Abstract
This study examined the relationship between white matter hyperintensities (WMH) and executive functioning on episodic memory in a group of older adults who were cognitively normal or diagnosed with MCI or dementia. Volumetric magnetic resonance imaging (MRI) measures of total brain volume, white matter hyperintensity volume, and hippocampal volume along with age, education, and gender were evaluated as predictors of episodic memory. WMH were found to influence both episodic memory and executive functioning independently of other variables. The influence WMH on episodic memory was mediated by executive functioning and was completely eliminated when the interaction between executive functioning and hippocampal volume was entered in the regression model. The results indicate that executive functioning mediates the effects of WMH on episodic memory but that executive functioning and hippocampal volume can also interact such that executive functioning can exacerbate or ameliorate the influence of hippocampal volume on episodic memory.
1. Introduction
White matter hyperintensities (WMH) are areas of hyperintense signal on T2-weighted magnetic resonance images (MRI) of the brain and are thought to be due to ischemic demylination, neuronal loss, and gliosis (Fazekas et al., 1993; Pantoni, 2002). WMH burden increases in healthy aging but is also related to risk factors such as hypertension, heart disease, and diabetes (Breteler et al., 1994; DeCarli et al., 1995; Debette et al., 2010). WMHs are typically greater in older adults with mild cognitive impairment (MCI) and dementia than in healthy older adults (Wu et al., 2002; Wolf et al., 2000), and have been related to cognition in all three groups (DeCarli et al., 2005; Elias et al., 2004; Lopez et al., 2003; Gunning-Dixon & Raz, 2000; Wu et al., 2002). Longitudinal data have supported the cross-sectional findings and have indicated a relationship between WMH burden and cognitive performance, with increasing WMH burden associated with decreasing cognitive functioning, particularly with regard to executive function and processing speed (DeGroot et al., 2001; Gunning-Dixon & Raz, 200; Kramer, Reed, Mungas, Weiner, Chui, 2002; Prins, Van Dijk, den Heijer, et al., 2005; Vannorsdall, Waldstein, Kraut, Pearlson, & Schretlen, 2009). Longitudinal reports have also shown that WMH can predict declines in global functioning (e.g., daily living activities such as housekeeping; Inzitari et al., 2007), motor performance, and the onset of dementia (Prins et al., 2004). Although early studies suggested that WMH may not be relevant to understanding cognition in older adults, the past 10 years of research have revealed a relationship between white matter integrity and cognitive function that plays an important role in age-related changes in cognition.
Older individuals commonly complain of impaired memory performance. Neuroimaging and neuropsychological research has shown that interactions between prefrontal cortex and medial temporal lobe structures are important for normal memory function (Dickerson, Miller, Greve, Dale, Albert, Schacter, & Sperling, 2007; Shimamura, Janowsky, & Squire, 1990; Simons et al., 2002; Simons & Spiers, 2003) and that the frontal lobes and hippocampus are particularly vulnerable to effects of aging and age-related disease processes, respectively (Moscovitch & Winnocur, 1995; Troyer, Graves, & Cullum, 1994; West, 1996; Wu et al., 2008). Neuroimaging studies of memory in young adults and neuropsychological tests in older adults have demonstrated that both frontally-mediated executive function and medial-temporal function are important predictors of memory and of age-related memory declines (e.g., Dickerson et al., 2007; Ferrer-Caja, Crawford, Bryan, 2002; Glisky, Polster, Routhieaux, 1995; Troyer et al., 1994).
The patterns found thus far between age, WMH, executive function and episodic memory parallel findings from recent investigations of frontal and medial temporal lobe functioning and age-related declines in memory and cognition. Specifically, age-related WMH are more prevalent in the frontal lobes than in posterior regions of the brain in cognitively normal older adults, and are more extensive throughout the brain in MCI and dementia patients (Fazekas et al., 1996; Wen & Sachdev, 2004; Yoshita et al., 2006). Moreover, WMH, irrespective of location, are associated with reduced frontal lobe metabolism (DeCarli, 1995; Tullberg et al. 2004) and recent work with cognitively normal individuals suggests that WMH are associated with disconnection of frontal lobe from functionally linked cortical areas (Nordahl et al., 2006). These findings converge with the frontal aging hypothesis (West, 1996; Buckner, 2004) and point to the possibility that frontal lobe dysfunction caused by WMH may lead to executive function impairment that is sufficient to affect episodic memory performance.
The relationship between WMH and executive functioning, however, appears to be more robust than that between WMH and episodic memory. Although a relationship between WMH burden and executive functioning is typically observed, a relationship between WMH and memory has not always been found (e.g., Parks, DeCarli, Jacoby, & Yonelinas, 2010). It may be the case, therefore, that multiple brain regions and types of cognition need to be considered in order to understand relationships between WMH and episodic memory. For instance, Brickman et al. (2006; 2007) found that frontal lobe white matter volume mediated the relationship between age and executive functioning as well as the relationship between age and memory, and Charlton et al. (2010) found that executive functioning partially mediated the effect of white matter integrity on memory performance in high-functioning healthy older controls.
To evaluate the hypothesis that WMH influence on episodic memory is mediated by impairments of executive function we examined the relationships between age, WMH volume, executive functioning and hippocampal volume. In addition, we specifically tested whether the effects of hippocampal volume and executive functioning on memory performance are interactive or additive.
2. Method
2.1 Subjects
All participants were evaluated at the University of California, Davis Alzheimer’s Disease Center. Participants included 422 individuals (264 women and 158 men; see Table 1 for demographic variables). The sample was fairly representative of the local population: 29.6% were African American, 26.1% were Hispanic, 40% were Caucasian, and 4.3% reported other ethnicities.
