Abstract
Pigmentation is a model trait for evolutionary and developmental analysis that is particularly amenable to molecular investigation in the genus Drosophila. To better understand how this phenotype evolves, we examined divergent pigmentation and gene expression over developmental time in the dark-bodied Drosophila americana and its light-bodied sister species Drosophila novamexicana. Prior genetic analysis implicated two enzyme-encoding genes, tan and ebony, in pigmentation divergence between these species, but questions remain about the underlying molecular mechanisms. Here, we describe stages of pupal development in both species and use this staging to determine when pigmentation develops and diverges between D. americana and D. novamexicana. For the developmental stages encompassing pigment divergence, we compare mRNA expression of tan and ebony over time and between species. Finally, we use allele-specific expression assays to determine whether interspecific differences in mRNA abundance have a cis-regulatory basis and find evidence of cis-regulatory divergence for both tan and ebony. cis-regulatory divergence affecting tan had a small effect on mRNA abundance and was limited to a few developmental stages, yet previous data suggests that this divergence is likely to be biologically meaningful. Our study suggests that small and developmentally transient expression changes may contribute to phenotypic diversification more often than commonly appreciated. Recognizing the potential phenotypic impact of such changes is important for a scientific community increasingly focused on dissecting quantitative variation, but detecting these types of changes will be a major challenge to elucidating the molecular basis of complex traits.
INTRODUCTION
Pigmentation is a classic model for investigating the links between gene sequence and morphology (e.g. McClintock 1967; Levis et al. 1985; Martin and Gerats 1993; Wittkopp et al. 2002; Linnen et al. 2009; Cooley et al. 2011), in part because it is one of the most variable traits within and between species. It is also easy to visualize and provides a direct readout of the activity level of the underlying pigment biosynthetic pathways. Finally, the genes comprising pigmentation pathways in invertebrates, vertebrates, and plants are well characterized (Clegg and Durbin 2003; True 2003; Hoekstra 2006; Cazzonelli and Pogson 2010), further contributing to the emergence of pigmentation as one of the premier systems for studying the evolution of development (Kopp 2009).
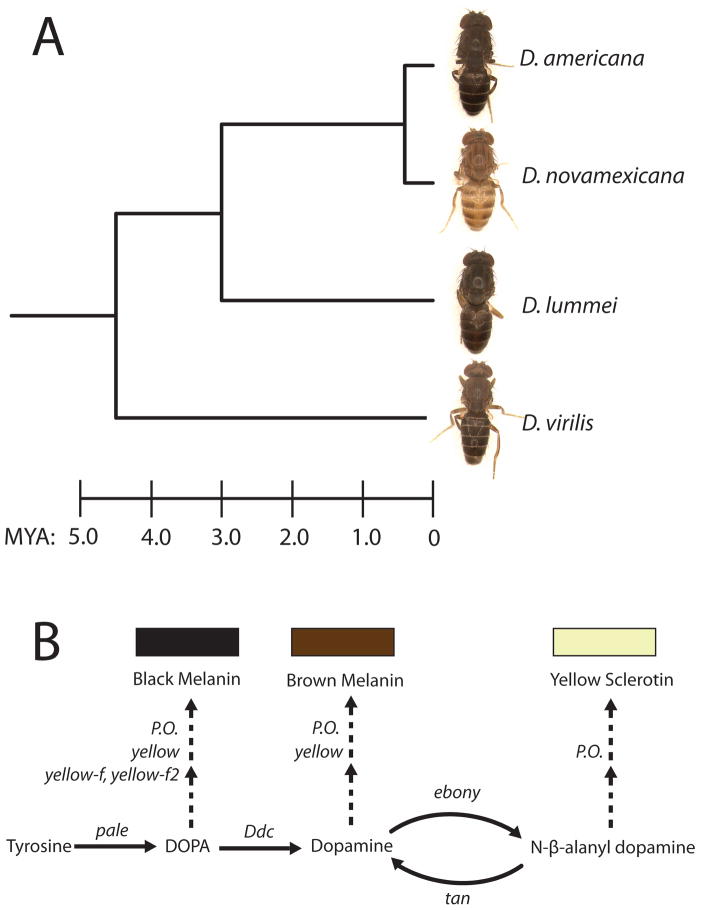
The genus Drosophila, which combines the advantages of a well-developed model system with substantial phenotypic diversity, has been a particular focus of pigmentation studies (Waddington 1942; Levis, Hazelrigg, and Rubin 1985; Hollocher et al. 2000; Dombeck and Jaenike 2004; Gompel et al. 2005; Jeong et al. 2006; Rebeiz et al. 2009; Telonis-Scott et al. 2011). Pigmentation variation in Drosophila includes changes in overall body color as well as changes in melanin patterns specific to the abdomen, wing, and thorax (reviewed in Wittkopp, Carroll et al. 2003). Here, we focus on a difference in overall body pigmentation between D. novamexicana and D. americana, members of the virilis species group. D. novamexicana has a derived light-colored phenotype that is both striking and recent (Fig. 1A). It is estimated to have evolved from the dark body color seen in its sister species, D. americana, during the last 0.4 million years (Caletka and McAllister 2004). These two species appear to have allopatric distributions with the dark-bodied D. americana found east of the Rocky Mountains and the light-bodied D. novamexicana found in sparse populations confined to the southwestern desert of the United States (Throckmorton 1982).
Figure 1. Pigmentation in the virilis group of Drosophila.
(A) The light body color of D. novamexicana is recently evolved and dramatically different from pigmentation in the other members of the virilis group. Estimated divergence times (MYA = millions of years ago), from Caletka and McAlllister (2004), are shown on the scale at the bottom. (B) A simplified depiction of the melanin and sclerotin biosynthesis pathway. Gene(s) controlling each enzymatic step are shown in italics. P.O., phenol oxidase-encoding genes. Arrows indicate chemical reactions, and branches with two consecutive dashed arrows indicate multiple enzymatic steps that are not completely known.
Prior genetic analysis has shown that changes linked to the pigmentation genes tan and ebony explain 87% of the body color difference between D. americana and D. novamexicana (Wittkopp et al. 2009). Interestingly, alleles contributing to this interspecific divergence also contribute to a longitudinal pigmentation cline in D. americana that is apparently maintained by natural selection (Wittkopp et al. 2009; Wittkopp et al. 2011). Biochemically and phenotypically, tan and ebony have opposite effects on pigmentation (Fig. 1B): the Ebony protein promotes the accumulation of light yellow sclerotin by catalyzing the conversion of dopamine into N-β-alanyl-dopamine (NBAD), while Tan promotes the accumulation of dark brown melanin via the reverse biochemical reaction, converting NBAD to dopamine (Wittkopp, Carroll, and Kopp 2003). Consistent with these functions, Ebony protein was reported to be more abundant in the lightly pigmented D. novamexicana (Wittkopp, Williams et al. 2003) whereas tan mRNA was reported to be slightly more abundant in the darkly pigmented D. americana (Wittkopp et al. 2009).
