Abstract
Inherited familial Alzheimer's disease (AD) is characterized by small increases in the ratio of Aβ42 versus Aβ40 peptide which is thought to drive the amyloid plaque formation in the brain of these patients. Little is known however whether ageing, the major risk factor for sporadic AD, affects amyloid beta-peptide (Aβ) generation as well. Here we demonstrate that the secretion of Aβ is enhanced in an in vitro model of neuronal ageing, correlating with an increase in γ-secretase complex formation. Moreover we found that peroxynitrite (ONOO−), produced by the reaction of superoxide anion with nitric oxide, promoted the nitrotyrosination of presenilin 1 (PS1), the catalytic subunit of γ-secretase. This was associated with an increased association of the two PS1 fragments, PS1-CTF and PS1-NTF, which constitute the active catalytic centre. Furthermore, we found that peroxynitrite shifted the production of Aβ towards Aβ42 and increased the Aβ42/Aβ40 ratio. Our work identifies nitrosative stress as a potential mechanistic link between ageing and AD.
Keywords: ageing, Alzheimer's disease, gamma-secretase, peroxynitrite, presenilin-1
INTRODUCTION
Despite the strong association between Alzheimer's disease (AD) and ageing, we still do not understand the underlying molecular mechanisms (reviewed in Kern & Behl, 2009). AD is characterized by the presence of extraneuronal senile plaques and intraneuronal neurofibrillary tangles (NFT), mainly composed of amyloid beta-peptide (Aβ) and deposits of tau protein, respectively. Mostly based on studies of families with inherited AD, the amyloid hypothesis states that the disorder is caused by Aβ accumulation in the parenchyma of the brain (Hardy & Selkoe, 2002). Aβ is generated by consecutive β- and γ-secretase cleavages of the single type I transmembrane amyloid precursor protein (APP).
The steady state level of Aβ is also determined by its clearance via transcytosis through the blood–brain barrier (BBB) and further degradation in the liver (reviewed in Zlokovic, 2008) and via direct proteolytic processing in the brain (reviewed in De Strooper, 2010). Thus Aβ levels in the central nervous system are ultimately the result of mechanisms that generate and mechanisms that clear the peptide. Apart from these quantitative considerations, it is important to take into account also qualitative elements. Indeed Aβ shows heterogeneity, a large part generated at its carboxy-terminus by γ-secretase cleavage (De Strooper et al, 1998, reviewed in De Strooper, 2010). Interestingly the longer Aβ peptides (especially Aβ42) are more prone to aggregate compared to Aβ40 and it is believed that increments in the Aβ42/Aβ40 ratio are pathogenic (Kuperstein et al, 2010), although the contribution of other Aβ species might be important as well (Saito et al, 2011).
The γ-secretases are therefore of central importance in the disease cascade. They are high molecular weight complexes composed of presenilin 1 (PS1) or presenilin 2 (PS2), nicastrin (Nct), anterior pharynx-defective phenotype 1 (Aph1) and PS enhancer 2 (Pen2) (reviewed in De Strooper, 2003). The catalytic activity of the complex resides on PS (De Strooper et al, 1998, 1999; Wolfe et al, 1999), a protein with nine transmembrane domains. At present more than 180 mutations in PS1 and 10 mutations in PS2 have been identified in patients with familial AD (FAD). Nevertheless, these mutations are found only in a small percentage of AD cases (<0.5%), while there is no definitive causal explanation for the rest of patients with late-onset or sporadic AD. Interestingly FAD mutations lead to an altered processing of APP and an increase in the Aβ42/Aβ40 ratio (Scheuner et al, 1996). New evidences suggest that a more rapid product release from the catalytic site in γ-secretase is behind the increase of the Aβ42/Aβ40 ratio (Chavez-Gutierrez et al, 2012). The insight that such partial loss of function (in contrast to full loss of function), underlies the disorder potentially changes our fundamental understanding of the role played by γ-secretase in sporadic AD as well (De Strooper, 2007; Wolfe, 2007). Indeed, as Aβ42 levels increase with ageing in humans (Funato et al, 1998), a possible link between ageing and the increased risk for AD might be found in age related alterations of γ-secretase. Therefore we asked whether molecular changes that occur during the ageing process could affect γ-secretase activity in similar ways as FAD mutations.
One of the most relevant hallmarks of the ageing process is the accumulation of reactive oxygen species (ROS) due to the impairment of cellular mechanisms that protect against oxidative stress (reviewed in Finkel & Holbrook, 2000). Neurons are especially susceptible to ROS as the brain consumes an inordinate fraction (20%) of the total oxygen consumption for its relatively small weight (2%). This high metabolism produces an increase of the mitochondrial respiratory chain activity and large amount of oxidants. Superoxide anion is mainly produced by the mitochondrial electron transport chain, though other sources also contribute to the generation of this radical species (Starkov, 2008). Superoxide anions react extremely fast with nitric oxide, which is generated by the mitochondrial nitric oxide synthase (NOS) or the neuronal NOS, to give peroxynitrite. Peroxynitrite at lower concentrations can alter the function of proteins by irreversibly reacting with the phenol ring of tyrosines to yield nitrotyrosine (Radi et al, 2002).
In agreement with the possibility that failure in the control of superoxide production participates in AD pathology, recent work reported that the overexpression of the mitochondrial anti-oxidant enzyme superoxide dismutase 2 (SOD2) in a mouse model of AD decreases the Aβ42/Aβ40 ratio and prevents the appearance of memory deficits (Massaad et al, 2009). In addition SOD2 but not the cytosolic enzyme SOD1, protects against glutamate-induced oxidative stress (Fukui & Zhu, 2010).
In the present work we set out to study the changes occurring in the γ-secretase complex in rat hippocampal neurons during ageing in vitro. Once seeded in the culture dish, hippocampal neurons undergo a whole series of morphological and functional maturation processes reflecting those of their counterparts in vivo. Thus, during the first week in vitro these neurons establish morphological and functional axons and dendrites; during the second week they establish synaptic activity and from the third week on they begin to show canonical signs of ageing, including accumulation of ROS, lipofuscin granules, heterochromatic foci, activation of the c-Jun N-terminal protein kinase (JNK) and the DNA repair p53/p21 pathways (Martin et al, 2008; Sodero et al, 2011). It was also shown that with time in vitro these neurons present increased protein oxidation, creatine kinase expression and calcium channel density, typical features of the ageing brain (Aksenova et al, 1999; Porter et al, 1997). Finally, hippocampal neurons in vitro undergo a time-associated increase in tubulin acetylation similarly to the in vivo situation and a time-associated increase in the phosphorylation of the microtubule-associated protein Tau (Sodero et al, 2011) similarly to that reported in aged human brains (Pikkarainen et al, 2009) and mouse models of senescence (Tomobe & Nomura, 2009). Our results support an AD-like change in the activity of γ-secretase triggered by nitrosative stress in vitro. We confirm similar changes in a Sod2 knockout mouse model and augmented nitrotyrosination of presenilin in the brains of individuals affected with sporadic AD, adding clinical value to the mechanistic association depicted in this work.
RESULTS
Total Aβ secretion as well as Aβ42/Aβ40 ratio increase during neuronal ageing in vitro
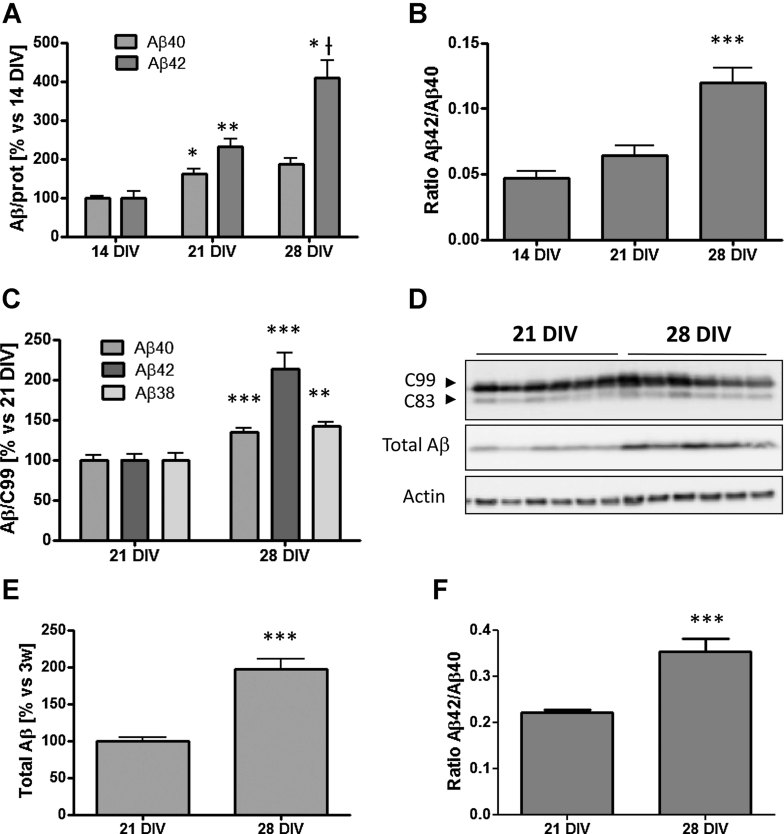
In order to determine if neuronal ageing itself can induce qualitative and quantitative changes in the profile of secreted Aβ, we cultured rat primary hippocampal neurons in vitro for 2, 3 or 4 weeks at 37°C and 5% CO2. These time-points were chosen because they represent terminal differentiation and early and late ageing, respectively, based on various criteria discussed in the introduction (see also Supporting Information Fig S1). At the day of experiment, the media was removed and substituted by fresh conditioned media. After 24 h the endogenous generated Aβ species present in the media (Aβ40 and Aβ42) were quantified by enzyme-linked immunosorbent assay (ELISA). As shown in Fig 1A, both Aβ40 (p < 0.05, n = 3) and Aβ42 (p < 0.01, n = 3) are elevated at 21 DIV compared to 14 DIV, without major changes in the Aβ42/Aβ40 ratio. Interestingly, between 21 DIV and 28 DIV a dramatic switch in the Aβ profile is observed with a strong increase in the more amyloidogenic species Aβ42 (p < 0.05, n = 3) (Fig 1A), elevating therefore the Aβ42/Aβ40 ratio (p < 0.01, n = 3) (Fig 1B). The change in the ratio points to a direct effect of ageing on γ-secretase. To directly demonstrate this, 14, 21 and 28 DIV hippocampal neurons were infected with an adenovirus expressing the human γ-secretase substrate huAPP-C99-3xFlag. This artificial substrate is equivalent to the APP carboxyterminal fragment generated by β-secretase cleavage (BACE1), and therefore this experiment allows excluding possible contributions of changes in BACE1 or APP expression on the Aβ ELISA results. Media were collected after 24 h and newly produced human Aβ species (Aβ38, Aβ40 and Aβ42) were determined by human peptide specific ELISAs or by Western blot (total Aβ). The media from non-infected neurons was used as a negative control to exclude possible interference by endogenous rat Aβ with the ELISA measurements. The results were normalized to the total C99 signal from the Western blot. As shown in Fig 1C–E, Aβ secretion increased indeed at 28 DIV compared to 21 DIV. In addition, the switch towards the more amyloidogenic Aβ42 peptide between 21 DIV and 28 DIV was confirmed (Fig 1F), stressing the possibility that ageing affects γ-secretase activity, raising the question to mechanisms.
