Abstract
Many neurodegenerative diseases are associated with accumulation of misfolded proteins in cells of the central nervous system (CNS). We have previously reported that accumulation of the precursor envelope protein gPr80env of ts1, a mutant of Moloney murine leukemia virus (MoMuLV), in the endoplasmic reticulum (ER) of infected astrocytes, results in ER stress, oxidative stress and cell death, subsequently leading to ts1-mediated neurodegeneration in infected mice. In the present study, we assessed whether treatments that reduce the accumulation of gPr80env in the ER of ts1-infected astrocytes provided a protective effect against ER stress and cell death. We show that treatment with phenylbutyric acid (PBA) can prevent the unfolded protein response (UPR), ER stress and cell death in cultured ts1-infected astrocytes. The protective effect of PBA is associated with its ability to reduce gPr80env accumulation and to increase the expression of proteins involved in protein folding in the ER, such as protein disulfide isomerase (PDI) and ERp44, rather than by decrease mRNA levels of gPr80env or alter the proteasomal degradation process for gPr80env. In infected mice treated with PBA we also noted a reduction in the severity of the neuropathology in brainstem tissues and a delayed onset of paralysis. These results show that PBA is a potentially effective drug for the treatment of neurodegeneration caused by protein accumulation in cells of the CNS.
Keywords: MoMuLV-ts1, ER stress, unfolded protein response, phenylbutyric acid, chemical chaperone
Introduction
The ts1 mutant of the Moloney murine leukemia virus (MoMuLV) causes a progressive neurodegeneration in infected mice. This mutant contains a single point mutation in the viral env gene, which results in misfolding of the viral encoded precursor envelope protein gPr80env. The misfolded gPr80env cannot be transported efficiently from the endoplasmic reticulum (ER) to the Golgi compartment in astrocytes, subsequently preventing the cleavage of gPr80env into the mature viral envelope proteins gp70 and PrP15E (Shikova et al. 1993; Szurek et al. 1990). In infected cells uncleaved gPr80env accumulates in the ER leading to the unfolded protein response (UPR), ER stress, and oxidative stress. In ts1-infected mice, these events lead to CNS cell death, neurodegeneration, hindlimb paralysis and eventually death (Kim et al. 2004; Liu et al. 2004). Thus, ts1 is a useful model to study protein accumulation associated neurodegeneration and prevention strategies.
Accumulation of misfolded proteins in the ER activates the UPR, a protective mechanism, to prevent ER stress. The major functions of the UPR are: (a) enhancing the capacity of ER folding, (b) slowing down general protein synthesis, and (c) facilitating protein degradation and trafficking out of the ER (Ron & Walter 2007). The UPR is initiated by three transmembrane ER stress sensors: the PRK-like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring enzyme 1 (IRE1). In stress-free ER, these three proteins are bound by a chaperone, BiP (also known as GRP78), on their luminal domains and are held inactive (Todd et al. 2008). Upon accumulation of misfolded proteins in the ER the ER stress sensors proteins are activated. Activation of PERK induces the phosphorylation of eukaryotic initiation factor α (elF2α), leading to activation of activating transcription factor 4 (ATF4). These events slow down the protein translation and activate the expression of other UPR-related genes, such as ER chaperones (DuRose et al. 2009; Martinez & Chrispeels 2003; Ron & Walter 2007; Yan et al. 2008; Bonapace et al. 2004). Activation of ATF6 results in its cleavage. The cleaved cytosolic fragment translocates to the nucleus where it activates transcription of UPR-responsive genes (Malhotra & Kaufman 2007; Haze et al. 1999; Haze et al. 2001). When the amount of misfolded protein in the ER overcomes the compensatory action of the UPR, apoptotic pathways are activated including transcription factor C/EBP homologous protein (CHOP, or GADD153), which downregulates Bcl2 and activates caspase 8 (Liu et al. 2006; McCullough et al. 2001).
The ER also contains a diverse pool of molecular chaperones that aid in protein folding. Some of these chaperones, such as BiP and calregulin, directly promote proper folding and subsequent trafficking of nascent proteins (Ni & Lee 2007). Both BiP and calregulin are upregulated by the UPR. Other ER resident proteins, particularly the protein disulfide isomerase (PDI) family members also facilitate protein folding (Wang & Tsou 1993; Santos et al. 2009). The PDI family consists of PDI and other ER proteins such as ER protein 44, 57 and 72 (ERp44, ERp57 and ERp72). The specific functions of these proteins are to catalyze the scheduled formation and reduction of disulfide bonds during oxidative protein folding and to facilitate protein trafficking out of the ER (Malhotra & Kaufman 2007; Fraldi et al. 2008).
Abnormal accumulation of proteins or lipids initiates ER stress leading to many disease conditions, such as neurodegeneration, cancer, diabetes, cardiac disease and immunodeficiency (Kim et al. 2008; Matus et al. 2008; Hotamisligil 2010). Treatment with chemical chaperones has been shown to reduce protein misfolding and ER stress, resulting in a positive therapeutic effect (Balch et al. 2008; Sawkar et al. 2002). One particular chemical chaperone, phenylbutyric acid (PBA), is an orally bioavailable short-chain unsaturated fatty acid that improves ER folding capacity and trafficking. Treatment with PBA reduces ER stress (Kanki et al. 2009; Welch & Brown 1996; Ozcan et al. 2006; Ono et al. 2009; Kubota et al. 2006; Yam et al. 2007) and restores proteostasis of the cystic fibrosis transductance regulator, which is prone to misfolding. Currently PBA is being tested in clinical trials for the treatment of cystic fibrosis (Balch et al. 2008).
In previous studies, we have shown that gPr80env accumulation initiates the UPR and a subsequent chain of events in the ER that causes apoptosis in ts1-infected astrocytes (Liu et al. 2004; Liu et al. 2006; Kim et al. 2002; Kim et al. 2004). In fact, expression of gPr80env is sufficient to induce apoptosis in gPr80env transfected cells (Yu et al. 1991; Yu et al. 1997; Zhao & Yoshimura 2008) and neuropathological lesion in ts1 env gene transgenic mice (Yu et al. 1997). Based on the above data accumulation of gPr80env is thought to be the primary insult in ts1-induced astrocyte dysfunction and neurodegeneration. In the present study, we investigated if PBA treatment reduces ts1 cytopathology in cultured astrocytes and if PBA treatment delays or prevents neurodegeneration in infected mice by reducing gPr80env accumulation and subsequent ER stress.
In this study, we first investigated whether PBA reduces gPr80env load in ts1-infected astrocytes. We then determined if treatment with PBA corrects the ER stress and UPR in these cells, thus prevents ts1-mediated astrocyte cell death. These studies provide a mechanistic insight into the role of accumulated misfolded protein-induced neurodegeneration, and a potential mechanism by which PBA could alleviate protein misfolding in neurodegenerative disease.
Materials and Methods
Cells, cell cultures and treatment
The immortalized C1 astrocytic cell line was developed in our laboratory (Lin et al. 1997). C1 cells and NIH3T3 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum. Primary cultured astrocytes (PCA) were isolated from 1- to 2-day-old FVB/N pups as described previously (Kuang et al. 2009; Shikova et al. 1993) and grown in DMEM-F-12 medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2.5 μg/ml fungizone. The media was changed every 3 days. After four or five passages, more than 99% of the cells in the cultures were positive for the astrocyte-specific glial fibrillary acid protein (GFAP) marker. C1 cells or primary astrocytes were infected with ts1 virus as described previously (Kuang et al. 2009). PBA (Sigma) was added to the cultures at different doses immediately after ts1 infection, and the cells were further incubated for the time periods indicated for each experiment. Tunicamycin (Sigma) was dissolved in ethanol and was added to the cultures at a concentration of 5 μg/ml as described in the figure legend.
1.1.1.1 Western blotting analysis
C1 astrocytes, primary astrocytes and NIH3T3 cells from different treatment groups were washed with PBS and lysed in RIPA buffer as described previously (Kuang et al. 2005). Whole cell lysates were cleared by centrifugation at 13000 x g for 20 min at 4°C. Protein concentrations were determined using the Bio-Rad Dc Protein Assay Reagent (Bio-Rad Laboratories, Hercules, CA). The lysates (30–50 μg total protein per sample) were separated on SDS-PAGE gels. The proteins were then transferred to PVDF membranes, and immunoblotted with primary antibodies as described previously (Kuang et al. 2005). The primary antibodies recognize gPr80env (Microbiology Associates; Burlingame, CA), cleaved caspase-3, phospho-p53, BiP, p-elF2α, phospho-PERK, ERp44, and PDI (Cell signaling), ATF4, Bcl2, Bax, CHOP, calregulin (Santa Cruz), ATF-6α (LifeSpan Bioscience). The immunoblotted membranes were then incubated with secondary antibodies, and immune complexes were detected on the membranes using enhanced chemiluminescence (NEN Life Science Products, Boston, MA), according to the manufacturer’s instructions. A monoclonal anti-β-actin antibody (Sigma) was used as a control for protein loading.