Table 1.
Demographic information, brain measures, and neuropsychological test scores for normal, MCI, and demented participants.
| Diagnostic Group |
||||
|---|---|---|---|---|
| Normal | MCI | Demented | p Value | |
| Participant Characteristics | ||||
| N (F,M) | 214 (145, 69) | 139 (74, 65) | 69 (45, 24) | |
| Age | 73.9 (7.2) | 75.6 (7.0) | 77.2 (7.3) | <.004 ‡ |
| Education | 12.7 (4.4) | 12.0 (5.2) | 10.9 (4.8) | <.03 ‡ |
| MMSE | 28.8 (8.7) | 25.9 (7.3) | 20.3 (5.1) | <.003 † |
| Vascular Risk | .25 (.2) | .28 (.2) | .32 (.2) | -- |
| Brain Measures | ||||
| Brain Volume/ TCV | .79 (.05) | .77 (.05) | .76 (.04) | <.002 ‡ |
| HC Volume/TCV | .0033 (.0005) | .0030 (.0006) | .0028 (.0006) | <.001 * |
| WMH volume /TCV§ | −5.46 (.96) | −5.27 (1.00) | −4.92 (1.00) | <.001‡ <.05~ |
| Neuropsychological Test Composite Scores | ||||
| Executive Functioning | .004 (.64) | −.38 (.60) | −1.02 (.75) | < .001 † |
| Episodic Memory | .07 (.81) | −.82 (.59) | −1.65 (.55) | <.001 † |
Note: Mean demographic and neuropsychological test values with standard deviation in parentheses. Brain volume, hippocampal volume, and WMH volume are relative to total cranial volume (TCV). Neuropsychological test scores are z-scores; p values are Bonferonni corrected values for pairwise comparisons following one-way ANOVA tests.
Normal vs. Demented
MCI vs. Demented
Normal vs. Demented, Normal vs. MCI
all groups significantly differ at this significance level or lower
Log transformed to normalize variance.
Participants received a thorough multidisciplinary clinical evaluation through the University of California, Davis Alzheimer’s Disease Center. Exams included medical history, a neurological exam, appropriate laboratory tests and neuropsychological testing with a standardized test battery. A bilingual physician examined Spanish speaking patients. Diagnosis of cognitive status (normal, MCI, or dementia) was made according to standard criteria. Mild cognitive impairment was diagnosed if a person did not meet the criteria for dementia but had a clinically significant impairment in at least one cognitive domain. Dementias were diagnosed using Diagnostic and Statistical Manual of Mental Disorders (Third Edition Revised) criteria for dementia, modified to exclude the requirement of memory impairment. Alzheimer’s disease was diagnosed using National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association criteria (McKhann et al., 1984). Underlying etiology of dementias were diagnosed but were not used for the current study. Of our 69 participants diagnosed as demented 61 met clinical diagnostic criteria for probable or possible Alzheimer’s Disease (AD), two met clinical criteria for possible or probable ischemic vascular disease, one met clinical criteria for probable dementia with lewy bodies, two met clinical criteria for frontal temporal dementia and a final two had a dementia with etiologic diagnosis deferred. Repeat analysis of our data excluding individuals with AD did not substantially change the main findings presented below.
2.2 Neuropsychological Measures
The Spanish and English Neuropsychological Assessment Scales (SENAS) were used to measure cognitive functioning. The SENAS tests are the result of an extensive process to develop English and Spanish measures of cognitive functioning in domains relevant to neuropsychological assessment of older adults and have been validated in several studies (e.g., Mungas, Reed, Crane, Haan, González, 2004; Mungas, Reed, Farias, & DeCarli, 2005; Mungas, Reed, Farias, & DeCarli, 2009). This study used a subset of the SENAS tests that were averaged within domains to create composite measures of executive function and episodic memory. The executive function composite was created from a set of fluency and working memory measures. The episodic memory composite was created from Word List Learning I and Word List Learning II.
Composite scores on the cognitive domains were z-transformed. Thus, scores of 0 indicate average performance, negative scores indicate below average performance and scores greater than zero indicate above average performance.
2.3 Vasular risk factors
The presence or absence of six cerebro-vascular risk factors (stroke, diabetes, hperlipidema, TIA, hypertension, and coronary heart disease) was reviewed for each participant and, in combination with a review of relevant medical records, was used to create a composite score that was the sum of factors present. Vascular risk scores range from 0 to 6 and are reported here as percentages.
2.4 Image acquisition
All brain imaging was obtained at the University of California at Davis Imaging Research Center on a 1.5T GE Signa Horizon LX Echospeed system. Two sequences were employed: a T1 weighted coronal 3D spoiled gradient recalled echo (SPGR) acquisition and a fluid attenuated inversion recovery (FLAIR) sequence designed to enhance WMH segmentation (Jack et al., 2001). The exact sequence parameters for the FLAIR imaging are as follows: Axial-oblique 2D Fluid Attenuated Inversion Recovery (FLAIR) Fast Spin Echo sequence: TE: 144 ms, TR: 11000 ms, TI: 2250 ms, Flip Angle: 90 deg, Slice thickness: 3 mm, slice spacing: 0.0 mm (Interleaved), FOV: 22 cm x 22 cm, NEX: 1, Matrix: 256 (freq) x 192 (phase), Bandwidth: 15.63 KHz, Phase FOV: 1.00, Freq Direction: A/P Options: Superior/Inferior saturation pulse On (80 mm thick).
2.4.1 WMH Segmentation
WMH volumes were measured across entire brain volumes. Segmentation of WMH was performed by a semi-automated procedure using a set of in-house computer algorithms and programs previously described (DeCarli, Fletcher, Ramey, Harvey, & Jagust, 2005; DeCarli, Massaro et al., 2005).