Expression differences between species can result from changes in either cis- or trans-acting sequences. The fact that genetic changes contributing to pigmentation divergence were found to be linked to tan and ebony suggests that the observed interspecific expression differences for these genes might be caused by divergent cis-regulatory sequences, although this has yet to be demonstrated. Consistent with this hypothesis, fine-scale genetic mapping has shown that noncoding changes in the 5′UTR and/or first intron of tan (gene regions that often harbor cis-regulatory elements in Drosophila) contribute to pigmentation divergence between D. americana and D. novamexicana (Wittkopp et al. 2009).
Here, we take a closer look at how pigment patterns and expression of pigmentation genes develop during the late pupal stages of D. americana and D. novamexicana. Specifically, we answer the following three questions: (1) How and when does pigment development visibly diverge between D. americana and D. novamexicana? (2) How are the pigmentation genes tan and ebony expressed in each species during this developmental window of time? And, (3) at each developmental stage examined, are differences in tan or ebony expression between species due to divergent cis-regulation? Answers to these questions provide a more comprehensive and integrated understanding of the mechanisms underlying pigmentation divergence. They also illustrate how tests for cis-regulatory divergence are influenced by the changing cellular environment over developmental time and suggest that small changes in gene regulation can have significant phenotypic consequences.
RESULTS
Pupal development of D. americana and D. novamexicana
In order to compare pigmentation development between species, we generated a chronology for the sequential appearance of developmental features in D. americana and D. novamexicana. The definition of discrete developmental stages, each marked by the appearance of one or more morphological features, is an important tool within the melanogaster group (e.g. Ashburner 1989; Warren et al. 2006; Ninov and Martin-Blanco 2007), but has never been established for any species in the virilis group. We timed the appearance of stage-defining morphological features (Table S1) in D. americana and D. novamexicana, with identically aged Drosophila melanogaster as a control, initiating data collection when the first pupae in the sample reached pupal stage P8 and continuing through adult stage A2.
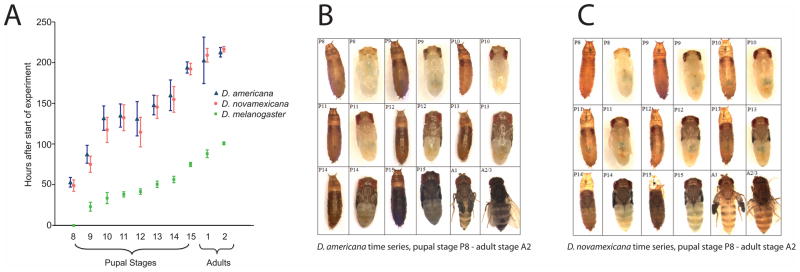
D. americana reached the A2 developmental stage 208.5 ± 5.7 hours (mean ± 95% CI) after the start of the experiment. D. novamexicana took a similar amount of time (212.1 ± 3.3 hours; Fig. 2A). Consistent with qualitative observations of the closely related species D. virilis (Markow and O’Grady 2005), these developmental times are more than twice that in D. melanogaster (98.9 ± 1.7 hours between start of experiment and occurrence of stage A2). Two-sample, two-sided t-tests showed that the time taken to reach stage A2 is significantly different between D. melanogaster and D. americana (P<0.0001) and between D. melanogaster and D. novamexicana (P<0.0001), but not between D. americana and D. novamexicana (P=0.287).
Figure 2. Developmental stages P13 to A1 encompass most of the visible accumulation of abdominal pigmentation in D. americana and D. novamexicana.
(A) D. americana and D. novamexicana develop more slowly than D. melanogaster. Beginning at stage P8 of D. melanogaster, samples of five developing flies per species were collected at regular time points, and their developmental stages were determined using morphological markers described by Bainbridge and Bownes 1981 (Table S1). The data were used to calculate the mean value (hours after initiation of P8 in D. melanogaster) of each developmental stage. Error bars indicate 95% confidence intervals. (B, C) Pigment accumulation differs between species in the late pupal and early adult stages. Photographs of D. americana (B) and D. novamexicana (C) were taken at pupal stages P8–P15 and adult stages A1–A2. For the eight pupal stages, flies were photographed both inside and outside of the pupal case (left and right photo, respectively, of each photo pair). Because the initial photos (taken for an earlier, unpublished project; L. Shefner, undergraduate senior thesis) showed an unusually dark stage P13 D. americana fly, we re-took the photos for that as well as the two surrounding stages (P12 and P14).
D. melanogaster accumulates melanic pigment during the latter half of pupal development (Walter et al. 1991), followed by yellow-pigmented sclerotization (Wright 1987). Similarly, visible abdominal pigmentation differences between D. americana and D. novamexicana accumulated between developmental stages P13 and A1 (Fig. 2B and 2C). The developing flies became darker in stages P13–P15, presumably reflecting melanization. They became more yellow at stage A1, implying the deposition of yellow sclerotin. This two-step process suggested that tan, which is necessary for melanization, might be transcriptionally active earlier in development than ebony, which is required for the production of yellow sclerotin. We tested this hypothesis by measuring changes in transcript abundance over developmental time for both genes.
Developmental profiles of tan and ebony differ over time and between species
To better understand the molecular changes underlying pigmentation divergence, we compared mRNA abundance of the tan and ebony genes between D. americana and D. novamexicana during the late pupal stages when the adult pigmentation differences are developing. mRNA levels were quantified using pyrosequencing, which can reliably detect expression differences as small as 10% between genotypes (Wittkopp et al. 2008). Because pyrosequencing directly measures the relative frequency of two alleles, we combined A1 stage flies from one species with flies from each pupal stage of interest from the other species (Fig. 3A). These A1 flies served as an internal reference that allowed us to compare expression within and between each species across developmental time, as described in the Methods.