Figure 1. Switch in the secreted Aβ profile during in vitro ageing of primary rat hippocampal neurons.
- At 21 DIV neurons secrete both more Aβ40 and Aβ42 in comparison to 14 DIV. However there is a dramatic increase of the levels of Aβ42 in the media of 28 DIV neurons in comparison to 21 DIV, without major changes in the levels of Aβ40. The endogenous Aβ peptides were determined in the media from three independent experiments by ELISA (*p < 0.05 and **p < 0.01 vs. 14 DIV; *†p < 0.05 vs. 21 DIV).
- The Aβ42/Aβ40 ratio is approximately two times higher in 28 DIV neurons in comparison to 14 DIV (***p < 0.001).
- Rat hippocampal neurons transduced for 48 h with an adenoviral vector driving expression of the 3xFlag-huAPP-C99 substrate for γ-secretase. huAβ42, huAβ40 and huAβ38 were measured by specific ELISA. Notice the dramatic increase in the levels of huAβ42 at 28 DIV. Values were normalized to total huAPP-C99 expression which is shown in panel D (n = 3; **p < 0.01, ***p < 0.001).
- Western blot analysis (4–12% Bis–Tris gel) of rat neurons expressing 3xFlag-huAPP-C99 using anti-Flag antibody. The next panel (total Aβ) shows Western blot with mAb 6E10 of media collected after 24 h from 3xFlag-huAPP-C99 infected neurons (4–12% Bis–Tris gel). Twenty-eight DIV neurons secrete higher amounts of total huAβ peptide in comparison to 21 DIV when normalized to huC99 expression. C99 refers to APP-C99 and C83 refers to APP-C83 which is generated by α-secretase cleavage from C99.
- Quantification from panel D by densitometry of the total secreted Aβ by 3xFlag-huAPP-C99 infected neurons (***p < 0.001).
- huAβ42/huAβ40 ratio in the media of 3xFlag-huAPP-C99 infected neurons. The data shown in panel C were used to calculate the ratio (***p < 0.001). Data are presented as mean ± SEM.
Old neurons show more γ-secretase complex formation and elevated expression of some of its components
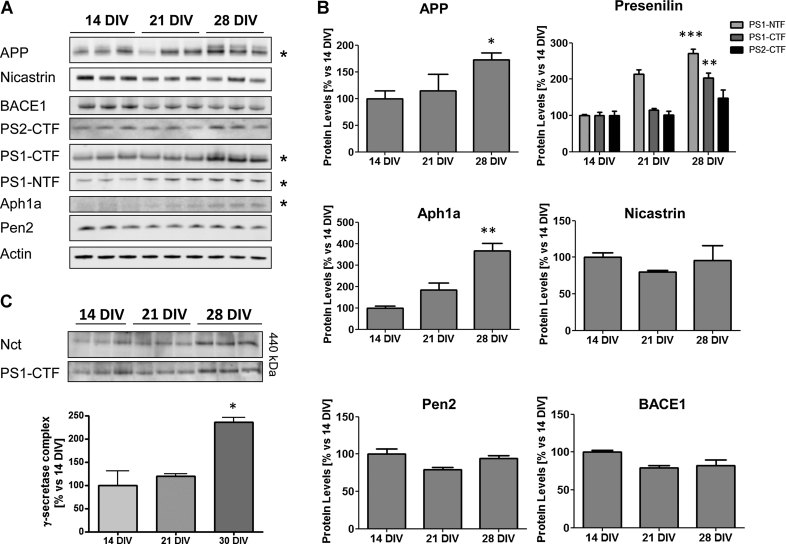
Next, we utilized the in vitro system of neuronal ageing to analyze the expression of the proteins involved in the amyloidogenic pathway, namely APP, BACE1 and the four components of γ-secretase. Western blot was used to check for changes of protein expression. We found at the protein level a significant increment of the presenilin fragments PS1-CTF and PS1-NTF, in 28 DIV neurons compared to 21 and 14 DIV (Fig 2A and B). Total APP (mature and immature) as well as Aph1a also showed higher expression levels in 28 DIV neurons (Fig 2A and B). On the contrary there was a slight reduction in BACE1 expression. We measured the amount of γ-secretase complex in blue native gel electrophoresis, which led us to conclude that the assembled complex was elevated twofold in old neurons (Fig 2C). This quantitative increase could account for the increase of the presenilin fragments, as these are generated by endoproteolytic cleavage of full-length presenilin once the four components are assembled (Thinakaran et al, 1996; reviewed in De Strooper, 2003). The presence of more complex formation or stability could also explain the higher Aβ production seen in these aged neurons. The question remains why the ratio of Aβ42/Aβ40 was altered.
Figure 2. Age-associated increase of proteins related to the amyloidogenic pathway and the γ-secretase complex.
- Western blot (4–12% Bis–Tris gel) of solubilized rat neurons. The panel shows the expression of the γ-secretase constituents as well as BACE1 and APP during in vitro neuronal ageing from three independent experiments. Fifteen micrograms of total protein was loaded per lane and actin was used as a loading control. The proteins showing a significant change after quantification (see panel B) are marked with a ‘*’. The antibodies used as well as the dilutions can be found in the Materials and Methods Section.
- Densitometric quantification of the Western blot in panel A shows a significant increase in the levels of presenilin fragments, APP and Aph1a, after normalization with the actin band (*p < 0.05, **p < 0.01, ***p < 0.001; n = 3).
- Blue native gel electrophoresis shows an increase of the γ-secretase complex in 28 DIV rat hippocampal neurons. Data are presented as mean ± SEM.
Elevated nitrosative stress with ageing causes increased Aβ42/Aβ40 ratio
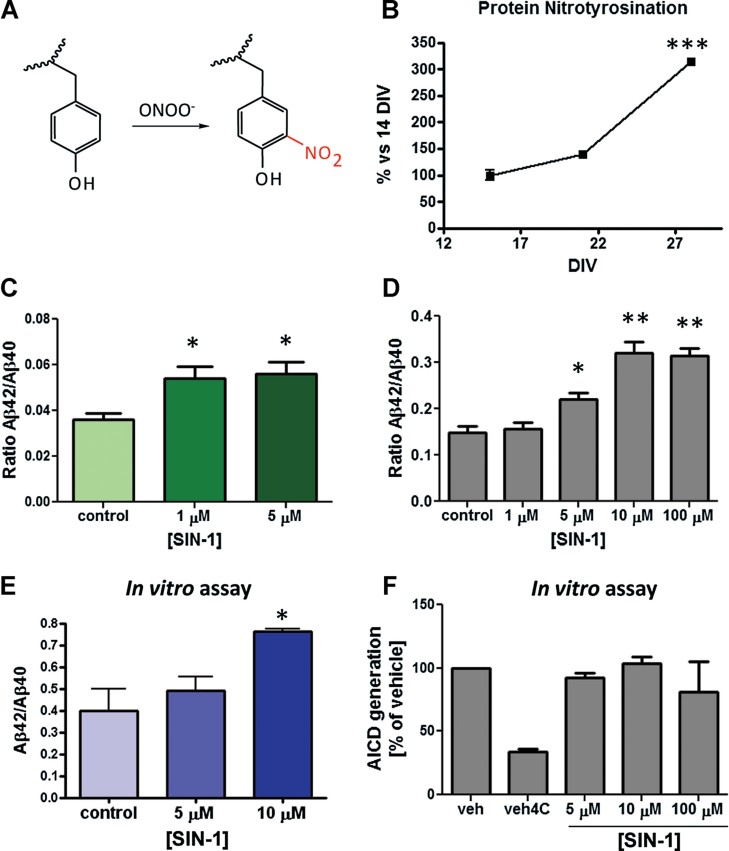
Ageing is partially due to the combination of ROS accumulation and reduced capacity of cells and tissues to efficiently remove them and/or protect against their deleterious consequences. Previous studies have demonstrated that stressful stimuli can promote the amyloidogenic pathway by increasing APP levels (Coma et al, 2008), or by promoting the β- or γ-secretase cleavage of APP (O'Connor et al, 2008; Quiroz-Baez et al, 2009). Nitrosative stress caused by the peroxynitrite during oxidative stress causes tyrosine modifications and therefore we wondered whether γ-secretase could be altered by this type of covalent modification during ageing. Peroxynitrite-triggered nitrotyrosination is especially relevant in AD. Widespread nitration has been found in AD brains (Smith et al, 1997). Nitrotyrosination has been implicated in AD pathogenesis (Guix et al, 2009; Kummer et al, 2011; Tran et al, 2003). In addition protein nitration is enhanced by a hydrophobic environment, such as the plasma membrane (reviewed in Szabó et al, 2007). We determined the levels of protein nitrotyrosination using a specific antibody (Guix et al, 2009) in Western blots of lysates of 14, 21 and 28 DIV neurons (Fig 3B and Supporting Information Fig S1). A small increase in protein nitrotyrosination was observed between 14 and 21 DIV (Fig 3B). However a threefold increase occurred between 21 and 28 DIV in comparison to 14 DIV (p < 0.001, n = 3). This also indicates that nitrosative stress increases dramatically in the 4th week of culture according with previous publications showing that age associated biochemical changes (e.g. protein carbonyls content) in the neurons become mainly manifest in this critical 4th week (Aksenova et al, 1999).