An analysis of regional WMH revealed that the distribution of WMH in participants in this study was similar to previous reports (Tullberg, et al., 2004; DeCarli, Fletcher, Ramey, Harvey, & Jagust, 2005), with average correlations between frontal, temporal, parietal, occipital and total WMH averaging about 0.9 with the exception of occipital to total WMH which was r = 0.67. In order to assess whether total WMH volume could account for the regional distribution of WMH, or whether WMH were distributed in a way that total volume could not account for, we performed a principle components analysis of the regional distribution of WMH that included total WMH volume. The results revealed a single eigenvector that explained 84.6% of the variance with weightings that were nearly balanced (total=0.40, frontal=0.45, occipital=0.30, parietal=0.64, temporal=0.37) with the possible exception of the parietal weighting which was slightly higher. Given the strong relationship between regional and total WMH volumes, we use total WMH volume in our statistical analyses.
2.4.2 Hippocampal Volume Measurement
Manual segmentation of hippocampus was performed by trained analysts according to a precise anatomical protocol as previously described (DeCarli, Reed, Jagust, Martinez, Ortega, & Mungas, 2008). Interater reliability using interclass correlation methods was extremely good ranging from 0.90-0.95.
2.4.3 MRI Normalization Method
All MRI measures were divided by total cranial volume to correct for differences in head size related to gender (DeCarli, Massaro et al., 2005.; Massaro et al., 2004.)
2.5 Statistical Analyses
One way analyses of variance (ANOVA) were conducted on demographic and brain variables to identify differences between diagnostic groups; Bonferonni corrections were used when testing pairwise group differences.
Relationships between brain measures and cognitive outcome measures were examined using multiple linear regression models. As a first step, episodic memory and executive function measures were regressed onto age, education, gender, ethnicity, brain volume, hippocampal volume, and log-transformed WMH volumes. (WMH volumes were log-transformed to obtain a normal distribution.) Brain volume was included as a control for non-specific atrophy. To test the hypothesis that executive functioning mediates the effects of WMH burden on episodic memory, we adopted the popular approach described by Baron and Kenney (1986) to test meditational relationships and added executive function to the model predicting episodic memory. Specifically, we examined whether the inclusion of executive functioning reduced or eliminated the significance of the β coefficient for WMH volumes. Finally, we examined whether the effects of executive functioning and hippocampal volumes on episodic memory interacted by including an interaction term in the regression model. We verified these relationships by testing the significance of the indirect effect of WMH on episodic memory as mediated by executive functioning using a bootstrap method to test whether the product of the unstandardized coefficients was significantly greater than zero (see Hayes, 2009 and Preacher and Hayes, 2008 for details). All tests used were two-sided, with α = .05. All statistical analyses were performed using SPSS (SPSS Inc., 2008).
3. Results
3.1 Subject Characteristics
Differences in age, education, brain measures, and neuropsychological test scores across diagnostic groups are presented in Table 1. Vascular risk measures were available for a subset of 386 participants. Significant differences between diagnostic groups were found for age, MMSE, education, normalized brain volume, normalized WMH volume, and normalized hippocampal volume (all Fs > 3, all ps < .03), but not for vascular risk factors (F < 1). Specific group differences are summarized in Table1.
3.2 Initial Multiple Linear Regression Analyses
Table 2 shows the results of the initial multiple regression analyses for episodic memory and executive function with age, gender, education, brain, WMH, and hippocampal volumes as predictor variables. The predictor variables accounting for 28.4% of the total variance of episodic memory (F (9, 412) = 18.2, p < .001) and 35.3% of the variance in executive functioning scores (F(9, 412) = 25.01, p < .001).
Table 2.
Initial Multiple Linear Regression Models
| Episodic Memory |
Executive Functioning |
|||
|---|---|---|---|---|
|
| ||||
| Total Model R2 | 0.284 | 0.353 | ||
|
| ||||
| β | p | β | p | |
|
|
||||
| Age | −0.035 | .501 | −0.091 | .069 |
| Education | 0.267 | .000 | 0.41 | .000 |
| Gender (male) | −0.153 | .000 | −0.011 | .781 |
| Ethnicity | ||||
| African American | 0.183 | .064 | 0.047 | .617 |
| Hispanic | −0.027 | .786 | −0.115 | .223 |
| White | 0.09 | .389 | 0.134 | .180 |
| Brain | 0.203 | .000 | 0.227 | .000 |
| Hippocampus | 0.193 | .000 | −0.004 | .919 |
| WMH | −0.151 | .001 | −0.153 | .000 |
Note: Table entries are standardized regression coefficients (beta weights, β) and associated significance values (p). Brain, brain volume relative to total cranial volume; hippocampus, hippocampal volume relative to total cranial volume; WMH, white matter hyperintensity volumes relative to total cranial volume and log transformed.
Significant predictors of episodic memory in order of importance were education, brain volume, hippocampal volume, gender, and WMH burden (see Table 2, and Figure 1a). Although there was a negative association between age and episodic memory with the demographic variables in the model, it lost significance once brain volume was entered into the model as a predictor. A similar pattern was found for executive functioning. Significant predictors in order of importance included education, brain matter volume, and WMH load (see Table 2), and an initial association between age and executive function that was eliminated when brain matter volume was entered into the model.
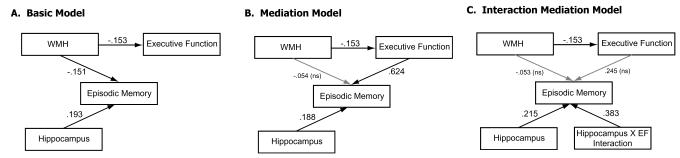
Figure 1.
