations of binary division before the further addition of fresh medium. At every transfer, the population size was ca. 2.5 × 103 cells. Growth rate and toxin cell quota, averaged for each strain, were checked every six months. Cultures were propagated until no significant changes in growth rate and toxin cell quota (tested by one-way analysis of variance [ANOVA]) were detected in three consecutive measurements (after 720 days, corresponding to ca. 250 and ca. 180 generations for strains AL1V and AL2V, respectively). Then, averaged growth rate and toxin cell quota of the evolved populations were newly measured (Fig. 2). Under all of the culture conditions used in the experiment, proliferation of cells was exclusively due to asexual reproduction.
The effects of adaptation, history, and chance on evolutionary change of growth rate and toxin cell quota were estimated by using the values measured at the start and at the end of the experiment (see Fig. 1). The effects of adaptation were defined by changes in the great mean value and 95% confidence limits were calculated by using the t distribution. The effects of history and chance were estimated by means of a two-level (two strains and 10 isolates within each strain, with three replicates of the growth rate or toxin cell quota measurement per isolate) nested ANOVA. Thus, the contribution of the history component corresponds to the variance measured among strains, whereas the chance component was estimated by the variance measured among isolates within the same strain. The homogeneity of variances was checked with the Bartlett test. The square root of the variance component for history and chance was reported in order to use units that were comparable to the mean change due to adaptation. Approximate 95% asymmetrical confidence limits were calculated for the variance components. All the tests were performed according to Zar (1999).
Acclimation versus genetic adaptation
In order to test whether adaptation resulted from acclimation or by selection of new genetic variants arising by mutations, the cells cultured in the control conditions (10 isolates from both strains, propagated for 720 days) were then transferred to the selective culture conditions (pH 7.5, 25°C) for 20 days (ca. eight generations in strain AL1V and ca. six generations in strain AL2V; Fig. 2). It must be highlighted that almost in bacteria, seven generations are enough to check if adaptation process is assured by means of acclimation or genetic mechanisms (Cooper 1991).
Genetic adaptation corresponds to the difference between growth rate of the derived cells under selective conditions and growth rate of the derived cells under control conditions and transferred for 20 days to selective conditions (i.e., growth rate data sets I–III in Fig. 2). Acclimation corresponds to any excess of the growth rate of the later in comparison to growth rate from the derived control cells (i.e., growth rate data sets III–II in Fig. 2).
Measurement of growth rate and toxin cell quota
Growth rate was measured under r-selection conditions in exponentially growing cultures according to Crow and Kimura (1970) as
where Nt and N0 are the cell number at time t= 5 and 0 day, respectively. The values of N0 and Nt were determined at 6 and 11 days after the transfer of cells to fresh medium. Three independent observers made cell counts by haemocytometer (Double Neubauer, ruling, Fortuna W. G. Co., Germany), obtaining a CV <5% on the same sample.
Toxin was measured using the Ridascreen® Fast PSP SC competitive enzyme immunoassay (R-Biopharm AG, Darmstadt, Germany) according to the instructions of the manufacturer. Toxin cell quota was computed as toxin content measured in the sample divided by the number of cells (measured by haemocytometer). Five pseudoreplicates on the same sample yielded a CV <3%.
Results
Growth rate
Ancestral and derived scores of growth rate in the two strains of A. minutum are shown in Fig. 3a. Adaptation was absent (by design) at the start of the experimental selective period but the initial scores of growth rate of A. minutum were significantly affected by history (F= 162.14; df = 1 and 18; P < 0.05) and, in a lower quantity, by chance (F= 4.94; df = 18 and 40; P < 0.05; Fig. 3b).
Figure 3.
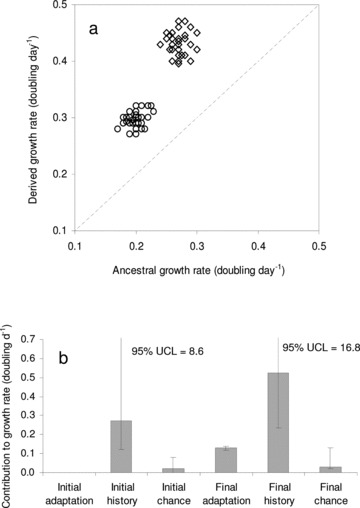
(a) Ancestral versus derived values for mean growth rate measurements in 10 isolates from strains AL1V (diamonds) and AL2V (circles) of Alexandrium minutum. Derived values were obtained after 720 days (ca. 250 and ca. 180 generations in strains AL1V and AL2V, respectively). Isocline (dotted line) represents the score location if no changes take place. (b) Contributions of adaptation, chance, and history to growth rate at the start and at the end of the experiment. Error bars represent 95% confidence limits; values of the upper confidence limits (UCL) for history are included.