Figure 3. Nitrosative stress affects Aβ42/Aβ40 ratio.
- Scheme showing the modification of tyrosine residues by peroxynitrite (ONOO−).
- Quantification from a Western blot (Supporting Information Fig 1) of tyrosine nitration (insert) as a marker of nitrosative stress. Nitrotyrosine levels are elevated in 28 DIV versus 14 DIV neurons (***p < 0.001, n = 3).
- Rat hippocampal neurons (14 DIV) treated with the peroxynitrite donator SIN-1 for 24 h increase the Aβ42/Aβ40 ratio in the medium as measured by ELISA (**p < 0.01, n = 3).
- The same effect of SIN-1 on Aβ42/Aβ40 ratio was achieved on HEK-swAPP cells treated for 24 h (*p < 0.05, **p < 0.01, n = 3).
- Cell-free γ-secretase activity carried out with microsomal extracts from HEK cells treated or untreated with SIN-1 for 24 h also showed an increased Aβ42/Aβ40 ratio (*p < 0.05, n = 3).
- AICD produced in vitro from membrane preparations from HEK-swAPP cells treated with vehicle only, or with different concentrations of SIN-1. No changes of AICD generation are observed in presence of SIN-1. Vh = vehicle control, Vh4C = reaction at 4°C (negative control). Values at 5, 10 and 100 µM are not statistically different. Data are presented as mean ± SEM.
To determine whether the excess of peroxynitrite modification could explain the changes in Aβ profile seen with ageing, we treated young rat hippocampal neurons (14 DIV) with low concentrations of the peroxynitrite donor 3-morpholinosyndnomine (SIN-1). After collecting conditioned media of 24 h, we analyzed the levels of Aβ40 and Aβ42. As shown in Fig 3C, Aβ42 increases after treatment with 1 and 5 µM of SIN-1, resulting in a dramatic alteration of the Aβ42/Aβ40 ratio. We obtained similar results in human embryonic kidney (HEK) cells overexpressing the human Swedish mutation FAD-linked form of APP (Fig 3D) treated with SIN-1, and in neuroblastoma-derived SH-SY5Y cells overexpressing the human wt APP (Supporting Information Fig S2A) treated with peroxynitrite. Subtoxic concentrations of the nitrating agent were used in both cell types as shown by the MTT reduction experiment (Supporting Information Fig S2B and C). The response was stronger in SH-SY5Y cells, where the highest increment of Aβ42 was already reached after the treatment with 1 µM SIN-1 (Supporting Information Fig S2A). This effect was specific for nitrosative stress, since treatment of HEK-swAPP cells with H2O2 at the same range of concentrations did not increase Aβ42/Aβ40 ratio (Supporting Information Fig S2D).
In order to determine if γ-secretase activity was changed by nitrosative stress, an in vitro assay was carried out with the solubilized microsomal fraction containing the γ-secretase complex, obtained from SIN-1 treated or untreated HEK cells. We used 0.5 µM of purified human APP-C99 triple tagged with a Flag sequence (3xFLAG-huAPP-C99) as substrate. At the concentration of 10 µM SIN-1, there was a nearly twofold increase of the Aβ42/Aβ40 ratio (Fig 3E), without changes of the AICD production (Supporting Information Fig S4C). Similarly no changes in the total AICD production were seen in HEK-swAPP membrane preparations incubated in the absence or the presence of 10 µM SIN-1 (Fig 3F), indicating no change in the ε-cleavage of C99.
The γ-secretase complex undergoes an internal conformational change by nitrosative stress
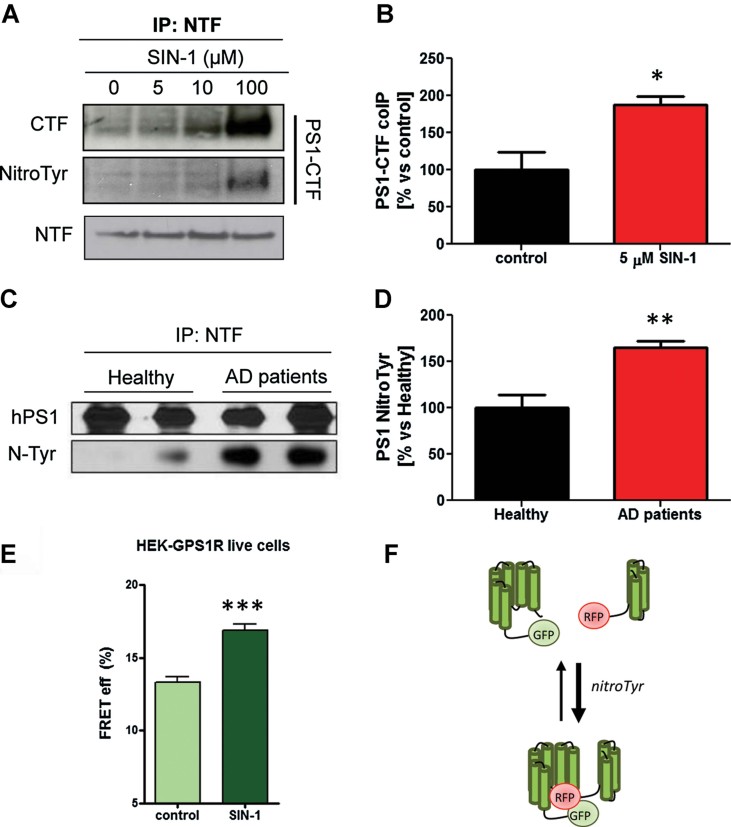
Interaction between PS1-CTF and NTF has been shown to modulate the Aβ42/40 ratio (Berezovska et al, 2005). We therefore co-immunoprecipitated PS1-CTF together with PS1-NTF from HEK cells pre-treated with increasing concentrations of SIN-1. A higher amount of PS1-CTF was pulled-down by the PS1-NTF antibody correlating with increasing SIN-1 concentrations under the experimental condition used (0.5% Triton-X-100) (Fig 4A and B and Supporting Information Fig S3A and B). Interestingly PS1-CTF and PS1-NTF appeared to coimmunoprecipitate under these conditions even in the presence of 2% Triton-X-100 which normally dissociates the two fragments (Supporting Information Fig S3C). At the same time, the PS1-CTF fragment showed an increased signal for the anti-nitrotyrosine antibody when cells had been treated with the nitrating agent (Fig 4A). Since SIN-1 can also generate little amounts of NO in addition to peroxynitrite, we repeated the experiment in cells pre-treated with sodium nitroprusside (SNP), a widely used NO donor. As shown in Fig S3A (Supporting Information), no differences in the co-precipitation of the presenilin fragments was observed. Then we investigated whether presenilin was modified by nitrotyrosination in the prefrontal cortex of brain autopsy samples from AD subjects. We immunoprecipitated PS1 with an antibody against PS1-NTF. In agreement with the possibility that the mechanism highlighted in vitro may operate in vivo, we observed high levels of nitrotyrosination of presenilin in patient material versus age-matched controls (Fig 4C and D).
Figure 4. Nitrosative stress promotes a stronger interaction between the PS1-CTF and the PS1-NTF fragments.
- PS1-NTF was immunoprecipitated with a monoclonal anti-PS1-NTF antibody from HEK-swAPP cells treated with increasing concentrations of SIN-1. Cells were extracted with 0.5% Triton-X-100. Material was resolved in 4–12% Bis–Tris gel and after transfer stained with PS1-CTF, PS1-NTF and N-tyr antibody. Notice the higher co-immunoprecipitation of PS1-CTF with PS1_NTF compared to untreated cells. PS1-CTF from HEK-swAPP cells treated with SIN-1 also shows higher levels of nitration, as detected with an anti-nitrotyrosine antibody.
- Quantification of PS1-CTF from untreated or SIN-1-treated HEK-swAPP cells co-immunoprecipitating with PS1-NTF as in panel A. Data are the mean ± SEM of four independent experiments. *p < 0.05 by Student's t-test analysis. AU = arbitrary unit.
- Nitration of PS1 inmunoprecipitated from the prefrontal cortex of two healthy or two AD patients. Presenilin was pulled down by inmunoprecipitation with a monoclonal antibody anti-PS1-NTF and after resolving the samples in a 4–12% Bis–Tris gel and transfer, an anti-nitrotyrosination antibody was used to detect nitration.
- Quantification by densitometry of Western blots from four healthy individuals and four AD patients. PS1 is more nitrated in AD patients compared to healthy individuals (*p < 0.05, n = 4 in each group). Data are presented as mean ± SEM.
- HEK293 were maintained in Opti-MEM (Invitrogen) supplemented with 5% foetal bovine serum (FBS). For the FLIM analysis of PS1 conformation, HEK293 cells were plated into eight-chamber slides and transiently transfected with the GFP-PS1 (negative control) or GFP-PS1-RFP (G-PS1-R) FRET reporter. Five to six hours after transfection, media was replaced with the treatment solution: 10 µM SIN-1 (Sigma) in 0.2% FBS, or only 0.2% FBS as a vehicle control. FLIM imaging was performed on living cells 24 h after the treatment. FRET efficiency was calculated using the following equation: %EFRET = 100 × (t1 − t2)/t1 and plotted on a graph. Higher FRET efficiencies represents a closer PS1-NT to TM6-7 loop proximity.
- Hypothetically different populations of the γ-secretase complex, with a closer or an open conformation, would co-exist. Nitrosative stress would bring the equilibrium towards the closer conformation.