Models of relationships between variables of primary interest. Arrows indicate hypothesized direction of effects and values are the standardized coefficients for the relationship between the variables. All relationships are significant except those indicated by the grey arrows and noted ns (non-significant). A. Relationships before investigating meditational effects of executive functioning, B. relationships with executive functioning as a mediator of the WMH influence on episodic memory, and C. with the interaction between executive functioning and hippocampal volumes mediating both WMH and executive functioning influences (EF, executive function).
3.3 Mediation Analyses
The initial regression analyses demonstrated that WMH load was a significant predictor of episodic memory and executive function after controlling for age, education, and other brain variables. Our next step was to examine whether executive function mediated the effects of WMH on episodic memory. Because ethnicity was consistently non-significant in the previous analyses we removed it from model and focused on age, education, gender, the brain measures, and executive function as predictors of episodic memory.
Table 3 and Figure 1b shows the results of the executive function mediation analysis. The initial regression model above accounted for 28.4% of the variance in episodic memory. Adding executive functioning as a predictor variable substantially increased the explained variance to 52.2% (F(7,414)=64.7, p < .001). With executive function in the model, predictors of episodic memory included executive function, hippocampal volume, and gender; WMH burden, education, and brain matter volumes were no longer significant predictors. These results support the mediation hypothesis and also show that controlling for executive functioning in a clinically diverse sample eliminates education and brain volume as significant predictors of episodic memory. A reduced model including only the significant predictors (gender, hippocampus, and executive functioning) accounted for approximately the same amount of variance (52.1%) as the full model.
Table 3.
Summary of the executive function mediation and interaction regression models of episodic memory.
| EF Mediation Model | EF Mediation Interaction Model |
|||
|---|---|---|---|---|
|
|
||||
| Total Model R2 | 0.522 | 0.527 | ||
|
| ||||
| β | p | β | p | |
|
|
||||
| Age | 0.021 | .619 | 0.015 | .724 |
| Education | 0.008 | .191 | 0.008 | .838 |
| Gender (male) | −0.159 | .000 | −0.154 | .000 |
| Brain | 0.068 | .109 | 0.069 | .104 |
| Hippocampus | 0.188 | .000 | 0.215 | .000 |
| WMH | −0.054 | .153 | −0.053 | .161 |
| Executive | 0.624 | .000 | 0.245 | .200 |
| HC.Exec Interaction | 0.383 | .043 | ||
Note: Table entries are standardized regression coefficients (beta weights, β) and associated significance values (p). EF, executive function; Brain, brain volume relative to total cranial volume; hippocampus, hippocampal volume relative to total cranial volume; WMH, white matter hyperintensity volumes relative to total cranial volume and log transformed; HC.Exec Interaction , hippocampus x executive function interaction term.
Because WMH and executive function are correlated, it is possible that this meditational relation could be an artifact. Thus, to verify the meaningfulness of the observed relationships and to overcome flaws in the Baron and Kenney (1986) approach, we also directly tested the significance of the indirect effect of WMH on episodic memory as mediated by executive functioning while controlling for age, gender, education, brain volume, hippocampal volume (see Hayes, 2009 and Preacher & Hayes, 2008 for details; SPSS and SAS macros for these procedures are described in Preacher & Hayes, 2004). The indirect effect in question is the product of the unstandardized coefficients (from WMH to executive functioning and executive functioning to episodic memory) obtained using ordinary least squares. If executive function accounts for the effect of WMH on episodic memory, then the product of these coefficients should be significantly different from zero; this is what we observed (indirect effect = −.086, 95% CI = −.143 - −.044). Additionally, the indirect effect in the alternative statistical model in which WMH is the proposed mediator and executive function is the independent variable was not significant (indirect effect = .012, 95% CI = −.003 -- .033). Thus, the finding that executive function accounts for the effects of WMH on episodic memory does not appear to be a result of overall high correlations between executive function and WMH.
3.4 Interaction Analysis
The previous analysis demonstrated that executive function mediates the effects of WMH burden on episodic memory in a diverse sample. Our final step was to investigate whether there was an interactive effect between hippocampal volume and executive function on episodic memory. Because both variables are known to be important to episodic memory performance, particularly when considering the aging process and age-related cognitive impairment, a natural question is whether they interact to predict memory and if that interaction may also account for the WMH effect on episodic memory. Predictor variables in the model included age, education, gender, brain, hippocampal and WMH volumes, executive functioning, and the interaction term between hippocampal volume and executive functioning. Results of the interaction analyses are presented in Table 3 and Figure 1c.