After ca. 250 generations in strain AL1V and ca. 180 generations in strain AL2V under selective conditions, ca. 20% of the evolutionary change was explained as adaptation (t= 25.461; df = 58; P < 0.05), ca. 75% was due to history (F= 342.83; df = 1 and 18; P < 0.05) and ca. 5% was a consequence of chance (F= 7.95; df = 18 and 40; P < 0.05; Fig. 3b). It must be highlighted that 95% upper confidence limits for both initial and final effects of history (i.e., the variance among strains) are very high because only two strains were used.
In order to disentangle genetic adaptation and acclimation as components of final adaptation, the growth rates corresponding to data sets I–III were used (see Fig. 2). Mean growth rates in the controls at pH 8.0 and 20°C at the outset of the experiment (data set II), were 0.27 ± 0.02 and 0.20 ± 0.01 doubling day–1 for the strains AL1V and AL2V, respectively. However, the growth rates from both strains significantly increased (t-test = 28.361 and 31.563 for strains Al1V and Al2V, respectively; df = 18; P < 0.05) when they were transferred for 20 days under selective conditions (data set III), yielding an overall mean value of 0.32 ± 0.02 and 0.23 ± 0.02 doubling day–1 for the strains AL1V and AL2V, respectively. These later values were significantly lower (t-test = 38.075 and 42.783 for strains Al1V and Al2V, respectively; df = 18; P < 0.05) than those found at the end of the experiment in the derived cells (0.43 ± 0.02 and 0.30 ± 0.02 for strains Al1V and Al2V, respectively; data set I). From the differences between the growth rate data sets, it was found that acclimation (data sets III–II) accounted ca. 32% of total adaptation, while the selection (data sets I–III) of new genetic variants was responsible for the rest ca. 68%.
Toxin cell quota
The scores of ancestral and derived toxin cell quota in strains AL1V and AL2V are shown in Figure 4a. Both history and chance significantly contributed to the initial scores of toxin cell quota of both strains of A. minutum (F= 325.07, df = 1 and 18, P < 0.05 for history; F= 4.78, df = 18 and 40, P < 0.05 for chance; Fig. 4b).
Figure 4.
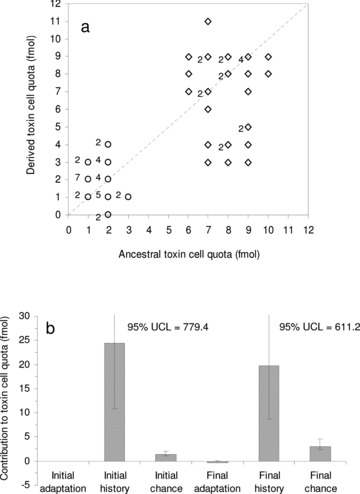
(a) Ancestral versus derived values for mean toxin cell quota measurements in 10 isolates from the strains AL1V (diamonds) and AL2V (circles) of Alexandrium minutum. Derived values were obtained after 720 days (ca. 250 and ca. 180 generations in strains AL1V and AL2V, respectively). Digits close to symbols indicate the number of similar scores. Isocline (dotted line) represents the score location if no changes take place. (b) Contributions of adaptation, chance, and history to toxin cell quota at the start and at the end of the experiment. Error bars represent 95% confidence limits; values of the upper confidence limits (UCL) for history are included.
The evolutionary change of toxin cell quota of A. minutum after ca. 250 generations in strain AL1V and ca. 180 generations in strain AL2V under the new conditions was not a consequence of adaptation (|t| = 1.463, df = 58, P= 0.14). However, the evolutionary change was significantly affected by both the historical contingency (F= 41.96; df = 1 and 18; P < 0.05) and the chance (F= 21.46, df = 18 and 40, P < 0.05; Fig. 4b).