Next we set out to get insight into the mechanism by which nitration of γ-secretase complex increases the Aβ42/Aβ40 ratio. We carried out cell-free assays using 1% 3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxy-1-propanesulfonate (CHAPSO) solubilized microsomal fractions from HEK cells as source of enzyme. Microsomal fractions were incubated for 24 h at 25°C in the presence or the absence of 10 µM of the nitrating reagent SIN-1. Detergent and lipids were added and kinetic experiments were carried out using increasing concentrations of the purified 3xFlag-huAPP-C99 as substrate. After 5 h of reaction, Aβ40 and Aβ42 levels were measured by specific ELISAs. AICD was determined after SDS–polyacrylamide gel electrophoresis (SDS–PAGE)/Western blot by incubating the membrane with a FlagM2 antibody and the bands were quantified by densitometry. Although total AICD did not significantly change in nitrated conditions at the substrate concentration of 0.6 µM, fitting our previous results (Fig 3F), we observed an increment in Aβ42 and no change in Aβ40 levels, which actually explains the increment of the Aβ42/Aβ40 ratio in the nitrated conditions (Supporting Information Fig S4A and B). At a substrate concentration close to Vmax (2.5 µM) (Supporting Information Table S1) the AICD shows a slight but significant increase (Supporting Information Fig S4C). Immunoprecipitation of the complex with a PS1-CTF antibody followed by SDS–PAGE/Western blot with an anti-nitrotyrosination specific antibody (Supporting Information Fig S4D) confirmed that γ-secretase complex is indeed nitrated in our conditions, suggesting a direct effect of the nitrosative stress on the protease. Interestingly, fluorescence lifetime microscopy (FLIM) carried out on HEK cells transfected with the previously validated fluorescent resonance energy transfer (FRET) reporter of PS1 conformation, green fluorescent protein (GFP)-PS1-RFP construct (Uemura et al, 2009), showed that nitrosative stress generated by 10 µM SIN-1 induced a conformational change of the γ-secretase complex that is similar to that reported for FAD Presenilin mutations (Fig 4E and F). Thus, we conclude that nitrosative stress shifts γ-secretase conformation towards a more close state, which has been related to an increased Aβ42/Aβ40 ratio in several conditions.
Increased peroxynitrite can be caused by deficient SOD2
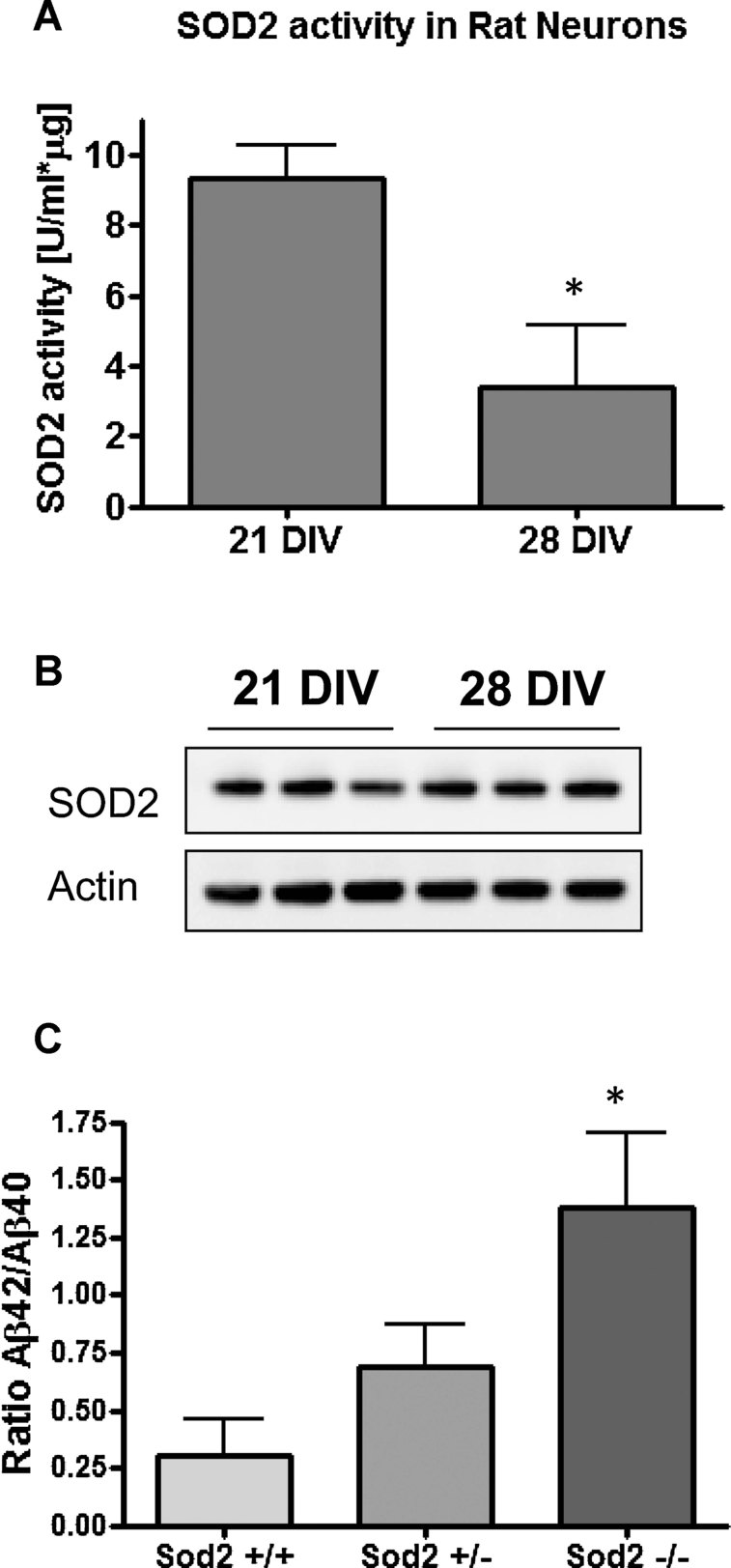
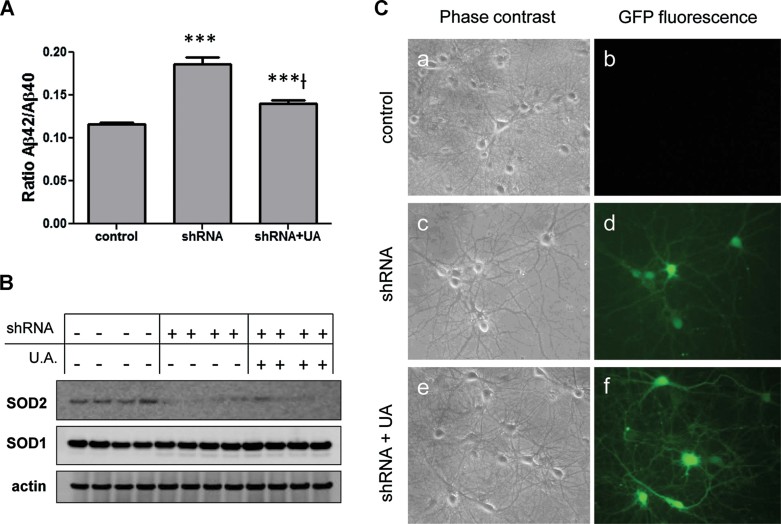
Increased nitrosative stress may be due to reduced radical scavenging capacity. To determine whether this is the cause of increased peroxynitrite in our in vitro ageing system, we measured the activity of the major ROS scavenging enzyme SOD2 in hippocampal neurons maintained in vitro for different periods of time. Fig 5A shows that SOD2 activity undergoes indeed a threefold decrease in 28 DIV compared to 21 DIV neurons, without changes at the protein level (Fig 5B). To directly assess whether reduced SOD2 activity plays a role in the excessive production of Aβ, we used neurons from SOD2 null mice. Consistently, SOD2 knockout neurons showed a five times higher Aβ42/Aβ40 ratio when compared to wt (Fig 5C). These results indicate that SOD2 deficiency in 28 DIV neurons per se can account for the increase of the Aβ42/Aβ40 ratio, but not for the higher levels of total Aβ. To confirm more directly that peroxynitrite mediates the increase of the Aβ42/Aβ40 ratio when the SOD2 activity is impaired, we measured both Aβ42 and Aβ40 by ELISA in the media of non-transfected neurons or transfected with a small hairpin RNA (shRNA) construct against SOD2. The ratio was increased after knocking down SOD2 (Fig 6A–C) and partially recovered with 100 µM of the physiological and powerful peroxynitrite scavenger uric acid (UA) (Hooper et al, 1998, 2000; Scott et al, 2005; Tran et al, 2003) (Fig 6A–C).
Figure 5. Reduced SOD2 activity in old neurons correlates with the increased Aβ42/Aβ40 ratio.
- SOD2 activity decreases threefold when neurons are kept for 28 DIV in vitro compared to 21 DIV-old neurons (*p < 0.05; n = 3 different cultures). SOD2 activity was specifically measured in neuronal lysates containing 10 µg of total protein each and 2 mM KCN in order to inhibit the activity of SOD1. A commercial kit based on the auto-oxidation of haematoxylin for 10 min was used to determine the activity (see Materials and Methods Section).
- The amount of SOD2 present in neuronal lysates was determined from 15 µg of total protein by Western blot using a 4–12% Bis–Tris gel and detection with a monoclonal antibody against the protein. The SOD2 levels measured by densitometry are not changed between 21 and 28 DIV, indicating that the decline in its activity is not due to less enzyme in old neurons.
- The Aβ42/Aβ40 ratio is elevated in the media of 10 DIV neurons derived from Sod2−/− mice in comparison to wt (*p < 0.05). Three independent cultures per genotype were used for the experiment. The Aβ species in the media were measured with ELISA. Data are presented as mean ± SEM.
Figure 6. Peroxynitrite mediates the increase of the Aβ42/Aβ40 ratio triggered by SOD2 depletion.
- Rat primary hippocampal neurons were left untransfected or transfected with an shRNA-enhanced GFP (EGFP) construct against SOD2. Half of the transfected cultures were immediately treated with 100 µM uric acid (UA). After 10 days in vitro, the media was collected and the Aβ42 and Aβ40 levels were analyzed by ELISA. Neurons transfected with the shRNA-EGFP construct showed an increased Aβ42/Aβ40 ratio (***n = 6, p < 0.001 vs control) that was partially recovered when their medium contained 100 µM UA (***†n = 6, p < 0.001 vs shRNA).
- SDS–PAGE/Western blot showing decreased SOD2 protein levels in shRNA-EGFP-transfected neurons and non-transfected ones. Actin was chosen to normalize the protein levels. The effect was specific for SOD2 since no differences in SOD1 levels were detected.