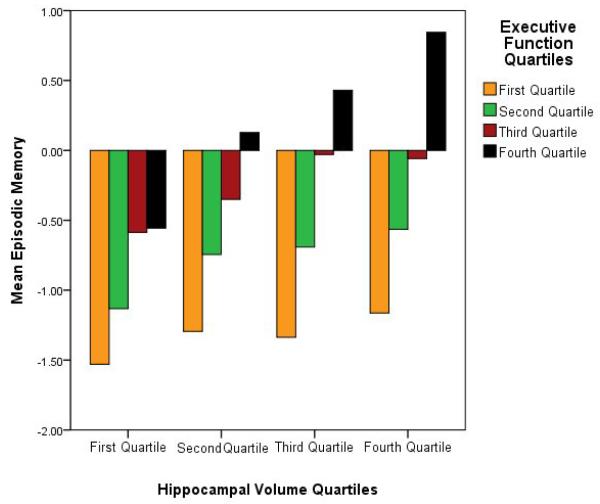
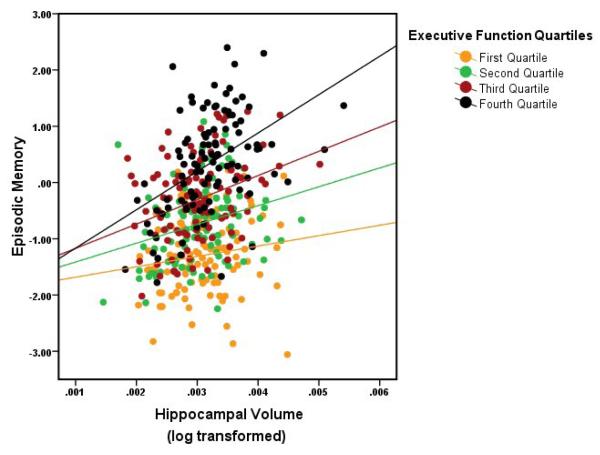
The interaction model accounted for 52.7% of the variance in episodic memory (F(8, 413) = 57.56, p < .001) and the interaction term was significant. Moreover, inclusion of the interaction term eliminated the WMH influence on episodic memory (see Table 3). The executive function-hippocampal interaction model resulted in gender, hippocampal volumes, and the executive-hippocampal interaction as significant predictors of episodic memory. We verified this effect using the Preacher and Hayes (2004; 2008) bootstrap technique and found that the indirect effect of WMH as mediated by the interaction of executive function and hippocampal volumes was significant (indirect effect = −.078, 95% CI = −.13 -- −.02). The interaction, depicted categorically in Figure 2, indicates that although hippocampal volume has a strong effect on episodic memory, the extent of that effect depends on executive function. To follow up on this interaction more specifically, we fitted simple linear regression models of hippocampal volume on episodic memory within each quartile of executive functioning (see Table 4) which revealed sequentially stronger associations between episodic memory performance and hippocampal volume with increasing executive function, as evident in Figure 2.
Figure 2.
Hippocampal X Executive function Interaction. A categorical depiction the interaction. Continuous hippocampal volumes and executive function scores were split into quartiles with “First Quartile” representing smallest volumes (hippocampus) or lowest scores (executive functioning) and “Fourth Quartile” representing largest hippocampal volumes and highest executive scores.
Table 4.
Summary of the simple linear regressions of hippocampal volume on episodic memory within executive function quartiles.
| Episodic |
|||
|---|---|---|---|
| β | p | R2 | |
|
|
|||
| Hippocampus in | |||
| EF First Quartile | 0.150 | 0.127 | 0.023 |
| EF Second Quartile | 0.240 | 0.012 | 0.058 |
| EF Third Quartile | 0.325 | 0.001 | 0.106 |
| EF Fourth Quartile | 0.446 | 0.000 | 0.199 |
4. General Discussion
Our primary aim in the current study was to test the hypothesis that executive function mediates the influence of WMHs on episodic memory. In line with that hypothesis, we found that controlling for executive function eliminated the effect of WMH on episodic memory, indicating that executive function completely mediated influences of WMH. Additionally though, that meditational effect was eliminated when the interaction between executive functioning and hippocampal volumes were considered, leaving the interaction as the best predictor of episodic memory performance in that model, and indicating that the effect of hippocampal volume on episodic memory depended on executive performance. Overall these findings provide support for the WMH/executive mediation model and suggest that the integrity of the frontal systems is important to episodic memory performance and apparently orthogonal to medial temporal hippocampal systems. Thus, the influence of WMH on episodic memory is an indirect effect, mediated by executive functioning and the interaction between executive functioning and hippocampal volumes.
Executive function completely mediated the effects of WMH on episodic memory, but even that very strong effect was eliminated when the interaction between executive function and hippocampal volumes was considered in a second model. Figures 2 and 3 illustrate the nature of the interaction in a categorical manner. Overall, the relationship between hippocampal volumes and episodic memory is very weak for those with the lowest degree of executive functioning (e.g., see Table 4 and Figure 2) indicating that hippocampal volume has little effect on episodic memory when executive functioning is low. Thus, the data suggest that severe frontal impairment may alter encoding or retrieval of material, making actual memory consolidation less important to performance on an episodic memory task. However, the effect of hippocampal volume becomes more important to episodic memory as executive function increases, suggesting that as the systems guiding encoding and retrieval improve, the influence of memory consolidation becomes increasingly important to episodic memory performance. Interestingly, this effect seems to be linear and continuous, with the strongest relationship between hippocampal volume and episodic memory found for those with the highest degree of executive function, those with intermediate levels of executive function showing a weaker relationship, and those with the lowest levels showing no significant relationship between hippocampal volume and episodic memory. We therefore conclude that, to the extent that WMH load is contributing to executive function deficits (as the overall regression analyses indicate, see Table 2), the functional effect of WMH on episodic memory can be quite large, particularly in cases where executive processes are substantially impaired, such as cases of substantial WMH burden. This supports previous a clinical study of amnestic MCI subjects with extensive WMH, but normal hippocampal volume (Nordahl et al., 2005).
Figure 3.
