Abstract
Perceiving biological motion is important for understanding the intentions and future actions of others. Perceiving an approaching person's behavior may be particularly important, because such behavior often precedes social interaction. To this end, the visual system may devote extra resources for perceiving an oncoming person's heading. If this were true, humans should show increased sensitivity for perceiving approaching headings, and as a result, a repulsive perceptual effect around the categorical boundary of leftward/rightward motion. We tested these predictions and found evidence for both. First, observers were especially sensitive to the heading of an approaching person; variability in estimates of a person's heading decreased near the category boundary of leftward/rightward motion. Second, we found a repulsion effect around the category boundary; a person walking approximately toward the observer was perceived as being repelled away from straight ahead. This repulsive effect was greatly exaggerated for perception of a very briefly presented person or perception of a chaotic crowd, suggesting that repulsion may protect against categorical errors when sensory noise is high. The repulsion effect with a crowd required integration of local motion and human form, suggesting an origin in high-level stages of visual processing. Similar repulsive effects may underlie categorical perception with other social features. Overall, our results show that a person's direction of walking is categorically perceived, with improved sensitivity at the category boundary and a concomitant repulsion effect.
Keywords: categorical perception, reference repulsion, biological motion, ensemble coding, motion repulsion
1. Introduction
Perceiving biological motion is useful for understanding and predicting the behaviors and intentions of other people (Frith & Frith, 2001). Perceiving an approaching person (e.g., Gurnsey, Roddy, & Troje, 2010; Neri, Morrone, & Burr, 1998) may be especially important, because oncoming biological motion is a good indicator that a social interaction is about to occur. Indeed, humans exhibit a “facing bias” when viewing a person's ambiguous direction of walking (e.g., Vanrie, Dekeyser, & Verfaillie, 2004), and they use gender (Brooks et al., 2008), kinematic (Schouten, Troje, & Verfaillie, 2011), and auditory information (Schouten, Troje, Vroomen, & Verfaillie, 2011; Wuerger, Crocker-Buque, & Meyer, 2012; Wuerger et al., 2012) to make judgments about whether a person is walking toward them. These findings converge to suggest that the visual system may devote extra resources for perceiving an approaching person's heading. If this were true, humans should show increased sensitivity for perceiving approaching headings, and as a result, a repulsive perceptual effect around the categorical boundary of leftward/rightward biological motion.
The visual system often devotes extra resources to sharpen perception around important category boundaries. This heightened sensitivity is a hallmark of categorical perception (Bornstein & Korda, 1984; Etcoff & Magee, 1992; Harnad, 1987; Liberman, Harris, Hoffman, & Griffith, 1957) and the presumed narrowed neural tuning responsible for this sensitivity has been shown to produce concomitant repulsive distortions for a range of low- and high-level features. For example, motion discrimination is best for horizontal and vertical trajectories (Ball & Sekuler, 1980, 1982; Ferrera & Wilson, 1990; Heeley & Buchanan-Smith, 1992; Matthews & Welch, 1997), and this sensitivity repels the perceived motion of a dot away from cardinal directions (e.g., Rauber & Treue, 1998). Similar increases in sensitivity at category boundaries produce repulsive distortions of facial identity (McKone, Martini, & Nakayama, 2001), and increased sensitivity from attention is even known to repulsively distort visual space (e.g., Suzuki & Cavanagh, 1997).
Although the idea of distorting a feature to improve perception may seem paradoxical, exaggeration away from the reference value (i.e., the category boundary) would reduce the likelihood of random sensory noise causing across-category perceptual errors (Kourtzi, 2010). This repulsive protection from noise may be especially useful for the perception of an approaching person's movement, for which across-category errors would lead to head-on collisions. If this were true, repulsive distortions of biological motion should be especially strong when sensory noise may be high, such as when a person is seen with only a fleeting glance, or seen as a member of a large and chaotically organized crowd.
Here, we determined whether or not humans show increased sensitivity for perceiving the approaching heading of a walking person, and as a result, a repulsive distortion around the categorical boundary of leftward/rightward motion. We also determined if reference repulsion was particularly strong for perception of a briefly viewed person or a crowd of people. Last, we determined if reference repulsion with a crowd occurred in high-level visual processing. For all experiments, we presented “point-light walkers” (Johansson, 1973). We used these stimuli because they harness the global movements of points of light to convey human form, and they could be easily and precisely manipulated for our experimental purposes.
2. Experiment 1: Does reference repulsion occur for perception of a single, briefly presented person?
Materials and Method
2.1. Observers
Five observers (three naïve) gave informed consent. All had normal or corrected-to-normal visual acuity, and were tested individually in a dimly lit room.
2.2. Stimuli
Point-light walkers were composed of configurations of twelve white dots (each dot: 0.11° × 0.11°, 149.5 cd/m2) presented against a black background (0.36 cd/m2). The dots were placed at different locations such that the overall configuration would be perceived as a human body. We created these walkers from a freely available set (Vanrie & Verfaillie, 2004). To create the impression of a walking human body, we generated “videos” from sets of twenty-one static frames in which the local position of each dot changed from frame-to-frame in a manner consistent with a natural human gait. Each gait cycle (i.e., one step by each foot) lasted 800 msec. The application to generate the videos was written in C# and interfaced with OpenGL via the Open Toolkit Library. We created forty-three videos, each with a distinct heading (i.e., direction of walking) toward the observer ranging from leftward (−90°) to rightward (90°) in 3° increments (see point-light stimuli in Fig. 1). To generate the different headings, we multiplied each point's 3D vector by a rotation matrix (before orthographic projection). Note that this 3° increment is less than the average just noticeable difference (5.778° - determined from trials in Experiment 2). We limited the range to forward headings (toward the observer) because backward headings can appear ambiguous (perceived as forward or backward) (Cavanagh, Labianca, & Thornton, 2001). A dot configuration with a completely leftward (−90°) or completely rightward (90°) heading subtended (1.9° × 2.91°) of visual angle at the full extension of the gait cycle (i.e., with ankles maximally extended) and (0.56° × 3.06°) at the minimum extension of the gait cycle (i.e., with ankles crossing the midline of the body). A dot configuration with a completely forward heading (0°) subtended (1.03° × 3.06°) of visual angle.
