Abstract
Background
Many patients exhibit multiple chronic disease risk behaviors. Research provides little information about advice that can maximize simultaneous health behavior changes.
Methods
To test which combination of diet and activity advice maximizes healthy change, we randomized 204 adults with elevated saturated fat and low fruit/vegetable intakes, high sedentary leisure time and low physical activity to one of four treatments: increase fruit/vegetable and physical activity; decrease fat and sedentary leisure; decrease fat and increase physical activity; increase fruit/vegetable and decrease sedentary leisure. Treatments provided three weeks of remote coaching supported by mobile decision support technology and financial incentives. During treatment, incentives were contingent on using the mobile device to self-monitor and attain behavioral targets; during follow-up they were contingent only on recording. The outcome was standardized, composite improvement on the four diet and activity behaviors at end of treatment and five month follow-up.
Results
Of those randomized, 200 (98%) completed follow-up. The increase fruit/vegetable and decrease sedentary leisure treatment improved more than the other 3 treatments (p<.001). Specifically, fruit/vegetables increased from 1.2 servings/day to 5.5; sedentary leisure decreased from 219.2 minutes/day to 89.3; saturated fat decreased from 12.0% of calories consumed to 9.5%. Differences between treatment groups were maintained through follow-up. Traditional dieting (decrease fat and increase physical activity) improved less than the other 3 treatments (p<.001).
Conclusions
Remote coaching supported by mobile technology and financial incentives holds promise to improve diet and activity. Targeting fruits/vegetables and sedentary leisure together maximizes overall adoption and maintenance of multiple healthy behavior changes.
Nonadherence with lifestyle change advice is cited as a major barrier to effective preventive care.1–2 Many physicians express skepticism that patients will change unhealthy behaviors; they also report lack of time and training to counsel patients effectively.3–4
Poor quality diet and inactivity are well-established behavioral risk factors for cardiovascular disease, cancer, and diabetes.5–8 Although healthy lifestyle change can reduce morbidity and premature mortality,9–12 fewer than 25% of U.S. adults meet dietary guidelines13 and 25% report no leisure time physical activity14. Suboptimal diet and a sedentary behavior pattern tend to cluster as risk behaviors,15–18 heightening disease risk19 and creating opportunity to intervene comprehensively, efficiently, and perhaps even synergistically on more than one risk behavior simultaneously. However, data are sparse regarding how to change multiple lifestyle behaviors simultaneously, especially when in-person contact time is limited, as in the medical encounter.
Increasingly, patients use mobile devices to manage activities across life domains, including health.20–21 This study’s interventions leveraged handheld technology to create efficient interventions that: make self-monitoring more convenient, extend decision support into life contexts where lifestyle choices are made21–22, and convey time-stamped behavioral data to paraprofessionals who provide coaching remotely. Participants received a handheld device and financial incentives initially to adopt recommended changes and subsequently to report behavior intermittently.
We designed the Make Better Choices trial to determine which combination of advice to change one dietary behavior (high saturated fat or low fruit/vegetable intake) and one activity behavior (high sedentary leisure or low physical activity) would maximize healthy diet and activity change during treatment and follow-up. Per behavioral choice theory,23–24 we predicted that increasing fruit/vegetables and decreasing sedentary leisure would maximize healthy change by fostering healthy substitution (of fruit/vegetables for fat and physical activity for sedentary leisure) and complementary behavior change (decreased fat accompanying decreased sedentary leisure). We also tested two alternative predictions about which treatment would maximize healthy change: traditional dieting (decrease fat and increase physical activity) because of its familiarity, or increase healthy behaviors (increase fruit/vegetables and physical activity) because it requires least inhibition of rewarding behaviors.24
Design and Methods
The study design and methods are discussed in detail elsewhere24 and will be described briefly.
Study Sample
Adults between ages 21 and 60 years were recruited through community advertisements. Eligible individuals were required to report all of the following: a) <5 fruit/vegetables/day25–26; b) >8% caloric intake from saturated fat; c) <60 min/day moderate/vigorous physical activity; and d) >90 min/day sedentary leisure (television, movies, recreational internet use, and videogames).
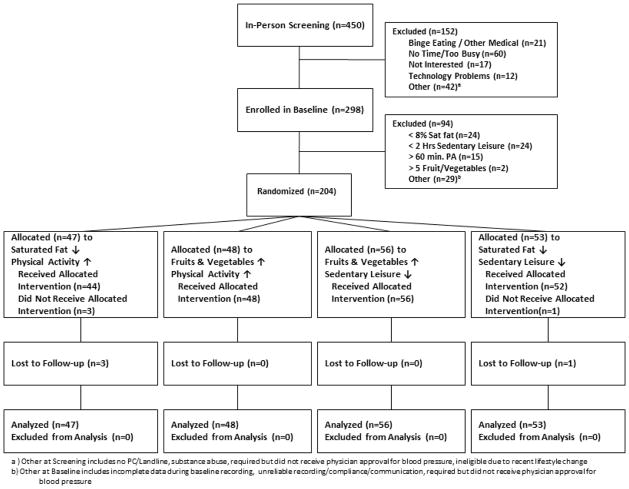
FIGURE 1 diagrams participant flow through the trial. All procedures were approved by the Institutional Review Boards of the University of Illinois at Chicago and Northwestern University.
