Summary
PNH is a rare clonal hematopoietic stem disorder clinically characterized by the triad of chronic complement-mediated hemolysis, thrombosis, and bone marrow failure. While median survival has improved when historical data are compared to more recent data, thrombosis, the major cause of death in PNH, is still observed in approximately 40% of patients. The symptoms associated with this disorder–including fatigue, pain, esophageal spasm, and erectile dysfunction–are often severe and disabling. While PNH may be a curiosity to the physician, it forces the majority of patients to significantly modify their lives. Transplantation represents a curative option; however, the risks associated with this option are not insignificant. Eculizumab has been shown to significantly reduce hemolysis, improve anemia, reduce transfusion requirements, and significantly improve fatigue and other QoL scores. Clearly, targeted complement inhibition by eculizumab has the promise to significantly improve the lives of patients with PNH.
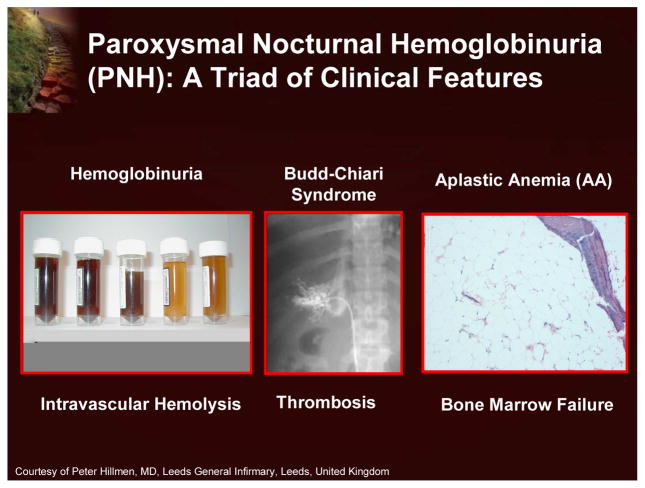
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired, clonal hematopoietic stem cell disorder that can manifest clinically as chronic hemolysis, a propensity for venous thrombosis, and bone marrow failure (Figure 1).1
Figure 1.
Paroxysmal Nocturnal Hemoglobinuria (PNH): A Triad of Clinical Features
While many physicians have little or no experience treating patients with PNH, there have been many recent advances in the management of this disease. These advances were discussed by a panel of international experts at a satellite symposium that preceded the 49th American Society of Hematology Annual Meeting in Atlanta, Georgia. This symposium was sponsored by an educational grant from Alexion Pharmaceuticals, Inc.
Introduction to the Pathophysiology of Paroxysmal Nocturnal Hemoglobinuria: Intravascular Hemolysis and Failed Hematopoiesis
Neal S. Young, MD, MACP
National Institutes of Health, Bethesda, Maryland
Co-Editor, Seminars in Hematology
Co-Editor, Clinical Hematology
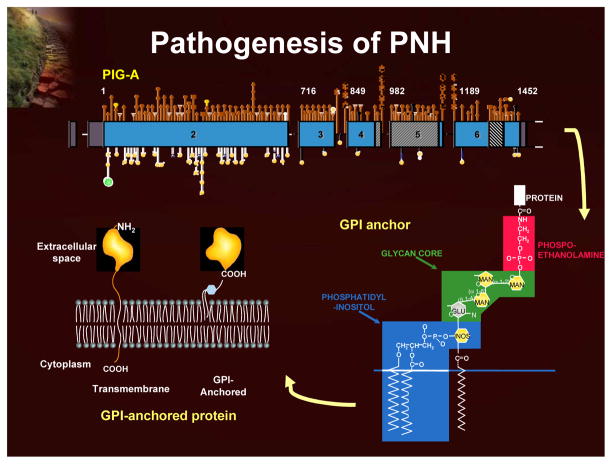
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired, clonal hematopoietic stem cell disorder that can manifest clinically as chronic complement-mediated hemolysis, bone marrow failure, and predisposition to thrombosis.1 The affected cells of the PNH clone have a deficiency of an entire class of cell surface proteins known as glycosylphosphatidylinositol-anchored proteins (GPI-AP). These proteins function as enzymes, receptors, complement regulators, and adhesion molecules. The GPI-AP defect in PNH results from a mutation in an X-linked gene (PIG-A; phosphatidylinositol glycan class A), the product of which is involved in an early step in the synthesis of the GPI anchor (Figure 2). Two GPI-AP that protect red blood cells from complement activity, CD55 (DAF or decay accelerating factor) and CD59 (MIRL or membrane inhibitor of reactive lysis) are lacking on the surface of PNH red blood cells, thereby leaving these cells highly sensitive to complement-mediated destruction.1
Figure 2.
Pathogenesis of PNH
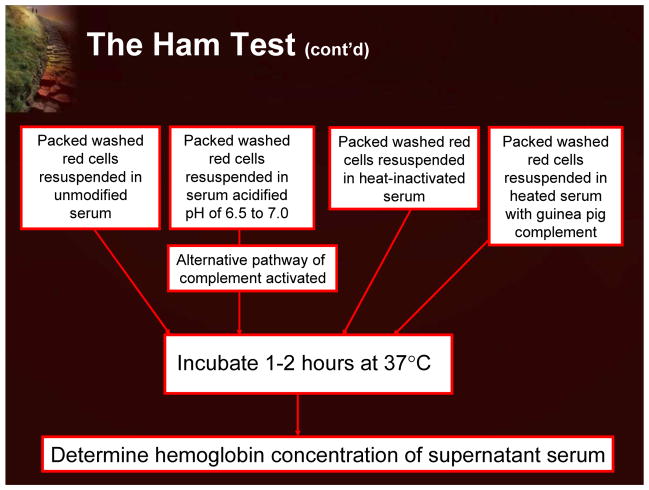
The early history of PNH is dominated by the characterization of the dark urine that was a hallmark of affected patients. It became clear that this was not the result of red blood cells itself, but actually the pigment (hemoglobin) inside red blood cells.2,3 Further work in the early part of the 20th century focused on comparing PNH as a hemolytic process with other more common types of hemolytic disease (eg, hemolytic anemia). It became clear that the defect was intrinsic in the PNH cells, with an additional factor(s) in the plasma required for this defect to become clinically manifested. The data established that the red blood cells in patients with PNH were more sensitive to lysis by the terminal components of complement.4 This work led to another milestone in understanding PNH that involved the development of a diagnostic test. Based on the classic observation that red cells could be lysed in the presence of acidified serum in a patient with PNH, a specific diagnostic test, the Ham test, was developed in the 1940s and 1950s.5 The Ham test was, until recently, used as a diagnostic tool in patients with PNH.