- Pictures of living rat hippocampal neurons obtained by light microscopy. Non-transfected neurons (a and b) were negative under the blue exciting beam, while neurons transfected with the shRNA-EGFP construct against SOD2 were fluorescent under the blue exciting beam, independently if they were untreated (c and d) or treated with 100 µM UA (e and f).
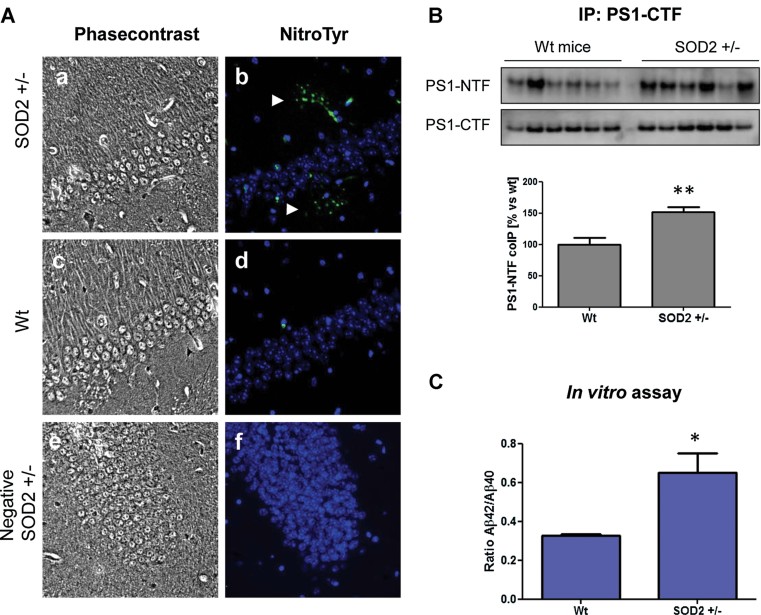
To test if reduced SOD2 activity plays a role in increased nitrosative stress and therefore in the observed peroxynitrite-mediated PS changes, we measured nitrotyrosine modifications in brains from Sod2+/− mice. Heterozygous mice had to be used due to the early lethality of the homozygous mice. We demonstrated first that all the heterozygote mice used in this study displayed a substantial 50% reduction of SOD2 protein levels compared to wt animals (Supporting Information Fig S5A). Histological analysis revealed the existence of widespread nitrotyrosination in the brains of Sod2+/− mice in comparison to age-matched wt (Fig 7A and Supporting Information Fig S5B), demonstrating that an impairment of the SOD2 activity is sufficient to increase the formation of peroxynitrite. We confirmed that more PS1-CTF fragments co-immunoprecipitated with the PS1-NTF when using 0.5% Triton-X-100 solubilized material from the cerebral cortex of Sod2+/− mice (Fig 7B). In order to determine the intrinsic activity of the complex, we carried out an in vitro assay on γ-secretase from microsomal fractions isolated from brain material (Serneels et al, 2009) comparing 18 months wt and Sod2+/− mice, and using as a substrate 0.5 µM of the purified 3xFLAG-huAPP-C99. As shown in Fig 7C, an elevated Aβ42/Aβ40 ratio was observed in material from the cerebral cortex of SOD2+/− mice compared to age-matched wt. These results demonstrate that γ-secretase processing of APP is shifted towards the production of Aβ42 when the anti-oxidant SOD2 activity is impaired.
Figure 7. Increased nitrotyrosination of γ-secretase in the brain of 18 months SOD2+/− mice correlates with a switch in its activity towards a higher Aβ42 production.
- Brains of 18 months Sod2+/− mice (a and b) show consistently higher nitrotyrosination signal (green) in the parenchyma compared to age-matched wt mice (c and d). The nucleus of the cells are stained with DAPI (blue) in (e) and (f) only secondary antibodies were used. The hippocampal area is shown.
- PS1-CTF was inmunoprecipitated from 0.5% Triton-X-100 solubilized extracts (f) the cerebral cortex of 18 months SOD2+/− mice. More PS1-NTF was co-immuneprecipitated as demonstrated by Western blot in SOD+/− compared to SOD+/+ (wt) controls (**p < 0.01, n = 6).
- Cell-free γ-secretase activity using microsomal extracts from the cerebral cortex of 18 months SOD2+/− or wt mice. 3xFlag-huAPP-C99 was used as a substrate at 0.5 µM. The Aβ42/Aβ40 ratio is increased (*p < 0.05, n = 6). Data are presented as mean ± SEM.
DISCUSSION
This study was designed to gain insight into the mechanism by which age predisposes to AD. We initially used rat hippocampal neurons cultured in vitro as an ageing model. Several lines of evidence from our laboratory (Supporting Information Fig S1A and B; Martin et al, 2008; Sodero et al, 2011) and others (Aksenova et al, 1999; Porter et al, 1997) and discussed extensively in the introduction, support their suitability for this purpose.
A first striking finding is that hippocampal neurons after 21 DIV show an increase in Aβ generation and a shift in the ratio of Aβ42/Aβ40 very similar to what is observed with cells transfected with APP or Presenilin carrying FAD mutations (Scheuner et al, 1996). The major shift in the Aβ ratio is observed in 28 DIV neurons, concomitant with dramatic increases in nitrosative levels as measured with a nitrotyrosine antibody and a decrease in the activity (although not expression) of one of the most important anti-oxidant enzymes, namely SOD2. These very similar changes were observed before in brains from elder individuals (Cardozo-Pelaez et al, 1999; Funato et al, 1998). Once we established that in vitro ageing of neurons is paralleled by one of the major signatures of FAD causing mutations, namely changes in the Aβ42/Aβ40 ratio, we moved on establishing a mechanism to explain these observations.
First we focused on the higher levels of Aβ secreted into the media by old neurons. We analyzed the expression of several components from the amyloidogenic pathway. We found that the expression levels of Aph1a and presenilin fragments are incremented in old neurons and that, using blue native gel electrophoresis, more fully assembled complex is present in the aged neurons. The elevation of the Aph1a levels could help to stabilize the complex, since increased expression might enhance γ-secretase assembly and stabilization (Pardossi-Piquard et al, 2009). In addition the overexpression of Aph1 in HEK cells has been shown to enhance the γ-secretase activity and the secretion of Aβ into the medium (Marlow et al, 2003). Aph1 competes with Rer1p to bind Nct in the ER, and thus higher levels of Aph1 could help the complex to assemble and escape from this control mechanism in the ER to the cell surface (Spasic et al, 2007). Changes in the lipid composition of membranes associated with ageing could also play a role in affecting the assembly and/or activity of the γ-secretase complex. During neuronal ageing those lipids which compose raft domains such as sphingomyelin (Trovò et al, 2011) and cholesterol (Martin et al, 2010) show altered levels. Two of the components of γ-secretase, Nct and Aph1, are palmitoylated and targeted into detergent resistant domains (DRM). However mutation of the palmitoylated cysteines does not prevent the full assembly of the complex (Cheng et al, 2009).
Importantly, independent evidence obtained with APP-C99 infection confirmed that the alterations we observed in the neuronal culture were likely caused by alterations in γ-secretase, and not by changes in APP expression or β-secretase activity. Obviously, this experiment does not exclude the possibility that also clearance factors were affected in our ageing neurons, but the dramatic shift in Aβ42/Aβ40 ratio points to additional important alterations in the γ-secretase complex itself, which was confirmed using several cell free assays.
Two main hallmarks of ageing, the first risk factor for AD, are the elevated levels of ROS and the altered pattern of gene expression, leading to the impairment of several cell functions associated with senescence (Butterfield et al, 1999). Mitochondria are the major sites for superoxide anion generation (Radi et al, 2002; Starkov, 2008). Superoxide anions react with nitric oxide (which is generated by the mitochondrial NOS) to give peroxynitrite (reviewed in Guix et al, 2005). Peroxynitrite can diffuse easily through the cell, having an action radius of about 100 µm (Radi et al, 2002). It can alter the function of proteins by irreversibly reacting with the phenol ring of tyrosines generating a nitrotyrosine residue or a covalent dityrosine bridge if two tyrosines are opposed closely to each other. Hence, we hypothesized that any decrease in superoxide scavenging activity in the ageing neurons could lead to a dysfunctional γ-secretase organization or activity due to damage from peroxynitrite. We provide here evidence that both in the cell cultures and in a γ-secretase cell free assay nitration of tyrosines in γ-secretase indeed affects the Aβ ratio. Increased nitrosative stress might be explained by loss of protective mechanisms in old neurons. One of these antioxidant defenses is SOD2, whose activity is dramatically decreased in the old neurons and such a decrease was accompanied by a higher nitrosative stress. In agreement with the possibility that low antioxidant defenses may be one of the causes for higher Aβ production, primary hippocampal neurons derived from mice lacking SOD2 produced more Aβ42 than wt neurons. In addition we demonstrated that this change is γ-secretase-dependent, as the effect on Aβ ratio is maintained in a cell free assay using γ-secretase complex extracted from the cerebral cortex of old Sod2+/− mice. We also demonstrated that the increase of the Aβ42/Aβ40 ratio induced by SOD2 depletion is, at least in part, due to peroxynitrite. Consistent with our results, in vivo experiments carried out in an AD mouse model crossed with a Sod2 overexpressing mice, have shown that Sod2 over-expression reduces the number of amyloid plaques (Esposito et al, 2006). Other works also support the notion that SOD2 deficiency may cause abnormal Aβ load. Li et al (2004) showed that the brains of APP mutant Sod2+/− knockout mice have increased plaque burden and Massaad et al (2009) that overexpressing Sod2 in a transgenic mice model of AD prevents memory deficits and more interestingly, decreases the Aβ42/Aβ40 ratio. In further support, an association between a polymorphism in the SOD2 gene and certain cases of AD has been suggested (Wiener et al, 2007). In summary, our work confirms the strong association between SOD2 activity and changes in Aβ secretion which then paved the way to define the underlying mechanism.