Figure 1.
Study Design & Participant Flow
Two-Week Baseline Phase (and Final Eligibility Screening)
Candidates who self-reported all four risk behaviors were screened by a Bachelor’s-level research assistant (coach). The coach trained participants to estimate accurately and use a handheld device to record and upload dietary intake, moderate-vigorous intensity physical activity, and targeted sedentary leisure. During the two-week baseline phase, participants wore an accelerometer, recorded diet and activity on the handheld device, and uploaded daily.
Randomization
Candidates who displayed all four risk behaviors, as evidenced by handheld and accelerometer data, were randomized (stratified by sex) using a computer-generated sequence of randomly permuted blocks.
Intervention (Behavioral Treatment) Phase
Coaches tailored behavioral strategies based on participants’ baseline data. For example, those asked to decrease fat were shown ten foods that supplied their greatest saturated fat grams and coached to reduce portion size or number for those foods. For the first week of treatment, daily goals were set mid-way between baseline behavior and the ultimate daily goal. From the second treatment week onward, full goals were set for the two targeted behaviors to which participant was randomized: 5 fruit/vegetable servings, saturated fat intake <8% of calories, physical activity ≥60 minutes, or sedentary leisure ≤ 90 minutes per day. Participants were expected to reach their behavioral targets during treatment week 2 and to maintain them during week 3. During the three treatment weeks, they uploaded data daily and communicated as needed with their coaches via telephone or e-mail, per preference, to problem-solve barriers.
Participants could earn a $175 incentive for meeting goals for both targeted behaviors during the treatment phase.
Follow-Up Phase
To explore the potential for maintenance of healthy behavior changes, the study included a 20-week follow-up. Immediately after the treatment period, participants were informed that attainment of diet and activity targets was no longer required; payment was now contingent solely upon recording and transmitting handheld data on a predetermined schedule. Recording was required daily for the first week post-treatment, for three consecutive days in post-treatment weeks two and three, bi-weekly for the next six weeks, then monthly until final follow-up. Participants could earn incrementally larger financial incentives (from $30 to $80) for uploading data during consecutive follow-ups. All incentives were received at end of follow-up.
Handheld Tool
Participants used a personal digital assistant to record and self-regulate their targeted behaviors. They were advised to carry the device and record immediately after executing a behavior. During treatment and follow-up, the handheld device displayed two decision support feedback “thermometers” – one for diet (fruit/vegetable or fat) and one for activity (physical activity or sedentary leisure) (See eFigure 1). Once activated, goal thermometers were continually updated in response to data entry. They also enabled participants to look up the potential impact of a food or activity choice.
Measures
Outcomes were assessed by daily self-report recordings on the handheld device. Fat and fruit/vegetable consumption were measured from dietary intake recordings. To prevent superfluous calories (e.g. in sweetened beverages) from inflating the fat gram allowance, the saturated fat goal for those randomized to decrease fat, was determined using the Harris-Benedict equation27 to estimate calories needed to maintain weight. Minutes of physical and sedentary activity were measured cumulatively by an end-of-day 24-hour activity log in which participants accounted for every 15 minute block of each day. Prior studies have established the validity of self-report diet and activity assessments administered via a mobile device28–32 To further encourage honest recording, we implemented a validated bogus pipeline protocol,33 whereby participants submitted grocery receipts, accelerometer data and urine samples that they believed would be used to evaluate their self-reports.
Composite Diet - Activity Improvement Score
Rather than measure only single behaviors, we wished to assess simultaneous overall change on all four behaviors. To place the four behaviors (fruit/vegetable, fat, physical activity, and sedentary leisure) on a common scale in order to quantify overall change, we developed a “Composite Diet-Activity Improvement Score,” weighting each behavior equally. We transformed all variables to better approximate normality, using square root transformation for the count outcomes (fruit/vegetable, physical activity, and sedentary leisure) and arc sine transformation for the percentage outcome, fat34. Then we standardized each individual health behavior using a modified z-score (where 1 unit represents a 1-standard deviation change), with higher values representing greater healthy lifestyle improvement. To reflect improvement relative to baseline, we standardized z-scores for time-points after baseline relative to the overall baseline distribution. We calculated the mean of all four individual z-scores at each time point, as recommended35, to derive a composite index that expressed each participant’s overall healthy change across multiple diet and activity behaviors.
Statistical Analysis
The primary analytic aim was to determine which behavioral treatment maximizes initiation of healthy diet and activity change, measured as improvement from baseline through treatment phase. The secondary aim was to examine maintenance of change on the Composite Diet-Activity Improvement Score. A sample size of 200 (50 per treatment) was projected to yield power of .85 to detect a 0.5 s.d. difference in diet and activity improvement between treatments.