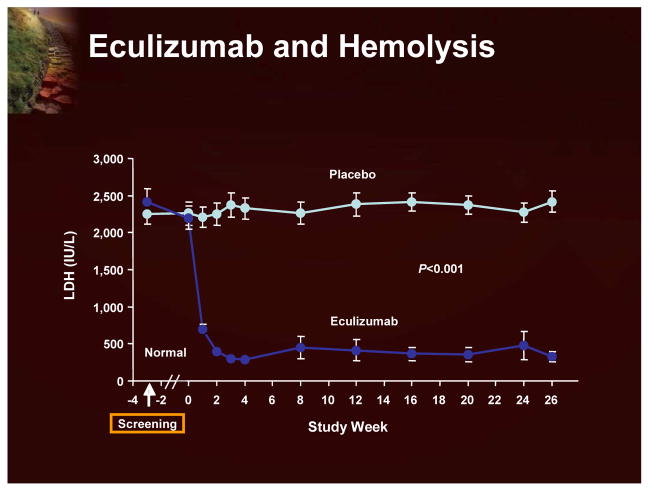
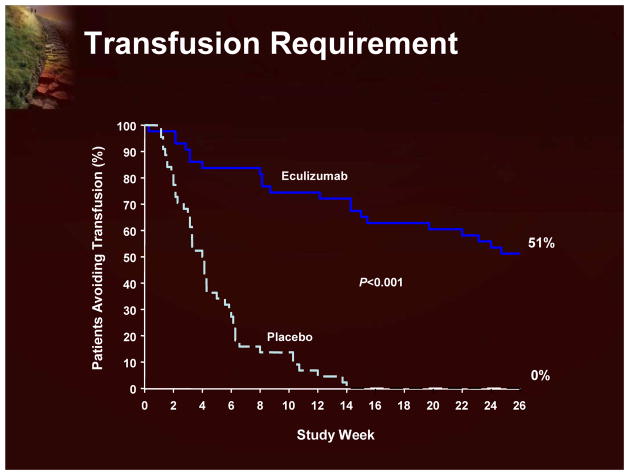
Although the classic treatment for hemolytic anemia has been corticosteroids, the basis for this treatment of the hemolytic component of PNH is not well established and is associated with significant side effects. There are, unfortunately, no randomized studies, and doubts remain about exactly how useful this therapy really is in PNH.1 A more recent therapeutic option is eculizumab, an antibody designed to block a late component of complement (C-5).6 This has been shown to have a significant effect on hemolysis. As shown in Figure 3, using LDH as a surrogate of intravascular hemolysis, eculizumab treatment significantly reduces hemolysis.7 Eculizumab also has a significant effect on transfusion requirements (Figure 4).7
Figure 3.
Eculizumab and Hemolysis
Figure 4.
Transfusion Requirement
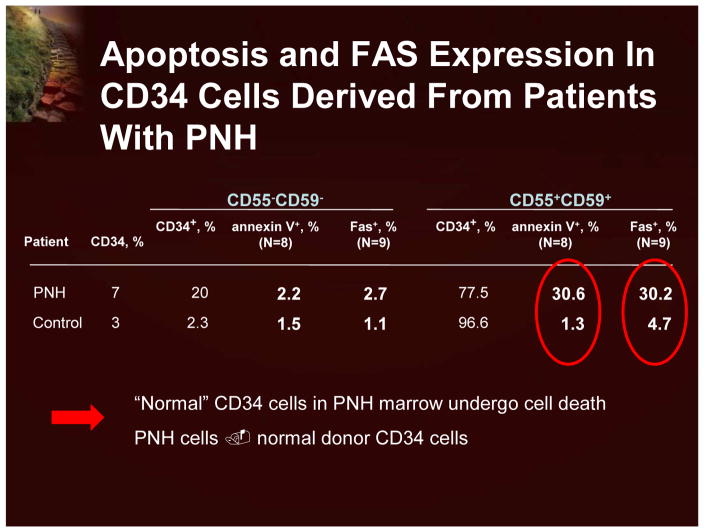
In contrast to the evolution of hemolysis and the resulting symptoms, the bone marrow failure characteristic in PNH patients is not well understood. Studies have shown that simply having a PNH molecular lesion (a PIG-A gene deletion) is not sufficient to cause PNH.1 There was no obvious proliferative or anti-apoptotic advantage to having the gene defect. Indeed, many individuals with no clinical symptoms have PNH cells. Clearly, other factors are necessary for the clonal expansion of PNH cells. Functional and microarray assays have demonstrated that the PNH clone looks extremely similar to normal CD-34 cells. It appears that the expansion of PNH cells is actually the result of phenotypically normal cells undergoing apoptosis in the PNH patient (Figure 5).8 There are thoughts that this is an immunologically driven process.
Figure 5.
Apoptosis and FAS Expression in CD34 Cells Derived From Patients With PNH
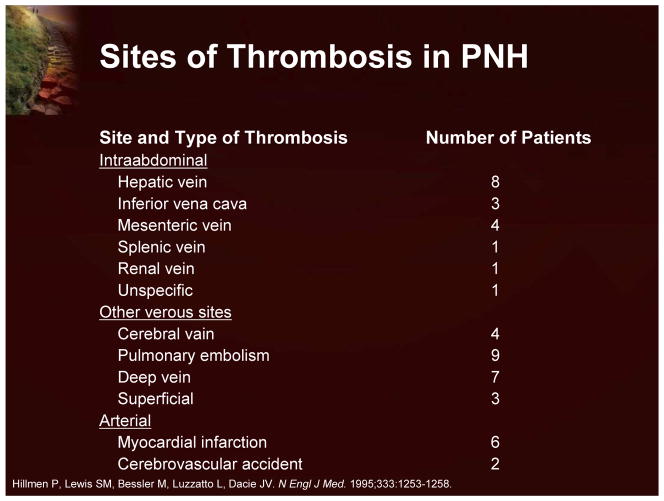
The third clinical manifestation of PNH is thrombosis. This is a very important clinical issue, as thrombosis is responsible for the majority of deaths among PNH patients.9 It is also significant to note that thrombosis occurs in unusual sites (Figure 6).