1.1.1.2 RNA extraction and real-time PCR
C1 astrocytes were infected with ts1 at a MOI of 10, and then PBA was added at a concentration of 10 mM. After 24 or 48 h of incubation, cellular total RNA was extracted using the RNeasy kit with optional DNaseI treatment (Qiagen, Valencia, CA). RNA was analyzed for integrity using the Agilent 2100 Bioanalyzer (Agilent Technologies, Inc. Santa Clara, CA). Total RNA (1 μg) was then used as template to synthesize cDNA with the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) and qPCR subsequently performed on the ABI 7900HT Fast Real Time PCR System (ABI) with primers specific to ts1 gPr80env using SYBR Green master mix (BioRad, Inc. Hercules, CA). RNA levels were normalized to the endogenous control gene GAPDH. Data analysis was performed using Sequence Detection System software from ABI, version 2.2.2. The experimental Ct (cycle thresh hold) was calibrated against the GAPDH control product. All amplifications were performed in duplicate. The DDCt method was used to determine the amount of product relative to that expressed by 24 h ts1-infected cells (ts1)-derived RNA (1-fold, 100%).
Hoechst/propidium iodide double staining for viability assay
C1 astrocytes were infected with or without PBA treatment as described above. Hoechst/propidium iodide double staining (Wise-Faberowski et al. 2004) was performed 48 h after ts1 infection at room temperature. To identify cells with disrupted membranes, cells were firstly exposed to 40 μg/mL of propidium iodide (Roche Applied Science) dissolved in PBS for 10 min. After washing with PBS, the cells were fixed with 4% paraformaldehyde for 10 min, followed by washing with PBS, and then being treated with 10 μg/mL Hoechst 33342 (Sigma) in PBS for 4 min in the dark. After washing, the cells were stored in Hank’s balanced salt solution (Invitrogen) and viewed under an inverted fluorescent microscope with the observer blinded to the treatment condition. Healthy cells were defined as those with no propidium iodide staining and without evidence of nuclear condensation. Three fields were chosen for each experimental condition and used for healthy cell counting. The average number of cells per field from uninfected control group was considered as to be 100%. The cell number counted from other groups were averaged and expressed as healthy cell % ± SEM relative to that of uninfected control group from three individual experiments.
Animals, virus inoculation and PBA treatment
FVB/N mice were obtained from Taconic Farms (Germantown, NY). The mice were divided into three groups: (a) uninfected, (b) ts1-infected (ts1), and (c) ts1-infected-PBA-treated (ts1+PBA). For infection, 3-day-old neonatal mice were injected intraperitoneally with 0.1 ml of ts1 viral suspension containing 107 of infection unit/ml per pup, as previously described (Choe et al. 1998). The ts1-infected-PBA-treated group (n=9) were treated with PBA intraperitoneally at 240 mg/kg daily, beginning at 5 days after birth (2 days post-infection, or dpi), until the infected untreated mice began to become paralyzed. Mice in the PBA-untreated ts1-infected group (n=11), or in the uninfected group were treated on the same schedule with the same volume of normal saline. All of the mice were observed carefully as signs of disease became evident, and the survival time was recorded when end-stage hindlimb paralysis appeared, as described previously (Jiang et al. 2006; Kuang et al. 2009). For histology and immunofluorescence analysis of CNS tissues, all animals were sacrificed at 30 dpi. These procedures were performed according to protocols approved by The University of Texas, MD Anderson Cancer Center Institutional Animal Care and Use Committee.
Histology and immunofluorescence analysis
Brainstem tissues frozen sections were prepared and immunostained as described previously (Kuang et al. 2009). Briefly, brainstem tissues from uninfected, ts1-infected untreated and ts1-infected PBA-treated animals were snap-frozen in Tissue-Tek OCT embedding medium (Electron Microscopy Sciences, Hatfield, PA) in liquid nitrogen, cut as 5 μm sections, and stained with hematoxylin and eosin (H&E) for histo-pathological analysis. For immunofluorescence analysis, the frozen sections were thawed, fixed in 3.7% paraformaldehyde for 10 minutes, followed by washing and blocking 40 min in 10% donkey serum. The sections were incubated with primary antibody goat anti-gPr80env overnight, and then washed three times and incubated 1 h in anti-goat IgG secondary antibody conjugated with DeLight 488 (Jackson Immunnoresearch, West Grove, PA). DAPI containing mounting medium (Invitrogen) was used to stain the nuclei. Control sections were incubated with purified goat IgGs prior to incubation in secondary antibody, or were incubated in secondary antibody alone. No nonspecific staining was observed on these control sections (data not shown).
For immunostaining of C1 astrocytes plated on chamberslides, the cells were fixed on the slides in 3.7% paraformaldehyde for 15 minutes, then washed and incubated in PBS containing 0.5% Triton X-100 for 5 min, and then in 10% donkey serum for 60 minutes. The slides were then incubated in primary antibody, which was goat anti-gPr80env. After incubation, the slides were washed and incubated with anti-goat IgG secondary antibody conjugated with DeLight 488 (Jackson Immunnoresearch, West Grove, PA), followed with DAPI mounting medium.
1.2 Statistical analysis
Data are presented as means ± SEM from three individual experiments. All cell culture experiments were conducted in triplicate. Statistical significance of the results was determined by analysis of variance (ANOVA) or Student’s t-test. p value of < 0.05 was considered statistically significant. For the survival curves, the cumulative incidence of hind limb paralysis for untreated vs. PBA-treated infected mice was determined by analysis of covariance comparing slopes of curves for the two groups.
1.3 Results
PBA decreases accumulation of gPr80env in ts1-infected cells in a dose-dependent manner
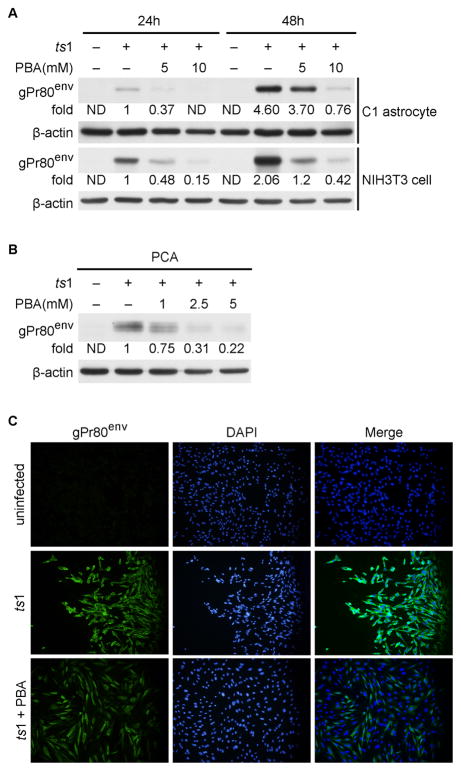
We previously reported that the envelope protein gPr80env is accumulated in the ER and induces ER stress in ts1-infected astrocytes, leading to ts1-induced cytopathicity (Kim et al. 2004; Liu et al. 2004; Shikova et al. 1993; Szurek et al. 1990; Wong et al. 1992; Yu et al. 1997; Qiang et al. 2004; Qiang et al. 2006). We hypothesized that an agent that attenuated gPr80env accumulation may provide a protective effect against ts1-induced cell death. For the current study we selected PBA because it attenuates ER stress as a chemical chaperone (Kanki et al. 2009; Kubota et al. 2006). Figure 1A shows that ts1 infection of cultured astrocytes and NIH3T3 cells is followed by accumulation of gPr80env in a time-dependent manner, but the addition of PBA can effectively decrease the accumulation of gPr80env in a dose-dependent manner (both in C1 astrocytes and in NIH3T3 cells). PBA also decreases the amount of gPr80env in ts1-infected PCAs in a dose-dependent fashion (Fig. 1B). Confirming the above results, we noted that untreated ts1-infected astrocytes were strongly immunoreactive for gPr80env, whereas PBA-treated ts1-infected cells were weakly immunoreactive for gPr80env (Fig. 1C). To quantify the gPr80env signal, we have counted and compared the percentages of gPr80env positive cell between untreated ts1-infected cultures and PBA-treated ts1-infected cultures. We found that 94.33 ± 1.202 % of untreated ts1-infected cells were gPr80env positive, whereas only 48.57 ± 1.257 % of PBA-treated ts1-infected cells were gPr80env positive, indicating that PBA-treated ts1-infected cells shown significantly less gPr80env signal (p < 0.001).