Discussion
Understanding the biological responses to global change will be one of the main tasks of evolutionary biology in coming decades (Bell and Collins 2008). Due to the implications for human health risks and economic losses caused by toxic algae (Hoagland et al. 2002), the assessment of the relative importance of the different components of evolution elicited by environmental forcing is crucial. The predictions about the growth rate and performance of microalgae at the prevailing conditions in seawater by 2100–2200 are based mostly on short-term experiments and they differ between species and taxonomic groups (Beardall and Raven 2004). For instance, it is known by short-term experiments that A. minutum shows higher growth rates at pH 7.5 than at pH 8.5, and 25°C seems to be an optimal environmental condition for cell growth in this species (Hwang and Lu 2000). However, the increment in growth rates found in our long-term experiments at pH 7.5 and 25°C is higher than the increment observed in short-term experiments at different pH values (Hwang and Lu 2000). Moreover, the increased growth rates in our experiment were mainly due to the selection of new genetic variants arising by mutation. This results point to the fact that long-term selection experiments are needed to understand the potential adaptation and evolution resulting from global change in the marine environment.
The evolution of growth rate in both strains of A. minutum was strongly constrained by the historical contingencies although adaptation was also involved. Genetic adaptation (i.e., selection of favored mutants) accounted two-thirds of total adaptation while one-third was due to acclimation. The selective effect of the increased temperature and slight drop in pH seemed to be strong enough to constrain the adaptation of all the experimental replicates in both strains, as suggested by the negligible contribution of chance. Since asexually growing clonal cultures were used, the evolutionary changes are due to new mutations, which occurred during propagation of derived cultures under the new environmental conditions. There should be a high number of mutants due to the huge number of cells growing in each culture. Some mutations increased growth rate and were selected, displacing the wild-type genotypes. Mutations decreasing growth rate were eliminated by natural selection.
The strong contribution of history suggests that disruptive selection could operate in A. minutum, because differences in mean growth rate scores between the strains increased under the selective conditions. The metaphor of the adaptive landscape (Wright 1932; Whitlock et al. 1995; Colegrave and Buckling 2005) could be applied to explain the different final growth rate scores between strains AL1V and ALV2: natural selection drove the strains to different final adaptive peaks differing in height. However, the disruptive selection pattern for the evolution of growth rate of A. minutum differs from the one found in other biological models in which directional selection (i.e., the convergence of the growth rate of experimental isolates) was the pattern found in viruses (Bull et al. 1997; Cuevas et al. 2002), bacteria (Travisano et al. 1995), protozoa (Pérez-Zaballos et al. 2005), the dinoflagellate Prorocentrum triestinum Schiller (Flores-Moya et al. 2008), the toxic cyanobacterium Microcystis aeruginosa (Kützing) Kützing (Rouco et al. 2011) and even digital organisms (Wagenaar and Adami 2004; in this case, award rate, a variable closely related to average growth rate, was used).
The phenotypic changes in toxin cell quota were due to chance but, as it occurred with evolution of the growth rate, they were strongly constrained by historical contingency. According to evolutionary theory, chance includes random genetic drift (Crow and Kimura 1970) and neutral mutations (Kimura 1983). Genetic drift events are a consequence of “sampling errors” when the number of individuals in populations becomes relatively low at a given moment (Crow and Kimura 1970); because the minimum cell density of A. minutum in the cultures was ca. 2.5 × 103 cells in 20 mL of culture medium, it can be hypothesized that the role of chance may be due to neutral mutations rather than to genetic drift events. Since each culture has a huge number of cells, numerous mutations should have arisen during cell growth in each isolate. Some mutations increased toxin production whereas other mutations decreased toxin production, suggesting that the effect of selection for toxin production in derived population was neutral. Thus, neither toxin-increased nor toxin-decreased mutants have selective advantage. Moreover, it could be supposed that wild-type ancestral genotypes have no selective advantage. Consequently, natural selection was not strong enough to constrain the evolution of toxin production in the experimental isolates. So, the effect of history was maintained during evolution of toxin production under new environmental conditions.