Figure 6.
Site of Thrombosis in PNH
The complement-mediated hemolysis and the resultant release of hemoglobin contributes to many of the clinical manifestations of PNH, including: fatigue, pain, esophageal spasm, erectile dysfunction, and possibly thrombosis. The treatment of patients with PNH who present with manifestations of bone marrow failure is typically directed toward improving the cytopenias and includes bone marrow transplantation or immunosuppression. Eculizumab, a humanized monoclonal antibody against C5 that inhibits terminal complement activation, has demonstrated a dramatic effect on intravascular hemolysis and transfusion requirements, in addition to improving patient quality of life (QoL). Eculizumab may also reduce the risk of life-threatening thrombotic events.1
PNH Diagnosis: A Rapidly Changing World
Gabrielle Meyers, MD
Oregon Health and Science University, Portland, Oregon
Paroxysmal nocturnal hemoglobinuria can present in a variety of clinical scenarios, including: Coombs’ negative hemolytic anemia, bone marrow failure (eg, aplastic anemia, MDS), hemoglobinuria, unusual or repetitive thromboses, or episodic dysphagia, or abdominal pain of unclear etiology associated with hemolysis. While PNH can be difficult to recognize, there are a number of new treatment options available and the use of reliable diagnostic tests is clearly clinically beneficial.
The Ham test, the first diagnostic test for PNH, was described in the 1930s and was based on the finding that PNH red blood cells were exquisitely sensitive to hemolysis in the setting of acidified serum (Figure 7).5
Figure 7.
The Ham Test
The Ham test is an elegant assay, as it is only positive in PNH and one other very different disorder (HEMPAS; hereditary erythroblastic multinuclearity with positive acidified serum lysis test). While the Ham test was the standard diagnostic assay for PNH for decades, the false negative rate represents a major drawback. The assay only evaluates red cells, and given the short life span of PNH red blood cells, a small population can easily be missed. Although other complement-dependent tests, such as the sucrose lysis test and the complement lysis sensitivity (CLS) test, were subsequently developed, the Ham test occupies a position of great historical importance as it directly led to further studies into the pathophysiology of the disease.10
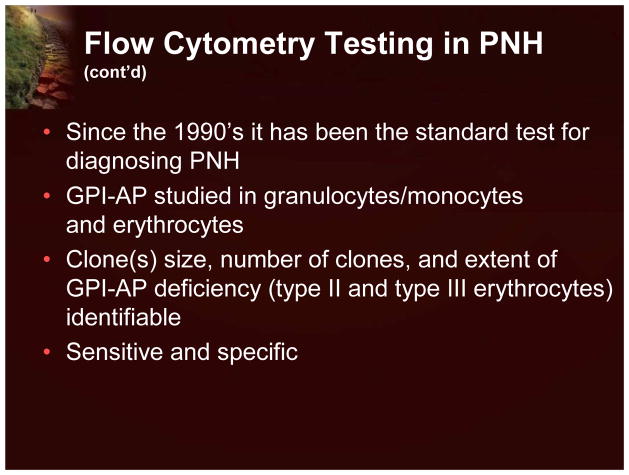
Flow cytometric analysis using labeled antibodies directed against GPI-AP currently represents the most sensitive and informative assay available for the diagnosis and screening of PNH.11 Indeed, as PNH is a disorder of cell surface proteins, it is ideally suited for a flow cytometric assay. The original flow cytometry studies in the 1990s demonstrated that granulocytes and lymphocytes, in addition to red blood cells, were deficient in GPI-AP. By evaluating these other cell types, it was possible to determine the size of the PNH clone. The flow cytometry assays typically evaluate at least 2 different GPI-AP on white cells (typically monocytes) and red blood cells. In addition to a diagnosis, one can also determine clone size, if there is more than one clone, and the extent of GPI-AP deficiency. Since peripheral blood can be used, the test is very easy to perform (Figure 8).12
Figure 8.
Flow Cytometry Testing in PNH
There are a number of additional flow-based assays that are of interest, albeit from a research point of view. The FLAER (fluorescently labeled aerolysin) assay utilizes aerolysin, an inactivated bacterial toxin. This toxin actually binds to GPI-AP, leading to membrane disruption and cell lysis. However, the fluorescently tagged inactivated form merely binds and tags them. As PNH cells lack GPI-AP, they are identified and separated from normal cells by being FLAER-negative.13
There are numerous molecular techniques that can be used to diagnose PNH, including the identification of mutations in genes (eg, PIG-A gene) through sequencing. Although standard sequencing techniques are time consuming and not suited for routine use, newer techniques allow for the easier identification of mutations in PNH patient samples. These new techniques include High Resolution Melting analysis that evaluates the melting points of PCR products (of usually less than 1,000 base pairs). This technique is highly sensitive and specific, and can identify single nucleotide changes, as well as deletions and insertions (Figure 9).14
Figure 9.
High Resolution Melting Analysis
PNH can be challenging to recognize because it may present in a variety of clinical scenarios. Testing for PNH with a reliable and sensitive assay will reduce missed diagnoses, help prognosticate disease in which PNH clones may also be present, and allow affected patients to receive new treatment options. The gold-standard test for the diagnosis of PNH is flow cytometry of the peripheral blood. Newer techniques are available, but are currently being used in the research setting rather than routine clinical practice.