Figure 1. PBA decreases accumulation of gPr80env in ts1-infected C1 astrocytes, primary astrocytes and NIH3T3 cells.
A. C1 astrocytes and NIH3T3 cells were infected with the ts1 virus at a MOI of 10, and PBA was added to the indicated cultures for 24 or 48 h after infection. Levels of gPr80env in the whole cell lysates were compared using Western blot analysis. ND: not detectable. B. PCAs were treated with PBA for 96 hours, and cell lysates were then subjected to Western blot analysis to compare their levels of gPr80env. C. C1 cells were infected with ts1 and treated with PBA as described above for 48 hours, and were then processed for immunofluorescent staining with gPr80env antibody.
1.3.1.1 PBA treatment does not affect mRNA levels of gPr80env in infected C1 astrocytes
We sought to determine if the reduction of gPr80env by PBA treatment was a result of reduced gPr80env mRNA levels. The effect of PBA treatment on gPr80env mRNA levels in ts1-infected C1 cells, was measured using real-time PCR. The results shown in Figure 2 demonstrates that treatment of ts1-infected astrocytes with PBA for 24 h or 48 h did not cause significant changes in gPr80env mRNA levels (p > 0.05). These results suggest PBA-induced reduction of gPr80env protein levels in ts1-infected cells is not due to decreased mRNA levels of gPr80env.
Figure 2. PBA treatment does not alter gPr80env mRNA levels in infected C1 astrocytes.
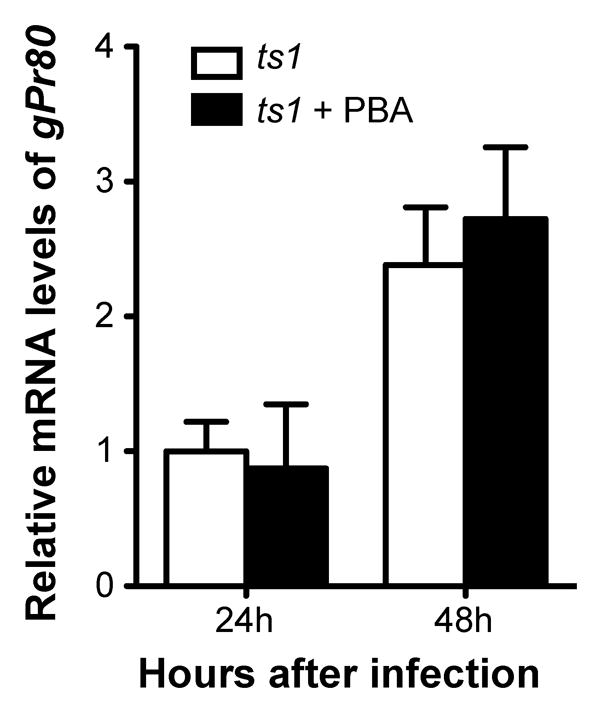
C1 astrocytes were infected with ts1 at a MOI of 10, and then PBA was added at a concentration of 10 mM. After 24 or 48 h, RNA was isolated and the gPr80env mRNA levels were quantitated by RT-PCR. p < 0.05 vs. control was considered statistically significant. Error bars represent mean ± SEM from three independent experiments.
PBA attenuates the UPR and ER stress in ts1-infected astrocytes
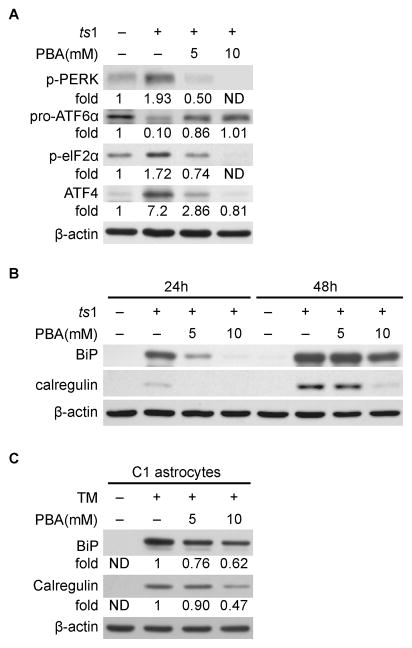
As noted above, the UPR is initiated by activation of the ER stress sensors PERK, ATF6 and IRE1α. To determine whether PBA can suppress UPR, we examined the effect of PBA on these ER stress sensors. Western blot analysis showed that levels of phospho-PERK (p-PERK) are upregulated in ts1-infected C1 astrocytes, while levels of 90KD full-length pro-ATF6α are decreased, implying that ATF6α protein cleavage and activation are occurring (Fig. 3A). These data indicate that the UPR is initiated in ts1-infected astrocytes as a consequence of gPr80env accumulation in the ER. Figure 3A also shows that levels of phospho-elF2α (p-elF2α) and ATF4, downstream effectors of p-PERK, are also increased. In contrast, levels of p-PERK, p-elF2α, and ATF4 are all reduced in a PBA dose-dependent manner, while normal levels of full-length pro-ATF6α are present in ts1-infected PBA-treated C1 cells. These results demonstrate that PBA treatment attenuates the UPR in ts1-infected astrocytes.
Figure 3. PBA reduces the UPR and ER stress in ts1-infected C1 astrocytes.
A. C1 astrocytes were treated as described above for 48 hours, and whole cell lysates were then subjected to Western blot analysis to compare their levels of p-PERK, ATF6, p-elF2α and ATF4. B. C1 astrocytes were treated as described above. Levels of BiP and calregulin in cell lysates were compared by Western blotting. ND: not detectable. C. C1 astrocytes were treated with 5 μg/ml tunicamycin (TM) with or without 5 or 10 mM PBA treatment for 24 hours. Levels of BiP and calregulin in whole cell lysates were compared by Western blotting. ND: not detectable.
Activation of the UPR may induce transcription of UPR-responsive genes, such as ER stress markers BiP and calregulin to aid in the refolding of the misfolded proteins (Kurokawa et al. 2009). Our laboratory has previously shown that ER stress is occurs in ts1-infected astrocytes (Kim et al. 2004; Liu et al. 2004). In light of the above findings we asked if PBA treatment affects the levels of these ER stress markers in ts1-infected C1 astrocytes. As shown in Figure 3B, both BiP and calregulin levels are upregulated in ts1-infected cells, and PBA suppresses the upregulation of BiP and calregulin. Activation of BiP in response to ER stress is generally viewed as the cytoprotective response in cells. Our results reveal a correlation between gPr80env levels (Fig.1A) and BiP or calregulin levels (Fig.3B) at 24 and 48 hours after ts1 infection in C1 astrocytes, suggesting that BiP or calregulin levels (ER stress markers) depend on the extent of gPr80env accumulation in the cells. Interestingly, PBA reduces both gPr80env (Fig.1A) and ER stress markers (Fig. 3B) in ts1-infected C1 astrocytes in a similar pattern. It is very likely that the reduction of ER stress by PBA is attributed to the ability of PBA to suppress gPr80env accumulation in the cells.
To further assess whether PBA affects the accumulation of other unknown misfolded proteins in the ER, we examined the effect of PBA on ER stress responses induced by tunicamycin. Tunicamycin is a known ER stress-inducer that mediates ER stress by inhibiting N-glucosylation and suppressing of protein maturation (Kubota et al. 2006). By examining the levels of the ER stress markers BiP and calregulin, we found that PBA partially attenuates tunicamycin-induced upregulation of BiP and calregulin (Fig 3C). These findings are consistent with the observation reported by Kubota et al (Kubota et al. 2006) that indicate PBA treatment inhibits tunicamycin-induced ER stress.