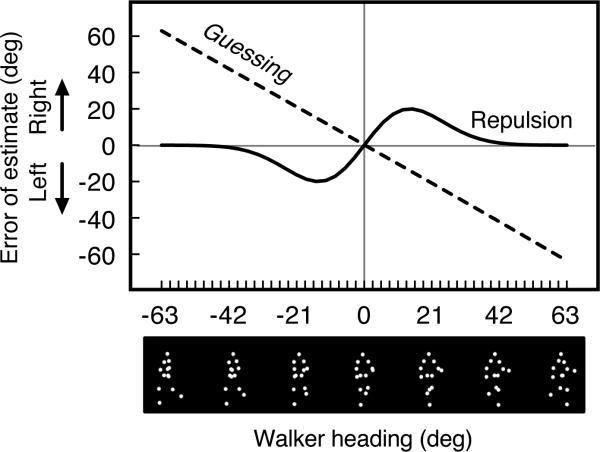
Fig. 1.
Patterns of response variability that could arise from cognitive or response biases, or from reference repulsion. If an observer were to randomly select a response heading on every trial, the pattern of response errors (dashed line) would decrease linearly and flip from rightward to leftward errors at the approaching heading. If an observer responded accurately, the magnitude or sign of response errors should not change across the range of test walker headings. If an observer categorically perceived the heading of the test walker and perceived a repulsion effect, response errors should resemble an s-shaped pattern around the category boundary, with the magnitude of exaggeration approaching zero at extreme headings (consistent with the derivative of a Gaussian function, solid line).
2.3. Walker heading selection
Across trials, the test walker's heading ranged from very leftward (−63°) to very rightward (63°) in 3° increments. The test walker was always presented at the center of the screen. Our displays did not include any depth cues; the size of each dot remained the same throughout each video, and the surface illumination of each dot was uniform (except for anti-aliased edges). Furthermore, we used a compositing mode that prevented overlapping dots from providing any occlusion cues. Consequently, our displays conveyed heading cues in the simplest way possible.
2.4. Procedure
Observers initiated each trial by pressing the space bar, followed immediately by a test walker presented for 200 ms at the center of the screen. Next, a blank black screen appeared for 1,000 ms and was followed by a single dynamic response walker presented at the center of the screen. The initial heading of the response walker was randomly chosen on each trial from a range of −90° to 90°. Observers adjusted the heading of the response walker to a value between −90° and 90° in 3° increments (left to right) to match the average heading of the test walker using the right and left arrows on the keypad. The response walker remained on the screen until the observer pressed the spacebar to end the trial. This adjustment procedure smoothly altered the heading without breaking the response walker's stride. An adjustment spanning the entire range of headings would have taken at least 3,200 ms, although no response required such a large adjustment. We note that although the time from the offset of the test walker to the end of the adjustment procedure may have introduced variability from a degraded memory trace into the recorded response, this added variability should have affected each heading equally (Blake, Cepeda, & Hiris, 1997).
Each test walker was shown five times at each of the 43 headings for a total of 215 trials. All stimuli were presented on a 61-cm LCD monitor at a viewing distance of 102 cm.
3. Results
3.1. Analyses
For each test heading (−63° through 63°), we calculated the difference between the perceived and actual heading of test walker (with negative values indicating a leftward error and positive values indicating a rightward error). We thus measured response errors as a function of the test walker's heading. We also constructed distributions of response errors binned across pairs of headings (e.g., response errors from −63° and −60° trials were binned into one distribution, errors from −57° and −54° were binned into another distribution, etc). We then calculated response variability as the standard deviation of each distribution. We thus measured response variability as a function of the test walker's heading. Headings for which an observer had the highest sensitivity would have a narrowed distribution (and a concomitant reduced SD) of response errors.
A few considerations about the patterns of response errors that could arise from cognitive or response biases are informative at this point. If an observer were to randomly select a response heading on every trial, the pattern of response errors across test walker headings would resemble the dashed line in Fig. 1; extremely rightward (or leftward) response errors for leftward (or rightward) test walkers with the magnitude of errors decreasing linearly and flipping direction at the directly approaching heading (we confirmed this pattern of errors using a Monte Carlo simulation). A bias to avoid the ends of the response range could have also produced such a pattern, although it would likely be less extreme than the pattern from random responding. If an observer responded accurately, then neither the magnitude nor the sign of response errors should change across the range of test walker headings. If an observer categorically perceived the heading of the test walker with an accompanying repulsion effect around the 0° heading, we should see an s-shaped pattern of exaggerated response errors (i.e., perceiving a leftward walker as more leftward than it actually was) around the category boundary, with the magnitude of exaggeration approaching zero at extreme headings (consistent with the derivative of a Gaussian function, solid line in Fig. 1). Importantly, demonstrating concomitant higher sensitivity for perception of headings near the category boundary would allow us to rule out a response bias as the source of this s-shaped pattern of results.
Note that some random responding or a response bias could occur independently of, and simultaneously with, a repulsion effect. In other words, the s-shaped repulsion pattern could be superimposed over the negatively sloped linear pattern. We thus included slope and y-intercept parameters in our fitting with the derivative of a Gaussian function. We were only interested in repulsion, so we normalized response errors around the linear fit across test headings in order to clearly display our main results in the figures. We verified that this normalization had no systematic relationship with the amplitude of repulsion; for each experiment, both slope and y-intercept were randomly distributed across observers and were unrelated to amplitude (all p-values > .175).