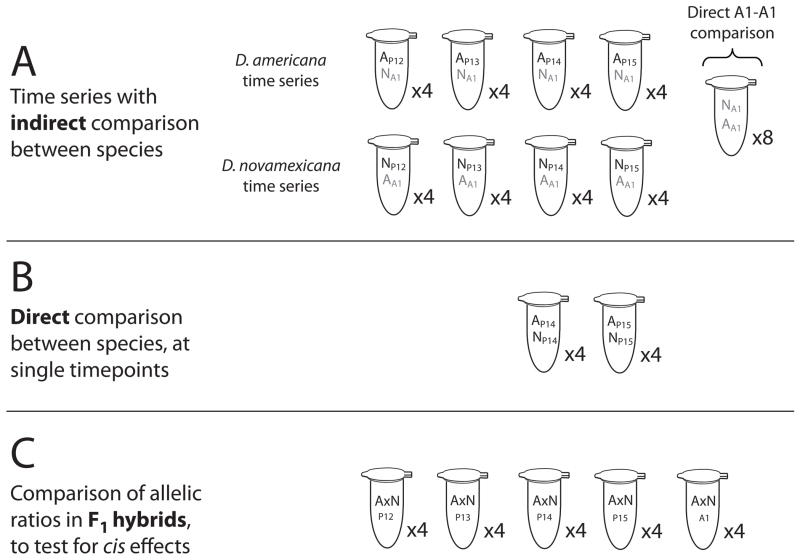
Figure 3. Experimental designs for pyrosequencing assays of mRNA expression.
Each tube represents a collection of six flies (three D. americana and three D. novamexicana, or six F1 hybrids). Four biological replicates were collected for each stage, with technical replicates obtained for each biological replicate as described in the Methods. DNA and RNA were extracted from each tube; RNA was converted to cDNA; and the relative abundance of D. americana and D. novamexicana alleles of tan and ebony in each tube was measured using pyrosequencing. (A) To measure changes in gene expression across time, each stage of D. americana (black font) was combined with D. novamexicana stage A1 reference flies (grey font). Conversely, each stage of D. novamexicana was compared to D. americana stage A1 reference flies. To enable an indirect comparison between the species, the ratio of the two references (D. novamexicana stage A1 and D. americana stage A1) was used as a correction factor for the D. americana time series, as described in the Methods. (B) Expression differences at stages P14 and P15 were further investigated using a direct comparison, of D. americana and D. novamexicana flies from the same stage. (C) D. americana x D. novamexicana F1 hybrids, collected at each of the five focal developmental stages, were used to determine whether the two alleles of each gene are still differentially expressed when they share a common trans-regulatory environment.
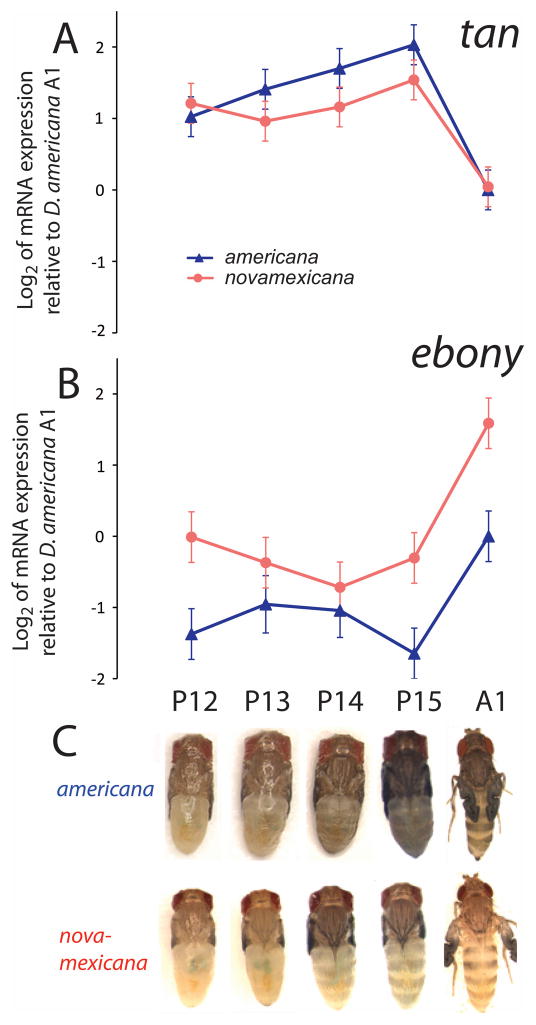
For tan, the P13 – P15 stages in which melanin visibly accumulated were marked by maximal gene expression in D. americana and relatively high gene expression in D. novamexicana (Fig. 4A, C). For ebony, expression increased dramatically at A1, coincident with the visible appearance of yellow sclerotin (Fig. 4B, C). Expression differences between the species were observed for both tan and ebony, in directions consistent with each gene’s biological function and previously published comparisons at single developmental time points (Wittkopp, Williams et al. 2003; Wittkopp et al. 2009). ebony expression was approximately two-fold higher in D. novamexicana than D. americana at each developmental time point (Fig. 4B; Table S2), whereas tan expression was generally higher in the dark-colored D. americana than in the light-colored D. novamexicana. The expression difference for tan was significant only at pupal stages P14–P15, with a marginally significant difference at stage P13 (Fig. 4A; Table S2).
Figure 4. Total mRNA expression of tan and ebony differs over developmental time and between species.
The log2 of mRNA abundance at tan (A) and ebony (B) are shown for developmental stages P12 - A1 (C). Each point represents the mean of four log2-transformed biological replicates of either D. americana (blue) or D. novamexicana (red) mRNA expression, relative to D. americana stage A1. D.americana stage A1, the internal reference point, was arbitrarily assigned a log2 value of zero for both genes. Error bars are the experimentwise 95% confidence intervals, calculated separately for tan and ebony.
To obtain more precise estimates of the relative mRNA abundance between species, we performed direct comparisons by combining D. americana and D. novamexicana flies at the same developmental stage, for the stages at which tan and ebony are highly expressed (Fig. 3B). This meant creating new pools of flies for stages P14 and P15, which showed the highest expression for tan. The A1 stage, which had the highest expression for ebony, was already directly measured as part of our initial experimental design (Fig. 3A). Relative to the indirect estimates obtained from the temporal experiment described above, the direct comparison revealed somewhat larger expression differences between the species (Fig. S1). Again, ebony was more highly expressed in D. novamexicana while tan was more highly expressed in D. americana. The direct-comparison data (stages P14, P15, and A1) revealed significant interspecific expression differences for ebony at all three stages, and for tan at stages P14 and P15, but not A1 (Table S2).
cis-regulatory variation contributes to divergent expression of both tan and ebony
Variation in gene expression can arise from differences in cis- or trans-regulation. In order to test whether cis-regulatory divergence contributes to the expression differences observed between D. americana and D. novamexicana, we quantified the relative mRNA abundance of the two alleles for each gene in a D. americana x D. novamexicana F1 hybrid at developmental stages P12 - A1 (Fig. 3C). Regulatory differences that act in cis cause allele-specific expression, whereas regulatory differences that act in trans affect expression of both alleles in a diploid cell. These two types of changes can therefore be differentiated by comparing allele-specific expression differences in an interspecific hybrid (Cowles et al. 2002; Wittkopp et al. 2004). Because both alleles in the hybrid are exposed to the same set of transcription factors, expression differences between the alleles are inferred to result from differences in cis-regulatory sequences.