Abiotic factors such as temperature, salinity, and inorganic nutrient availability (Anderson et al. 1990) have a clear effect on both toxin production and growth rate in dinoflagellates (Boyer et al. 1987; Ogata et al. 1987; Anderson et al. 1990; Parkhill and Cembella 1999). However, growth rate and toxin production are not usually correlated in dinoflagellates. For instance, Guisande et al. (2002) found that growth rate and toxin production were not associated in A. minutum. The reason for the lack of relationship between growth rate and toxin production could be due to the toxin synthesis that is not a constitutive component of algal metabolism; they are synthesized from low molecular weight metabolites while cell growth is a much more complex process. At present, it is not yet clear why some marine dinoflagellate produce toxins and the possible adaptive advantages. The evolutionary approach followed in this work could be useful to clarify the possible adaptive value of toxins in dinoflagellates. It is well established in evolutionary theory that traits that are strongly correlated with fitness (such as growth rate) evolve by adaptation (selection of favorable mutations) whereas traits that are not (or are very weakly) correlated with fitness evolve by chance (Kimura 1983; Spiess 1989). In fact, these were results found in the study and, consequently, it could be hypothesized that toxicity in A. minutum had no adaptive value. Chance could also explain the transformation of a daughter line of a single parental culture of the toxic strain 18-1T from A. minutum (sub Alexandrium lusitanicum Balech), to the nontoxic phenotype, then renamed strain 18-1NT (Martins et al. 2004). The toxic strain was isolated in 1962; in 1992, a subculture was sent to a different laboratory. It was found that the loss of toxicity occurred between 1995 and 2000 and it was argued that it could be due to genetic mutations or the effects of prolonged treatment of the nontoxic strain with antibiotics (Martins et al. 2004). Although the role of antibiotics cannot be rejected, the hypothesis of the role of neutral mutations in the loss of toxicity seems to be more plausible.
Taken together the results from the changes in growth rate and toxin cell quota under a scenario of global change of increased temperature and a pH drop of 0.5 units, it could be hypothesized that red tides of toxic A. minutum could increase but the toxicity of the cells may be unpredictable. Of course, the present study is an oversimplification of reality but it is a novel way to explore one of the main deficits in our knowledge of the long-term evolution of toxic algae. In order to add more knowledge on the future performance of toxic algae, more studies including other ecological factors involved in global change must be addressed.
Acknowledgments
This work was financially supported by the Spanish Ministry of Science and Innovation by the grant CGL2008–00652/BOS, and Junta de Andalucía Research Group RNM-115. E. C. Henry kindly revised the English style and usage. The suggestions and critical reading by S. Collins are deeply acknowledged.
References
- Anderson DM. Toxic algal blooms and red tides: a global perspective. In: Okaichi T, Anderson DM, Nemoto T, editors. Red tides: biology, environmental science, and toxicology. New York, NY: Elsevier; 1989. pp. 11–16. [Google Scholar]
- Anderson DM, Kullis DM, Sullivan JJ, Hall S, Lee C. Dynamics and physiology of saxitoxin production by the dinoflagellates Alexandrium spp. Mar. Biol. 1990;104:511–524. [Google Scholar]
- Beardall J, Raven JA. The potential effects of global climate change on microalgal photosynthesis, growth and ecology. Phycologia. 2004;43:26–40. [Google Scholar]
- Belin C. Distribution of Dinophysis spp. and Alexandrium minutum along French coasts since 1984 and their DSP and PSP toxicity levels. In: Smayda TJ, Shimizu Y, editors. Toxic phytoplankton blooms in the sea. Amsterdam, The Netherlands: Elsevier; 1993. pp. 469–474. [Google Scholar]