3.2. Increased sensitivity to approaching headings
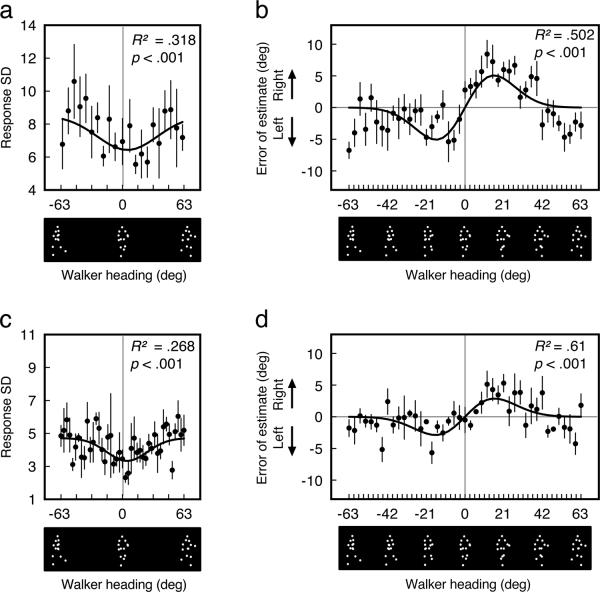
Observers were most sensitive to the heading of a briefly presented approaching walker; response variability decreased as headings approached 0° (Fig. 2A). An analysis using Akaike's Information Criterion (AICc) confirmed that a Gaussian function (R2 = 0.318, p < .001) fit the data better than a linear function (R2 = 0.068, n.s.). The AICc determines the likelihood that one fit is more appropriate than another when different numbers of fitting parameters are used, and it favored the derivative of a Gaussian with 87.22% likelihood. The amplitude of the Gaussian fit indicates the magnitude of the sensitivity boost around the category boundary – the heading sensitivity effect (M = −2.087, SEM = 0.777). The heading sensitivity effect even reached statistical significance separately for two observers (p-values < .026).
Fig. 2.
Reference repulsion for perception of a single walker (a) Observers were most sensitive to the heading of a briefly presented approaching walker. Response variability decreased for headings near the category boundary, as shown by a good fit from a Gaussian function to the average of all observers. (b) A briefly presented walker's heading was perceived as exaggerated around the leftward-rightward category boundary (0°). The average difference between the perceived and actual heading is shown for each walker heading (with negative values indicating a leftward error and positive values indicating a rightward error). The data (filled circles) were well fit by the derivative of a Gaussian (black line), which characterized the highly ordered tuning of the exaggerations near the category boundary. (c) When a walker was presented for a longer duration, observers were still most sensitive to the headings of approaching crowds although the heading sensitivity effect was reduced. (d) Reference repulsion still occurred for perception of a walker shown for a long duration, albeit of a smaller amplitude. Error bars represent ±1 SEM.
3.3. Reference repulsion of a single walker
A briefly presented walker's heading appeared exaggerated away from the leftward-rightward category boundary (Fig. 2B). For example, a walker with a −15° leftward heading might have appeared to have a −19° heading. The magnitude of these exaggerations followed an “s-shaped” pattern across the range of headings that was well fit by the derivative of a Gaussian function (R2 = 0.502, p < .001). An AICc analysis confirmed with 100% certainty that the derivative of a Gaussian characterized the pattern of data better than a linear function (linear R2 = .077, n.s.). The “s-shape” of the function is consistent with a flip in the direction of exaggeration around the 0° heading (directly toward the observer), and the half-amplitude of the function indicates the maximum amount of heading exaggeration – the repulsion effect (bootstrapped M = 5.92°, bootstrapped SEM = .9107°). The repulsion effect occurred for all five observers, and it was even statistically significant separately for four of these observers (p-values < .001). The “s-shape” shows that exaggeration was not uniform across the range of headings, but only affected headings near the category boundary. The increased sensitivity for approaching headings (see above) provides evidence against a response bias as the source of this pattern; a response bias would not have produced a change in sensitivity. The heading sensitivity and repulsion effects suggest, instead, that the visual system optimizes perception nearby the category boundary where heading discrimination may be most important.
4. Experiment 2: Does reference repulsion decrease for perception of a single person presented for a longer duration?
If categorical perception and reference repulsion function to prevent sensory noise from causing across-category errors, then repulsive effects should be reduced when encoding noise is low, such as when a stimulus is presented for a long duration. Here, we tested this hypothesis by presenting a test walker for 1000 ms instead of 200 ms.
Materials and Method
4.1. Observers
Four trained psychophysical observers (3 from Experiment 1 and 1 naïve) gave informed consent. All had normal or corrected-to-normal visual acuity, and were tested individually in a dimly lit room.
4.2. Stimuli and Procedure
The stimuli and procedure were identical to those used in Experiment 1, except that a walker was shown for 1000 ms, and we did not bin the values of response variability across headings.
5. Results
5.1. Increased sensitivity to approaching headings
As expected, response variability was lower with the longer duration stimuli (median SD = 4.282) compared to the shorter duration stimuli (median SD = 6.866). Observers were most sensitive to the heading of an approaching walker; response variability decreased as headings approached 0° (Fig. 2C). An AICc analysis confirmed with 98.29% likelihood that a Gaussian function (R2 = 0.268, p < .001) fit the data (for the average of all observers) better than a linear function (R2 = 0.0003, n.s.). The heading sensitivity effect was separately significant for all four observers (p-values < .04). As predicted, the heading sensitivity effect (M = −1.383, SEM = 0.383) was smaller with the longer duration than with the shorter duration.
5.2. Reference repulsion of a single walker
A single walker's heading appeared exaggerated away from the leftward-rightward category boundary (Fig. 2D). The magnitude of these exaggerations was well fit by the derivative of a Gaussian function (for the average of all four observers, R2 = 0.61, p < .001). An AICc analysis confirmed with 99.84% certainty that the derivative of a Gaussian characterized the pattern of data better than a linear function (linear R2 = 0.398). The repulsion effect was even separately significant for two observers (p-values < .003) and marginally significant for the other two observers (p-values < .1). As predicted, the repulsion effect with the longer duration (bootstrapped M = 3.359°, bootstrapped SEM = 0.56° for the average of all observers) was smaller than with the shorter duration (compare Fig. 2D with Fig. 2B). Overall, these data suggest that reference repulsion around the category boundary may be especially strong when encoding noise is high, and it suggests that the magnitude of repulsion increases with the relative sensitivity to approaching headings.