Statistical analyses were performed using SAS (version 9.2; SAS Institute Inc, Cary, North Carolina). Study hypotheses were tested using three a-priori planned contrasts comparing the predicted best treatment to all others combined: (1) decrease fat and increase physical activity versus others combined (2) increase fruit/vegetable and physical activity versus others combined, or (3) increase fruit/vegetable and decrease sedentary versus others combined. Analyses used a linear mixed model for longitudinal data36 with the Composite Diet-Activity Improvement Score as the dependent variable. For the within-participants factor of time (baseline, intervention weeks 1, 2–3, and follow-ups 1 through 8), we tested two comparisons for the intervention phase (intervention week 1 versus baseline, and intervention weeks 2–3 versus week 1) and three for the follow-up phase (an average of all follow-ups versus the final treatment phase time point, and linear and quadratic trends during the follow-up). These five time comparisons were included in all analyses. We also created interaction terms for each hypothesis by each time contrast. Inferences focused on treatment by time interactions, which compare the difference in behavior change over time between treatments, specifically testing for the components of the treatment by time interaction in a hierarchical manner (i.e., treatment by follow-up first, then treatment by intervention phase). For the variance-covariance structure of the longitudinal data, we used a heterogeneous Toeplitz structure36 that allowed the variances to vary across time and the correlations to vary across time lags. All analyses were carried out on an intent-to-treat basis and included sex as a covariate. Across the 11 time points, the proportion of missing data ranged from 4 to 11%.
We used separate mixed-effects regression models to predict each participant’s change from baseline through treatment phase for each of the four behaviors. We then correlated these four individual change estimates to examine whether changes in targeted behaviors were correlated with changes in untargeted behaviors.
Results
Study Sample
The final sample of 204 adults included 48 males; 46.6% with non-white race/ethnicity; 25% with no more than a high school education; and had a mean age of 33 years (s.d. = 11). Demographic information is summarized in TABLE 1. Except for one individual, all participants attained behavioral targets; the majority did so promptly. The median time taken to achieve consumption of five fruit/vegetables was nine days (i.e., two days after the five fruit/vegetable goal was set). The median time taken to attain the sedentary, fat, and physical activity targets was eight days (i.e., one day after the targeted amount was set).
Table 1.
Baseline Characteristics of Participants Assigned to Each Treatment Group*
| Fruits & Vegetables↑ | Saturated Fat↓ | Fruits & Vegetables↑ | Saturated Fat↓ | ||||
|---|---|---|---|---|---|---|---|
| Total | Physical Activity↑ | Sedentary Leisure↓ | Sedentary Leisure↓ | Physical Activity↑ | Test | P Value | |
| (n = 204) | (n = 48) | (n = 53) | (n = 56) | (n = 47) | (by treatment) | ||
| Age, y | |||||||
| Mean (SD) | 32.8 (11.0) | 33.4 (10.8) | 30.8 (10.8) | 35.0 (12.1) | 31.9 (9.7) | F=1.563 | 0.200 |
| BMI | |||||||
| Mean (SD) | 28.3 (7.3) | 28.6 (7.0) | 27.0 (6.6) | 28.3 (6.1) | 29.4 (9.3) | F=0.864 | 0.461 |
| Gender, No. (%) | |||||||
| Male | 48 (23.5) | 14 (29.2) | 12(22.6) | 14 (25.0) | 8(17.0) | ||
| Female | 156 (76.5) | 34 (70.8) | 41 (77.4) | 42 (75.0) | 39 (83.0) | χ2=2.05 | 0.563 |
| Ethnicity, No. (%) | |||||||
| White | 109 (53.4) | 22 (45.8) | 32 (60.4) | 33 (58.9) | 22 (46.8) | ||
| Black | 47 (23.0) | 15 (31.3) | 6 (11.3) | 12 (21.4) | 14 (29.8) | ||
| Hispanic/Latino | 18 (8.8) | 3 (6.3) | 5 (9.4) | 6 (10.7) | 4 (8.5) | ||
| Asian | 23 (11.3) | 7 (14.6) | 7 (13.2) | 4 (7.1) | 5 (10.6) | ||
| Other or multiple | 7 (3.5) | 1 (2.1) | 3 (5.7) | 1 (1.8) | 2 (4.3) | χ2=10.93 | 0.535 |
| Education, No. (%) | |||||||
| College degree | 151 (74.0) | 31 (64.6) | 41 (77.4) | 44 (78.6) | 35 (74.5) | ||
| No college degree | 53 (26.0) | 17 (35.4) | 12 (22.6) | 12 (21.4) | 12 (25.5) | χ2=3.14 | 0.371 |
Abbreviations: ↑ indicates increase; ↓ indicates decrease; n indicates number; y indicates years; SD indicates standard deviation
Treatment Effects on Composite Diet-Activity Improvement
The treatment by intervention phase interaction was highly significant (χ2 =38.1, df=6, p<.001), indicating that the treatment groups differed significantly on the Composite Diet-Activity Improvement Score. Further, this treatment effect remained significant throughout the subsequent follow up period (observed means graphed in FIGURE 2a).
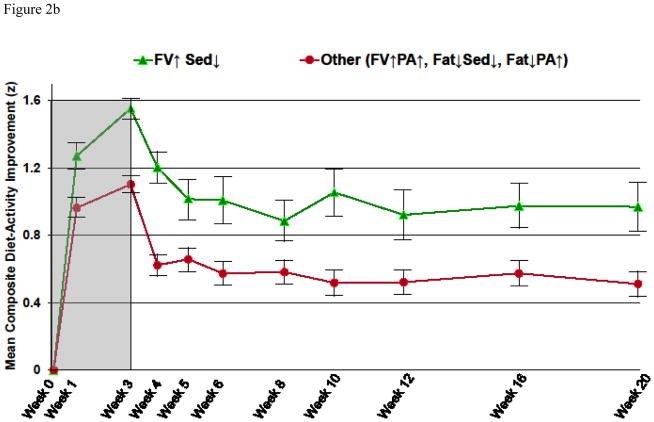
Figure 2.