Nitrosative stress has been considered as an etiopathogenic factor for AD (Coma et al, 2008; Guix et al, 2009; Tamagno et al, 2002). Firstly, it was shown that peroxynitrite modifies the tyrosines in the glycolytic enzyme triosephosphate isomerase, an event that triggers the formation of NFT, one of the hallmarks of the disease (Guix et al, 2009). Secondly, Kummer et al (2011) showed that nitration of the Tyr-10 of Aβ enhances its aggregation and plaque formation. Since a dramatic increase of nitrotyrosination occurs in old neurons, we reasoned that the increment of the nitrosative stress, as a consequence of a reduced SOD2 activity, could trigger the increase in the Aβ42/Aβ40 ratio. In the present work we have demonstrated that peroxynitrite itself is able to shift Aβ production, favouring the generation of Aβ42. Other oxidants (such as H2O2) have already been shown to increase APP cleavage by raising the expression of BACE-1 and, as a consequence, to enhance the production of both Aβ42 and Aβ40 (Coma et al, 2008; Shen et al, 2008; Tamagno et al, 2002; O'Connor et al, 2008). However, to date, no satisfactory mechanism has been provided to explain the increase in the Aβ42/Aβ40 ratio that occurs during ageing and in sporadic AD pathology and in the absence of any mutation or polymorphism in presenilin or APP genes. Our finding that peroxynitrite alters the ratio of Aβ42 to Aβ40 suggests the involvement of the γ-secretase complex, since its activity determines the length of the Aβ species generated. In fact kinetic studies carried out with enzyme nitrated in vitro demonstrated that nitration increases the production of Aβ42 without changing the levels of the Aβ40 peptide. The band corresponding to the quartet complex in the blue native gel electrophoresis gel showed a mildly slower electrophoretic mobility in old neurons, which suggests an internal structural change (Fig 2C). Indeed peroxynitrite treatment nitrotyrosinates and increases the interaction between the PS1-NTF and PS1-CTF fragments as assessed by immunoprecipitation, which was further confirmed in an independent FLIM analysis showing that nitrosative stress brings γ-secretase into a closer conformational state resembling that of complexes bearing FAD mutations. This conformational change has been related to the increase in the Aβ42/Aβ40 ratio (Berezovska et al, 2005; Uemura et al, 2009). We suggest therefore that the modification of γ-secretase by peroxynitrite induces a conformational change that mimics the effects of FAD mutations. We cannot rule out that peroxynitrite, apart from the effect on the γ-secretase, could also have an effect on the clearance of Aβ, which would hypothetically potentiate the effect we see.
In summary, our data supports the involvement of γ-secretase as a key link between ageing and AD. The activity of the complex switches towards the more amyloidogenic Aβ42 as the neurons get older. Our results points to the impairment of the mitochondrial SOD2 and the increase of nitrosative stress as major effectors of this molecular event. Therefore we conclude that nitrosative stress is an ethiopathogenic factor in AD, and gives an age-related mechanistic explanation for γ-secretase malfunction in absence of mutations.
MATERIALS AND METHODS
Cell cultures
HEK-swAPP cells (gift from Dr. C. Haass) were grown in Dulbecco's modification of Eagles medium (DMEM)/F12 medium supplemented with 10% foetal bovine serum (FBS) (Gibco BRL, Rockville, MD, USA), 1% penicillin and 1% streptomycin. The human neuroblastoma cell line SH-SY5Y overexpressing the wild type human APP (SH-SY5Y-wtAPP cells) were grown in DMEM medium supplemented with 15% FBS, 1% penicillin and 1% streptomycin. Primary cultures of rat hippocampal neurons were obtained from Wistar rat embryos at day 18–19 as described by Kaech and Banker (2006). The procedure was carried out in accordance with the Ethic Committee of K. Leuven University (Ethische Commissie Dierproeven, KULeuven). Briefly, hippocampi were aseptically dissected and trypsinized for 20 min. After centrifugation for 1 min, cells were seeded in phenol-red-free DMEM plus 10% horse serum into 0.1 mg/ml poly-l-lysine coated plates. After 120 min, medium was removed and minimal essential medium with N2 supplement (MEM-N2) (GIBCO N2-supplement, Invitrogen, Carlsbad, CA) was added containing streptomycin and penicillin. At day 5 the media was replaced by MEM containing B27 supplement (MEM-B27). Cultured hippocampal cells were used for the experiments on day 14, 21 or 28.
Sod2 knockout mice
The B6.129S7-Sod2tm1Leb mice were purchased from The Jackson Laboratory. They were kept in-bred in heterozygote condition. All the experiments were reviewed and approved by the Local Ethical Committee. Mice were genotyped by PCR using the followings primers for Sod2: 5′-CCGAGGAGAAGTACCACGAG-3′ and 5′-CTGTGTTTTCCGGGATAGGA-3′. Knockouts were identified with primers to detect the inserted gene Hprt: 5′-TGTTCTCCTCTTCCTCATCTCC-3′ and 5′-ACCCTTTCCAAATCCTCAGC-3′.
ELISA
For detection of rat Aβ, the media was probed with an ELISA kit from Wako (Japan). For detection of human Aβ, an in-house ELISA sandwich was carried out. Briefly, 96-wells Nunc-Immuno plates (Nunc, Denmark) were coated overnight at 4°C with JRF cAb040/28 antibody for Aβ40 or JRF Ab042/26 antibody for A42 (Janssen Pharmaceutica), both used at 1.5 mg/ml in phosphate buffered saline (PBS) containing 0.1% casein (Casein Buffer). Plates were washed five times with washing buffer (PBS-0.05% Tween 20) before the addition of the samples or the standard curve made with consecutive dilutions (from 100 to 0.0003 ng/ml) of Aβ40 or Aβ42 (rPeptide). After overnight incubation at 4°C and five time washes with the washing buffer, the samples were developed with a 0.02% tetramethylbenzidine (TMB) solution in sodium acetate (100 mM pH 4.9) containing 0.03% H2O2. The reaction was stopped with 0.2 N H2SO4 and read at 450 nm.
Infection of rat hippocampal neurons
Rat hippocampal neurons kept for 21 or 28 DIV were infected with an Adenoviral vector expressing the human APP-C99 triple tagged with a Flag peptide (AV-huAPP-C99-3xFlag) at a MOI of 100. After 48 h the medium was replaced by fresh MEM-B27 and kept for 24 h before processing of the media and the neurons for ELISA and Western blot, respectively.
Western blot
Total cell lysates were prepared in lysis buffer (50 mM HEPES pH 7.4, 150 mM NaCl, 1% NP40 (unless indicated otherwise) and complete protease inhibitors (Roche Applied Science)). Post-nuclear fractions were taken, and protein concentrations were determined using standard BCA assay (Pierce). Twenty micrograms of protein was separated on NuPAGE 4–12% Bis–Tris gels (Invitrogen) and transferred to nitrocellulose membranes for Western blot analysis. After blocking the membrane with 1% non-fat milk in T-TBS 1× (0.05 M Tris, pH 7.5; 0.15 M NaCl; 0.1% Tween20), the proteins were detected with the commercial antibodies MAB5232 anti-NTF-PS1 (1/5000; Chemicon International, Temecula, CA, USA), MAB1563 anti-CTF-PS1 (1/5000; Chemicon/Millipore Billerica, MA, USA), 9C3 anti-Nct (1/5000), monoclonal anti-nitrotyrosine antibody (1/1000; Abcam), monoclonal anti-SOD2 (1/1000; Abcam) 6E10 anti-Aβ and the following in-house made antibodies: B63 for APP (1/10,000), B80 for Aph1a (1/1000), B126 for Pen2 (1/2000) and 10B8 for BACE1. Signals were detected using the chemiluminescence detection with Renaissance (PerkinElmer Life Sciences). Quantifications were performed by densitometry.
Blue-native gel electrophoresis
Microsomal membrane fractions were prepared in lysis buffer containing 0.5% dodecyl maltoside, 20% glycerol and 25 mM Bis–Tris/HCl, pH 7. Upon ultracentrifugation (55,000 rpm), supernatant was taken; protein concentrations were measured, and 5 µg of protein was supplemented with 5× concentrated sample buffer (2.5% Coomassie Blue G-250, 100 mM Bis–Tris/HCl, 500 mM 6-aminocaproic acid, pH 7, 15% sucrose). Samples were loaded on a 5–16% polyacrylamide gel and run for 4 h at 4°C. Subsequently, the gel was incubated with 0.1% SDS in transfer buffer for 10 min at room temperature (RT) and transferred to a polyvinylidene difluoride membrane. Membranes were destained in water/methanol/acetic acid (60:30:10, v/v/v) and incubated with antibodies to detect the γ-secretase complex.
Peroxynitrite treatment of cells
Neurons seeded at 10,000 dish−1 were treated at DIV10 with 1, 5 and 10 µM of a peroxynitrite donor (3-morpholino-sydnonimine; SIN-1; Sigma, St Louis, MO, USA) for 24 h and the Aβ40 and Aβ42 was measured directly from the media by ELISA as discussed above. HEK-swAPP and SH-SY5Y-wtAPP cells were seeded on 6-well plate at a density of 500,000 cells/well. After 24 h the medium was replaced by fresh 0.2% FBS supplemented with DMEM for SH-SY5Y-wtAPP cells and DMEM/F12 for HEK-swAPP cells, with our without increasing concentrations of SIN-1. After 24 h Aβ species were analyzed by ELISA.
The paper explained
PROBLEM:
Despite the fact that AD is strongly associated with increased age, we still lack a molecular explanation to link ageing with any of the known pathways causing AD. AD is characterized by the accumulation of the Aβ and Tau protein in the brain. Aβ is produced from APP by consecutive cleavages. The proteases responsible for these activities have been called β-secretase and γ-secretase. Aβ is a heterogeneous mixture of Aβ peptides, the major ones being Aβ40 and the more aggregation prone Aβ42 which also exerts higher neurotoxicity. A low percentage of AD cases (<0.5%) are caused by inherited mutations in APP or the γ-secretase complex (FAD mutations), which have a common effect: a relative increase of the Aβ42 species versus the Aβ40 species. However the vast majority of patients with AD (late-onset AD) do not carry mutations in these genes, raising the question why they accumulate Aβ in the brain. We hypothesized that molecular changes associated with ageing could mimic the effect of the FAD mutations on γ-secretase, causing a relative increase of the Aβ42/Aβ40 ratio.