- Bell G, Collins S. Adaptation, extinction and global change. Evol. Appl. 2008;1:3–16. doi: 10.1111/j.1752-4571.2007.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount ZD, Borland CZ, Lenski RE. Historical contingency and the evolution of a key innovation in an experimental population of Escherichia coli. Proc. Natl. Acad. Sci. USA. 2008;105:7899–7906. doi: 10.1073/pnas.0803151105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer GL, Sullivan JJ, Andersen RJ, Harrison PJ, Taylor FJR. Effects of nutrient limitation on toxic production and composition in the marine dinoflagellate Protogonyaulax tamarensis. Mar. Biol. 1987;96:123–128. [Google Scholar]
- Bull JJ, Badgett MR, Whicman HA, Huelsenbeck JP, Hillis DM, Gulati A, Ho C, Molineaux IJ. Exceptional convergent evolution in a virus. Genetics. 1997;147:1497–1507. doi: 10.1093/genetics/147.4.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldeira K, Wickett ME. Anthropogenic carbon and ocean pH. Nature. 2003;425:325. doi: 10.1038/425365a. [DOI] [PubMed] [Google Scholar]
- Colegrave N, Buckling A. Microbial experiments on adaptive landscapes. BioEssays. 2005;27:1167–1173. doi: 10.1002/bies.20292. [DOI] [PubMed] [Google Scholar]
- Collins S, Bell G. Phenotypic consequences of 1,000 generations of selection at elevated CO2 in a green alga. Nature. 2004;431:566–569. doi: 10.1038/nature02945. [DOI] [PubMed] [Google Scholar]
- Collins S, Sültemeyer D, Bell G. Rewiding the tape: selection of algae adapted to high CO2 at current and Pleistocene levels of CO2. Evolution. 2006;60:1392–1401. [PubMed] [Google Scholar]
- Cooper S. Bacterial growth and division. Biochemistry and regulation of prokaryotic and eukaryotic division cycles. San Diego, CA: Academic; 1991. [Google Scholar]
- Costas E, López-Rodas V. Identification of marine dinoflagellates using fluorescent lectins. J. Phycol. 1994;30:987–990. [Google Scholar]
- Costas E, López-Rodas V. Enumeration and separation of the toxic dinoflagellate Alexandrium minutum from natural samples using immunological procedures with blocking antibodies. J. Exp. Mar. Biol. Ecol. 1996;198:81–87. [Google Scholar]
- Costas E, Zardoya R, Bautista J, Garrido A, Rojo C, López-Rodas V. Morphospecies vs. genospecies in toxic marine dinoflagellates: an analysis of Gymnodinium catenatumGyrodinium impudicum and Alexandrium minutumA. lusitanicum using antibodies, lectins, and gene sequences. J. Phycol. 1995;31:801–807. [Google Scholar]
- Crow JS, Kimura M. An introduction to population genetics theory. New York, NY: Harper and Row; 1970. [Google Scholar]
- Cuevas JM, Elena SF, Moya A. Molecular basis of adaptive convergence in experimental populations of RNA viruses. Genetics. 2002;162:533–542. doi: 10.1093/genetics/162.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbrachter M. Exotic flagellates of coastal North Sea waters. Helgolander Wissenschaftliche Meeresuntersuchungen. 1999;52:235–242. [Google Scholar]
- Fahnenstiel G, Hong Y, Millie D, Doblin MA, Johengen T, Reid D. Marine dinoflagellate cysts in the ballast tank sediments of ships entering the Laurentian Great Lakes. Verhandlungen Internationale Verein Limnology. 2009;30:1035–1038. [Google Scholar]
- Flores-Moya A, Costas E, López-Rodas V. Roles of adaptation, chance and history in the evolution of the dinoflagellate Prorocentrum triestinum. Naturwissenschaften. 2008;95:697–703. doi: 10.1007/s00114-008-0372-1. [DOI] [PubMed] [Google Scholar]
- Flynn K, Franco JM, Fernández P, Reguera B, Zapata M, Wood G, Flynn KJ. Changes in toxin content, biomass and pigments of the dinoflagellate Alexandrium minutum during nitrogen reefeding and growth into nitrogen or phosphorus stress. Mar. Ecol. Prog. Ser. 1994;111:99–109. [Google Scholar]
- Franco JM, Fernández P, Reguera B. Toxin profiles of natural populations and cultures of Alexandrium minutum Halim from Galician (Spain) coastal waters. J. Appl. Phycol. 1994;6:275–279. [Google Scholar]