Illustration of simple linear regressions of hippocampal volume on episodic memory within executive function quartiles. Hippocampus becomes increasingly more related to episodic memory as executive function increase. (First Quartile = lowest 25% executive function, Fourth Quartile = highest 25% executive function)
Two limitations of the current study are worth considering. First and most obvious is the cross-sectional nature of the data. Research examining longitudinal changes in WMH burden, executive function, hippocampal volume, and episodic memory will be important to identifying causal relationships among these variables. Another potential limitation is that we examined WMH relationships in a clinically diverse sample but did not examine how those effects differed between diagnostic groups; in fact, such an analysis was not possible here due to restricted ranges and small numbers within diagnostic categories. More important, however, is whether the influence of WMH differs based on whether a person is healthy or demented (or on the kind of dementing pathology that is present). This question is beyond the scope of our current data, but accumulating evidence suggests that the most parsimonious answer is that WMH is related to executive dysfunction in general and that the nature of the relationship does not differ, at least not between individuals with and without AD. First, one reason to suspect that the nature of WMH relationships might differ as a function of dementia is because WMH extend to more posterior regions in individuals with AD than in non-demented individuals. However, recent findings indicate that the greater posterior extent of WMH in AD is not actually related to AD pathology (Jagust, et al., 2008) and is more likely due to an interaction of vascular factors that occur with degeneration (Yoshita, et al., 2006 ). Additionally, measures of cerebral metabolism and behavior both indicate that the effects of WMH on frontal systems are continuous (DeCarli, et al. 1995; Tullberg, et al., 2004). These effects have also been found using specific cognitive control tasks in amnestic MCI subjects assumed to have early Alzheimer’s pathology (Nordhal et al., 2005). Overall then, the evidence to date indicates that WMH directly affects executive function which in turn affects episodic memory and that this pattern is characteristic of individuals with and without AD.
Our findings raise the question as to how WMH influence episodic memory through impaired executive functioning. The mediation and interaction effects found in the current study suggest that WMH may have the effect of disconnecting specific brain regions involved in the distributed memory system, either by direct disruption of white matter pathways or by decreasing the efficiency of processing amongst critical regions. Thus, these data provide a foundation for future research to investigate the influence of WMH burden on functional connectivity between the multiple regions of the memory system in clinically diverse samples.
Moreover, these data suggest that WMH influence on episodic memory likely occurs at the stage of either encoding or retrieval, or both. Because the effects of WMH are mediated completely by executive functioning, it follows that memory functions most reliant on executive processes will be most vulnerable to WMH effects. Thus, the degree to which one may expect to see WMH influences on episodic memory are likely dependent on the degree to which the memory task recruits executive processes. For example, tasks with heavy working-memory loads at either the encoding or the retrieval stage are the most likely to be vulnerable to WMH influences. Because consolidation is thought to be primarily a hippocampal process, or a process of transfer from the hippocampus to cortical regions, it is a less likely candidate for explaining WMH influences on episodic memory. This, of course, is speculative. Therefore, to the extent that frontal-hippocampal communication may be important to consolidation, WMH may have some indirect influence on consolidative processes as well. Overall though, the evidence suggests that WMH effects will be most evident in any episodic memory task that requires a substantial working memory load or that requires some other executive processes at encoding or retrieval.
Notably, these results align well with the cognitive aging and WMH literature. The mediation effects are consistent with recent findings using different measures of WMH or white matter volume in cognitively normal middle-aged and older adults (e.g., Charlton et al., 2009). The mediation of WMH by executive function is also consistent with behavioral findings in the cognitive aging literature which show that episodic memory performance is consistently related to executive function (e.g., Ferrer-Caja et al., 2002; Glisky et al., 2000; Park et al., 2002). At a more general level, these results are consistent with current theories regarding the influence of executive function on memory performance. For instance, medial temporal and prefrontal lesions have larger effects on recall tests than on recognition, consistent with findings that recall tests place heavier demands on executive processes than most recognition tests (e.g., Schacter et al., 1984; Simons et al., 2002; Warrington & Wiskrantz, 1982). Age-related effects are also larger in recall than in recognition tests (e.g., Craik & McDowd, 1987). Thus, the current findings are not only consistent with theories of executive (or frontal) function in cognitive aging (e.g., West, 1996), but also point toward WMH load as a one potential mechanism underlying age-related declines in executive functioning.
How WMH affect executive function remains an unanswered question. Limited cognitive research suggests the possibility that WMH disconnect frontal systems from functional targets at other cortical locations (Nordahl et al., 2006). There is a vast literature in animal research suggesting that working memory involves direct neuronal connections between parietal cortex and prefrontal cortex (Chafee & Goldman-Rakic, 2000; Selemon & Goldman-Rakic, 1988). Recent data suggest that parietal regions are an important component of the network of brain areas that mediate the short-term storage and retrieval of verbal material (Jonides et al., 1998; Honey, Bullmore, & Sharma, 2000) and it has been suggested that the human posterior parietal cortex is the node for the storage capacity limit of visual short-term memory (Todd & Marois, 2005). WMH, which are commonly located along rostral-caudal white matter pathways overlapping with the superior longitudinal fasciculus (Schmahmann & Pandya, 2006) which connects parietal with prefrontal cortex (Selemon & Goldman-Rakic, 1988), therefore, may cause degradation of these connections leading to impaired cognitive performance. Unfortunately, research in this area is extremely limited and further investigation is necessary to confirm or refute this hypothesis.
Overall, these data indicate that age-related vascular disease, assumed to be the primary cause of WMH burden among the subjects of our sample, is important to long-term episodic memory function in older adults. This effect is mediated by the effect of WMH on executive functioning, suggesting that the memory effects that do occur are related to deficits in encoding or retrieval or both. Finally, these data suggest that memory tasks with lesser executive processing demands may be less affected by WMH load than those with larger executive processing demands. Unfortunately, however, daily activities of many older adults include memory tasks with high executive processing demands. For instance, simply taking daily medications can lead to a great deal of proactive interference that may need to be resolved in order to figure out if a medication has been taken today or not. Thus, albeit a mediated influence, WMH load stands to have significant practical influences on the daily activities of older adults.
Research Highlights.