RESULTS:
We studied the changes in the Aβ profile occuring in rat hippocampal neurons during ageing in vitro. With ageing, the neurons secrete more Aβ into the media and this is accompanied by increased γ-secretase complex formation. More interestingly, neurons switch also the Aβ profile during ageing, increasing the relative amounts of Aβ42 versus the Aβ40 species. This is paralleled by the decrease of the antioxidant enzyme SOD2 activity and the increase of nitrosative stress. We identified nitrosative stress as a major effector of the increased Aβ42/Aβ40 ratio in old neurons through the modification of γ-secretase by peroxynitrite. These results were confirmed in a Sod2 knockout mice model and in human samples from AD cerebral cortex.
IMPACT:
Our study provides a molecular explanation to the important question how ageing predisposes to AD. More concretely, our findings show that the age-associated increase of nitrosative stress drives γ-secretase towards a switch of the Aβ profile favouring the Aβ42 species. This effect on the complex mimics the characteristic treat of FAD-mutations and sets the γ-secretase as a target not only for inherited but also for late-onset AD.
As a negative control for the co-immunoprecipitation experiments, HEK-swAPP cells seeded on 6-well plates containing 0.2% FBS DMEM/F12 medium were treated for 24 h with a nitric oxide donor (SNP; Sigma).
Immunohistofluorescence
Cerebral slides from 18 months wt or Sod2+/− mice were deparaffinizated with Clear Rite 3 and subsequent washes with decreasing ethanol dilutions. Slides were microwaved at 350 W in boiling Na-citrate buffer for 5 min. After 1 h of quenching endogenous peroxidase activity with a 3% H2O2 solution, slides were blocked for 1 h at RT with 5% normal goat serum in TNB blocking buffer (0.1 M Tris-HCl pH 7.5, 0.15 M NaCl, 0.5% Blocking Reagent). The same solution was used to incubate the samples overnight at 4°C with the mouse anti-nitrotyrosine primary antibody (1:50). The goat anti-mouse-HRP secondary antibody was diluted (1:200) in TNB blocking solution without serum. Slides were developed by 6-min incubation with the Tyr-FITC reagent at darkness (1:50 in 1× reagent diluent). The Tyramide Signal Amplification kit (Perkin-Elmer) was used following the instructions given by the company. Briefly, all the washes were performed with PBS-T 1× buffer. DAPI (1:5000) was used to detect the nuclei and the slices were mounted with Mowiol solution.
Fluorescence lifetime imaging microscopy, FLIM
HEK293 were maintained in Opti-MEM (Invitrogen) with 5% foetal bovine serum (FBS). For the FLIM analysis of PS1 conformation cells were plated into eight-chamber slides and transfected with the GFP-PS1 or GFP-PS1-RFP (G-PS1-R) FRET reporter probe using Lipofectamine 2000 reagent (Invitrogen). FLIM analysis was performed as previously described (Uemura et al, 2009). GraphPad Prism for Windows, version 5.03 (GraphPad Software, Inc.) was used to perform statistical analysis using two-tailed unpaired Student's t-test. Samples were considered significantly different at p < 0.05.
Immunoprecipitation
Five hundred micrograms of total solubilized protein (50 mM HEPES pH 7.4, 150 mM NaCl and 0.5% Triton-X-100, unless a distinct concentration is indicated for the experiment) from HEK-swAPP lysates or human or mice cerebral cortex were incubated overnight at 4°C with 1.25 µg of MAB5232 anti-NTF-PS1 (Chemicon International, Temecula, CA, USA) or MAB1563 anti-CTF-PS1 (Chemicon/Millipore Billerica, MA, USA) monoclonal antibodies. Following protein G-sepharose addition, samples were shaken for 2 h at RT. Aggregates were collected by centrifugation at 10,000 rpm for 10 min and washed five times. Protein G and Abs were removed from the immunoprecipitated proteins by boiling the samples for 6 min at 100°C.
SOD2 activity
The SOD-560 colorimetric assay kit (Applied Bioanalytical Labs, USA) was used to determine the activity of SOD2 in lysates from rat hippocampal neurons according to the instructions of the manufacturer. Ten micrograms of total protein from 21 or 28 DIV neurons was used in each assay. In a 96-well plate, 10 µl of assay buffer (blank) or sample previously diluted with sample dilution buffer were added to 230 µl of Assay Buffer containing 2 mM KCN to inhibit SOD1. After 2 min incubation at 25°C, 10 µl haematoxylin was added per well and its auto-oxidation rate was recorded by absorbance at 560 nm for 10 min at 25°C. The SOD concentration in the sample was calculated as follows: Ci(SOD2 U/ml) = 125 × (100%-ri), being Ci the SOD2 concentration of the sample and ri the ratio between auto-oxidation rate of the sample versus the auto-oxidation rate of blank.
Statistical analysis
Data are expressed as the mean ± SEM of the values from the number of experiments as indicated in the corresponding figures. Data were evaluated statistically by using the Student's t-test.
For more detailed Materials and Methods see the Supporting Information.
Acknowledgments
This work was made possible by grants from the Fund for Scientific Research, Flanders; the K.U.Leuven; the VIB, Methusalem (K.U.Leuven and the Flemisch government), the Foundation for Alzheimer Research (SAO/FRMA), the European Research council (BDS), NIH AG15379 (OB), Spanish Ministry of Science and Innovation SAF 2010-14906, Consolider 2010-00045 (CGD), Spanish Ministery of Health (Fondo de Investigación Sanitaria-PI10/00587 and Red HERACLES RD06/0009); The European FEDER Fundings; and Fundació La Marató de TV3 (Catalonia; Spain; no. 100310). We would like to acknowledge the Banc de Teixits Neurologics de l' Hospital Clinic de Barcelona and the Unidad de Neuropatología y Banco de Cerebros of Fundación Hospital Alcorcón for providing the brain samples. BDS is the Arthur Bax and Anna Vanluffelen chair for AD. FG obtained a IEF fellowship of the Marie Curie Actions program in FP7 and a Beatriu de Pinos grant of the Generalitat de Catalunya, Spain. TW was supported by EMBO and DFG long-term fellowships. We thank Miguel A. Valverde for his contribution at the discussion.
While this work was under review a related manuscript was published. (Oxidative lipid modification of nicastrin enhances amyloidogenic gsecretase activity in Alzheimer's disease, Gwon et al, Ageing Cell, in press.)
Supporting Information is available at EMBO Molecular Medicine Online.
The authors declare that they have no conflict of interest.
Author contributions
FXG, BDS and CGD designed and wrote the manuscript. TW and KV did the experiments concerning the ageing of hippocampal neurons. AS did the immunostainings. GIR, LCG, ERF and FJM assisted the experiments and provided inputs to the work. CGL and AL determined the endogenous AICD production under nitrosative stress. MA and OB did the FLIM analysis of γ-secretase under nitrosative stress. All authors have read the manuscript and provided inputs.
Supplementary material
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
References
- Aksenova MV, Aksenov MY, Markesbery WR, Butterfield DA. Aging in a dish: age-dependent changes of neuronal survival, protein oxidation, and creatine kinase BB expression in long-term hippocampal cell culture. J Neurosci Res. 1999;58:308–317. [PubMed] [Google Scholar]
- Berezovska O, Lleo A, Herl LD, Frosch MP, Stern EA, Bacskai BJ, Hyman BT. Familial Alzheimer's disease presenilin 1 mutations cause alterations in the conformation of presenilin and intarections with amyloid precursor protein. J Neurosci. 2005;25:3009–3017. doi: 10.1523/JNEUROSCI.0364-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Howard B, Yatin S, Koppal T, Drake J, Hensley K, Aksenov M, Aksenova M, Subramaniam R, Varadarajan S, et al. Elevated oxidative stress in models of normal brain aging and Alzheimer's disease. Life Sci. 1999;65:1883–1892. doi: 10.1016/s0024-3205(99)00442-7. [DOI] [PubMed] [Google Scholar]
- Cardozo-Pelaez F, Song S, Parthasarathy A, Hazzi C, Naidu K, Sanchez-Ramos J. Oxidative DNA damage in the aging mouse brain. Mov Disord. 1999;14:972–980. doi: 10.1002/1531-8257(199911)14:6<972::aid-mds1010>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Chavez-Gutierrez L, Bammens L, Benilova I, Vandersteen A, Benurwar M, Borgers M, Lismont S, Zhou L, Van Cleynenbreugel S, Esselmann H, et al. The mechanism of γ-Secretase dysfunction in familial Alzheimer disease. EMBO J. 2012 doi: 10.1038/emboj.2012.79. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Vetrivel KS, Drisdel RC, Meckler X, Gong P, Leem JY, Li T, Carter M, Chen Y, Nguyen P, et al. S-palmitoylation of gamma-secretase subunits nicastrin and APH-1. J Biol Chem. 2009;284:1373–1384. doi: 10.1074/jbc.M806380200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coma M, Guix FX, Ill-Raga G, Uribesalgo I, Alameda F, Valverde MA, Muñoz FJ. Oxidative stress triggers the amyloidogenic pathway in human vascular smooth muscle cells. Neurobiol Aging. 2008;29:969–980. doi: 10.1016/j.neurobiolaging.2007.01.009. [DOI] [PubMed] [Google Scholar]
- De Strooper B. Aph-1, Pen-2, and nicastrin with presenilin generate an active gamma-secretase complex. Neuron. 2003;38:9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- De Strooper B. Loss-of-function presenilin mutations in Alzheimer disease. Talking point on the role of presenilin mutations in Alzheimer disease. EMBO Rep. 2007;8:141–146. doi: 10.1038/sj.embor.7400897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B. Proteases and proteolysis in Alzheimer disease: a multifactorial view on the disease process. Physiol Rev. 2010;90:465–494. doi: 10.1152/physrev.00023.2009. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- Esposito L, Raber J, Kekonius L, Yan F, Yu GQ, Bien-Ly N, Puoliväli J, Scearce-Levie K, Masliah E, Mucke L. Reduction in mitochondrial superoxide dismutase modulates Alzheimer's disease-like pathology and accelerates the onset of behavioral changes in human amyloid precursor protein transgenic mice. J Neurosci. 2006;26:5167–5179. doi: 10.1523/JNEUROSCI.0482-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Fukui M, Zhu BT. Mitochondrial superoxide dismutase SOD2, but not cytosolic SOD1, plays a critical role in protection against glutamate-induced oxidative stress and cell death in HT22 neuronal cells. Free Radic Biol Med. 2010;48:821–830. doi: 10.1016/j.freeradbiomed.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funato H, Yoshimura M, Kusui K, Tamaoka A, Ishikawa K, Ohkoshi N, Namekata K, Okeda R, Ihara Y. Quantitation of amyloid beta-protein (A beta) in the cortex during aging and in Alzheimer's disease. Am J Pathol. 1998;152:1633–1640. [PMC free article] [PubMed] [Google Scholar]