Figure 2a: Standardized Composite Diet-Activity Improvement over Time for Each Treatment*
*Abbreviations: FV↑ indicates increase fruit/vegetable; Fat↓ indicates decrease saturated fat; PA↑ indicates increase physical activity; Sed ↓ indicates decrease sedentary leisure. Gray background indicates treatment phase (weeks 0–3); white background indicates follow-up without treatment (weeks 4–20).
Figure 2b: Mean (and SD¥) Standardized Composite Diet-Activity Improvement over Time for Increase Fruits/Vegetables, Decrease Sedentary Leisure versus All Other Treatments Combined*
*Abbreviations: ¥SD indicates standard deviation. FV↑ indicates increase fruit/vegetable; Fat↓ indicates decrease saturated fat; PA↑ indicates increase physical activity; Sed↓ indicates decrease sedentary leisure. Gray background indicates treatment phase (weeks 0–3); white background indicates follow-up without treatment (weeks 4–20).
The increase fruit/vegetable and decrease sedentary leisure treatment increased Composite Diet-Activity Improvement Scores more than the alternative treatments in the first week of intervention [t(383) = 3.16, p = .002]. The superiority of the increase fruit/vegetable and decrease sedentary leisure treatment, evident after one week, was maintained through the end of the three week treatment period and through follow-up week 20, (FIGURE 2b).
The decrease fat and increase physical activity treatment decreased Composite Diet-Activity Improvement Scores, as compared to other treatments in the first week of intervention [t(382) = −2.17, p = .031], a disadvantage that persisted through the end of treatment and follow-up.
Treatment Effects on Individual Diet and Activity Behaviors
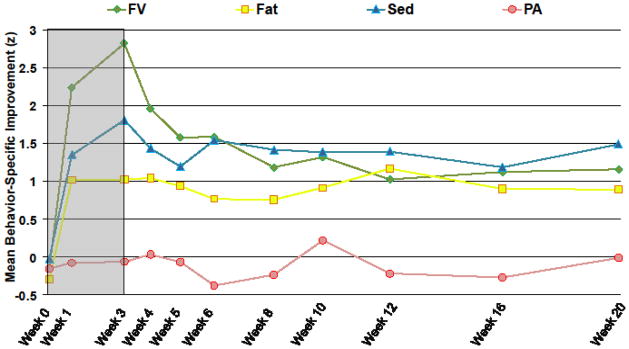
FIGURE 3 shows the effect of the increase fruit/vegetable and decrease sedentary leisure treatment on standardized improvement over time in the four behaviors. Raw (unstandardized) change is included, indicating that average fruit/vegetable intake changed from 1.2 (s.d. = .9) servings per day at baseline to 5.5 (s.d. = 1.0) at end of treatment, and 2.9 (s.d. = 2.3) at end of follow-up. Average minutes per day of sedentary leisure changed from 219.2 (s.d. = 93.8) at baseline to 89.3 (s.d. = 65.5) at end of treatment, and 125.7 (s.d. = 108.7) at end of follow-up. Average percentage of daily calories from saturated fat changed from 12.0 (s.d. = 2.2) at baseline to 9.4 (s.d. = 1.9) at end of treatment, and 9.9 (s.d. = 3.4) at end of follow-up.
Figure 3.
Standardized Improvement in Each Behavior Produced by Increase Fruits/Vegetables, Decrease Sedentary Leisure Treatment*
*Abbreviations: FV indicates fruits/vegetables; Fat indicates saturated fat; PA indicates physical activity; Sed indicates sedentary leisure. Gray background indicates treatment phase (weeks 0–3); white background indicates follow-up without treatment (weeks 4–20).
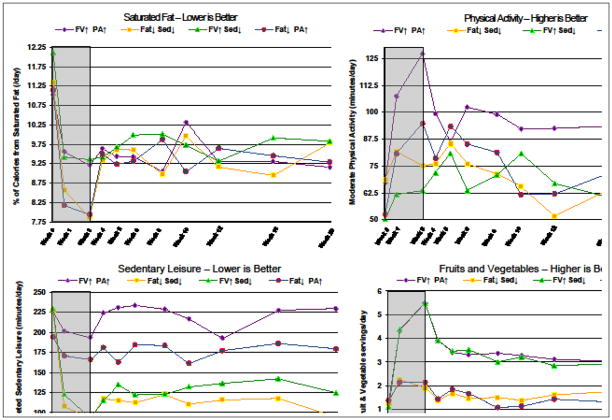
Group means for each behavior in natural units (i.e., minutes/day, servings, or percent of daily caloric intake) are presented in TABLE 2 for all treatments and are graphed in FIGURE 4.
Table 2.