PBA enhances the expression of the ER folding proteins PDI and ERp44 in ts1-infected astrocytes
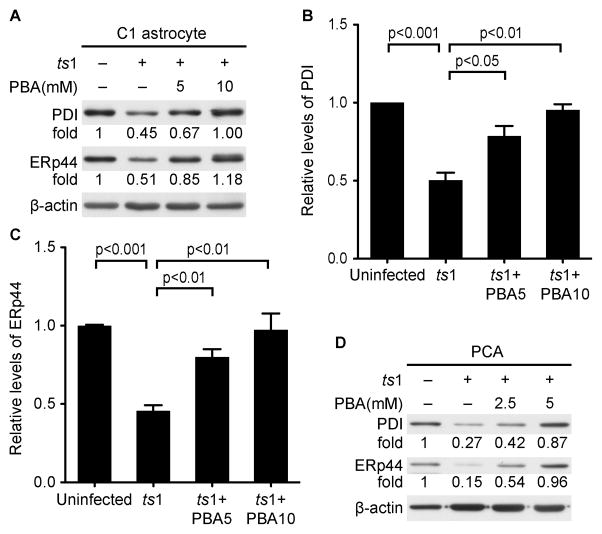
PBA treatment effectively reduced the accumulation of gPr80env (Fig. 1), which lead us to ask if the reduction is due to its ability to enhance the protein-folding capacity of the ER. The ER proteins PDI and ERp44 have been shown to facilitate protein folding. We therefore compared the amounts of PDI and ERp44 in PBA-treated vs. untreated ts1-infected C1 astrocytes. Figure 4A-D shows that protein levels of both PDI and ERp44 are significantly decreased, in both ts1-infected C1 astrocytes and primary astrocytes, whereas PBA treatment was found to prevent the reduction of PDI and ERp44 levels in these cells. These data clearly show that downregulation of ER folding proteins is accompanied with a gPr80env folding deficit in ts1-infected astrocytes, while PBA treatment enhances the expression of ER folding proteins in these cells.
Figure 4. PBA enhances the expression of ER folding proteins in ts1-infected cells.
A. C1 astrocytes were infected with ts1 at a MOI of 10, and then PBA was added at a concentration of 5 or 10 mM for infected C1 cells. At 48 hours post infection, whole cell lysates were subjected to Western blotting to compare the expression of PDI and ERp44. Relative protein levels of PDI (B) and ERp44 (C) from three independent experiments described above were presented. Kodak Image Station was used to obtain intensity of band from Western blotting. PBA5: PBA at a concentration of 5 mM; PBA10: PBA at a concentration of 10 mM; D. PCAs were infected with ts1 at a MOI of 10, and then PBA was added at a concentration of 2 or 4 mM for 96 hours. The protein levels of PDI and ERp44 were compared by Western blotting.
PBA treatment does not affect the proteasomal degradation process for gPr80env in ts1-infected astrocytes
To determine whether PBA treatment prevents the accumulation of gPr80env in ts1-infected astrocytes by accelerating its proteasomal degradation, we incubated ts1-infected astrocytes in 2.5 μM of the proteasomal inhibitor MG132, following PBA treatment. MG132 treatment blocked proteasomal degradation of gPr80env in ts1-infected PBA-untreated C1 cells, but MG132 treatment did not block the reduction of gPr80env expression in ts1-infected PBA-treated C1 cells as shown in Figure 5. This observation suggests that PBA treatment decreases the accumulation of gPr80env in infected cells by promoting the ER folding capacity or accelerating the transport of gPr80env, rather than by facilitating its degradation in the PBA-treated cells.
Figure 5. PBA treatment does not affect proteasomal degradation for gPr80env in ts1-infected astrocytes.
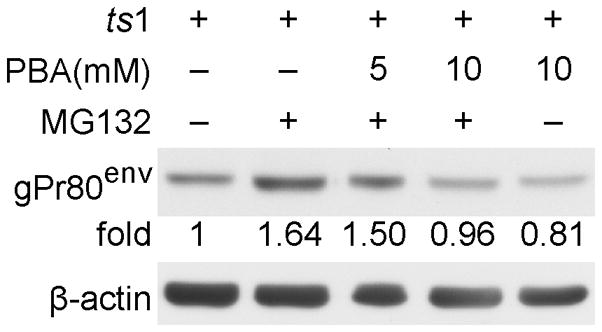
C1 cells were infected with ts1 at a MOI of 10 for 24 hours, and then were treated with 2.5 μM of MG132, either alone or together with 5 or 10 mM PBA. After 16 hours, the whole cell lysates were immunoblotted with gPr80env antibody.
PBA treatment prevents apoptosis in ts1-infected astrocytes
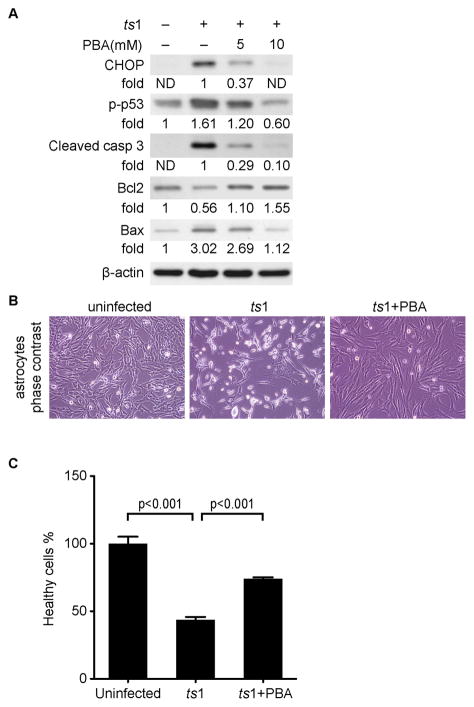
We have previously shown that ER stress induced by ts1-infection causes apoptosis of infected C1 cells (Liu et al. 2004). In light of the above results, we asked whether prevention of ER stress by PBA treatment prevents apoptosis in ts1-infected cells. We compared levels of ER stress activated apoptotic proteins in untreated versus PBA-treated ts1-infected astrocytes. Figure 6A shows that levels of CHOP, phospho-p53, Bax and cleaved caspase 3 are increased, while Bcl2 is decreased, in ts1-infected astrocytes, which confirms the results of previous studies (Kim et al. 2002; Kuang et al. 2005; Liu et al. 2004). In contrast, levels of CHOP, phospho-p53, Bax and cleaved caspase 3 are decreased, and levels of the anti-apoptotic protein Bcl2 are restored in PBA-treated ts1-infected astrocytes (Fig. 6A). The phase contrast images of astrocytes in Figure 6B and the hoechest 33342/propidium iodide double staining viability data in Figure 6C, taken together show that PBA treatment prevents cell death in ts1-infected astrocytes. This result is consistent with previous work from our laboratory showing that PBA promotes survival of ts1-infected astrocytes (Liu et al. 2002).
Figure 6. PBA prevents apoptosis in ts1-infected astrocytes, and also protects primary neurons exposed to spent medium from these cells.
A. C1 astrocytes were treated as described above for 48 hours. Levels of the apoptotic pathway-related proteins CHOP, p-p53, cleaved caspase 3, Bcl-2 and Bax from whole cell lysates were analyzed by Western blotting. ND: not detectable. B. Phase contrast photo-microscopy shows that PBA protects ts1-infected astrocytes. C1 astrocytes were infected with ts1 at a MOI of 10, and PBA was added at a concentration of 10 mM. Images were taken at 48 hours post infection. C. Cell viability assay with or without PBA treatment. C1 astrocytes were infected and treated as described above for 48 hours. Cells were stained firstly with propidium iodide and then with Hoechst 33342 as described in “Material and methods”. Cell viabilities from different treatments were compared using a healthy cell count excluding cells with propidium iodide staining. An average healthy cell number from uninfected control group was considered as to be100%. The healthy cell number counted from other groups were averaged and expressed as healthy cell % ± SEM relative to that of uninfected control group from three individual experiments.