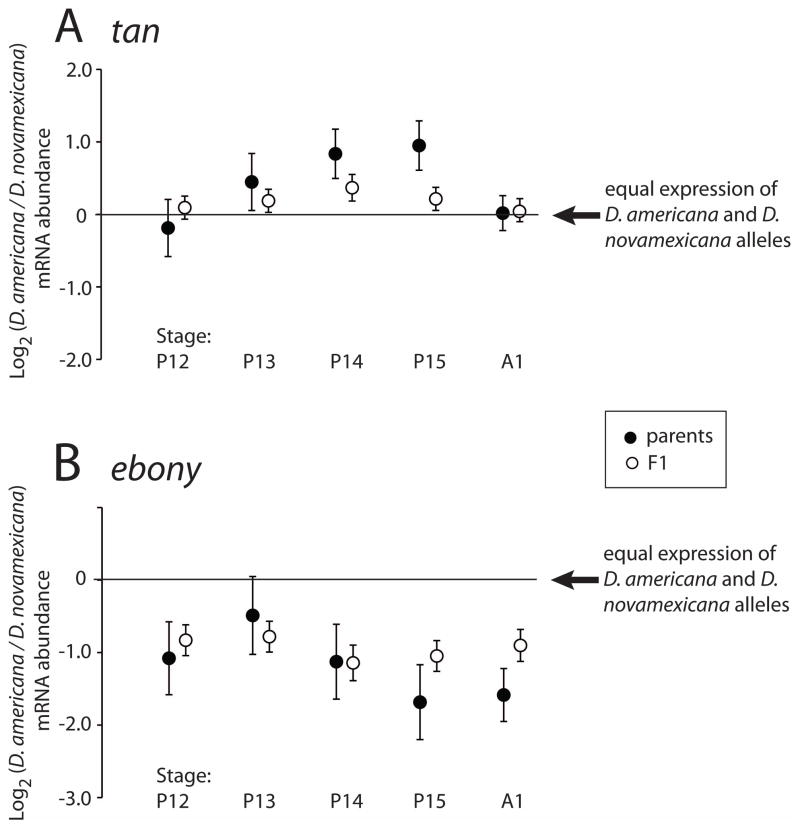
In the F1 hybrids, the D. americana allele of tan was expressed more highly than the D. novamexicana allele (Fig. 5A). This difference was nominally significant (p < 0.05) for stages P13 – P15, suggesting differences in cis-regulatory activity, although only stage P14 survived a (conservative) Bonferroni correction for multiple testing (Table S2). For ebony, the D. americana allele was expressed approximately 2-fold lower than the D. novamexicana allele, at each developmental stage (Fig. 5B). The cis-regulatory contribution to the expression differences between the two species was significant at each developmental stage (Table S2). Divergent cis-regulation explained the majority of the interspecific expression difference for ebony, but less than half of the interspecific expression difference at tan (Fig. 5). This suggests that trans-regulatory changes also contribute to expression divergence between D. americana and D. novamexicana, especially for tan.
Figure 5. Species- and allele-specific differences in tan and ebony mRNA expression over developmental time.
The log2 of mRNA abundance at tan (A) and ebony (B) are shown for developmental stages P12 - A1 (x-axis). Filled circles represent the ratio of D. americana to D. novamexicana gene expression between the species (“parental data”). Open circles represent the ratio of expression of the two alleles within F1 hybrids. A value of 0 corresponds to no expression difference between the two alleles; alleles are significantly differentially expressed (P < 0.05) at all except stages P12 and A1 (both parental and F1 data) for tan, and stage P13 (parental data only) for ebony. Parental data were obtained using indirect comparisons for stages P12 and P13, and direct comparisons for stages P14 - A1. Each point shows the mean of four biological replicates. Error bars are experiment-wide 95% confidence intervals, calculated separately for each dataset (parental indirect; parental direct; and F1 hybrid).
DISCUSSION
The recent and dramatic difference between the dark-bodied D. americana and the light-bodied D. novamexicana presents an ideal opportunity for better understanding the mechanisms of trait divergence. The visibility of the phenotype, the lack of strong mating barriers between the two species, and the extensive history of Drosophila pigmentation research all make this system especially useful for genetic, molecular, and developmental investigation.
Developmental genetic changes underlying pigmentation divergence
Most of the body color evolution between D. americana and D. novamexicana is explained by genetic variants linked to two biosynthetic enzymes, tan and ebony (Wittkopp, Williams et al. 2003; Wittkopp et al. 2009). The causal variant(s) at each locus could be amino acid changes that affect protein function, or noncoding changes that affect mRNA or protein abundance in either a cis-regulatory (allele specific) or trans-regulatory (non-allele-specific) fashion. Neither Tan nor Ebony protein sequences contain fixed differences between D. americana and D. novamexicana (Wittkopp et al. 2009), suggesting that nonsynonymous mutations are unlikely to be responsible for the fixed difference in pigmentation.
At tan, fine mapping has identified a functionally divergent region that contains no coding differences. The fine mapped region spans 2.7 kb, from the 5′UTR to the end of the first intron (Wittkopp et al. 2009). It contains 56 single nucleotide polymorphisms (SNPs) and 19 insertions or deletions (indels) in the first intron (which is a common location for regulatory elements in Drosophila) and one SNP just upstream of the first exon. These data show that the pigmentation phenotype must be controlled at least in part by noncoding changes in tan. The location of these divergent sites - 5′UTR and first intron – suggests, but does not prove, that an allele-specific (cis-regulatory) mechanism such as a mutation in a transcription factor binding site is responsible for divergent tan activity. A cis-regulatory difference was previously looked for but, surprisingly, not found (Wittkopp et al. 2009). In the present study, however, we sampled developmental stages more broadly and precisely and found evidence of significant cis-regulatory differences between the D. americana and D. novamexicana tan alleles, although the difference observed was small and limited to a narrow window of developmental time.