- Guix FX, Uribesalgo I, Coma M, Muñoz FJ. The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol. 2005;76:126–152. doi: 10.1016/j.pneurobio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Guix FX, Ill-Raga G, Bravo R, Nakaya T, de Fabritiis G, Coma M, Miscione GP, Villà-Freixa J, Suzuki T, Fernàndez-Busquets X, et al. Amyloid-dependent triosephosphate isomerase nitrotyrosination induces glycation and tau fibrillation. Brain. 2009;132:1335–1345. doi: 10.1093/brain/awp023. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:2209. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hooper DC, Spitsin S, Kean RB, Champion JM, Dickson GM, Chaudhry I, Koprowski H. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci USA. 1998;95:675–680. doi: 10.1073/pnas.95.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper DC, Scott GS, Zborek A, Mikheeva T, Kean RB, Koprowski H, Spitsin SV. Uric acid, a peroxynitrite scavenger, inhibits CNS inflammation, blood-CNS barrier permeability changes, and tissue damage in a mouse model of multiple sclerosis. FASEB J. 2000;14:691–698. doi: 10.1096/fasebj.14.5.691. [DOI] [PubMed] [Google Scholar]
- Kaech S, Banker G. Culturing hippocampal neurons. Nat Protoc. 2006;1:2406–2415. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- Kern A, Behl C. The unsolved relationship of brain aging and late-onset Alzheimer disease. Biochim Biophys Acta. 2009;1790:1124–1132. doi: 10.1016/j.bbagen.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Kummer MP, Hermes M, Delekarte A, Hammerschmidt T, Kumar S, Terwel D, Walter J, Pape HC, König S, Roeber S, et al. Nitration of tyrosine 10 critically enhances amyloid β aggregation and plaque formation. Neuron. 2011;71:833–844. doi: 10.1016/j.neuron.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Kuperstein I, Broersen K, Benilova I, Rozenski J, Jonckheere W, Debulpaep M, Vandersteen A, Segers-Nolten I, Van Der Werf K, Subramaniam V, et al. Neurotoxicity of Alzheimer's disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO J. 2010;29:3408–3420. doi: 10.1038/emboj.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Calingasan NY, Yu F, Mauck WM, Toidze M, Almeida CG, Takahashi RH, Carlson GA, Flint Beal M, Lin MT, et al. Increased plaque burden in brains of APP mutant MnSOD heterozygous knockout mice. J Neurochem. 2004;89:1308–1312. doi: 10.1111/j.1471-4159.2004.02455.x. [DOI] [PubMed] [Google Scholar]
- Marlow L, Canet RM, Haugabook SJ, Hardy JA, Lahiri DK, Sambamurti K. APH1, PEN2, and nicastrin increase Abeta levels and gamma-secretase activity. Biochem Biophys Res Commun. 2003;305:502–509. doi: 10.1016/s0006-291x(03)00797-6. [DOI] [PubMed] [Google Scholar]
- Martin MG, Perga S, Trovò L, Rasola A, Holm P, Rantamäki T, Harkany T, Castrén E, Chiara F, Dotti CG. Cholesterol loss enhances TrkB signaling in hippocampal neurons aging in vitro. Mol Biol Cell. 2008;19:2101–2112. doi: 10.1091/mbc.E07-09-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Dotti CG, Ledesma MD. Brain cholesterol in normal and pathological aging. Biochim Biophys Acta. 2010;1801:934–944. doi: 10.1016/j.bbalip.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Massaad CA, Washington TM, Pautler RG, Klann E. Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2009;106:13576–13581. doi: 10.1073/pnas.0902714106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor T, Sadleir KR, Maus E, Velliquette RA, Zhao J, Cole SL, Eimer WA, Hitt B, Bembinster LA, Lammich S, et al. Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis. Neuron. 2008;60:988–1009. doi: 10.1016/j.neuron.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardossi-Piquard R, Yang SP, Kanemoto S, Gu Y, Chen F, Böhm C, Sevalle J, Li T, Wong PC, Checler F, et al. APH1 polar transmembrane residues regulate the assembly and activity of presenilin complexes. J Biol Chem. 2009;284:16298–16307. doi: 10.1074/jbc.M109.000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikkarainen M, Kauppinen T, Alafuzoff I. Hyperphosphorylated tau in the occipital cortex in aged nondemented subjects. J Neuropathol Exp Neurol. 2009;68:653–660. doi: 10.1097/NEN.0b013e3181a6ee45. [DOI] [PubMed] [Google Scholar]
- Porter NM, Thibault O, Thibault V, Chen KC, Landfield PW. Calcium channel density and hippocampal cell death with age in long-term culture. J Neurosci. 1997;17:5629–5639. doi: 10.1523/JNEUROSCI.17-14-05629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz-Baez R, Rojas E, Arias C. Oxidative stress promotes JNK-dependent amyloidogenic processing of normally expressed human APP by differential modification of alpha-, beta- and gamma-secretase expression. Neurochem Int. 2009;55:662–670. doi: 10.1016/j.neuint.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002;33:1451–1464. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- Saito T, Suemoto T, Brouwers N, Sleegers K, Funamoto S, Mihira N, Matsuba Y, Yamada K, Nilsson P, Takano J, et al. Potent amyloidogenicity and pathogenicity of Aβ43. Nat Neurosci. 2011;14:1023–1032. doi: 10.1038/nn.2858. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, et al. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Scott GS, Cuzzocrea S, Genovese T, Koprowski H, Hooper DC. Uric acid protects against secondary damage after spinal cord injury. Proc Natl Acad Sci USA. 2005;102:3483–3488. doi: 10.1073/pnas.0500307102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serneels L, Van Biervliet J, Craessaerts K, Dejaegere T, Horré K, Van Houtvin T, Esselmann H, Paul S, Schäfer MK, Berezovska O. g-Secretase heterogeneity in the Aph1 subunit: relevance for Alzheimer's disease. Science. 2009;324:639–642. doi: 10.1126/science.1171176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Chen Y, Liu H, Zhang K, Zhang T, Lin A, Jing N. Hydrogen peroxide promotes Abeta production through JNK-dependent activation of gamma-secretase. J Biol Chem. 2008;283:17721–17730. doi: 10.1074/jbc.M800013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G. Widespread peroxynitrite-mediated damage in Alzheimer's disease. J Neurosci. 1997;17:2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodero AO, Trovò L, Iannilli F, Van Veldhoven P, Dotti CG, Martin MG. Regulation of tyrosine kinase B activity by the Cyp46/cholesterol loss pathway in mature hippocampal neurons: relevance for neuronal survival under stress and in aging. J Neurochem. 2011;116:747–755. doi: 10.1111/j.1471-4159.2010.07079.x. [DOI] [PubMed] [Google Scholar]
- Spasic D, Raemaekers T, Dillen K, Declerck I, Baert V, Serneels L, Füllekrug J, Annaert W. Rer1p competes with APH-1 for binding to nicastrin and regulates gamma-secretase complex assembly in the early secretory pathway. J Cell Biol. 2007;176:629–640. doi: 10.1083/jcb.200609180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkov AA. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann N Y Acad Sci. 2008;1147:37–52. doi: 10.1196/annals.1427.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Bardini P, Obbili A, Vitali A, Borghi R, Zaccheo D, Pronzato MA, Danni O, Smith MA, Perry G, et al. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis. 2002;10:279–288. doi: 10.1006/nbdi.2002.0515. [DOI] [PubMed] [Google Scholar]
- Thinakaran G, Borchelt DR, Lee MK, Slunt HH, Spitzer L, Kim G, Ratovitsky T, Davenport F, Nordstedt C, Seeger M, et al. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996;17:181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- Tomobe K, Nomura Y. Neurochemistry, neuropathology, and heredity in SAMP8: a mouse model of senescence. Neurochem Res. 2009;34:660–669. doi: 10.1007/s11064-009-9923-x. [DOI] [PubMed] [Google Scholar]
- Tran MH, Yamada K, Nakajima A, Mizuno M, He J, Kamei H, Nabeshima T. Tyrosine nitration of a synaptic protein synaptophysin contributes to amyloid beta-peptide-induced cholinergic dysfunction. Mol Psychiatry. 2003;8:407–412. doi: 10.1038/sj.mp.4001240. [DOI] [PubMed] [Google Scholar]
- Trovò L, Van Veldhoven PP, Martín MG, Dotti CG. Sphingomyelin upregulation in mature neurons contributes to TrkB activity by Rac1 endocytosis. J Cell Sci. 2011;124:1308–1315. doi: 10.1242/jcs.078766. [DOI] [PubMed] [Google Scholar]
- Uemura K, Lill CM, Li X, Peters JA, Ivanov A, Fan Z, DeStrooper B, Bacskai BJ, Hyman BT, Berezovska O. Allosteric modulation of PS1/gamma-secretase conformation correlates with amyloid beta(42/40) ratio. PLoS One. 2009;4:e7893. doi: 10.1371/journal.pone.0007893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener HW, Perry RT, Chen Z, Harrell LE, Go RC. A polymorphism in SOD2 is associated with development of Alzheimer's disease. Genes Brain Behav. 2007;6:770–775. doi: 10.1111/j.1601-183X.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- Wolfe MS. When loss is gain: reduced presenilin proteolytic function leads to increased Abeta42/Abeta40. Talking point on the role of presenilin mutations in Alzheimer disease. EMBO Rep. 2007;8:136–140. doi: 10.1038/sj.embor.7400896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- Zlokovic B. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.