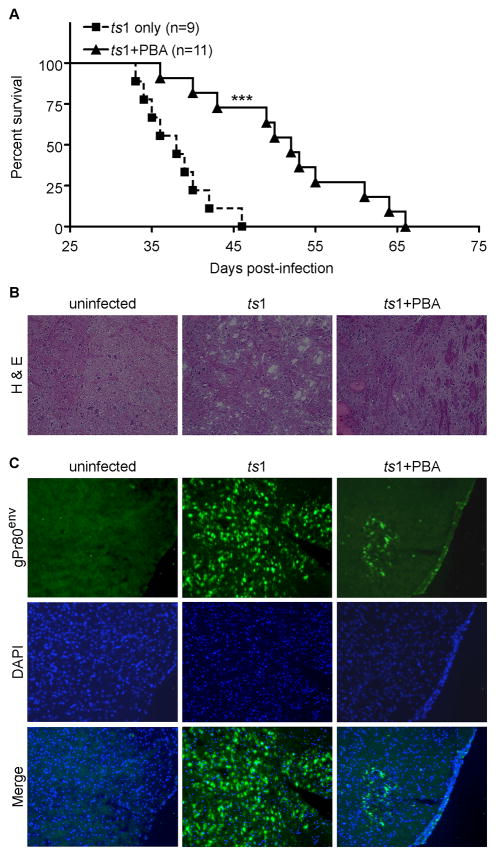
PBA treatment delays the onset of paralysis in ts1-infected FVB/N mice
We administered PBA to ts1-infected FVB/N mice to determine whether PBA treatment of ts1-infected mice decreases CNS accumulation of gPr80env, and to find out if PBA can prevent ts1-induced neurodegeneration. The survival curves in Figure 7A show that untreated ts1-infected (ts1 only) mice developed paralysis at 35 dpi, whereas paralysis is significantly delayed in PBA-treated ts1-infected (ts1-PBA) mice. In brainstem tissue sections stained with H&E, uninfected mice show normal neuropil cytological structures, whereas sections from the same region of ts1-infected CNS tissues contain large spongiform lesions. However, brainstem sections from infected, PBA-treated mice have nearly normal neuropil structure, without discrete spongiform foci (Fig. 7B). To determine whether PBA treatment reduces the accumulation of gPr80env in CNS tissues of infected mice, we immunostained the same brainstem sections with anti-gPr80env antibody. Figure 7C shows that gPr80env staining was present and widespread in ts1-infected untreated brainstems, while few cells were stained with gPr80env in sections from brainstems of PBA-treated ts1-infected mice. This result is consistent with our in vitro data shown in Figure 1C, indicating that PBA treatment reduces gPr80env accumulation.
Figure 7. PBA delays paralysis and spongiform lesion formation in ts1-infected mice.
A) Survival curves for PBA-treated ts1-infected mice (ts1+PBA, n=11) against survival curves for untreated ts1-infected mice (ts1 only, n=9). ***p<0.001. B). Frozen sections of brainstem from uninfected, ts1-infected untreated and PBA-treated mice were stained with H&E. Spongiform lesions are apparent in brainstems of ts1-infected untreated mice, while brainstems from PBA-treated mice showed much less vacuolation. C). Strong gPr80env immunoreactivity is evident in ts1-infected untreated mice brainstems at 30 dpi, while brainstems from PBA-treated infected mice show considerably less gPr80env at this time point.
1.4 Discussion
Many neurodegenerative conditions are associated with protein misfolding, protein accumulation and ER stress in CNS cells (Matus et al. 2008; Kim et al. 2008; Scheper & Hoozemans 2009; Winklhofer et al. 2008; Hashimoto et al. 2003; Hoozemans et al. 2006; Tan et al. 2009; Lindholm et al. 2006; Ono et al. 2009; Inden et al. 2007; Kubota et al. 2006; Castilla et al. 2004; Hetz & Soto 2006; Ilieva et al. 2007; Kanekura et al. 2009). When the early cytoprotective machinery (the UPR or antioxidant defenses) fails to correct protein misfolding or protein accumulation in CNS cells, apoptotic pathways are activated (Boyce & Yuan 2006; Zhang & Kaufman 2008a; Zhang & Kaufman 2008b). Thus, approaches that minimize or correct protein misfolding are important for the treatment of neurodegeneration associated with the accumulation and aggregation of misfolded proteins. In the present study, we have identified that accumulation of the gPr80env protein in ts1-infected astrocytes induces the UPR, ER stress and apoptosis, and that the chemical chaperone PBA ameliorates the death of infected astrocytes and delays the onset of paralysis in ts1-infected mice by reducing the accumulation of gPr80env in infected astrocytes.
Using real-time PCR, we demonstrate that ts1 gPr80env mRNA levels are not altered with PBA treatment in ts1-infected astrocytes. The experiments employing the proteasomal degradation inhibitor MG132 also showed that PBA does not exert its effects by promoting proteasomal degradation of gPr80env. Furthermore, PBA suppresses ER stress induced by tunicamycin, which prevents protein transport from the ER to the Golgi through inhibiting protein N-glycosylation, resulting in protein accumulation in the ER. Taken together these data suggests that PBA suppresses ts1-induced ER stress and reduces the amount of misfolded protein, including gPr80env. PBA likely acts as a chemical chaperone to enhance the capacity of ER protein folding, rather than to reduce ts1 gPr80env mRNA levels or to promote proteasomal degradation of gPr80env. Further support for PBA acting as a chemical chaperone is documented in a number of recent reports (Bonapace et al. 2004; Kubota et al. 2006; Sawkar et al. 2002; Kanki et al. 2009; Inden et al. 2007; Ozcan et al. 2006; Ono et al. 2009).
The ER proteins PDI and ERp44, which are associated with protein folding, are maintained at normal levels in PBA-treated infected cells, whereas the levels of these proteins are significantly reduced in untreated ts1-infected cells. We suggest two possible mechanisms for these events. First, PDI is cleaved by caspase 3 and caspase 7 during apoptosis (Na et al. 2007), subsequently ts1-infection-induced activation of caspase 3 may lead to cleavage of PDI, thus reducing its levels and its availability for preventing of gPr80env accumulation. Second, accumulation of misfolded gPr80env may exhaust available levels of PDI and ERp44 during the process of disulfide bond formation or folding. Regardless of mechanisms, the data presented in this study show that decreased levels of PDI and ERp44 correlate with gPr80env accumulation and that the effect of PBA in maintaining the levels of PDI and ERp44 is likely to be associated with its ability to decrease gPr80env accumulation. We noted that BiP and calregulin levels correlates with gPr80env in ts1-infected astrocytes in presence or absence of PBA treatment. BiP has been shown to bind to the precursor envelope protein of another neurovirulent murine retrovirus, suggesting its function as ER chaperone (Portis et al. 2009). Overexpression of BiP prevents activation of ER stress transducer (Bertolotti et al. 2000). These further support the notion that BiP plays a protective role under ER stress. The mechanisms for the opposite regulation of PDI and ERp44 with respect to BiP and calregulin in ts1-infected cells are unclear at present and need further investigation. We suspect that PDI and ERp44 chaperones are regulated by distinct pathways of the UPR compared to the regulation of BiP and calregulin following ts1 infection and PBA treatment.
Based on this study and previous studies generated by our laboratory, we believe that PBA may play multiple roles against ts1-induced neurodegeneration. We have shown that PBA is a peroxisome proliferator receptor promoter and that this activity increases levels of catalase, an enzyme that converts hydrogen peroxide to water and oxygen (Liu et al. 2002). Among these effects, decreasing the accumulation of gPr80env is likely the primary mechanism associated with its neuroprotectve effects. Since oxidative stress can activate ER stress and vice versa, a treatment that targets one of these effects may also alleviate the other. This hypothesis is supported by other studies showing that reactive oxygen species can be produced by the accumulation of misfolded proteins, and that antioxidants reduce ER stress and improve protein secretion, thereby reducing protein accumulation (Malhotra et al. 2008). We have also previously demonstrated that treatment of ts1-infected mice with the antioxidant compound monosodium luminol (GVT or MSL) extended their survival by decreasing gPr80env accumulation in T cells (Scofield et al. 2009a; Scofield et al. 2009b) and astrocytes (Kuang X & Wong PK unpublished data). This may be due to the fact that GVT, in addition to acting as a free radical scavenger, also upregulates the cellular antioxidant system with activation and nuclear translocation of nuclear factor erythroid 2-related factor 2 (Nrf2) (Reddy et al. 2010). These studies suggest that PBA and GVT may share common mechanisms responsible for ameliorating the effects of gPr80env. In both cases, CNS and T cell protection may be associated with the prevention of gPr80env accumulation and the accompanying prevention of ER and oxidative stress.
Among known chemical chaperones, PBA has a high in vivo safety profile and has already been approved by US Food and Drug Administration for use in the treatment of urea-cycle disorders, thalassemia and cystic fibrosis (Collins et al. 1995; Rubenstein & Zeitlin 1998). Recently, PBA has also been shown to have beneficial effects on several mouse models of neurodegeneration. In a transgenic mouse model of Alzheimer's diseases that expresses the human mutant isoform of amyloid precursor protein PBA reversed spatial learning and memory deficits (Ricobaraza et al. 2009). PBA attenuates neuropathogenic effects in mouse models of Parkinson’s disease induced by human α-synuclein A30P + A53T transgene (Ono et al. 2009). In another mouse model of Parkinson’s disease induced by oral administration of rotenone, PBA significantly inhibits α-synuclein accumulation and aggregation (Inden et al. 2007).