Qualitative differences in the abundance of the Ebony protein have been reported between D. americana and D. novamexicana (Wittkopp, Williams et al. 2003); ebony mRNA abundance has not previously been examined in these species. The difference in Ebony expression could be due to genetic changes at ebony itself or at other (trans-acting) loci. Genetic mapping has shown that variation linked to ebony contributes to pigmentation divergence; however, in the population used for mapping, ebony was linked to ~20% of the second chromosome because of a chromosomal inversion between species (Wittkopp, Williams et al. 2003; Wittkopp et al. 2009). Consequently, genetic mapping between D. americana and D. novamexicana cannot be used to discriminate genetic changes at ebony from genetic changes at other loci within the inversion. The cis-regulatory difference affecting ebony mRNA expression we report here therefore provides the most direct evidence to date that genetic changes at ebony itself contribute to pigmentation divergence.
Small changes in mRNA abundance may have functional consequences
With many studies now using gene expression (i.e., mRNA and/or protein abundance) as a way to understand how genotypes are converted into phenotypes, it is important to understand the relationship between changes in gene expression and changes (or lack thereof) in phenotype. We found that cis-regulatory divergence for tan is modest, increasing expression of the D. americana allele by only 29% compared to the D. novamexicana allele, yet multiple lines of evidence suggest that these cis-acting changes contribute to pigmentation divergence (Wittkopp et al. 2009). This suggests that even small and hard-to-detect differences in expression can be biologically meaningful, although we cannot definitively rule out a precise combination of cis- and trans-regulatory changes between species that cause a smaller difference in relative allelic expression in hybrids than between species (Takahasi et al. 2011). Small yet highly repeatable expression changes have also been observed in other studies, for example in response to experimental diet manipulations in mice and rats (de Boer et al. 2006; Patsouris et al. 2006; Rodenburg et al. 2008) and among wild-caught Fundulus fish (Oleksiak et al. 2005). In the latter case, small differences in gene expression were found to have just as much predictive power for individual metabolic trait variation as larger differences. These small differences in RNA abundance might translate into small differences in protein abundance that nevertheless have large effects on cellular function, but it is also possible that small differences in RNA abundance might be amplified to produce larger differences in protein abundance, perhaps by producing stable proteins that accumulate overtime. Regardless of the mechanism, the focus of molecular geneticists on increasingly on complex, quantitative traits suggests that the ability to accurately detect small as well as temporally limited changes in expression is likely to be ever more critical.
cis-regulatory mutations can have temporally restricted effects
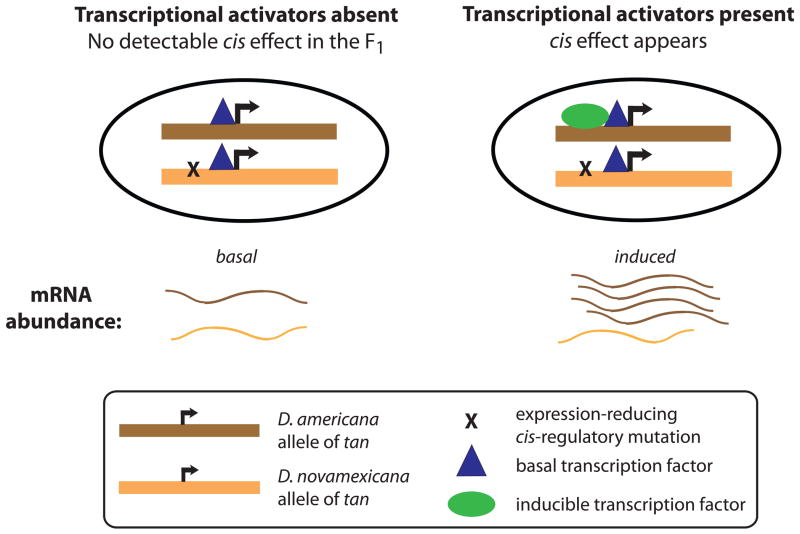
Although genomic sequence remains constant throughout an individual’s life, the effects of cis-regulatory sequence variants may only be seen in certain transcriptional contexts, such as a particular sex (Bhasin et al. 2008), tissue type (Petretto et al. 2006), or environmental condition (Li et al. 2006; Tirosh et al. 2009). Ontogeny creates, from the perspective of a single cell, a dynamic environment within a single individual. For any cell, the surrounding tissue types, extracellular molecules, and/or intracellular regulatory factors are subject to change over developmental time, and these changes cause temporally dynamic gene expression. This is illustrated by the temporal specificity of cis-regulatory divergence at tan. The allele-specific expression difference is visible at developmental stages P13 – P15, but is absent at P12 and A1 (Fig. 5) -- despite the fact that the gene is still expressed at those times. The ephemeral nature of the allele-specific expression difference may result from a cis-regulatory mutation(s) that affects a binding site(s) for a transcription factor(s) that is important primarily at stages P13–P15 (Fig. 6). Other cis-regulatory sequences, conserved between species, presumably control expression at other times during development (Fig. 6). This illustrates that although cis-regulatory mutations with phenotypic effects can be mapped genetically, assessing the impact of these mutations on gene expression is dependent on the environmental and developmental context.
Figure 6. cis-regulatory mutations are constant, but their effects can be ephemeral.
Here we illustrate a hypothetical example in which two alleles of the tan gene are functionally differentiated by a single SNP in the cis-regulatory region of the gene. The derived allele, in D. novamexicana, contains a mutation that reduces the binding affinity of a stage-specific transcription factor (green oval), leading to reduced gene expression. The blue triangle represents a transcription factor controlling basal levels of expression that binds similarly to each species’ allele. When this type of scenario occurs, the effects of the cis-regulatory mutation may only be noticed in environmentally- or developmentally-specific environments, when a sufficient amount of the relevant transcription factor is active.