6. Experiment 3: Is reference repulsion also strong for perception of a crowd?
Crowd behavior is common for many species (Sumpter, 2006) and is important for survival (Bode, Faria, Franks, Krause, & Wood, 2010). Perceiving crowds is important too. In fact, humans are equipped with specialized ensemble coding mechanisms for efficiently perceiving the “gist” of a crowd's heading (Sweeny, Haroz, & Whitney, in press). Categorically perceiving a crowd's heading may be especially important, because crowds are often chaotically organized (e.g., in a panic situation, Helbing, Farkas, & Vicsek, 2000; Low, 2000), and repelling a crowd's heading away from the leftward/rightward category boundary would reduce the likelihood of chaos within the crowd from causing across-category errors. In this experiment, we determined if high sensitivity to approaching headings and concomitant repulsive effects occurred for perception of a crowd. We also determined if the repulsion effect was stronger for crowds with high variability in the headings of their members.
Method
6.1. Observers
The same four observers that participated in Experiment 2 participated in Experiment 3.
6.2. Crowd heading selection
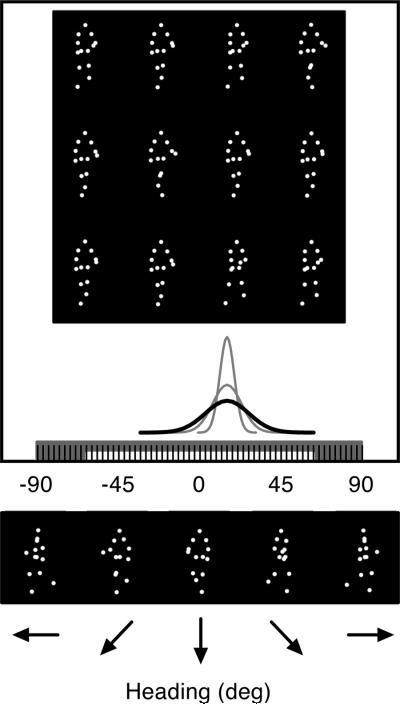
A crowd consisted of 12 individual walkers with various headings (i.e., walkers within a crowd had identical or increasingly variable headings; see Fig. 3). As with single walkers, average crowd headings ranged from very leftward (−63°) to very rightward (63°) in 3° increments, and the headings of individuals in crowds ranged from extremely leftward (−90°) to extremely rightward (90°). On a given trial, we randomly selected twelve headings from a continuous Gaussian distribution centered at one of the forty-three headings. The peak of the distribution determined the average heading of the crowd and the width of the distribution determined the heading variability within the crowd. The standard deviations of the sampling distribution included 0° (resulting in a homogenous group), 2°, 4°, 6°, 8°, 10°, and 12°. We used a truncated range of average crowd headings so that values from the tails of a distribution centered at −63° or 63° would not exceed −90° or 90°. Because our stimulus set contained walkers with discrete headings (e.g., −63°, −60°, −57°, etc.), we sorted each of the twelve outputs from the continuous Gaussian distribution into 3° bins centered at the 60 possible walker headings between −90° and 90°. For example, a sampled heading of −61.6° would generate a walker with a −63° heading, and a sampled heading of −61.4° would generate a walker with a −60° heading.
Fig. 3.
Crowd generation procedure. A heterogeneous crowd of walking people was generated by sampling 12 individual headings from a Gaussian distribution centered at one of 43 headings. The width of the distribution varied from narrow to wide (SDs = 0°, 2°, 4°, 6°, 8°, 10°, and 12°) to create seven levels of crowd variability. SDs of 4°, 8°, and 12° (in black) are shown. The white and gray bars along the x-axes indicate the range of possible crowd headings and individual headings, respectively.
6.3. Crowd configurations
We presented walkers randomly placed among twelve non-overlapping locations in a 4×3 grid subtending 15.6° × 8.36° of visual angle (measured from the center of each walker) with an average horizontal inter-walker distance of 2.34° and an average vertical inter-walker distance of 0.612°. We used an orthographic projection (i.e., discounting linear perspective such that a −45° walker on the left side of the screen was identical to a −45° walker on the right side of the screen). Walkers were presented at randomly selected locations within the grid.
6.4. Procedure
All procedures were identical to those from Experiment 2, with the following exceptions. We paired each value of heading variability (SDs = 0°, 2°, 4°, 6°, 8°, 10°, and 12°) with each mean heading (43 values) five times for a total of 1,505 trials run across 5 blocks, and we presented crowds for 1,000 ms.
7. Results
7.1. Increased sensitivity to approaching headings
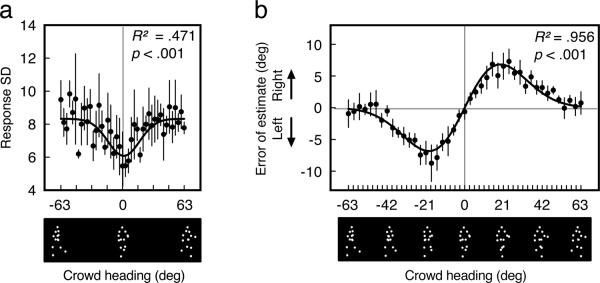
Observers were most sensitive to the headings of approaching crowds. When data were collapsed across crowds with low levels of variability (SDs = 0°, 2°, and 4°), response variability decreased as headings approached 0°. We analyzed the heading sensitivity effect using these low-variability crowds because (1) they were less likely than the high-variability crowds to contain walkers with deviant headings and were thus more likely to reflect the underlying sensitivity to crowd heading, and (2) we previously showed that heading differences within a crowd are not likely to be perceived at these low levels of variability (Sweeny, et al., in press). The heading sensitivity effect was large for crowds (M = −2.488°, SEM = 0.881, Fig. 4A), and an AICc analysis confirmed with 100% likelihood that a Gaussian function (R2 = 0.471, p < .001) fit the data (for the average of all observers) better than a linear function (R2 = 0.004, n.s.). The heading sensitivity effect occurred for all four observers, and it was separately significant for three of these observers (p-values < .001).