Mean (and SD) for Each Observed Behavior in Natural Units as a function of Treatment Group and Time*
| Sedentary Leisure (minutes) | Physical Activity (minutes) | Fruits & Vegetables (servings) | Calories from Sat Fat (%) | |
|---|---|---|---|---|
| Fruits & Vegetables↑ | ||||
| Physical Activity↑ | ||||
| (n = 48) | ||||
|
| ||||
| Week 0 | 228.1 (100.8) | 70.4 (46.8) | 1.3 (1.1) | 11.2 (2.0) |
|
| ||||
| Week 3 | 206.2 (135.2) | 127.1 (88.6) | 5.6 (1.1) | 9.3 (2.1) |
|
| ||||
| Week 20 | 245.8 (161.8) | 85.1 (73.3) | 2.8 (1.9) | 9.8 (3.2) |
|
| ||||
| Saturated Fat↓ | ||||
| Sedentary Leisure↓ | ||||
| (n = 53) | ||||
|
| ||||
| Week 0 | 232.1 (91.6) | 66.0 (59.8) | 1.4 (1.1) | 11.3 (2.3) |
|
| ||||
| Week 3 | 89.5 (75.2) | 76.8 (85.7) | 1.9 (1.6) | 7.8 (1.8) |
|
| ||||
| Week 20 | 103.2 (77.1) | 63.7 (58.8) | 1.5 (1.5) | 9.6 (4.2) |
|
| ||||
| Fruits & Vegetables↑ | ||||
| Sedentary Leisure↓ | ||||
| (n = 56) | ||||
|
| ||||
| Week 0 | 219.2 (93.8) | 49.6 (36.3) | 1.2 (0.9) | 12.0 (2.2) |
|
| ||||
| Week 3 | 89.3 (65.5) | 64.0 (78.0) | 5.5 (1.0) | 9.5 (1.9) |
|
| ||||
| Week 20 | 125.7 (108.7) | 74.5 (87.6) | 2.9 (2.3) | 9.9 (3.4) |
|
| ||||
| Saturated Fat↓ | ||||
| Physical Activity↑ | ||||
| (n = 47) | ||||
|
| ||||
| Week 0 | 199.0 (82.3) | 50.7 (47.4) | 1.1 (0.9) | 11.4 (1.9) |
|
| ||||
| Week 3 | 182.3 (100.4) | 95.5 (65.9) | 1.7 (1.1) | 7.8 (1.9) |
|
| ||||
| Week 20 | 188.3 (114.5) | 78.1 (75.6) | 1.3 (1.4) | 9.1 (4.1) |
Abbreviations: ↑ indicates increase; ↓ indicates decrease. Week 3 is end of treatment. Week 20 is end of no treatment follow-up.
Figure 4.
Effects of the 4 Treatments on Changes Over Time in Each Behavior Expressed in Natural Units*
*Abbreviations: FV indicates fruits/vegetables; Fat indicates saturated fat; PA indicates physical activity; Sed indicates sedentary leisure. Gray background indicates treatment phase (weeks 0–3); white background indicates follow-up without treatment (weeks 4–20)
Within the increase fruit/vegetable and decrease sedentary leisure treatment group, we examined correlations among the individual behavior change estimates to determine whether changes in targeted behaviors were correlated with changes in untargeted behaviors. The degree to which participants decreased their sedentary leisure time (targeted) correlated positively with the degree to which they also reduced their fat intake (untargeted), r(52) = .29, p = .04. Correlations among other behavior change pairs were nonsignificant and ranged from r(52) = −.14 to .15.
Comment
This study demonstrates the feasibility of changing multiple unhealthy diet and activity behaviors simultaneously, efficiently, and with minimal face-to-face contact by using mobile technology, remote coaching, and incentives. The increase fruit/vegetable and decrease sedentary leisure treatment maximized healthy lifestyle change compared with the other interventions. In addition to producing targeted improvements in fruits/vegetables and sedentary leisure time, the treatment produced untargeted improvement in saturated fat intake. The superiority of the increase fruit/vegetable and decrease sedentary leisure treatment was present after 1 week of intervention persisted through the end of the 3-week treatment phase and was maintained. As expected, since participants were no longer asked to maintain healthy changes, lifestyle gains did diminish once treatment ended. Nevertheless, substantial improvements (1 s.d. compared to baseline) in fruit/vegetables, sedentary leisure, and fat persisted through the 5-month follow-up. From baseline to end of treatment to end of follow-up, respectively, mean fruits/vegetables changed from 1.2 to 5.5 to 2.9; mean minutes/day sedentary leisure from 219.2 to 89.3 to 125.7; and percent daily calories from saturated fat from 12.0 to 9.4 to 9.9. Even though they were neither asked nor reinforced to maintain eating or activity improvements, 86% of the 185 participants from whom exit interviews were obtained said they “definitely” or “somewhat” tried to maintain gains.
Consistent with behavioral choice theory,23–24 decreasing recreational screen time was complemented by a reduction in saturated fat. Previous research has demonstrated that manipulating screen time changes energy and fat intake in children.38 This is the first study to show that reducing sedentary leisure time decreases fat intake in adults. Increased interest in sedentary behavior has been driven by epidemiological data associating sedentarism with cancer39, obesity40–41, type 2 diabetes 19, 40–42, depression43, cardiovascular events19,44, and increased mortality 19, 44–45, independent of physical activity. Reduced screen time may be an important behavioral target not only to reverse direct adverse effects of prolonged sitting46 but also to disrupt pairing of screen time with high-fat snacking.