MATERIALS AND METHODS
Timing of developmental stages
Three fly strains - D. melanogaster Canton S; D. americana A00 (strain 15010-0951.01); and D. novamexicana N14 (strain 15010-1031.14) - were raised on standard cornmeal media at room temperature. Sixty wandering third instar larvae of each strain were removed from their vials and distributed into new vials, with larval density standardized to 20 per vial. Vials were incubated at 20° C until pupal cases had formed and the pupae had hardened. Pupae were affixed to pieces of tape placed inside clear boxes, which were returned to the 20° C incubator. Boxes were checked every two to six hours, and each time a sample of five pupae from each species was removed for analysis. The stage of each fly was determined according to the traits listed in Table S1 by examination under a dissecting microscope, removing the pupa from its case when necessary for unambiguous staging. Data were recorded from stage P8 onward; time 0 was defined as the time at which the first fly reached stage P8. One individual from D. americana and one individual from D. novamexicana was photographed for each pupal stage using a Leica MZ6 microscope and a Scion color digital camera, model CFW-1308C. Both males and females were used in this experiment, as pigment patterning is not sexually dimorphic in D. americana or D. novamexicana (Wittkopp et al. 2011). Because the initial photos (taken for an earlier, unpublished project; L. Shefner, undergraduate senior thesis) showed an unusually dark Stage P13 D. americana fly, we re-took the photos for that as well as the two surrounding stages (P12 and P14) in D. americana. The P12 and P14 stages were used as references to verify that lighting conditions were consistent between the two photo sets.
Experimental design for three types of gene expression analyses
We used three experimental designs to investigate different aspects of tan and ebony mRNA abundance: over time, between species, and in F1 hybrids (Fig. 3). In all three cases, ratios of D. americana:D. novamexicana gene expression were determined using pyrosequencing.
Gene expression over time
To track changes in expression over time, we measured mRNA abundance of each D. americana developmental stage relative to D. novamexicana A1-stage flies, and mRNA abundance of each D. novamexicana developmental stage relative to D. americana A1-stage flies. In order to compare the time courses between the species, we wished to plot all of the time points relative to D. americana A1. Because the raw pyrosequencing data provided the ratio of D. americana alleles at each stage relative to D. novamexicana alleles at stage A1, a transformation was necessary to make D. americana A1 the common denominator:
where AstageX = the mean D. americana value for stage X and NA1 = the mean D. novamexicana value for stage A1. This is simply the log equivalent of:
Gene expression differences between species
To more directly quantify differences in mRNA abundance between the species, at developmental time points of particular interest, we combined D. americana and D. novamexicana flies from the same stage and measured their relative mRNA abundance. This assay was performed for stages P14 and P15. A direct comparison of mRNA abundance at the A1 stage was obtained from the previous experiment (Fig. 3A).
Gene expression in F1 hybrids
To test whether cis-regulatory divergence contributes to the total expression difference observed between the species, the relative mRNA abundance of D. americana: D. novamexicana alleles was measured in F1 hybrids (Fig. 3B). While the total expression difference between the species can reflect both cis- and trans-regulatory divergence, allelic expression differences in hybrids represent only the cis-regulatory component, because both alleles in the hybrid share a common trans-regulatory environment.
Fly collection and processing
D. americana A00 and D. novamexicana N14 lines for gene expression analysis were raised on standard cornmeal media at 20° C. F1 hybrid females were obtained by mating virgin female D. americana flies with male D. novamexicana flies (3 of each gender per vial). Parents were discarded prior to hybrid eclosion, in order to prevent backcrossing. Prior work shows that female hybrids from reciprocal crosses have comparable pigmentation (Wittkopp et al. 2003).
D. americana, D. novamexicana, and F1 hybrid females were collected at stages P12 - A1. Stages P12 – P15 were first removed from pupal cases. A1 flies were collected within 15 min of eclosion. Because we wished to enrich for abdominal expression of tan and ebony, and wings are visibly pigmented from stage P12 on, wings were removed and discarded. Heads were also removed because ebony is expressed strongly in the eyes (Hovemann et al. 1998; Richardt et al. 2002) and both tan and ebony are expressed in the brain (True et al. 2005). The remaining tissue (thorax, abdomen, and legs) was frozen on dry ice and stored at −70° C until used for DNA and RNA extraction.
Each sample, containing either three flies of each species or six F1 hybrids, was homogenized and used for sequential RNA and DNA extractions followed by cDNA synthesis, as in Wittkopp et al. (2004). The success of each extraction and cDNA synthesis was verified by visualizing 5 uL of final product on an agarose gel.
Pyrosequencing analyses of gene expression
In order to quantify species differences in tan and ebony mRNA expression, we compared the abundance of D. americana and D. novamexicana alleles using pyrosequencing (Ahmadian 2000). PCR and pyrosequencing primers were developed for both tan and ebony, in each case surrounding a site that differs between D. americana and D. novamexicana (Table S3). PCR and pyrosequencing reactions were performed as described in Wittkopp et al. (2008), using four biological replicates per developmental stage. For each biological replicate, cDNA was measured in triplicate. Genomic DNA, used to correct for allelic differences in extraction or PCR efficiency, was measured in duplicate for the indirect (Fig. 3A) and direct (Fig. 3B) parental comparisons. Genomic DNA from the F1 hybrids (Fig. 3C) was measured once per biological replicate, and measurements were pooled across all four biological replicates for analysis purposes. We took this approach because there is no concern about disproportional representation of the two alleles within F1 hybrids – genomic DNA from each hybrid is expected to contain the same number of D. novamexicana and D. americana alleles. Pyrograms were examined by eye to remove failed or poor-quality reactions. Ratios of allelic abundance were calculated as described in the Supplementary Methods.
Statistical analyses
Pyrosequencing expression data were analyzed using PROC MIXED in SAS v9.2 (Cary, NC, USA), with models fitted using restricted maximum likelihood. The “indirect” dataset (Fig. 3A) was fitted with the following model to test for differences between species and over time:
where Yijkl is the log2 of the ratio of D. americana to D. novamexicana mRNA abundance, corrected using genomic DNA controls. Si and Tj are fixed effects of species and time point (developmental stages P12-A1), respectively. STij is a fixed-effect interaction of species and time; PSTijk is the random effect of biological replicate (P) nested within species and developmental time point; and eijkl is the residual error.
The “direct” and “F1” datasets (Fig. 3B and 3C) were fitted to the same set of variables, minus the effect of species. (For these two datasets a single ratio comparing the two species was obtained, whereas in the “indirect” comparison, the two species were measured separately.) The model used was:
with variable names as above. Least-squares means and 95% confidence intervals of the log-transformed gene expression ratios were calculated and used to plot Figures 4, 5, and S1. For the “direct” and “F1” datasets, one-sample t-tests were performed within PROC MIXED to test the least-squares means for a significant difference from zero; that is, for significant difference in D. americana versus D. novamexicana allele abundance. For the “indirect” dataset, the D. americana and D. novamexicana data were compared at each stage using two-sample t-tests, again within PROC MIXED.
Supplementary Material
Figure S1. Direct measurements confirm indirect estimates of species expression differences, but provide more precise estimates. For both tan (A) and ebony (B), direct estimates (dark blue) indicated a slightly greater expression difference between species than the data obtained from indirect estimation (light blue).