Optimizing Patient Outcomes in PNH
Prof. Dr. Hubert Schrezenmeier
Universität Ulm, Ulm, Germany
PNH is characterized by the triad of acquired corpuscular hemolysis, thrombophilia, and bone marrow failure. The intravascular destruction of PNH red blood cells causes chronic, often disabling symptoms of fatigue and intermittent episodes of dysphagia, abdominal pain, and hemoglobinuria. Hemolytic anemia often makes the patient transfusion dependent, and symptoms of PNH, including risk of thromboembolic complications and aplastic anemia, significantly affect the patient’s QoL.1
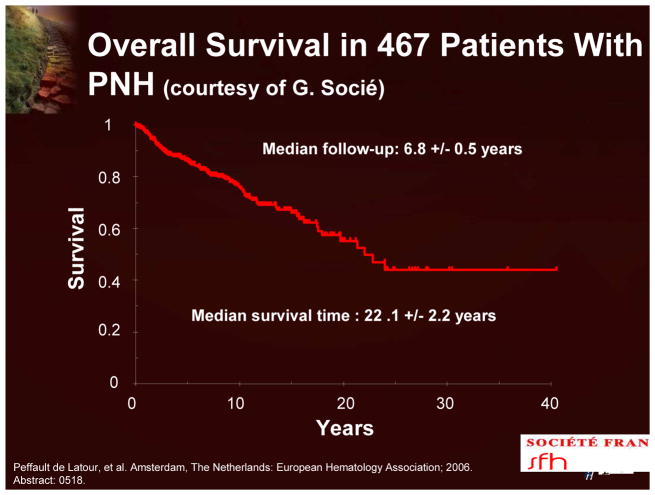
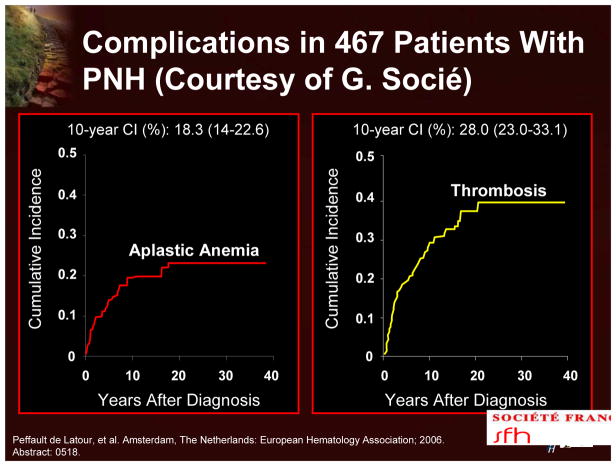
The course of PNH is highly variable. It is usually chronic, but spontaneous remissions do occur. Current data indicate that the median survival is 22 years (Figure 10). The 10-year cumulative incidence of aplastic anemia and thrombosis is 18% and 28%, respectively.15 Although there have been recent significant improvements in survival, for most patients PNH is a chronic and often life-threatening disease.
Figure 10.
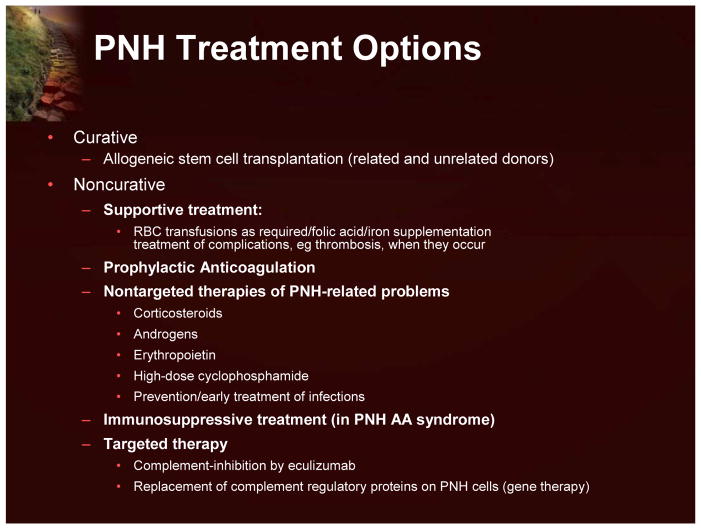
The current available treatment options for patients with PNH are shown in Figure 11.
Figure 11.
PNH Treatment Options
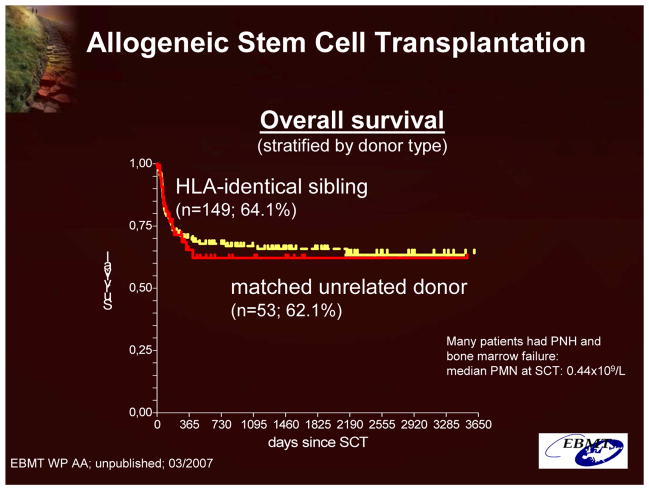
The only truly curative option is allogeneic stem cell transplantation from related or unrelated donors (Figure 12). In light of the related morbidity and mortality, stem cell transplantation is typically recommended for patients with an underlying bone marrow abnormality, major PNH complications (eg, major life-threatening thromboembolic episodes), or refractory transfusion-dependent hemolytic anemia.16
Figure 12.
Allogeneic Stem Cell Transplantation: Overall Survival (Data From the Working Party on Aplastic Anemia of the European Group for Blood and Marrow Transplantation (EBMT)
Noncurative treatment options include supportive treatment with red blood cell transfusions and iron supplementation. The transfused cells are resistant to complement-mediated lysis, elevate hemoglobin, transiently ameliorate the hemolysis by suppressing hematopoiesis, and result in the passive transfer of GPI-AP. Transfusions are typically given as required based on the symptoms of anemia and vary greatly from patient to patient and during the course of the disease.16
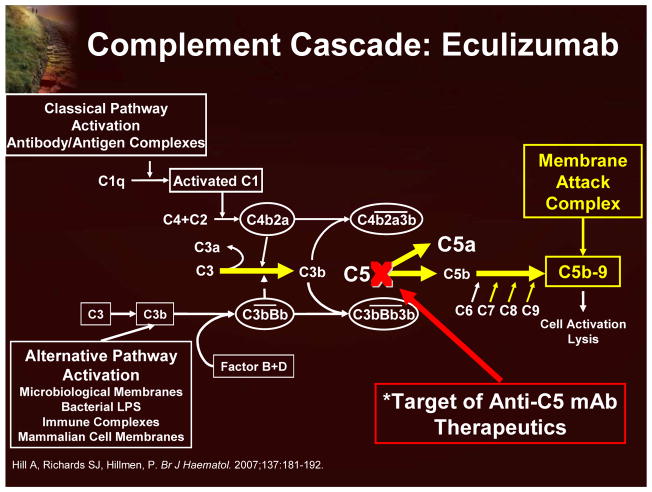
Eculizumab is a humanized monoclonal antibody that specifically targets the complement protein C5 and prevents its cleavage (Figure 13). Consequently, PNH red blood cells are not subject to complement-mediated lysis.17
Figure 13.