Notably, the traditional dieting regimen (decrease fat and increase physical activity) produced less healthy change than other treatments: it only improved two behaviors, and increased physical activity did not persist. The requirement to inhibit rewarding behaviors had no systematic impact on diet-activity change: the increase fruit/vegetables and physical activity treatment was no more advantageous than other treatments and the decrease fat and sedentary leisured was no more disadvantageous. 24
These results are germane to physicians trying to help patients improve multiple health risk behaviors. Physicians play an important role by advising and assisting patients to accomplish healthy behavioral changes,9,47 especially since a trusting relationship with a provider is associated with greater adherence to advice.3 However, limits on physicians’ time combined with movement towards new systems of patient-centered, team-based care48 create an opportunity to reconsider the optimal locus and configuration of health behavior change counseling. Results suggest feasibility and potential benefit of a systems reconfiguration that reinforces health behavior change by connecting patients with mobile technology, incentives, and remote, non-physician coaches.
A number of study limitations warrant consideration. Generalizability of the findings is limited by the constraints that the study was conducted in a research setting and only a quarter of the sample was male. Use of a screening phase to confirm the presence of the risk behaviors may additionally limit generalizability to those with entrenched unhealthy diet and activity behaviors. Also, the amount of the financial incentive was larger than would be feasible for some settings. It remains to be determined whether such rapid and full acquisition of behavior change targets would occur with smaller incentives. Further, the fact that primary outcome measures were self-reported raises the possibility that participants might have overstated their behavioral improvements to earn incentives. We find that unlikely for several reasons. First, treatment differences remained after controlling for the effects of financial motivation and social desirability. Second, the sample ranked financial motives lowest among their reasons to join the trial. Third, maintaining diet and activity improvements yielded no financial reward during follow-up, an altered contingency made apparent to participants by staff reminders and by discontinuation of study procedures (urine samples, accelerometry, grocery receipts) that could have verified self-reports. Yet participants maintained substantial improvements and most said they did so intentionally.
Finally, although physical activity was increased by treatments that targeted it, it was the one behavior not improved by the increase fruit/vegetable and decrease sedentary leisure treatment. We currently are testing whether all four risk behaviors can be improved by targeting physical activity simultaneously or sequentially with fruit/vegetables and sedentary leisure.
Strengths of the study include the ethnic diversity of the sample and minimal loss to follow-up. Also, the sample was deliberately chosen to present challenges for behavior change. The requirement that participants unremittingly display all four risk behaviors throughout baseline screening, even while self-monitoring, yielded a sample with risk behaviors that were refractory to lower intensity behavioral intervention. A key innovation was the use of mobile technologies that connect and provide decision support to patients and coaches, reducing the need for professionals to perform counseling. Another strength was the conservative, comparative research design that contrasts active treatments.
Interventions that target multiple, prevalent, covariant risk behaviors simultaneously have the potential to be powerfully efficient and cost effective. Yet many multiple behavior change interventions have achieved limited success,49–51 presumably because their interventions were insufficiently intensive.52 As mobile technologies become increasingly ubiquitous, they afford a scalable platform to extend continuing support for healthy behavior change pervasively into the environment with potential to improve population health.
Supplementary Material
Acknowledgments
The Make Better Choices trial was supported by National Institutes of Health (NIH) grant HL075451 to Dr. Spring, by the Robert H. Lurie Comprehensive Cancer Center grant (NIH P30 CA060553), and by NIH F31 MH070107 to Dr. Schneider. We thank Michael J. Coons, Sherry Pagoto, Christine Dutton Pellegrini, and Alex Pictor for technical assistance.
Footnotes
Trial Registration: Make Better Choices, R01 HL0756451 (Spring), 09/01/04 – 01/10, NIH/NHLBI.
Author Contributions: Dr Spring had full access to all of the study data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Spring, Schneider, Epstein, Hedeker
Acquisition of data: Schneider, Vaughn, Kozak, Smith, DeMott
Analysis and interpretation of data: Hedeker, Spring, Siddique, Moller, Schneider, McFadden
Drafting of the manuscript: Moller, Spring, Schneider, Kozak
Critical revision of the manuscript for intellectual content: Kozak, Hedeker, Epstein, Lloyd-Jones
Statistical analysis: Hedeker, Siddique, Spring, Moller, Schneider, McFadden
Obtained funding: Spring
Administrative, technical, or material support: McFadden, Vaughn, Smith
Study supervision: Spring
References
- 1.Colditz GA, Jenkins PR. Prevention trials: their place in how we understand the value of prevention strategies. Ann Rev Pub Hlth. 2010;31:105–20. doi: 10.1146/annurev.publhealth.121208.131051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith TW, Orleans CT, Jenkins CD. Prevention and health promotion: decades of progress, new challenges, and an emerging agenda. Health Psychol. 2004;23:126–31. doi: 10.1037/0278-6133.23.2.126. [DOI] [PubMed] [Google Scholar]
- 3.Bazata DD, Robinson JG, Fox KM, Grandy S SHIELD Study Group. Affecting behavior change in individuals with diabetes: findings from the Study to Help Improve Early Evaluation and Management of Risk Factors Leading to Diabetes (SHIELD) Diabetes Educator. 2008;34:1025–1036. doi: 10.1177/0145721708325767. [DOI] [PubMed] [Google Scholar]
- 4.Vogt F, Hall S, Marteau TM. General practitioners’ and family physicians’ negative beliefs and attitudes towards discussing smoking cessation with patients: a systematic review. Addiction. 2005;100:1423–1431. doi: 10.1111/j.1360-0443.2005.01221.x. [DOI] [PubMed] [Google Scholar]
- 5.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Department of Agriculture, U.S. Department of Health and Human Services. Dietary Guidelines for Americans. 7. Washington, DC: U.S. Government Printing Office; 2010. [Google Scholar]
- 7.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans (ODPHP Publication No U0036) Washington, DC: U.S. Government Printing Office; 2008. Available online: http://www.health.gov/paguidelines/pdf/paguide.pdf. [Google Scholar]
- 9.Lin JS, O’Connor E, Whitlock EP, Beil TL. Behavioral counseling to promote physical activity and a healthful diet to prevent cardiovascular disease in adults: A systematic review for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2010;153:736–750. doi: 10.7326/0003-4819-153-11-201012070-00007. [DOI] [PubMed] [Google Scholar]
- 10.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Svetkey LP, Harsha DW, Vollmer WM, Stevens VJ, Obarzanek E, Elmer PJ, et al. Premier: a clinical tria modification for blood pressure control: rationale, design and baseline characteristics. Ann Epidemiol. 2003;13:462–471. doi: 10.1016/s1047-2797(03)00006-1. [DOI] [PubMed] [Google Scholar]
- 12.Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemiö K, et al. Sustained reduction incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368(9548):1673–1679. doi: 10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC) Prevalence and Trends Data 2009. [Accessed May 30, 2011];Behavioral Risk Factor Surveillance System. 2011 Available at http://apps.nccd.cdc.gov/BRFSS.