Given that about 8% of the human genome is composed of sequences of human endogenous retroviruses (HERVs) (Lander et al. 2001; Griffiths 2001) and that these HERV gene sequences are able to express biologically active envelope proteins (Cheynet et al. 2005), it is not surprising that some of these HERV envelope proteins may have cellular functions. Unfortunately, under certain conditions, activation, overexpression or mutations in these env gene sequences could result in a spectrum of disease phenotypes, including neurodegeneration (Antony et al. 2004; Antony et al. 2007). In addition to HERVs noted above, a number of retroviruses including HIV and ts1 have been shown to cause a spectrum of nervous system diseases (Gonzalez-Scarano 1995; Power 2001). In lieu of a designated mouse model for HIV-associated dementia (HAD), the ts1 mouse model of neurodegeneration has been considered a suitable surrogate (Clark et al. 2001; Gonzalez-Scarano 1995). Recent reports show that astrocyte infection is much more extensive than previously reported in human patients with HAD (Churchill et al. 2009) and that ER stress occurs in the CNS of HIV-positive individuals (Lindl et al. 2007). Thus, ER stress resulting from the oxidative stress may still be a possible cause of astrocyte damage and neuronal cell loss in the CNS resulting in HAD. The fact that other proteins such as β-amyloid accumulates in HAD brain cells (An & Scaravilli 1997; Esiri et al. 1998; Rempel & Pulliam 2005; Achim et al. 2009) provides a strong support for the notion that accumulation of β-amyloid and other proteins could be the result of oxidative stress and ER stress in HIV-infected CNS cells (Lindl et al. 2007). Interestingly, in neurons of ts1 infected brainstem, we also observed the presence of “Lewy bodies”, which are formed as a result of accumulation of α-synuclein, a pathologic hallmark of Parkinson’s disease (Stoica et al. 2000). Since ts1 does not infect neurons, the accumulation of these Lewy bodies is not due to virus infection but rather indirectly caused by ts1-mediated oxidative stress, which leads to accumulation of α-synuclein in neurons. Interestingly, PBA has also been shown to attenuate the pathogenic potency of human α-synuclein accumulation in the transgenic mouse model of Parkinson disease (Ono et al. 2009).
In conclusion, the data presented in the current study on the pathogenic mechanism of the ts1-induced neurodegeneration and its prevention with PBA treatment may provide new insights into the utilization of PBA as a potential therapeutic molecule. PBA treatment is likely a beneficial intervention to prevent neurological diseases not only in human retrovirus associated neurodegeneration, but also in many neurodegenerative diseases that are associated with protein accumulation and/or aggregation and ER stress. 1.5 Acknowledgements This work was supported in part by NIH Grants RO1 MH071583, RO1 NS043984 (to P. K. Wong), NIEHS center grant P30 ES007784, the National Cancer Institute core Grant P30 CA016672 and by funds from The Longevity Foundation in Austin, Texas. We thank Dr. John Repass for his assistance in real-time PCR analysis, Dr. Virginia Scofield, Dr. William S. Lynn and Dr. Joanne M. Ajmo and Hilary Graham for their critical review of the manuscript, Christine Brown for preparing the figures. We are also most grateful to Mrs. Lifang Zhang for technical assistance.
Research Highlights.
In this study, we assessed whether treatments that reduce the accumulation of precursor envelope protein gPr80env of ts1, a mutant of Moloney murine leukemia virus (MoMuLV), in the ER of infected astrocytes provided a protective effect against ER stress and cell death. We show that treatment with phenylbutyric acid (PBA) can prevent the unfolded protein response (UPR), ER stress and cell death in cultured ts1-infected astrocytes. The protective effect of PBA is associated with its ability to reduce gPr80env accumulation and to increase the expression of proteins involved in protein folding in the ER, such as protein disulfide isomerase (PDI) and ERp44, rather than by decrease mRNA levels of gPr80env or alter the proteasomal degradation process for gPr80env. In infected mice treated with PBA we also noted a reduction in the severity of the neuropathology in brainstem tissues and a delayed onset of paralysis. These results show that PBA is a potentially effective drug for the treatment of neurodegeneration caused by misfolded protein accumulation in cells of the CNS.
Abbreviation used
- ER
endoplasmic reticulum
- UPR
unfolded protein response
- PBA
Phenylbutyric acid
- MoMuLV
Moloney murine leukemia virus
- PERK
PRK-like ER kinase
- ATF6
activating transcription factor 6
- IRE1
inositol-requiring kinase 1
- elF2α
eukaryotic initiation factor α
- ATF4
activating transcription factor 4
- CHOP
the transcription factor C/EBP homologous protein
- PDI
protein disulfide isomerase
- ERp44
ERp57 and ERp72, endoplasmic reticulum proteins 44, 57 and 72
- DMEM
Dulbecco’s modified Eagle’s medium
- PCA
Primary cultured astrocytes
- GFAP
glial fibrillary acid protein
- MOI
multiplicity of infection
- FITC
fluorescein
- dpi
days post-infection
- H&E
hematoxylin and eosin
- DAPI
4,6'-diamidino-2-phenylindole
- HERV
human endogenous retroviruses
- HAD
HIV-associated dementia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achim CL, Adame A, Dumaop W, Everall IP, Masliah E. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol. 2009;4:190–199. doi: 10.1007/s11481-009-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An SF, Scaravilli F. Early HIV-1 infection of the central nervous system. Arch Anat Cytol Pathol. 1997;45:94–105. [PubMed] [Google Scholar]
- Antony JM, Ellestad KK, Hammond R, Imaizumi K, Mallet F, Warren KG, Power C. The human endogenous retrovirus envelope glycoprotein, syncytin-1, regulates neuroinflammation and its receptor expression in multiple sclerosis: a role for endoplasmic reticulum chaperones in astrocytes. J Immunol. 2007;179:1210–1224. doi: 10.4049/jimmunol.179.2.1210. [DOI] [PubMed] [Google Scholar]
- Antony JM, van Marle G, Opii W, et al. Human endogenous retrovirus glycoprotein-mediated induction of redox reactants causes oligodendrocyte death and demyelination. Nat Neurosci. 2004;7:1088–1095. doi: 10.1038/nn1319. [DOI] [PubMed] [Google Scholar]
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- Bonapace G, Waheed A, Shah GN, Sly WS. Chemical chaperones protect from effects of apoptosis-inducing mutation in carbonic anhydrase IV identified in retinitis pigmentosa 17. Proc Natl Acad Sci U S A. 2004;101:12300–12305. doi: 10.1073/pnas.0404764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 2006;13:363–373. doi: 10.1038/sj.cdd.4401817. [DOI] [PubMed] [Google Scholar]
- Castilla J, Hetz C, Soto C. Molecular mechanisms of neurotoxicity of pathological prion protein. Curr Mol Med. 2004;4:397–403. doi: 10.2174/1566524043360654. [DOI] [PubMed] [Google Scholar]
- Cheynet V, Ruggieri A, Oriol G, Blond JL, Boson B, Vachot L, Verrier B, Cosset FL, Mallet F. Synthesis, assembly, and processing of the Env ERVWE1/syncytin human endogenous retroviral envelope. J Virol. 2005;79:5585–5593. doi: 10.1128/JVI.79.9.5585-5593.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe W, Stoica G, Lynn W, Wong PK. Neurodegeneration induced by MoMuLV-ts1 and increased expression of Fas and TNF-alpha in the central nervous system. Brain Res. 1998;779:1–8. doi: 10.1016/s0006-8993(97)00929-3. [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, Gorry PR. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann Neurol. 2009;66:253–258. doi: 10.1002/ana.21697. [DOI] [PubMed] [Google Scholar]
- Clark S, Duggan J, Chakraborty J. Tsl and LP-BM5: a comparison of two murine retrovirus models for HIV. Viral Immunol. 2001;14:95–109. doi: 10.1089/088282401750234475. [DOI] [PubMed] [Google Scholar]