- Godhe A, Otta SK, Rehnstam-Holm AS, Karunasagar I, Karunasagar I. Polymerase chain reaction in detection of Gymnodinium mikimotoi and Alexandrium minutum in field samples from southwest India. Mar. Biotechnol. 2001;3:152–162. doi: 10.1007/s101260000052. [DOI] [PubMed] [Google Scholar]
- Gould SJ. Wonderful life: the Burgess Shale and the nature of history. New York, NY: Norton; 1989. [Google Scholar]
- Gould SJ. The structure of evolutionary theory. Cambridge, MA: Harvard Univ. Press; 2002. [Google Scholar]
- Gould SJ, Lewontin RC. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc. R. Soc. Lond. B. 1979;205:581–598. doi: 10.1098/rspb.1979.0086. [DOI] [PubMed] [Google Scholar]
- Guisande C, Frangópulos M, Maneiro I, Vergara AR, Riveiro I. Ecological advantages of toxin production by the dinoflagellate Alexandrium minutum under phosphorus limitation. Mar. Ecol. Prog. Ser. 2002;225:169–176. [Google Scholar]
- Hallegraeff GM. A review of harmful algal blooms and their apparent global increase. Phycologia. 1993;32:79–99. [Google Scholar]
- Hallegraeff GM. Impacts of climate change on harmful algal blooms. SciTopics. 2009 Available at http://www.scitopics.com/Impacts_of_Climate_Change_on_Harmful_Algal_Blooms.html. [Google Scholar]
- Hallegraeff GM, Steffensen DA, Wetherbee R. Three estuarine Australian dinoflagellates that can produce paralytic shellfish toxins. J. Plankton Res. 1988;10:533–541. [Google Scholar]
- Hansen J, Sato M, Ruedy R, Lacis A, Oinas V. Global warming in the twenty-first century: an alternative scenario. Proc. Natl. Acad. Sci. USA. 2000;97:9875–9880. doi: 10.1073/pnas.170278997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen G, Daugbjerg N, Franco JM. Morphology, toxin composition and LSU rDNA phylogeny of Alexandrium minutum (Dinophyceae) from Denmark, with some morphological observations on other European strains. Harmful Algae. 2003;2:317–335. [Google Scholar]
- Hoagland P, Anderson DM, Kaoru Y, White AW. The economic effects of harmful algal blooms in states: estimates, assessment issues, and information. Estuaries. 2002;25:819–837. [Google Scholar]
- Hwang DF, Lu YH. Influence of environmental and nutritional factors son growth, toxicity, and toxin profile of dinoflagelate Alexandrium minutum. Toxicon. 2000;38:1491–1503. doi: 10.1016/s0041-0101(00)00080-5. [DOI] [PubMed] [Google Scholar]
- IPCC, Intergovermental Panel on Climate Change. Contribution of working group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge Univ. Press; 2007. [Google Scholar]
- Kimura M. The neutral theory of molecular evolution. Cambridge, UK: Cambridge Univ. Press; 1983. [Google Scholar]
- Lenski RE, Travisano M. Dynamics of adaptation and diversification: a 10.000 generation experiment with bacterial populations. Proc. Natl. Acad. Sci. USA. 1994;91:6808–6814. doi: 10.1073/pnas.91.15.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly EL, Kulis DM, Gentien P, Anderson DM. Paralytic shellfish poisoning toxins in France linked to a human-introduced strain of Alexandrium catenella from the western Pacific: evidence from DNA and toxin analysis. J. Plankton Res. 2002;24:443–452. [Google Scholar]
- Martins CA, Kulis D, Franca S, Anderson DM. The loss of PSP toxin production in a formerly toxic Alexandrium lusitanicum clone. Toxicon. 2004;43:195–205. doi: 10.1016/j.toxicon.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Mendoza H, López-Rodas V, González-Gil S, Aguilera A, Costas E. The use of polyclonal antisera and blocking of antibodies in the identification of marine dinoglafellates: species-specific and clone-specific antisera against Gymnodinium and Alexandrium. J. Exp. Mar. Biol. Ecol. 1995;186:103–115. [Google Scholar]
- Nehring S. Non-indigenous phytoplankton species in the North Sea: supposed region of origin and possible transport vector. Arch. Fish. Mar. Res. 1998;46:181–194. [Google Scholar]