- 14.Centers for Disease Control and Prevention. State Indicator Report on Physical Activity. Atlanta, GA: U.S. Department of Health and Human Services; 2010. [Google Scholar]
- 15.Emmons KM, Marcus BH, Linnan L, Rossi JS, Abrams DB. Mechanisms in multiple risk factor interventions: Smoking, physical activity, and dietary fat intake among manufacturing workers. Prev Med. 1994;23:481–489. doi: 10.1006/pmed.1994.1066. [DOI] [PubMed] [Google Scholar]
- 16.Fine LJ, Philogene GS, Gramling R, Coups EJ, Sinha S. Prevalence of multiple chronic disease risk factors: 2001 national health interview survey. Am J Prev Med. 2004;27(2S):18–24. doi: 10.1016/j.amepre.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Gillman MW, Pinto BM, Tennstedt S, Glanz K, Marcus B, Friedman RH. Relationship of physical activity with dietary behaviors among adults. Prev Med. 2001;32:295–301. doi: 10.1006/pmed.2000.0812. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien K. Bulletin No 24. Canberra: Australian Institute of Health and Welfare; 2005. Living dangerously: Australians with multiple risk factors for cardiovascular disease. [Google Scholar]
- 19.Grøntved A, Hu FB. Television Viewing and Risk of Type 2 Diabetes, Cardiovascular Disease, and All-Cause Mortality: A Meta-analysis. JAMA. 2011;305:2448–455. doi: 10.1001/jama.2011.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pew Research Center. Internet and American Life Project: Tracking Survey. Washington, DC: Pew Research Center; 2011. [Google Scholar]
- 21.Baumgart D. Smartphones in clinical practice, medical education, and research. Arch Intern Med. 2011;171:1294–1296. doi: 10.1001/archinternmed.2011.320. [DOI] [PubMed] [Google Scholar]
- 22.Spring B. Health decision making: lynchpin of evidence-based practice. Med Decis Making. 2008;28:866–872. doi: 10.1177/0272989X08326146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bickel WK, Vuchinich RE, editors. Reframing health behavior change with behavioral economics. New Jersey: Lawrence Erlbaum Associates, Inc; 2000. [Google Scholar]
- 24.Spring B, Schneider K, McFadden HG, Vaughn J, Kozak AT, Smith M, et al. Make Better Choices (MBC): Study design of a randomized controlled trial testing optimal technology-supported change in multiple diet and physical activity risk behaviors. BMC Public Health. 2010;10:586. doi: 10.1186/1471-2458-10-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Cancer Institute (US) 5 a Day for Better Health Program. Bethesda, MD: National Institutes of Health, National Cancer Institute; 2001. [Google Scholar]
- 26.United States Department of Agriculture, US Department of Health and Human Services. Nutrition and your health: dietary guidelines for Americans. Washington, DC: US Government Printing Office; 1995. [Google Scholar]
- 27.Harris J, Benedict F. A biometric study of basal metabolism in man. Washington D.C: Carnegie Institute of Washington; 1919. [Google Scholar]
- 28.Beasley J, Riley WT, Jean-Mary J. Accuracy of a PDA-based dietary assessment program. Nutrition. 2005 Jun;21:672–7. doi: 10.1016/j.nut.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Fukuoka Y, Kamitani E, Dracup K, Jong SS. New Insights Into Compliance With a Mobile Phone Diary and Pedometer Use in Sedentary Women. J Phys Act Health. 2011 Mar;8:398–403. doi: 10.1123/jpah.8.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sternfeld B, Jiang SF, Picchi T, Chasan-Taber L, Ainsworth B, Quesenberry CP., Jr Evaluation of a Cell Phone-Based Physical Activity Diary. Med Sci Sports Exerc. 2011 Aug 12; doi: 10.1249/MSS.0b013e3182325f45. [DOI] [PubMed] [Google Scholar]
- 31.Clark BK, Sugiyama T, Healy GN, Salmon J, Dunstan DW, Owen N. Validity and reliability of measures of television viewing time and other non-occupational sedentary behaviour of adults: a review. Obes Rev. 2009 Jan;10:7–16. doi: 10.1111/j.1467-789X.2008.00508.x. 2008 Jul 8. [DOI] [PubMed] [Google Scholar]
- 32.Long JD, Littlefield LA, Estep G, Martin H, Rogers TJ, Boswell C, Shriver BJ, Roman-Shriver CR. Evidence review of technology and dietary assessment. Worldviews Evid Based Nurs. 2010 Dec 7;:191–204. doi: 10.1111/j.1741-6787.2009.00173.x. [DOI] [PubMed] [Google Scholar]