- Collins AF, Pearson HA, Giardina P, McDonagh KT, Brusilow SW, Dover GJ. Oral sodium phenylbutyrate therapy in homozygous beta thalassemia: a clinical trial. Blood. 1995;85:43–49. [PubMed] [Google Scholar]
- DuRose JB, Scheuner D, Kaufman RJ, Rothblum LI, Niwa M. Phosphorylation of eukaryotic translation initiation factor 2alpha coordinates rRNA transcription and translation inhibition during endoplasmic reticulum stress. Mol Cell Biol. 2009;29:4295–4307. doi: 10.1128/MCB.00260-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esiri MM, Biddolph SC, Morris CS. Prevalence of Alzheimer plaques in AIDS. J Neurol Neurosurg Psychiatry. 1998;65:29–33. doi: 10.1136/jnnp.65.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraldi A, Zito E, Annunziata F, et al. Multistep, sequential control of the trafficking and function of the multiple sulfatase deficiency gene product, SUMF1 by PDI, ERGIC-53 and ERp44. Hum Mol Genet. 2008;17:2610–2621. doi: 10.1093/hmg/ddn161. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Nathanson N, Wong PKY. Retrovirus and the nervous system. In: Levy J, editor. The retrovirus. Vol. 4. San Francisco: 1995. p. 409. [Google Scholar]
- Griffiths DJ. Endogenous retroviruses in the human genome sequence. Genome Biol. 2001;2:REVIEWS1017. doi: 10.1186/gb-2001-2-6-reviews1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Rockenstein E, Crews L, Masliah E. Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer's and Parkinson's diseases. Neuromolecular Med. 2003;4:21–36. doi: 10.1385/NMM:4:1-2:21. [DOI] [PubMed] [Google Scholar]
- Haze K, Okada T, Yoshida H, Yanagi H, Yura T, Negishi M, Mori K. Identification of the G13 (cAMP-response-element-binding protein-related protein) gene product related to activating transcription factor 6 as a transcriptional activator of the mammalian unfolded protein response. Biochem J. 2001;355:19–28. doi: 10.1042/0264-6021:3550019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz CA, Soto C. Stressing out the ER: a role of the unfolded protein response in prion-related disorders. Curr Mol Med. 2006;6:37–43. doi: 10.2174/156652406775574578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoozemans JJ, Stieler J, van Haastert ES, Veerhuis R, Rozemuller AJ, Baas F, Eikelenboom P, Arendt T, Scheper W. The unfolded protein response affects neuronal cell cycle protein expression: implications for Alzheimer's disease pathogenesis. Exp Gerontol. 2006;41:380–386. doi: 10.1016/j.exger.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Endoplasmic reticulum stress and atherosclerosis. Nat Med. 2010;16:396–399. doi: 10.1038/nm0410-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilieva EV, Ayala V, Jove M, et al. Oxidative and endoplasmic reticulum stress interplay in sporadic amyotrophic lateral sclerosis. Brain. 2007;130:3111–3123. doi: 10.1093/brain/awm190. [DOI] [PubMed] [Google Scholar]
- Inden M, Kitamura Y, Takeuchi H, et al. Neurodegeneration of mouse nigrostriatal dopaminergic system induced by repeated oral administration of rotenone is prevented by 4-phenylbutyrate, a chemical chaperone. J Neurochem. 2007;101:1491–1504. doi: 10.1111/j.1471-4159.2006.04440.x. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Scofield VL, Yan M, Qiang W, Liu N, Reid AJ, Lynn WS, Wong PK. Retrovirus-induced oxidative stress with neuroimmunodegeneration is suppressed by antioxidant treatment with a refined monosodium alpha-luminol (Galavit) J Virol. 2006;80:4557–4569. doi: 10.1128/JVI.80.9.4557-4569.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekura K, Suzuki H, Aiso S, Matsuoka M. ER stress and unfolded protein response in amyotrophic lateral sclerosis. Mol Neurobiol. 2009;39:81–89. doi: 10.1007/s12035-009-8054-3. [DOI] [PubMed] [Google Scholar]
- Kanki K, Kawamura T, Watanabe Y. Control of ER stress by a chemical chaperone counteracts apoptotic signals in IFN-gamma-treated murine hepatocytes. Apoptosis. 2009;14:309–319. doi: 10.1007/s10495-009-0318-x. [DOI] [PubMed] [Google Scholar]
- Kim HT, Tasca S, Qiang W, Wong PK, Stoica G. Induction of p53 accumulation by Moloney murine leukemia virus-ts1 infection in astrocytes via activation of extracellular signal-regulated kinases 1/2. Lab Invest. 2002;82:693–702. doi: 10.1097/01.lab.0000017373.82871.45. [DOI] [PubMed] [Google Scholar]
- Kim HT, Waters K, Stoica G, Qiang W, Liu N, Scofield VL, Wong PK. Activation of endoplasmic reticulum stress signaling pathway is associated with neuronal degeneration in MoMuLV-ts1-induced spongiform encephalomyelopathy. Lab Invest. 2004;84:816–827. doi: 10.1038/labinvest.3700104. [DOI] [PubMed] [Google Scholar]
- Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- Kuang X, Scofield VL, Yan M, Stoica G, Liu N, Wong PK. Attenuation of oxidative stress, inflammation and apoptosis by minocycline prevents retrovirus-induced neurodegeneration in mice. Brain Res. 2009;1286:174–184. doi: 10.1016/j.brainres.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang X, Yan M, Liu N, Scofield VL, Qiang W, Cahill J, Lynn WS, Wong PK. Control of Atm-/- thymic lymphoma cell proliferation in vitro and in vivo by dexamethasone. Cancer Chemother Pharmacol. 2005;55:203–212. doi: 10.1007/s00280-004-0870-6. [DOI] [PubMed] [Google Scholar]
- Kubota K, Niinuma Y, Kaneko M, et al. Suppressive effects of 4-phenylbutyrate on the aggregation of Pael receptors and endoplasmic reticulum stress. J Neurochem. 2006;97:1259–1268. doi: 10.1111/j.1471-4159.2006.03782.x. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Hideshima M, Ishii Y, Kyuwa S, Yoshikawa Y. Aortic ER stress in streptozotocin-induced diabetes mellitus in APA hamsters. Exp Anim. 2009;58:113–121. doi: 10.1538/expanim.58.113. [DOI] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Lin YC, Chow CW, Yuen PH, Wong PK. Establishment and characterization of conditionally immortalized astrocytes to study their interaction with ts1, a neuropathogenic mutant of Moloney murine leukemia virus. J Neurovirol. 1997;3:28–37. doi: 10.3109/13550289709015790. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13:385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- Lindl KA, Akay C, Wang Y, White MG, Jordan-Sciutto KL. Expression of the endoplasmic reticulum stress response marker, BiP, in the central nervous system of HIV-positive individuals. Neuropathol Appl Neurobiol. 2007;33:658–669. doi: 10.1111/j.1365-2990.2007.00866.x. [DOI] [PubMed] [Google Scholar]
- Liu N, Kuang X, Kim HT, Stoica G, Qiang W, Scofield VL, Wong PK. Possible involvement of both endoplasmic reticulum- and mitochondria-dependent pathways in MoMuLV-ts1-induced apoptosis in astrocytes. J Neurovirol. 2004;10:189–198. doi: 10.1080/13550280490448043. [DOI] [PubMed] [Google Scholar]
- Liu N, Qiang W, Kuang X, Thuillier P, Lynn WS, Wong PK. The peroxisome proliferator phenylbutyric acid (PBA) protects astrocytes from ts1 MoMuLV-induced oxidative cell death. J Neurovirol. 2002;8:318–325. doi: 10.1080/13550280290100699. [DOI] [PubMed] [Google Scholar]
- Liu N, Scofield VL, Qiang W, Yan M, Kuang X, Wong PK. Interaction between endoplasmic reticulum stress and caspase 8 activation in retrovirus MoMuLV-ts1-infected astrocytes. Virology. 2006;348:398–405. doi: 10.1016/j.virol.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez IM, Chrispeels MJ. Genomic analysis of the unfolded protein response in Arabidopsis shows its connection to important cellular processes. Plant Cell. 2003;15:561–576. doi: 10.1105/tpc.007609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus S, Lisbona F, Torres M, Leon C, Thielen P, Hetz C. The stress rheostat: an interplay between the unfolded protein response (UPR) and autophagy in neurodegeneration. Curr Mol Med. 2008;8:157–172. doi: 10.2174/156652408784221324. [DOI] [PubMed] [Google Scholar]
- McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na KS, Park BC, Jang M, Cho S, Lee do H, Kang S, Lee CK, Bae KH, Park SG. Protein disulfide isomerase is cleaved by caspase-3 and -7 during apoptosis. Mol Cells. 2007;24:261–267. [PubMed] [Google Scholar]
- Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–3651. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Ikemoto M, Kawarabayashi T, Ikeda M, Nishinakagawa T, Hosokawa M, Shoji M, Takahashi M, Nakashima M. A chemical chaperone, sodium 4-phenylbutyric acid, attenuates the pathogenic potency in human alpha-synuclein A30P + A53T transgenic mice. Parkinsonism Relat Disord. 2009;15:649–654. doi: 10.1016/j.parkreldis.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis JL, Askovich P, Austin J, Gutierrez-Cotto Y, McAtee FJ. The degree of folding instability of the envelope protein of a neurovirulent murine retrovirus correlates with the severity of the neurological disease. J Virol. 2009;83:6079–6086. doi: 10.1128/JVI.02647-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C. Retroviral diseases of the nervous system: pathogenic host response or viral gene-mediated neurovirulence? Trends Neurosci. 2001;24:162–169. doi: 10.1016/s0166-2236(00)01737-9. [DOI] [PubMed] [Google Scholar]
- Qiang W, Cahill JM, Liu J, et al. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol. 2004;78:11926–11938. doi: 10.1128/JVI.78.21.11926-11938.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang W, Kuang X, Liu J, et al. Astrocytes survive chronic infection and cytopathic effects of the ts1 mutant of the retrovirus Moloney murine leukemia virus by upregulation of antioxidant defenses. J Virol. 2006;80:3273–3284. doi: 10.1128/JVI.80.7.3273-3284.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PV, Lungu G, Kuang X, Stoica G, Wong PK. Neuroprotective effects of the drug GVT (monosodium luminol) are mediated by the stabilization of Nrf2 in astrocytes. Neurochem Int. 2010;56:780–788. doi: 10.1016/j.neuint.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel HC, Pulliam L. HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS. 2005;19:127–135. doi: 10.1097/00002030-200501280-00004. [DOI] [PubMed] [Google Scholar]
- Ricobaraza A, Cuadrado-Tejedor M, Perez-Mediavilla A, Frechilla D, Del Rio J, Garcia-Osta A. Phenylbutyrate ameliorates cognitive deficit and reduces tau pathology in an Alzheimer's disease mouse model. Neuropsychopharmacology. 2009;34:1721–1732. doi: 10.1038/npp.2008.229. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Rubenstein RC, Zeitlin PL. A pilot clinical trial of oral sodium 4-phenylbutyrate (Buphenyl) in deltaF508-homozygous cystic fibrosis patients: partial restoration of nasal epithelial CFTR function. Am J Respir Crit Care Med. 1998;157:484–490. doi: 10.1164/ajrccm.157.2.9706088. [DOI] [PubMed] [Google Scholar]
- Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- Sawkar AR, Cheng WC, Beutler E, Wong CH, Balch WE, Kelly JW. Chemical chaperones increase the cellular activity of N370S beta -glucosidase: a therapeutic strategy for Gaucher disease. Proc Natl Acad Sci U S A. 2002;99:15428–15433. doi: 10.1073/pnas.192582899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheper W, Hoozemans JJ. Endoplasmic reticulum protein quality control in neurodegenerative disease: the good, the bad and the therapy. Curr Med Chem. 2009;16:615–626. doi: 10.2174/092986709787458506. [DOI] [PubMed] [Google Scholar]
- Scofield VL, Yan M, Kuang X, Kim SJ, Crunk D, Wong PK. The drug monosodium luminol (GVT) preserves thymic epithelial cell cytoarchitecture and allows thymocyte survival in mice infected with the T cell-tropic, cytopathic retrovirus ts1. Immunol Lett. 2009a;122:159–169. doi: 10.1016/j.imlet.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Scofield VL, Yan M, Kuang X, Kim SJ, Wong PK. The drug monosodium luminol (GVT) preserves crypt-villus epithelial organization and allows survival of intestinal T cells in mice infected with the ts1 retrovirus. Immunol Lett. 2009b;122:150–158. doi: 10.1016/j.imlet.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Shikova E, Lin YC, Saha K, Brooks BR, Wong PK. Correlation of specific virus-astrocyte interactions and cytopathic effects induced by ts1, a neurovirulent mutant of Moloney murine leukemia virus. J Virol. 1993;67:1137–1147. doi: 10.1128/jvi.67.3.1137-1147.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica G, Tasca SI, Wong PK. Motor neuronal loss and neurofilament-ubiquitin alteration in MoMuLV-ts1 encephalopathy. Acta Neuropathol. 2000;99:238–244. doi: 10.1007/pl00007433. [DOI] [PubMed] [Google Scholar]
- Szurek PF, Yuen PH, Ball JK, Wong PK. A Val-25-to-Ile substitution in the envelope precursor polyprotein, gPr80env, is responsible for the temperature sensitivity, inefficient processing of gPr80env, and neurovirulence of ts1, a mutant of Moloney murine leukemia virus TB. J Virol. 1990;64:467–475. doi: 10.1128/jvi.64.2.467-475.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JM, Wong ES, Lim KL. Protein misfolding and aggregation in Parkinson's disease. Antioxid Redox Signal. 2009;11:2119–2134. doi: 10.1089/ars.2009.2490. [DOI] [PubMed] [Google Scholar]
- Todd DJ, Lee AH, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol. 2008;8:663–674. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- Wang CC, Tsou CL. Protein disulfide isomerase is both an enzyme and a chaperone. FASEB J. 1993;7:1515–1517. doi: 10.1096/fasebj.7.15.7903263. [DOI] [PubMed] [Google Scholar]
- Welch WJ, Brown CR. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones. 1996;1:109–115. doi: 10.1379/1466-1268(1996)001<0109:iomacc>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklhofer KF, Tatzelt J, Haass C. The two faces of protein misfolding: gain- and loss-of-function in neurodegenerative diseases. EMBO J. 2008;27:336–349. doi: 10.1038/sj.emboj.7601930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise-Faberowski L, Aono M, Pearlstein RD, Warner DS. Apoptosis is not enhanced in primary mixed neuronal/glial cultures protected by isoflurane against N-methyl-D-aspartate excitotoxicity. Anesth Analg. 2004;99:1708–1714. doi: 10.1213/01.ANE.0000136474.35627.FF. table of contents. [DOI] [PubMed] [Google Scholar]
- Wong PK, Shikova E, Lin YC, Saha K, Szurek PF, Stoica G, Madden R, Brooks BR. Murine leukemia virus induced central nervous system diseases. Leukemia. 1992;6(Suppl 3):161S–165S. [PubMed] [Google Scholar]
- Yam GH, Gaplovska-Kysela K, Zuber C, Roth J. Sodium 4-phenylbutyrate acts as a chemical chaperone on misfolded myocilin to rescue cells from endoplasmic reticulum stress and apoptosis. Invest Ophthalmol Vis Sci. 2007;48:1683–1690. doi: 10.1167/iovs.06-0943. [DOI] [PubMed] [Google Scholar]
- Yan M, Shen J, Person MD, Kuang X, Lynn WS, Atlas D, Wong PK. Endoplasmic reticulum stress and unfolded protein response in Atm-deficient thymocytes and thymic lymphoma cells are attributable to oxidative stress. Neoplasia. 2008;10:160–167. doi: 10.1593/neo.07935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Kamps CA, Yuen PH, Wong PK. Construction and characterization of expression systems for the env gene of ts1, a mutant of Moloney murine leukemia virus-TB. Virus Res. 1991;19:83–92. doi: 10.1016/0168-1702(91)90096-e. [DOI] [PubMed] [Google Scholar]
- Yu YE, Choe W, Zhang W, Stoica G, Wong PK. Development of pathological lesions in the central nervous system of transgenic mice expressing the env gene of ts1 Moloney murine leukemia virus in the absence of the viral gag and pol genes and viral replication. J Neurovirol. 1997;3:274–282. doi: 10.3109/13550289709029468. [DOI] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008a;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ. Identification and characterization of endoplasmic reticulum stress-induced apoptosis in vivo. Methods Enzymol. 2008b;442:395–419. doi: 10.1016/S0076-6879(08)01420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Yoshimura FK. Expression of murine leukemia virus envelope protein is sufficient for the induction of apoptosis. J Virol. 2008;82:2586–2589. doi: 10.1128/JVI.02291-07. [DOI] [PMC free article] [PubMed] [Google Scholar]