Fig. 4.
Reference repulsion for perception of a crowd of walkers. Note that for both panels, crowd heading is depicted using a single walker. (a) Observers were most sensitive to the headings of approaching crowds. Response variability decreased for crowd headings near the category boundary, as shown by a good fit from a Gaussian function to the average of all observers (solid black). (b) Reference repulsion was very strong for the perception of a crowd. Error bars represent ±1 SEM.
7.2. Reference repulsion with a crowd
A crowd's heading was greatly exaggerated away from the leftward-rightward category boundary (when data were collapsed across the seven levels of crowd variability, Fig. 4B). The magnitude of these exaggerations was well fit by the derivative of a Gaussian function (R2 = 0.956, p < .001), which an AICc analysis favored with 100% likelihood over a linear function (linear R2 < 0.268, n.s.). The repulsion effect was even separately significant for all four observers (p-values < .001). As expected, the repulsion effect was greater for perception of a crowd (bootstrapped M = 7.99°, bootstrapped SEM = 0.37°) than for perception of a single walker shown for the same duration (compare Fig. 4B with Fig. 2D).
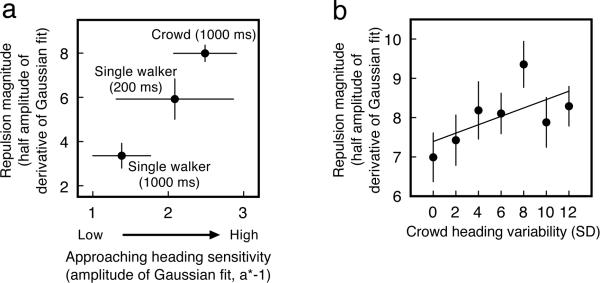
When comparing the results from Experiments 1, 2, and 3, it is clear that increased sensitivity to approaching headings was associated with an increase in the magnitude of reference repulsion (Fig. 5A), = −1, p < .01. This is consistent with the hypothesis that narrowed tuning of channels that respond to feature values near the category boundary directly determines the magnitude of reference repulsion (Gros, Blake, & Hiris, 2003; Suzuki & Cavanagh, 1997).
Fig. 5.
(a)Reference repulsion increased with sensitivity to approaching headings. (b) The magnitude of reference repulsion in a crowd increased with variability in a crowd's heading. Error bars represent ±1 SEM.
7.3. Reference repulsion with crowd variability
To determine if the reference repulsion effect depended on the heading variability within the crowd, we compared the repulsion effect across the seven levels of crowd variability averaged across all observers. The magnitude of repulsion increased linearly with the variability within the crowd, which we confirmed with a contrast in which we assigned values of (−3, −2, −1, 0, +1, +2, +3) to bootstrapped half-amplitude values from the different crowd variability conditions (SDs = 0°, 2°, 4°, 6°, 8°, 10°, and 12°), p < .05 (Fig. 5B). This suggests that reference repulsion may function to increase signal strength when natural sources of crowd noise, like heading variability, are prevalent.
8. Experiment 4: Does crowd reference repulsion occur in high-level vision? Control for a low-level motion account
Perception of a point-light walker's heading can rely on low-level cues like the movements of individual dots (e.g., Chang & Troje, 2009; Thurman & Grossman, 2008; Troje & Westhoff, 2006), but most likely requires integration of this local motion information with human form (Giese & Poggio, 2003; Peuskens, Vanrie, Verfaillie, & Orban, 2005), which presumably occurs in high-level visual processing (e.g., superior temporal sulcus) (e.g., Grossman, Battelli, & Pascual-Leone, 2005; Grossman & Blake, 2001; Peuskens, et al., 2005). Previous investigations have shown reference repulsion for the perception of moving dots without human configurations (e.g., Rauber & Treue, 1998), presumably in lower-level visual areas. We conducted a control experiment to determine if the reference repulsion we found with a crowd was based on physical motion alone, or if instead, it required integration of human configurations and motion, presumably in high-level visual areas. To accomplish this, we presented moving clusters of dots without human configurations, but with local motion identical to the local motion in Experiment 3 (i.e., point-scrambled walkers, see Fig. 6A). We used crowds instead of single walkers because it was with crowds that we found the most convincing evidence of reference repulsion (based on R2 values from fitting with the derivative of a Gaussian).
Fig. 6.
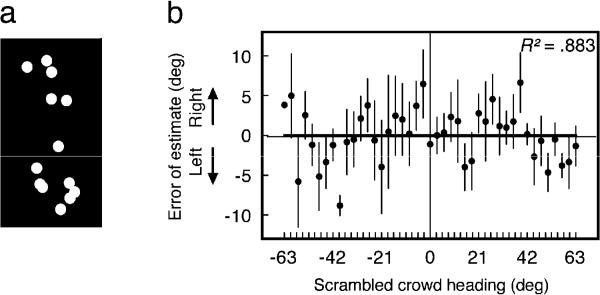
Reference repulsion with a crowd required integration of motion and human form. (a) Example of a scrambled walker with a heading of 54 deg (see section 8.2). (b) Reference repulsion did not occur for perception of a crowd of scrambled walkers. A linear fit characterized this pattern better than the derivative of a Gaussian function. Error bars represent ±1 SEM.
Materials and Method
8.1. Observers
The same observers who participated in Experiments 2 and 3 participated in Experiment 4.