White matter hyperintensities (WMH) influence both episodic memory and executive function
Executive function completely mediates the influence of WMH on episodic memory
The influence of hippocampal volumes on episodic memory depends on executive function
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baron RM, Kenny DK. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical consideration. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Breteler MM, van Swieten JC, Bots ML, Grobbee DE, Claus JJ, van den Hout JH, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology. 1994;44(7):1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- Brickman AM, et al. Regional white matter and neuropsychological functioning across the adult lifespan. Biological Psychiatry. 2006;60:444–453. doi: 10.1016/j.biopsych.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Habeck C, Zarahn E, Flynn J, Stern Y. Structural MRI covariance patterns associated with normal aging and neuropsychological functioning. Neurobiology of Aging. 2007;28:284–295. doi: 10.1016/j.neurobiolaging.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Buckner R. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, et al. Age-related slowing of memory retrieval: Contributions of perceptual speed and cerebral white matter integrity. Neurobiology of Aging. 2008;29(7):1070–1079. doi: 10.1016/j.neurobiolaging.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, Markus HS, Morris RG. The relationship between episodic long-term memory and white matter integrity in normal aging. Neuropsychologia. 2010;48(1):114–122. doi: 10.1016/j.neuropsychologia.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Inactivation of Parietal and Prefrontal Cortex Reveals Interdependence of Neural Activity During Memory-Guided Saccades. Journal of Neurophysiology. 2000;83:1550–1566. doi: 10.1152/jn.2000.83.3.1550. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Mcdowd JM. Age-Differences in Recall and Recognition. Journal of Experimental Psychology-Learning Memory and Cognition. 1987;13(3):474–479. [Google Scholar]
- Davidson PS, Glisky EL. Neuropsychological correlates of recollection and familiarity in normal aging. Cogn Affect Behav Neurosci. 2002;2(2):174–186. doi: 10.3758/cabn.2.2.174. [DOI] [PubMed] [Google Scholar]
- Debette S, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham offspring study. Stroke. 2010;41 doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Murphy DG, Tranh M, Grady CL, Haxby JV, Gillette JA, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45(11):2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham Heart study: establish what is normal. Neurobiology of Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical Mapping of White Matter Hyperintensities (WMH): Exploring the Relationships between Periventricular WMH, Deep WMH, and Total WMH Burden. Stroke. 2005;36:50–55. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Reed BR, Jagust W, Martinez O, Ortega M, Mungas D. Brain behavior relationships among African Americans, Whites, and Hispanics. Alzheimer Disease and Associated Disorders. 2008;22:382–391. doi: 10.1097/wad.0b013e318185e7fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot JC, de Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MMB. Cerebral white matter lesions and subjective cognitive dysfunction: The Rotterdam scan study. Neurology. 2001;56:1539–1545. doi: 10.1212/wnl.56.11.1539. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Miller SL, Greve DN, Dale AM, Albert MS, Schacter DL, Sperling RA. Prefrontal-hippocampal-fusiform activity during encoding predicts intraindividual differences in free recall ability: An event-related functional-anatomic MRI study. Hippocampus. 2007;17:1060–1070. doi: 10.1002/hipo.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias MF, et al. Framingham stroke risk profile and lowered cognitive performance. Stroke. 2004;35:404–409. doi: 10.1161/01.STR.0000103141.82869.77. [DOI] [PubMed] [Google Scholar]
- Ferrer-Caja E, Crawford JR, Bryan J. A structural modeling examination of the executive decline hypothesis of cognitive aging through reanalysis of Crawford et al.’s (2000) data. Aging Neuropsychology and Cognition. 2002;9(3):231–249. [Google Scholar]
- Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43(9):1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- Glisky EL, Polster MR, Routhieaux BC. Double dissociation between item and source memory. Neuropsychology. 1995;9:229–235. [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14(2):224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Beyond Baron and Kenney: Statistical mediation analysis in the new millennium. Communication Monographs. 2009;76:408–420. [Google Scholar]
- Honey GD, Bullmore ET, Sharma T. Prolonged Reaction Time to a Verbal Working Memory Task Predicts Increased Power of Posterior Parietal Cortical Activation. Neuroimage. 2000;12:495–503. doi: 10.1006/nimg.2000.0624. [DOI] [PubMed] [Google Scholar]
- Inzitari D, et al. Risk of rapid global functional decline in elderly patients with severe cerebral age-related white matter changes. Archives of Internal Medicine. 2007;167:81–88. doi: 10.1001/archinte.167.1.81. [DOI] [PubMed] [Google Scholar]
- Jagust WJ, Zheng L, Harvey DJ, et al. Neuropathological basis of magnetic resonance images in aging and dementia. Annals of Neurology. 2008;63:72–80. doi: 10.1002/ana.21296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., O’Brien PC, Rettman DW, Shiung MM, Xu Y, Muthupillai R, Manduca A, Avula R, Erickson BJ. FLAIR histogram segmentation for measurement of leukoaraiosis volume. Journal of Magnetic Resonance Imaging. 2001;14:668–676. doi: 10.1002/jmri.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, Marshuetz C, Willis CR. The Role of Parietal Cortex in Verbal Working Memory. Journal of Neuroscience. 1998;18:5026–5034. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Reed BR, Mungas D, Weiner MW, Chui HC. Executive dysfunction in subcortical sichaemic vascular disease. Journal of Neurology, Neurosurgery, & Psychiatry. 2002;72:217–220. doi: 10.1136/jnnp.72.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez OL, Jagust WJ, Dulberg C, et al. Risk factors for mild cognitive impairment in the Cardiovascular Health and Cognition Study: part 2. Archives of Neurology. 2003;60:1394–1399. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- Massaro JM, D’Agostino RB, Sullivan LM, Beiser A, DeCarli C, Au R, Elias MF, Wolf PA. Managing and analyzing data from a large-scale study on Framingham Offspring relating brain structure to cognitive function. Statistics in Medicine. 2004;23:351–367. doi: 10.1002/sim.1743. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s Disease: Report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. Frontal lobes, memory, and aging. Annals of the New York Academy of Sciences. 1995;769:119–150. doi: 10.1111/j.1749-6632.1995.tb38135.x. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Crane PK, Haan MN, González H. Spanish and English Neuropsychological Assessment Scales (SENAS): Further development and psychometric characteristics. Psychological Assessment. 2004;16:347–359. doi: 10.1037/1040-3590.16.4.347. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Farias S, DeCarli C. Criterion-referenced validity of a neuropsychological test battery: Equivalent performance in elderly Hispanics and Non-Hispanic Whites. Journal of the International Neuropsychological Society. 2005;11:620–630. doi: 10.1017/S1355617705050745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Farias S, DeCarli C. Age and education effects on relationships of cognitive test scores with brain struction in demographically diverse older persons. Psychology and Aging. 2009;24:116–128. doi: 10.1037/a0013421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CC, Ranganath C, Yonelinas AP, DeCarli C, Fletcher E, Jagust WJ. White matter changes compromise prefrontal cortex function in healthy elderly individuals. Journal of Cognitive Neuroscience. 2006;18:418–429. doi: 10.1162/089892906775990552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Ranganath C, Yonelinas AP, DeCarli C, Reed BR, Jagust WJ. Different mechanisms of episodic memory failure in mild cognitive impairment. Neuropsychologia. 2005;43:1688–1697. doi: 10.1016/j.neuropsychologia.2005.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoni L. Pathophysiology of age-related cerebral white matter changes. Cerebrovascular Disease. 2002;13:7–10. doi: 10.1159/000049143. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychology and Aging. 2002;17(2):299–320. [PubMed] [Google Scholar]
- Parks CM, DeCarli C, Jacoby LL, Yonelinas AP. Aging effects on recollection and familiarity: The role of white matter hyperintensities. Aging, Neuropsychology, and Cognition. 2010;17:432–438. doi: 10.1080/13825580903469838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, and Computers. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotoic and reseampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Prins ND, et al. Cerebral white matter lesions and the risk of dementia. Archives of Neruology. 2004;10:1531–1534. doi: 10.1001/archneur.61.10.1531. [DOI] [PubMed] [Google Scholar]
- Prins ND, Van Dijk EJ, den Heijer T, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 2005;128:2034–2041. doi: 10.1093/brain/awh553. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. Journal of Neuroscience. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. University Press; Oxford: 2006. [Google Scholar]
- Shimamura AP, Janowsky JS, Squire LR. Memory for the temporal order of events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia. 1990;28(8):803–813. doi: 10.1016/0028-3932(90)90004-8. [DOI] [PubMed] [Google Scholar]
- Simons JS, et al. Recollection-based memory in frontotemporal dementia: implications for theories of long-term memory. Brain. 2002;125:2523–2536. doi: 10.1093/brain/awf247. [DOI] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nature Neuroscience. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Simons JS, Verfaellie M, Galton CJ, Miller BL, Hodges JR, Graham KS. Recollection-based memory in frontotemporal dementia: implications for theories of long-term memory. Brain. 2002;125(Pt 11):2523–2536. doi: 10.1093/brain/awf247. [DOI] [PubMed] [Google Scholar]
- SPSS for Windows, Rel. 17. SPSS Inc.; Chicago: 2008. [Google Scholar]
- Todd JJ, Marois R. Posterior parietal cortex activity predicts individual differences in visual short-term memory capacity. Cognitive, affective & behavioral neuroscience. 2005;5:144–155. doi: 10.3758/cabn.5.2.144. [DOI] [PubMed] [Google Scholar]
- Troyer AK, Graves RE, Cullum M. Executive functioning as a mediataor of the relationship between age and episodic memory in healthy aging. Aging, Neuropsychology, and Cognition. 1994;1:45–53. [Google Scholar]
- Tullberg M, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63:246–253. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannorsdall TD, Waldstein SR, Kraut M, Pearlson GD, Schretlen DJ. White matter abnormalities and cognition in a community sample. Archives of clinical neuropsychology. 2009;24:209–207. doi: 10.1093/arclin/acp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK, Weiskrantz L. Amnesia - a Disconnection Syndrome. Neuropsychologia. 1982;20(3):233–248. doi: 10.1016/0028-3932(82)90099-9. [DOI] [PubMed] [Google Scholar]
- Wen W, Sachdev P. The topography of white matter hyperintensities on brain MRI in healthy 60- to 64-year-old individuals. Neuroimage. 2004;22:144–154. doi: 10.1016/j.neuroimage.2003.12.027. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120(2):272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Wolf H, Ecke GM, Bettin S, Dietrich J, Gertz H. Do white matter changes contribute to the subsequent development of dementia in patients with mild cognitive impairment? A longitudinal study. International Journal of Geriatric Psychiatry. 2000;15:803–812. doi: 10.1002/1099-1166(200009)15:9<803::aid-gps190>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Wu W, Brickman AM, Luchsinger J, Ferrazzano P, Pichiule P, Yoshita M, et al. The brain in the age of old: the hippocampal formation is targeted differentially by diseases of late life. Ann Neurol. 2008;64(6):698–706. doi: 10.1002/ana.21557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Mungas D, Petkov CI, Eberling JL, Zrelak PA, Buonocore MH, et al. Brain structure and cognition in a community sample of elderly Latinos. Neurology. 2002;59(3):383–391. doi: 10.1212/wnl.59.3.383. [DOI] [PubMed] [Google Scholar]
- Yoshita M, et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67:2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]