Acknowledgments
The authors thank Ulises Rosas for developing pyrosequencing primers to tan and ebony, Elizabeth Walker for assistance with fly collection, and members of the Wittkopp lab for feedback on the manuscript.
This work was supported by grants from the National Science Foundation [DEB-0640485] and National Institutes of Health [5-R01-GM-089736-03] to P.J.W. and an NIH Ruth Kirschstein Award [1 F32 GM087928-01] to A.C. P.J.W. is an Alfred P. Sloan Research Fellow.
References
- Ahmadian A. Single-nucleotide polymorphism analysis by pyrosequencing. Analytical Biochemistry. 2000;280:103–110. doi: 10.1006/abio.2000.4493. [DOI] [PubMed] [Google Scholar]
- Ashburner M. A laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. Drosophila. [Google Scholar]
- Bainbridge SP, Bownes M. Staging the metamorphosis of Drosophila melanogaster. J Embr Exper Morphol. 1981;66:57–80. [PubMed] [Google Scholar]
- Bhasin JM, Chakrabarti E, Peng DQ, Kulkarni A, Chen X, Smith JD. Sex specific gene regulation and expression QTLs in mouse macrophages from a strain intercross. PLoS One. 2008;3:e1435. doi: 10.1371/journal.pone.0001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caletka BC, McAllister BF. A genealogical view of chromosomal evolution and species delimitation in the Drosophila virilis species subgroup. Molecular Phylogenetics and Evolution. 2004;33:664–670. doi: 10.1016/j.ympev.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Cazzonelli CI, Pogson BJ. Source to sink: regulation of carotenoid biosynthesis in plants. Trends in Plant Science. 2010;15:266–274. doi: 10.1016/j.tplants.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Clegg MT, Durbin ML. Tracing floral adaptations from ecology to molecules. Nature Reviews Genetics. 2003;4:206–215. doi: 10.1038/nrg1023. [DOI] [PubMed] [Google Scholar]
- Cooley AM, Modliszewski JL, Rommel M, Willis JH. Gene duplication in Mimulus underlies parallel floral evolution via independent trans-regulatory changes. Current Biology. 2011;21:700–704. doi: 10.1016/j.cub.2011.03.028. [DOI] [PubMed] [Google Scholar]
- Cowles CR, Hirschhorn JN, Altshuler D, Lander ES. Detection of regulatory variation in mouse genes. Nature Genetics. 2002;32:432–437. doi: 10.1038/ng992. [DOI] [PubMed] [Google Scholar]
- de Boer VC, van Schothorst EM, Dihal AA, van der Woude H, Arts IC, Rietjens IM, Hollman PC, Keijer J. Chronic quercetin exposure affects fatty acid catabolism in rat lung. Cellular and Molecular Life Sciences. 2006;63:2847–2858. doi: 10.1007/s00018-006-6316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombeck I, Jaenike J. Ecological genetics of abdominal pigmentation in Drosophila falleni: A pleiotropic link to nematode parasitism. Evolution. 2004;58:587–596. [PubMed] [Google Scholar]
- Gompel N, Prud’homme B, Wittkopp PJ, Kassner VA, Carroll SB. Chance caught on the wing: cis-regulatory evolution and the origin of pigment patterns in Drosophila. Nature. 2005;433:481–487. doi: 10.1038/nature03235. [DOI] [PubMed] [Google Scholar]
- Hoekstra HE. Genetics, development and evolution of adaptive pigmentation in vertebrates. Heredity. 2006;97:222–234. doi: 10.1038/sj.hdy.6800861. [DOI] [PubMed] [Google Scholar]
- Hollocher H, Hatcher JL, Dyreson EG. Evolution of abdominal pigmentation differences across species in the Drosophila dunni subgroup. Evolution. 2000;54:2046–2056. doi: 10.1111/j.0014-3820.2000.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Hovemann BT, Ryseck RP, Walldorf U, Störtkuhl KF, Dietzel ID, Dessen E. The Drosophila ebony gene is closely related to microbial peptide synthetases and shows specific cuticle and nervous system expression. Gene. 1998;221:1–9. doi: 10.1016/s0378-1119(98)00440-5. [DOI] [PubMed] [Google Scholar]
- Jeong S, Rokas A, Carroll SB. Regulation of body pigmentation by the abdominal-B Hox protein and its gain and loss in Drosophila evolution. Cell. 2006;125:1387–1399. doi: 10.1016/j.cell.2006.04.043. [DOI] [PubMed] [Google Scholar]
- Kopp A. Metamodels and phylogenetic replication: A systematic approach to the evolution of developmental pathways. Evolution. 2009;63:2771–2789. doi: 10.1111/j.1558-5646.2009.00761.x. [DOI] [PubMed] [Google Scholar]
- Landry CR, Wittkopp PJ, Taubes CH, Ranz JM, Clark AG, Hartl DL. Compensatory cis-trans evolution and disregulation of gene expression in interspecific hybrids of Drosophila. Genetics. 2005;171:1813–1822. doi: 10.1534/genetics.105.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R, Hazelrigg T, Rubin GM. Separable cis-acting control elements for expression of the white gene of Drosophila. EMBO Journal. 1985;4:3489–3499. doi: 10.1002/j.1460-2075.1985.tb04108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Álvarez OA, Gutteling EW, Tijsterman M, Fu J, Riksen JAG, Hazendonk E, Prins P, Plasterk RHA, Jansen RC, Breitling R, Kammenga JE. Mapping determinants of gene expression plasticity by genetical genomics in C. elegans. PLoS Genetics. 2006;2:e222. doi: 10.1371/journal.pgen.0020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnen CR, Kingsley EP, Jensen JD, Hoekstra HE. On the origin and spread of an adaptive allele in deer mice. Science. 2009;325:1095–1098. doi: 10.1126/science.1175826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markow TA, O’Grady PM. Drosophila: A guide to species identification and use. London: Academic Press; 2005. [Google Scholar]
- Martin C, Gerats T. Control of pigment biosynthesis genes during petal development. Plant Cell. 1993;5 (10):1253–1264. doi: 10.1105/tpc.5.10.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock B. Twenty-Sixth Symposium of the Society for Developmental Biology. In: Locke M, editor. The role of the nucleus: Genetic systems regulating gene expression during development. New York and London: Academic Press; 1967. [Google Scholar]
- Ninov N, Martin-Blanco E. Live imaging of epidermal morphogenesis during the development of the adult abdominal epidermis of Drosophila. Nature Protocols. 2007;2:3074–3080. doi: 10.1038/nprot.2007.417. [DOI] [PubMed] [Google Scholar]