Complement Cascade and Eculizumab
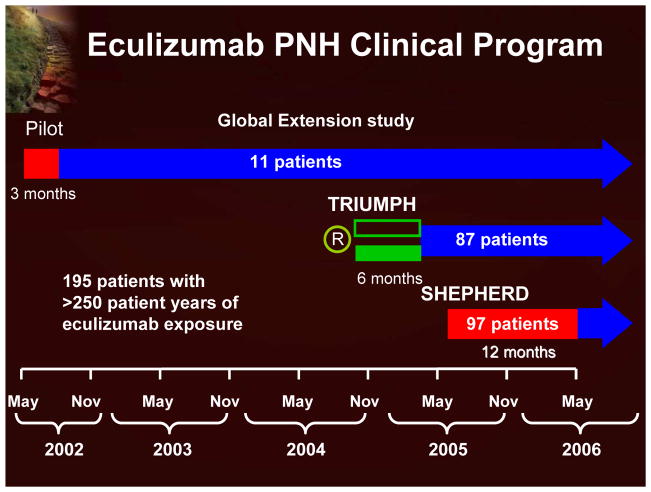
The eculizumab clinical program (Figure 14) includes 3 studies (a pilot study, TRIUMPH, and SHEPHERD; each followed by an extension study), with 195 patients and >250 patient years of eculizumab exposure.7,18–20 The data from these clinical trials clearly demonstrate that eculizumab significantly reduces hemolysis (using LDH as a surrogate marker; Figure 15) and leads to improvement in anemia with reduction in red blood cell transfusion requirements and stabilization of hemoglobin. Transfusion independence is achieved by half of the patients treated with eculizumab. The reduction in intravascular hemolysis with eculizumab was associated with significant improvement in fatigue and other QoL measurements. Recent evidence also indicates that eculizumab reduces the risk of thrombosis.18
Figure 14.
Eculizumab PNH Clinical Program
Figure 15.
Levels of LDH During Eculizumab Treatment
Importantly, eculizumab was well tolerated with rates of adverse events and infections generally similar to those observed in the placebo-treated patients in the clinical trials. However, eculizumab may increase a patient’s susceptibility to meningococcal infections, so patients should be vaccinated 2 weeks prior to the initiation of therapy. Nevertheless, eculizumab treatment offers significant clinical benefit to a broad and diverse population of patients with PNH.
With the advent of new therapies have come a number of questions. These include the choice and sequence of combination therapies, the necessary reevaluation of stem cell transplantation in the era of targeted complement inhibition by eculizumab, the role of immunosuppressive therapy and eculizumab, the future role of prophylactic anticoagulation, and the use of eculizumab in specific patient populations (eg, pregnancy).
PNH and Thrombosis: Who to Treat and How
Peter Hillmen, MB, ChB, PhD, FRCP, FRCPath
Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom
The most frequent complication and cause of death due to PNH is thrombosis.9 Recent data, demonstrating a median survival of 20 years for patients with PNH, also indicate that thrombosis develops in about 40% of patients (Figure 16).15 Thrombosis in PNH patients is commonly observed in the venous system with a particular predilection for the hepatic veins, other intra-abdominal veins, such as the mesenteric veins, and the cerebral veins. The relative risk of dying after a patient’s first thrombosis increases tenfold. Recent data indicate that there may also be an increased risk of thrombosis in the arterial system with cerebrovascular accidents occurring in relatively young people.9
Figure 16.
Complications in 467 Patients With PNH
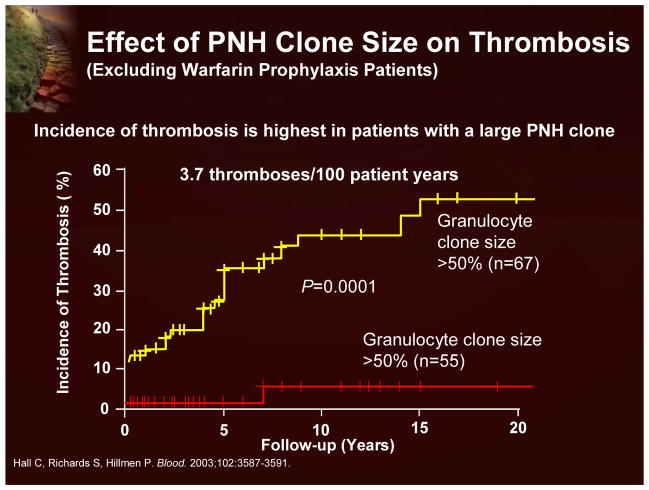
While there are some well known factors that increase the risk of thrombosis (eg, pregnancy, oral contraceptives, or the presence of a lupus anticoagulant), recent studies have suggested that the most accurate predictor of thrombosis in PNH is the size of the PNH clone (Figure 17). Patients with >50% PNH neutrophils are considered to be at the high risk.21 A number of additional studies have also demonstrated that patients with large PNH clones predominantly develop thrombosis.
Figure 17.
Effect of PNH Clone Size on Thrombosis
The cause of thrombosis in PNH is certainly multifactorial, but it is thought to be a direct result of intravascular hemolysis (consumption of nitric oxide by free hemoglobin) the result of which is increased thrombosis and platelet activation by the unopposed action of complement.21
In terms of management, prompt initiation of thrombolytic therapy is critical in cases of organ- or life-threatening thrombosis, especially large-vessel thrombosis such as that which can occur in the hepatic or portal vein. In acute thrombosis, such thrombolytic therapy can be administered directly into the affected vessel through a catheter. Prophylaxis against thromboembolic events in PNH is a matter of debate. While there are no randomized, prospective studies available, prophylaxis with warfarin has been evaluated in patients with a >50% PNH clones size.21 Although such therapy did appear to reduce the risk of first thrombosis, there are risks involved. Such therapy is associated with a risk of hemorrhage, particularly in those patients with thrombocytopenia (a common condition in PNH patients), and fatal bleeds have been reported. In addition, when patients controlled on warfarin have a severe PNH crisis, they frequently may not eat for several days. The combination of low platelets, nausea, and anorexia make anticoagulation control in these patients very difficult to manage.