8.2. Stimuli and Procedure
The stimuli were identical to those used in Experiment 3, with the following exceptions. We randomly positioned the location of each dot in a 3D bounding box with an aspect ratio comparable to that of a human configuration. We generated these scrambled dot locations separately for each heading (−90° through 90°) for each observer. The local motion of each dot was centered about its randomly selected location (rather than a location on the walker's body). For example, a dot representing an ankle in Experiment 3 would appear with the same frame-to-frame local motion and heading in Experiment 4, but at a different location and without any predictable spatial relationship to nearby dots. The response walker in Experiment 4 always had a dynamic human configuration so that any differences in response error between the experiments could not be attributed to increased difficulty adjusting a scrambled response walker. Experiment 4 was identical to Experiment 3 in all other respects.
9. Results
Overall, observers perceived the headings of scrambled crowds with very little sensitivity. Response variability for extreme headings was near chance level (SD of ~37, determined from Monte Carlo methods), and even the most precisely perceived scrambled headings produced response variability greater than twice of that from the worst performance with coherent crowds.
Reference repulsion did not occur for perception of scrambled crowds (Fig. 6B). An AICc analysis confirmed with 79.35% likelihood that perceptual errors across the range of headings were better fit by a linear function (R2 = 0.883, n.s.) than the derivative of a Gaussian function (R2 = 0.889, n.s.). Moreover, a bootstrapped interaction confirmed that the repulsion effect was stronger with intact crowds (Experiment 3) than with the point-scrambled crowds (Experiment 4, p < .05). This clearly shows that our main finding — reference repulsion of a crowd of moving people — was not simply due to the physical motion of individual dots. We have shown that reference repulsion with a crowd is a high-level visual phenomenon because it depends on the integration of multiple trajectories of motion with human form.
10. Simulating reference repulsion with a model of population coding
Across all experiments with normally configured walkers, we observed greater sensitivity for perception of approaching headings. This could have occurred because neural channels tuned to approaching headings are narrower than those tuned to extreme headings, or it could have occurred because there are more channels that respond to approaching headings. In either case, this increased sensitivity was associated with greater amounts of reference repulsion (Fig. 5A). Narrowed tuning has been suggested as a mechanism for reference repulsion of low-level motion (Gros, et al., 2003) and for attention-based repulsion of visual space (e.g., Suzuki & Cavanagh, 1997). Here, we used a population-coding simulation to demonstrate how narrowed tuning is likely to have produced reference repulsion for the perception of a person or a crowd's heading. We simulated perception of crowds because (1) it was with crowds that we found the most convincing evidence of reference repulsion (e.g., Fig. 4B), and (2) we wanted to use response variability data from single walker trials to guide our estimates of the fitting parameters.
We simulated perception of a crowd's heading as the weighted-average of the outputs of a hypothetical population of heading-tuned channels. This approach is both simple and biologically plausible. First, sensitivity to specific headings in point-light walkers has been shown among populations of cells in the Superior Temporal Polysensory area (STPa) of the macaque (Oram & Perrett, 1994). Second, adaptation-based aftereffects, a hallmark of population based central-tendency coding (e.g., Suzuki, 2005), have been demonstrated for the perception of biological motion (Troje, Sadr, Geyer, & Nakayama, 2006).
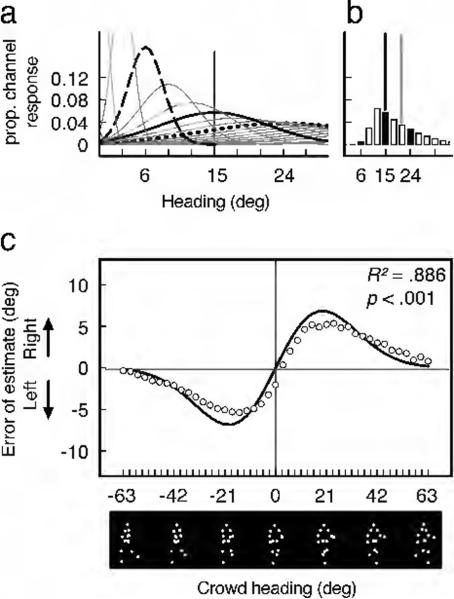
The mechanisms through which channel narrowing could produce reference repulsion are straightforward. For a given approaching heading (e.g., 15°), widely-tuned channels most sensitive to extreme headings would respond more strongly than narrowly-tuned channels sensitive to approaching headings (Fig. 7A), and thus contribute more strongly to the population response. This would skew the weighted-average of the population activity away from the category boundary (Fig. 7B) and cause an approaching crowd's heading to be repelled away from the category boundary (see Suzuki & Cavanagh, 1997, for further discussion of this reasoning). Following this same logic, greater channel density near the category boundary could not have produced our repulsion effect. Abundant approach-tuned channels would contribute more heavily to the weighted population activity and skew the perception of a person or crowd toward the category boundary, producing the opposite pattern.
Fig. 7.
Hypothetical simulation of perceived crowd heading based on narrowed channel tuning near the category boundary. Channel narrowing produced a good fit to the data. (a and b) With narrowing near the category boundary, the 24°-tuned channel (the dotted line) would respond more strongly than the 6°-tuned channel (the dashed line), and would pull the mean of the population response (the gray vertical line in panel b) away from the actual mean of the crowd (the black vertical line in panel b) and the category boundary. (c) Simulated crowd perception errors (open circles) provided an excellent fit to the pattern of errors from Experiment 3 (black line). Crowd heading is depicted in this panel using a single walker.
For our purposes, we defined “channels” as sub-populations of neurons with similar tuning characteristics. We assumed that each channel had a Gaussian-shaped tuning function (see Figs. 7A and 7B for illustrations). We used the best Gaussian fit to the average response variability (the SD) across all headings from briefly presented single-walker trials from Experiment 1 as a starting point for estimating the width of each channel's tuning. For our models, our “population” included 121 channels with peak sensitivities ranging from −180° to 180°. It was necessary to include channel peaks beyond the range of “forward facing” walkers used in our experiments (−90° to 90°) to ensure that population responses to extreme walkers (e.g., −63°) were not unfairly skewed toward the middle of the range.