- Ogata T, Ishimaru T, Kodama M. Effect of water temperature and light on growth rate and toxicity change in Protogonyaulax tamarensis. Mar. Biol. 1987;95:217–220. [Google Scholar]
- Parkhill JP, Cembella AD. Effects of salinity, light and inorganic nitrogen on growth and toxigenicity of the marine dinoflagellate Alexandrium tamarense from northeastern Canada. J. Plankton Res. 1999;21:939–955. [Google Scholar]
- Pérez-Zaballos FJ, Ortega-Mora LM, Álvarez-García G, Collantes-Fernández E, Navarro-Lozano V, García-Villada L, Costas E. Adaptation of Neospora caninum isolates to cell-culture changes: an argument in favor of its clonal population structure. J. Parasitol. 2005;91:507–510. doi: 10.1645/GE-381R1. [DOI] [PubMed] [Google Scholar]
- Persich GR, Kulis DM, Lilly EL, Anderson DM, García VMT. Probable origin and toxin profile of Alexandrium tamarense (Lebour) Balech from southern Brazil. Harmful Algae. 2003;5:36–44. [Google Scholar]
- Persson A, Godhe A, Karlson B. Dinoflagellate cysts in recent sediments from the west coast of Sweden. Bot. Mar. 2000;43:69–79. [Google Scholar]
- Pitcher GC, Cembella AD, Joyce LB, Larsen J, Probyn TA, Ruiz Sebastián C. The dinoflagellate Alexandrium minutum in Cape Town harbour (South Africa): bloom characteristics, phylogenetic analysis and toxin composition. Harmful Algae. 2007;6:823–836. [Google Scholar]
- Raven JA, Caldeira K, Elderfielf H, Hoegh-Guldberg O, Liss P, Riebessell U, Shepperd J, Turley C, Watson A, Heap R, et al. Ocean acidification due to increasing atmospheric carbon dioxide. London, U.K: Royal Society; 2005. Available at http://www.scar.org/articles/Ocean_Acidification%281%29.pdf. [Google Scholar]
- Reboud X, Bell G. Experimental evolution in Chlamydomonas. III. Evolution of specialist and generalist types in environments that vary in space and time. Heredity. 1996;78:507–514. [Google Scholar]
- Rost B, Zondervan I, Wolf-Gladrow D. Sensitivity of phytoplankton to future changes in ocean carbonate chemistry: current knowledge, contradictions and research directions. Mar. Ecol. Prog. Ser. 2008;373:227–237. [Google Scholar]
- Rouco M, López-Rodas V, Flores-Moya A, Costas E. Evolutionary changes in growth rate and toxin production in the cyanobacterium Microscytis aeruginosa under a scenario of eutrophication and temperature increase. Microb. Ecol. 2011;62:265–273. doi: 10.1007/s00248-011-9804-0. [DOI] [PubMed] [Google Scholar]
- Spiess EB. Genes in populations. New York, NY: Wiley; 1989. [Google Scholar]
- Travisano M, Mongold JA, Bennet FA, Lenski RE. Experimental tests of the roles of adaptation, chance and history in evolution. Science. 1995;267:87–90. doi: 10.1126/science.7809610. [DOI] [PubMed] [Google Scholar]
- Usup G, Pin LC, Ahmad A, Teen LP. Alexandrium (Dinophyceae) species in Malaysian waters. Harmful Algae. 2002;1:265–275. [Google Scholar]
- Vila M, Garcés E, Masó M, Camp J. Is the distribution of the toxic dinoflagellate Alexandrium catenella expanding along the NW Mediterranean coast? Mar. Ecol. Prog. Ser. 2001;222:73–83. [Google Scholar]
- Wagenaar DA, Adami C. Influence of chance, history and adaptation on digital evolution. Artif. Life. 2004;10:181–190. doi: 10.1162/106454604773563603. [DOI] [PubMed] [Google Scholar]
- Whitlock MC, Phillips PC, Moore FBG, Tonsor SJ. Multiple fitness peaks and epistasis. Annu. Rev. Ecol. Syst. 1995;26:601–629. [Google Scholar]
- Wright S. The roles of mutation, inbreeding, crossbreeding and selection in evolution. Proc. 6th Int. Congress Genet. 1932;1:356–366. [Google Scholar]
- Yoshida M, Ogata T, Thuoc CV, Matsuoka K, Fukuyo Y, Hoi NC, Kodama M. The first finding of toxic dinoflagellate Alexandrium minutum in Vietnam. Fish. Sci. 2000;66:177–179. [Google Scholar]
- Zar JH. Biostatistical analysis. 4th ed. Upper Saddle River, NJ: Prentice Hall; 1999. [Google Scholar]
- Zeebe RE, Wolf-Gladrow D. CO2 in seawater: equilibrium, kinetics, isotopes. Elsevier oceanographic series. Amsterdam, The Netherlands: Elsevier; 2001. [Google Scholar]