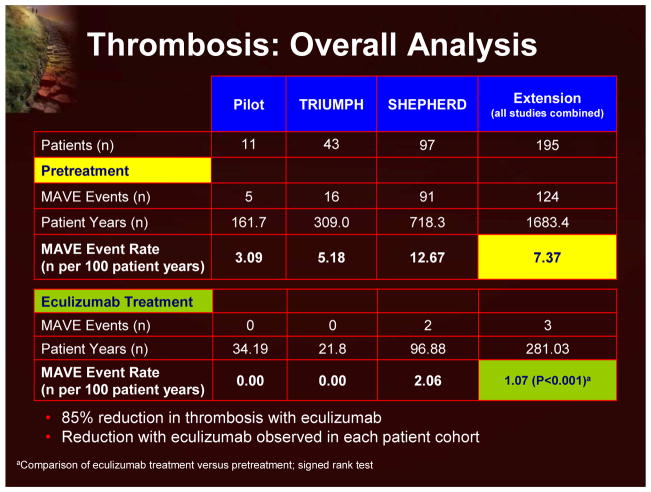
There are emerging data that eculizumab treatment results in a decrease in thrombotic risk in patients with PNH. In an overall analysis of the available eculizumab clinical trials, the thrombotic event rate significantly decreased from 7.37 thromboses per 100 patient years in the pre-eculizumab period to 1.07 in the posteculizumab period (Figure 18).18 While these data are impressive, it is also important to note that the patients enrolled in these trials did appear to be on the more severe end of the PNH spectrum (195 patients, 1,683 follow-up years, 124 thrombotic events=7.37 thromboses per 100 patient years). Further sub-analyses demonstrated that eculizumab lowered the rate of thromboses in patients receiving anticoagulants, in patients with elevated hemoglobin levels, and in lower-risk patients (ie, lower transfusion requirements).
Figure 18.
Thrombosis: Overall Analysis
The identification of high-risk patients (ie, >50% PNH neutrophils) is important in the management of thrombosis in patients with PNH. Patients who do not require transfusions, are relatively asymptomatic from hemolysis, and have no contraindication to warfarin should be considered for primary thromboprophylaxis with warfarin. Patients who do require transfusions or are symptomatic from intravascular hemolysis should be considered for eculizumab, which will protect against thrombosis as well as improving other PNH-related symptoms. Patients who present with a thrombosis should be considered for thrombolysis (if appropriate), initially anticoagulated with heparin, and considered for eculizumab therapy.
In conclusion, thrombosis represents the major cause of mortality and morbidity in PNH, in spite of adequate anticoagulation, particularly after the initial thrombosis. Eculizumab significantly reduces the risk of thrombosis in PNH and appears to have a robust effect across all patients.
Improving the Quality of Life for Patients With PNH
Anita Hill, MB, ChB (Hons), MRCP, FRCPath
Bradford Teaching Hospitals NHS Foundation Trust, Bradford, United Kingdom
The impact of PNH on patients’ lives is often underestimated by many physicians. PNH does not have to cause a thrombosis or require monthly transfusions to have a major adverse impact on a patient’s life. The consequences of hemolysis, including dark urine, pain, dysphagia, and erectile dysfunction, can be devastating and serve as constant reminders to the patient that they are ill. However, the fatigue that affects many patients with PNH is often the most disabling symptom. The fatigue experienced is typically out of proportion with the level of hemoglobin; patients feel worse than would be expected from the degree of anemia.
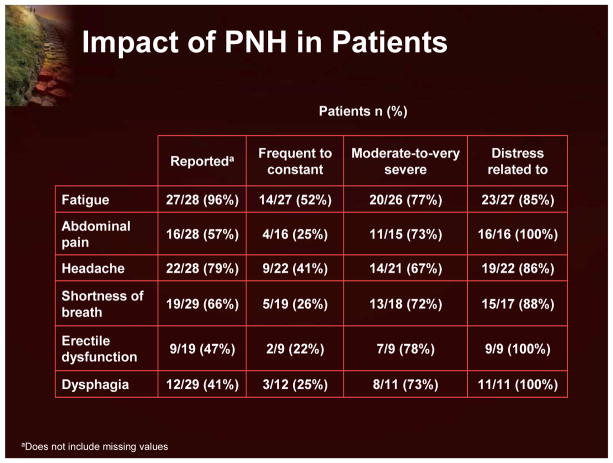
The prevalence and severity of PNH symptoms were studied in 29 PNH patients using 2 instruments: Functional Assessment of Chronic Illness Therapy - Fatigue (FACIT-F) and the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire 30 (EORTC QLQ-C30) (Figure 19).22 There was a significant burden of disease within this population: 76% were forced to modify their daily activities and 17% were unemployed due to their disease. Nearly all of the patients reported fatigue; erectile dysfunction and dysphagia were also common. More than half reported abdominal pain, headache, and shortness of breath. In most patients these symptoms were characterized as moderate-to-very severe, and were associated with distress. This fatigue and poor QoL is thought to be due to chronic hemolysis and the negative consequences of the release of free hemoglobin and the subsequent depletion of nitric oxide.
Figure 19.
Impact of PNH in Patients
There are number of strategies that can improve the lives of patients with PNH (Figure 20). Listening to patients is an important start, even if their symptoms are not thought by the physician to be significant. Physicians typically place greater importance on pain as a factor to control, whereas patients are actually often more concerned with fatigue. The amelioration of fatigue in patients with PNH would significantly improve their QoL. While transfusions can improve fatigue, albeit temporarily, by increasing the hemoglobin level, they do not impact the other consequences of chronic hemolysis that also contribute to fatigue. Some patients feel their QoL is actually diminished by transfusion therapy because of the inconvenience.
Figure 20.