In each simulation, we started by genera ting a heterogeneous crowd of walkers using the same sampling and binning methods from Experiment 3. Because we previously demonstrated that estimates of a crowd's heading were based on an ensemble code that pools headings of multiple, but not necessarily all walkers in a crowd (Sweeny, et al., in press), we then selected a subset of 5 of the 12 walkers and calculated the linear average of this subset's heading (Parkes, Lund, Angelucci, Solomon, & Morgan, 2001). We then computed the weighted average of the response from each of the 121 channels to this subset's heading and compared it to the actual heading of the full crowd. We iteratively calculated response errors 400 times for each of the headings from the full range (from −63° to 63°) and compared the pattern of these average simulated response errors to those from Experiment 3. Recall that we based our initial estimates of channel widths on the Gaussian fit to the pattern of response variability from Experiment 1. To find the best fit to the crowd data, we used a gradient-descent method to find the combination of gain and narrowing applied to this underlying distribution that produced the lowest sums-of-squared errors value against the actual response errors from Experiment 3.
Narrowed tuning produced an excellent fit to the data (R2 = 0.886, p < .0001) (Fig. 7C). The purpose of this simulation was simply to illustrate how narrowed tuning of channels that respond to approaching headings could cause a reference repulsion effect. In this regard, our simulation was successful. We note, however, that our simulation was far from exhaustive and is intended merely to complement our behavioral findings. It included two free parameters – gain and narrowing applied to our first estimate of the Gaussian distribution that determined channel widths. We were limited by the necessity of using response variability as our best estimate of channel widths, and future neurophysiological investigations of the tuning properties of heading-sensitive neurons will undoubtedly provide better estimates. Overall, this simulation offers a simple illustration of why channel narrowing is a plausible source of the increased sensitivity to approaching headings, and it clearly shows how this narrowing could have caused a repulsion effect.
11. Discussion
We demonstrated that the visual system is especially sensitive to approaching biological motion, and we showed that this increased sensitivity produced a repulsive perceptual effect in which a person's direction of walking was exaggerated away from the category boundary of leftward/rightward motion. This repulsive distortion occurred for perception of a single person and for a crowd, it was especially strong when encoding was noisy. We showed that repulsion with a crowd required integration of low-level motion and human form, suggesting a neural origin in high-level stages of visual processing (de Gelder, 2006; Grossman, et al., 2005; Vaina, Lemay, Bienfang, Choi, & Nakayama, 1990). Overall, we showed that biological motion is categorically perceived, and we demonstrated that this sensitivity produces a repulsive perceptual distortion that may be important for everyday social behavior, like avoiding head-on collisions.
Our finding of sensitive perception of approaching headings bears the hallmark of categorical perception – increased sensitivity around a category boundary (Bornstein & Korda, 1984; Etcoff & Magee, 1992; Harnad, 1987; Liberman, et al., 1957). Typical demonstrations of categorical perception infer that such changes in sensitivity distort the perception of a given feature. We directly measured this distortion as reference repulsion around the approaching heading. Our findings converge with investigations of motion (e.g., Rauber & Treue, 1998) and facial identity (McKone, et al., 2001) to show that increased sensitivity does indeed produce a measurable repulsive distortion away from the category boundary. Moreover, our results suggest that reference repulsion should occur for several other important social features for which categorical perception has been shown (e.g., facial expression: Calder, Young, Perrett, Etcoff, & Rowland, 1996; vocal emotional expression: Laukka, 2005; shape: Newell & Bülthoff, 2002; and familiarity: Rossion, Schiltz, Robaye, Pirenne, & Crommelinck, 2001).
Reference repulsion was strongest when encoding was noisy, such as when a single person was viewed briefly or seen among other people in a chaotic crowd. These findings are consistent with the idea that categorical perception may be a mechanism for mitigating the effect of sensory noise near a category boundary (Kourtzi, 2010). Indeed, repulsive exaggerations only occurred near the category boundary (where variability in a person's or a crowd's heading would have been most likely to cause an across-category error) rather than uniformly across the range of headings (as would have occurred with a response bias). This repulsive protection from noise would be useful for avoiding head-on collisions while navigating around an approaching pedestrian or a crowd, especially in a panic situation (Helbing, et al., 2000; Low, 2000).
What are the mechanisms of reference repulsion of biological motion? The current results rule out one explanation and suggest a plausible alternative. First, reference repulsion is unlikely to be due to lateral inhibitory interactions that are typically thought to produce repulsion effects when multiple features are simultaneously presented (Gibson, 1937; Losada & Mullen, 1995; Magnussen & Kurtenbach, 1980; Mareschal, Morgan, & Solomon, 2008; Perkins & Landy, 1991; Solomon, 2000, 2002; Sweeny, Grabowecky, Kim, & Suzuki, 2011; see the Discussion section of Sweeny, Grabowecky, & Suzuki, 2011, for a review). Although inhibition of approaching heading-tuned channels could cause reference repulsion by shifting the mean of a weighted-population response away from the leftward/rightward category boundary, such inhibition would also cause increased response variability and decreased accuracy for directly approaching headings (i.e., at 0°). We found the opposite pattern – decreased response variability and increased accuracy for directly approaching headings, an indication of narrowed tuning of heading-sensitive neurons or channels near the category boundary. Earlier investigations of low-level motion (Gros, et al., 2003) and attention-induced distortions of visual space (Suzuki & Cavanagh, 1997) suggested that narrowed tuning could produce a reference repulsion effect. We simulated the crowd repulsion effect with a simple and biologically plausible model and found that, indeed, a sufficient amount of narrowed tuning near the category boundary could have produced our results.