- 33.Roese NJ, Jamieson DW. Twenty years of bogus pipeline research: A critical review and meta-analysis. Psychological Bulletin. 1993;114:363–375. [Google Scholar]
- 34.Mosteller F, Tukey JW. Data analysis and regression: a second course in statistics. Reading, MA: Addison-Wesley; 1977. [Google Scholar]
- 35.Prochaska JJ, Velicer WF, Nigg CR, Prochaska JO. Methods of quantifying change in multiple risk factor interventions. Prev Med. 2008;46:260–265. doi: 10.1016/j.ypmed.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hedeker D, Gibbons RD. Longitudinal data analysis. Hoboken, NJ: Wiley and Sons; 2006. [Google Scholar]
- 38.Epstein LH, Roemmich JN, Paluch RA, Raynor HA. Influence of changes in sedentary behavior on energy and macronutrient intake in youth. Am J Clin Nutr. 2005;81:361–366. doi: 10.1093/ajcn.81.2.361. [DOI] [PubMed] [Google Scholar]
- 39.Gierach GL, Chang SC, Brinton LA, Lacey JV, Jr, Hollenbeck AR, Schatzkin A, et al. Physical activity, sedentary behavior, and endometrial cancer risk in the NIH-AARP diet and health study. Inter J Cancer. 2009;124:2139–2147. doi: 10.1002/ijc.24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. J Am Med Assoc. 2003;289:1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 41.Healy GN, Wijndaele K, Dunstan DW, et al. Objectively measured sedentary time, physical activity, and metabolic risk: The Australian diabetes, obesity and lifestyle study (AusDiab) Diabetes Care. 2008;31:369–371. doi: 10.2337/dc07-1795. [DOI] [PubMed] [Google Scholar]
- 42.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–79. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 43.Hamer M, Stamatakis E, Mishra GD. Television and screen-based activity and mental well-being in adults. Am J Prev Med. 2010;38:375–380. doi: 10.1016/j.amepre.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 44.Stamatakis E, Hamer M, Dunstan DW. Screen-based entertainment time, all-cause mortality, and cardiovascular events: Population-based study with ongoing mortality and hospital events follow-up. J Am Col Cardiol. 2011;57:292–300. doi: 10.1016/j.jacc.2010.05.065. [DOI] [PubMed] [Google Scholar]
- 45.Patel AV, Bernstein L, Deka A, et al. Leisure time spent sitting in relation to total mortality in a prospective cohort of US adults. Am J Epidemiol. 2010;172:419–429. doi: 10.1093/aje/kwq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamilton MT, Healy GN, Dunstan DW, Zderic TW, Owen N. Too little exercise and too much sitting: Inactivity physiology and the need for new recommendations on sedentary behavior. Curr Cardiovasc Risk Rep. 2009;2:292–298. doi: 10.1007/s12170-008-0054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldstein MG, Whitlock EP, DePue J. Planning Committee of the Addressing Multiple Behavioral Risk Factors in Primary Care Project. Am J Prev Med. 2004;27(2 Suppl):61–79. doi: 10.1016/j.amepre.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 48.Schuetz B, Mann E, Everett W. Educating Health Professionals Collaboratively For Team-Based Primary Care. Health Affairs. 2010;29:1476–1480. doi: 10.1377/hlthaff.2010.0052. [DOI] [PubMed] [Google Scholar]
- 49.Multiple Risk Factor Intervention Trial Research Group. Multiple Risk Factor Intervention Trial: Risk Factor Changes and Mortality Results. JAMA. 1982;248:1465–1477. [PubMed] [Google Scholar]
- 50.Fortmann SP, Varady AN. Effects of a Community-wide Health Education Program on Cardiovascular Disease Morbidity and Mortality: the Stanford Five-City Project. Am J Epidemiol. 2000;152:216–232. doi: 10.1093/aje/152.4.316. [DOI] [PubMed] [Google Scholar]
- 51.Luepker RV, Råstam L, Hannan PJ, Murray DM, Gray C, Baker WL, et al. Community education for cardiovascular disease prevention: morbidity and mortality results from the Minnesota Heart Health Program. Am J Epidemiol. 1996;144:351–62. doi: 10.1093/oxfordjournals.aje.a008936. [DOI] [PubMed] [Google Scholar]
- 52.Spring B. Translational Behavioral Medicine: A pathway to better health. Translational Behavioral Medicine. 2011 Mar;1:1–3. doi: 10.1007/s13142-011-0029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.