Strategies to Improve QoL
Clearly, therapies for PNH that can prevent hemolysis, and thereby improve fatigue, pain, shortness of breath, dysphagia, and erectile dysfunction will be of great benefit to the patient. While transplantation may result in a cure for the patient, there are significant side effects that can be long-lasting and negatively impact QoL. The humanized monoclonal antibody eculizumab has been shown to prevent intravascular hemolysis, and importantly, has been shown in PNH clinical trials to result in clinically meaningful improvements in patient-reported outcomes, including fatigue, global health status, patient functioning, and disease-related symptoms. In the original pilot study, eculizumab was administered for 12 weeks to 11 patients, who then continued into extension studies. Using the EORTC QLQ-C30 instrument, significant improvements in many health domains were observed, including global status, physical functioning, and fatigue. Six of the 11 patients reported 1 or more of these symptoms regularly prior to treatment, and all demonstrated improvements with eculizumab treatment.23
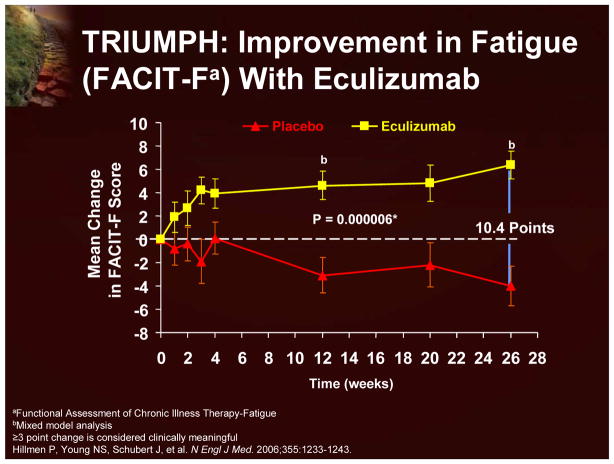
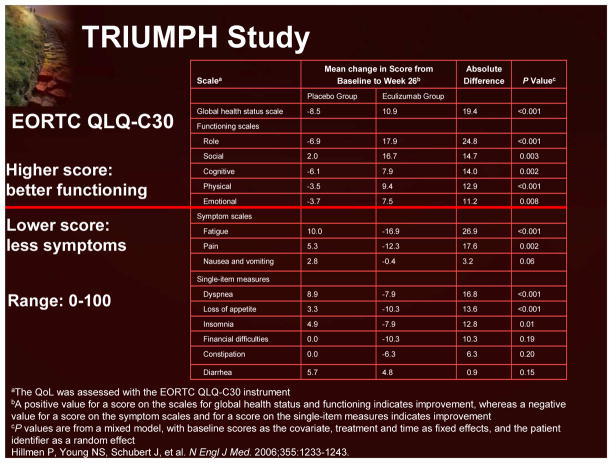
In the Phase 3 Triumph study, in which patients were randomized to receive either eculizumab or placebo for 6 months, the effects upon fatigue and other QoL measures were prospectively examined using the FACIT-F and EORTC QLQ-C30 instruments. Eculizumab treatment resulted in clinically meaningful improvements in fatigue (using both the FACIT-F; Figure 21 and EORTC QLQ-C30 instruments) and global health status, and patient functioning (Figure 22). Interestingly, treatment-independent univariant analyses demonstrated that a reduction in intravascular hemolysis (demonstrated by a decrease in LDH levels) and an improvement in anemia (demonstrated by increased hemoglobin levels) were both significantly associated with an improvement in fatigue. Multivariant analyses indicated that a reduction in hemolysis was more predictive of improvements in fatigue that an improvement in anemia.7
Figure 21.
TRIUMPH: Improvement in Fatigue (FACIT-F) With Eculizumab
Figure 22.
TRIUMPH Study: EORTC QLQ-C30
The impact of PNH on the patient can be immense and in many cases, quite debilitating. It is, unfortunately, often underestimated by physicians. However, data from the eculizumab trial indicate that the resolution of intravascular hemolysis can result in meaningful improvements in QoL and patient-reported outcomes, including fatigue, global health status, patient functioning, and disease-related symptoms. This has the potential to dramatically improve the lives of patients with PNH.
Case study.
A 36-year-old man was referred to Leeds in June 1999. In February 1994, he presented with severe abdominal pain that required opiate pain killers. He was given an initial diagnosis of Crohns Disease. Over the next 4 years, he suffered frequent attacks of abdominal pain (4–6 per year) characterized by severe central abdominal pain with nausea and vomiting. The patient lost weight, could not work, experienced feelings of guilt, and became opiate dependent. In December 1998, he developed dark urine associated with an attack of abdominal pain, and PNH was finally diagnosed. The patient was then referred to Leeds. He was started on warfarin to combat thrombosis and folic acid for the increased consumption from the high cellular turnover. His QoL was so bad that transplantation was discussed. He returned to Leeds in May 2000 with continuing episodes of severe abdominal pain and was actually hospitalized for 8 months. He remained opiate-dependent, with attacks occurring every 2–3 weeks accompanied by dark urine. In 2002, the patient became transfusion dependent. His pain was constant with severe flare-ups, forcing him to retire from work. He sought counseling due to his opiate dependence and depression.
He entered the TRIUMPH eculizumab trial in October 2004, and received his first infusion on January 20, 2005. His urine color improved very quickly and by April his abdominal pain had resolved. He reduced his opiate use and returned to work at the beginning of April 2005, having not worked for over 10 years. He ate more, put on weight, and began to socialize. He says that this drug transformed his life and he is now working full-time. He continues on treatment.
Footnotes
| Name of Faculty or Presenter | Reported Financial Relationship |
|---|---|
| Anita Hill, MD | Lecture Fees: Alexion Pharmaceuticals |
| Peter Hillmen, MB, ChB, PhD, FRCP, FRCPath | Consulting Fees: Alexion Pharmaceuticals Paid Research: Alexion Pharmaceuticals |
| Gabrielle Meyers, MD | Lecture Fees: Alexion Pharmaceuticals, Inc; MGI Pharma, Inc. |
| Prof. Dr. Hubert Schrezenmeier | No financial interest/relationships with financial interests relating to the topic of this activity |
| Neal S. Young, MD, MACP | No financial interest/relationships with financial interests relating to the topic of this activity |
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Neal S. Young, Email: youngns@nhlbi.nih.gov, Hematology Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland.