- Oleksiak MF, Roach JL, Crawford DL. Natural variation in cardiac metabolism and gene expression in Fundulus heteroclitus. Nature Genetics. 2005;37:67–72. doi: 10.1038/ng1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsouris D, Reddy JK, Muller M, Kersten S. Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression. Endocrinology. 2006;147:1508–1516. doi: 10.1210/en.2005-1132. [DOI] [PubMed] [Google Scholar]
- Petretto E, Mangion J, Dickens NJ, Cook SA, Kumaran MK, Lu H, Fischer J, Maatz H, Kren V, Pravenec M, Hubner N, Aitman TJ. Heritability and tissue specificity of expression quantitative trait loci. PLoS Genetics. 2006;2:e172. doi: 10.1371/journal.pgen.0020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz M, Pool JE, Kassner VA, Aquadro CF, Carroll SB. Stepwise modification of a modular enhancer underlies adaptation in a Drosophila population. Science. 2009;326:1663–1667. doi: 10.1126/science.1178357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardt A, Rybak J, Strortkuhl KF. Ebony protein in the Drosophila nervous system: Optic neuropile expression in glial cells. Journal of Comparative Neurology. 2002;452:93–102. doi: 10.1002/cne.10360. [DOI] [PubMed] [Google Scholar]
- Rodenburg W, Heidema AG, Boer JMA, Bovee-Oudenhoven IMJ, Feskens EJM, Mariman ECM, Keijer J. A framework to identify physiological responses in microarray-based gene expression studies: selection and interpretation of biologically relevant genes. Physiological Genomics. 2008;33:78–90. doi: 10.1152/physiolgenomics.00167.2007. [DOI] [PubMed] [Google Scholar]
- Takahasi KR, Matsuo T, Takano-Shimizu-Kouno T. Two types of cis-trans compensation in the evolution of transcriptional regulation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15276–15281. doi: 10.1073/pnas.1105814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telonis-Scott M, Hoffmann AA, Sgrò CM. The molecular genetics of clinal variation: a case study of ebony and thoracic trident pigmentation in Drosophila melanogaster from eastern Australia. Molecular Ecology. 2011;20:2100–2110. doi: 10.1111/j.1365-294X.2011.05089.x. [DOI] [PubMed] [Google Scholar]
- Throckmorton LH. The virilis species group. In: Ashburner M, Carson HL, Thompson JN, editors. The Genetics and Biology of Drosophila. New York: Academic Press; 1982. [Google Scholar]
- Tirosh I, Reikhav S, Levy AA, Barkai N. A yeast hybrid provides insight into the evolution of gene expression regulation. Science. 2009;324:659–662. doi: 10.1126/science.1169766. [DOI] [PubMed] [Google Scholar]
- True JR. Insect melanism: the molecules matter. Trends in Ecology & Evolution. 2003;18:640–647. [Google Scholar]
- True JR, Yeh SD, Hovemann BT, Kemme T, Meinertzhagen IA, Edwards TN, Liou SR, Han Q, Li J. Drosophila tan encodes a novel hydrolase required in pigmentation and vision. PLoS Genetics. 2005;1 (5):551–562. doi: 10.1371/journal.pgen.0010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington CH. Body-colour genes in Drosophila. Proceedings of the Zoological Society of London Series A. 1942;111:173–180. [Google Scholar]
- Walter MF, Black BC, Afshar G, Kermabon AY, Wright TRF, Biessmann H. Temporal and spatial expression of the yellow gene in correlation with cuticle formation and DOPA decarboxylase activity in Drosophila development. Developmental Biology. 1991;147:32–45. doi: 10.1016/s0012-1606(05)80005-3. [DOI] [PubMed] [Google Scholar]
- Warren JT, Yerushalmi Y, Shimell MJ, MBOC, LLR, LIG Discrete pulses of molting hormone, 20-hydroxyecdysone, during late larval development of Drosophila melanogaster: Correlations with changes in gene activity. Developmental Dynamics. 2006;235:315–326. doi: 10.1002/dvdy.20626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittkopp PJ, Carroll SB, Kopp A. Evolution in black and white: Genetic control of pigment patterns in Drosophila. Trends in Genetics. 2003;19:495–504. doi: 10.1016/S0168-9525(03)00194-X. [DOI] [PubMed] [Google Scholar]
- Wittkopp PJ, Haerum BK, Clark AG. Evolutionary changes in cis and trans gene regulation. Nature. 2004;430:85–88. doi: 10.1038/nature02698. [DOI] [PubMed] [Google Scholar]
- Wittkopp PJ, Haerum BK, Clark AG. Regulatory changes underlying expression differences within and between Drosophila species. Nature Genetics. 2008;40:346–350. doi: 10.1038/ng.77. [DOI] [PubMed] [Google Scholar]
- Wittkopp PJ, Smith-Winberry G, Arnold LL, Thompson EM, Cooley AM, Yuan D, Song Q, McAllister BF. Local adaptation for body color in Drosophila americana. Heredity. 2011;106:592–602. doi: 10.1038/hdy.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittkopp PJ, Stewart EE, Arnold LL, Neidert AH, Haerum BK, Thompson EM, Akhras S, Smith-Winberry G, Shefner L. Intraspecific polymorphism to interspecific divergence: Genetics of pigmentation in Drosophila. Science. 2009;326 (5952):540–544. doi: 10.1126/science.1176980. [DOI] [PubMed] [Google Scholar]
- Wittkopp PJ, Vaccaro K, Carroll SB. Evolution of yellow gene regulation and pigmentation in Drosophila. Current Biology. 2002;12:1547–1556. doi: 10.1016/s0960-9822(02)01113-2. [DOI] [PubMed] [Google Scholar]
- Wittkopp PJ, Williams BL, Selegue JE, Carroll SB. Drosophila pigmentation evolution: divergent genotypes underlying convergent phenotypes. Proceedings of the National Academy of Science of the United States of America. 2003;100:1808–1813. doi: 10.1073/pnas.0336368100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TRF. The genetics of biogenic amine metabolism, sclerotization, and melanization in Drosophila melanogaster. Advances in Genetics. 1987;24:127–222. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Direct measurements confirm indirect estimates of species expression differences, but provide more precise estimates. For both tan (A) and ebony (B), direct estimates (dark blue) indicated a slightly greater expression difference between species than the data obtained from indirect estimation (light blue).