Gabrielle Meyers, Center for Hematologic Malignancies, Oregon Health and Science University, Portland, Oregon.
Prof. Dr. Hubert Schrezenmeier, Institut fur Klinische Transfusionsmedizin und Ummungenetik Ulm, Institut fur Transfusionsmedizin, Universitat Ulm, Ulm, Germany
Peter Hillmen, Leeds Teaching Hospital NHS Trust, Leeds, United Kingdom.
Anita Hill, Bradford Teaching Hospitals, NHS Foundation Trust and University of Leeds, Bradford, United Kingdom.
References
- 1.Brodsky RA. New insights into paroxysmal nocturnal hemoglobinuria. Hematology Am Soc Hematol Educ Program. 2006:24–8,516. doi: 10.1182/asheducation-2006.1.24. [DOI] [PubMed] [Google Scholar]
- 2.Rosse W. A brief history of PNH. In: Young NS, Moss J, editors. Paroxysmal Nocturnal Hemoglobinuria and the GPI-linked Proteins. New York, NY: Academic Press; 2000. pp. 1–20. [Google Scholar]
- 3.Crosby WH. Paroxysmal nocturnal hemoglobinuria; a classic description by Paul Strübing in 1882, and a bibliography of the disease. Blood. 1951;6(3):270–284. [PubMed] [Google Scholar]
- 4.Rosse WF. Variations in the red cells in paroxysmal nocturnal haemoglobinuria. Br J Haematol. 1973;24(3):327–342. doi: 10.1111/j.1365-2141.1973.tb01657.x. [DOI] [PubMed] [Google Scholar]
- 5.Ham TH. Chronic hemolytic anemia with paroxysmal nocturnal hemoglobinuria: A study of the mechanism of hemolysis in relation to acid-base equilibrium. N Engl J Med. 1937;217:915–918. [Google Scholar]
- 6.Thomas TC, Rollins SA, Rother RP, et al. Inhibition of complement activity by humanized anti-C5 antibody and single-chain Fv. Mol Immunol. 1996;33(17–18):1389–1401. doi: 10.1016/s0161-5890(96)00078-8. [DOI] [PubMed] [Google Scholar]
- 7.Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355:1233–1243. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- 8.Chen G, Kirby M, Zeng W, Young NS, Maciejewski JP. Superior growth of glycophosphatidy linositol-anchored protein-deficient progenitor cells in vitro is due to the higher apoptotic rate of progenitors with normal phenotype in vivo. Exp Hematol. 2002;30(7):774–782. doi: 10.1016/s0301-472x(02)00811-1. [DOI] [PubMed] [Google Scholar]
- 9.Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333(19):1253–1258. doi: 10.1056/NEJM199511093331904. [DOI] [PubMed] [Google Scholar]
- 10.Richards SJ, Hillmen P. Advances in the laboratory diagnosis of paroxysmal nocturnal hemoglobinuria. Clin Appl Immunol Rev. 2001;1(6):315–330. [Google Scholar]
- 11.Hall SE, Rosse WF. The use of monoclonal antibodies and flow cytometry in the diagnosis of paroxysmal nocturnal hemoglobinuria. Blood. 1996;87(12):5332–5340. [PubMed] [Google Scholar]
- 12.Richards SJ, Rawstron AC, Hillmen P. Application of flow cytometry to the diagnosis of paroxysmal nocturnal hemoglobinuria. Cytometry. 2000;42(4):223–233. [PubMed] [Google Scholar]
- 13.Brodsky RA, Mukhina GL, Li S, et al. Improved detection and characterization of paroxysmal nocturnal hemoglobinuria using fluorescent aerolysin. Am J Clin Pathol. 2000;114:459–466. doi: 10.1093/ajcp/114.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reed GH, Kent JO, Wittwer CT. High-resolution DNA melting analysis for simple and efficient molecular diagnostics. Pharmacogenomics. 2007;8:597–608. doi: 10.2217/14622416.8.6.597. [DOI] [PubMed] [Google Scholar]
- 15.Peffault de Latour R, Mary JY, Salanoubat C, et al. Paroxysmal nocturnal hameoglobinuria: long-term epidemiological study. Presented at: 11th Congress of the European Hematology Association; June 15–18, 2006; Amsterdam, The Netherlands. p. Abstract #0518. [Google Scholar]
- 16.Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106(12):3699–3709. doi: 10.1182/blood-2005-04-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill A, Richards SJ, Hillmen P. Recent developments in the understanding and management of paroxysmal nocturnal hemoglobinuria. Br J Haematol. 2007;137(3):181–192. doi: 10.1111/j.1365-2141.2007.06554.x. [DOI] [PubMed] [Google Scholar]
- 18.Hillmen P, Muus P, Dührsen U, et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007;110(12):4123–4128. doi: 10.1182/blood-2007-06-095646. [DOI] [PubMed] [Google Scholar]
- 19.Hillmen P, Hall C, Marsh JC, et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2004;350(6):525–559. doi: 10.1056/NEJMoa031688. [DOI] [PubMed] [Google Scholar]
- 20.Young NS, Antonioli E, Rotoli B, et al. Safety and efficacy of the terminal complement inhibitor eculizumab in patients with paroxysmal nocturnal hemoglobinuria: Interim Shepherd Phase III Clinical Study [abstract]. Presented at: American Society of Hematology 48th Annual Meeting and Exhibition; December 9–12, 2006; Orlando, Fl. [Google Scholar]
- 21.Hall C, Richards S, Hillmen P. Primary prophylaxis with warfarin prevents thrombosis in paroxysmal nocturnal hemoglobinuria (PNH) Blood. 2003;102(10):3587–3591. doi: 10.1182/blood-2003-01-0009. [DOI] [PubMed] [Google Scholar]
- 22.Meyers G, Weitz I, Lamy T, et al. Disease-related symptoms reported across a broad population of patients with paroxysmal nocturnal hemoglobinuria. [abstract]. Presented at: American Society of Hematology 49th Annual Meeting and Exhibition; December 8–11, 2007; Atlanta, GA. [Google Scholar]
- 23.Hill A, Rother RP, Hillmen P. Improvement in the symptoms of smooth muscle dystonia during eculizumab therapy in paroxysmal nocturnal hemoglobinuria. Haematologica. 2005;90(12 suppl):ECR40. [PubMed] [Google Scholar]