The movements of the human body provide a window into the future behaviors, intentions, and minds of other people (Frith & Frith, 2001). We have shown that the visual system is optimized for perceiving biological motion, producing categorical perception of a person's behavior and a concomitant repulsion effect. More generally, our findings underscore the importance of perceiving biological motion for typical social interaction and everyday life.
Highlights
Humans are especially sensitive to an approaching person's direction of walking
This sensitivity underlies repulsive categorical perception of biological motion
Sensitivity and repulsion effects are exaggerated when sensory noise is high
Categorical perception of biological motion is important for social interaction
Acknowledgments
This study was supported in part by the National Institutes of Health grants T32 EY007043 and R01 EY018216, and National Science Foundation grant NSF 0748689.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ball K, Sekuler R. Models of stimulus uncertainty in motion perception. Psychological Review. 1980;87(5):435–469. [PubMed] [Google Scholar]
- Ball K, Sekuler R. A specific and enduring improvement in visual motion discrimination. Science. 1982;218(4573):697–698. doi: 10.1126/science.7134968. [DOI] [PubMed] [Google Scholar]
- Blake R, Cepeda NJ, Hiris E. Memory for visual motion. Journal of ExperimentalPpsychology. Human Perception and Performance. 1997;23(2):353–369. doi: 10.1037//0096-1523.23.2.353. [DOI] [PubMed] [Google Scholar]
- Bode NW, Faria, Franks DW, Krause J, Wood AJ. How perceived threat increases synchronization in collectively moving animal groups. Proceedings. Biological sciences / The Royal Society. 2010;277(1697):3065–3070. doi: 10.1098/rspb.2010.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Korda NO. Discrimination and matching within and between hues measured by reaction times: some implications for categorical perception and levels of information processing. Psychological Research. 1984;46:207–222. doi: 10.1007/BF00308884. [DOI] [PubMed] [Google Scholar]
- Brooks A, Schouten B, Troje NF, Verfaillie K, Blanke O, van der Zwan R. Correlated changes in perceptions of the gender and orientation of ambiguous biological motion figures. Current Biology. 2008;18(17):R728–R729. doi: 10.1016/j.cub.2008.06.054. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Young AW, Perrett DI, Etcoff NL, Rowland D. Categorical perception of morphed facial expressions. Visual Cognition. 1996;3(2):81–117. [Google Scholar]
- Cavanagh P, Labianca AT, Thornton IM. Attention-based visual routines: sprites. Cognition. 2001;80:47–60. doi: 10.1016/s0010-0277(00)00153-0. [DOI] [PubMed] [Google Scholar]
- Chang DH, Troje NF. Acceleration carries the local inversion effect in biological motion perception. Journal of Vision. 2009;9(1):19, 11–17. doi: 10.1167/9.1.19. [DOI] [PubMed] [Google Scholar]
- de Gelder B. Towards the neurobiology of emotional body language. Nature Reviews Neuroscience. 2006;7(3):242–249. doi: 10.1038/nrn1872. [DOI] [PubMed] [Google Scholar]
- Etcoff NL, Magee JJ. Categorical perception of facial expressions. Cognition. 1992;44(3):227–240. doi: 10.1016/0010-0277(92)90002-y. [DOI] [PubMed] [Google Scholar]
- Ferrera VP, Wilson HR. Perceived direction of moving two-dimensional patterns. Vision Research. 1990;30(2):273–287. doi: 10.1016/0042-6989(90)90043-k. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith C. The biological basis of social interaction. Current Directions in Psychological Science. 2001;10:151–155. [Google Scholar]
- Gibson JJ. Adaptation, after-effect and contrast in the perception of tilted lines: II. Simultaneous contrast and areal restriction of the aftereffect. Journal of Experimental Psychology. 1937;20:553–569. [Google Scholar]
- Giese MA, Poggio T. Neural mechanisms for the recognition of biological movements. Nature reviews. Neuroscience. 2003;4(3):179–192. doi: 10.1038/nrn1057. [DOI] [PubMed] [Google Scholar]
- Gros BL, Blake R, Hiris E. Anisotropies in visual motion perception: a fresh look. Journal of the Optical Society of America. A, Optics, Image Science, and Vision. 2003;15(8):2003–2011. doi: 10.1364/josaa.15.002003. [DOI] [PubMed] [Google Scholar]
- Grossman ED, Battelli L, Pascual-Leone A. Repetitive TMS over posterior STS disrupts perception of biological motion. Vision Research. 2005;45(22):2847–2853. doi: 10.1016/j.visres.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Grossman ED, Blake R. Brain activity evoked by inverted and imagined biological motion. Vision Research. 2001;41(10–11):1475–1482. doi: 10.1016/s0042-6989(00)00317-5. [DOI] [PubMed] [Google Scholar]
- Gurnsey R, Roddy G, Troje NF. Limits of peripheral direction discrimination of point-light walkers. Journal of Vision. 2010;10(2):15, 11–17. doi: 10.1167/10.2.15. [DOI] [PubMed] [Google Scholar]
- Harnad S. Psychophysical and cognitive aspects of categorical perception. In: Harnad S, editor. Categorical Perception: The Groundwork of Cognition. Cambridge University Press; New York: 1987. [Google Scholar]
- Heeley DW, Buchanan-Smith HM. Directional acuity for drifting plaids. Vision Research. 1992;32(1):97–104. doi: 10.1016/0042-6989(92)90117-2. [DOI] [PubMed] [Google Scholar]
- Helbing D, Farkas I, Vicsek T. Simulating dynamical features of escape panic. Nature. 2000;407(6803):487–490. doi: 10.1038/35035023. [DOI] [PubMed] [Google Scholar]
- Johansson G. Visual perception of biological motion and a model for its analysis. Perception & Psychophysics. 1973;14(2):201–211. [Google Scholar]
- Kourtzi Z. Visual learning for perceptual and categorical decision in the human brain. Vision Research. 2010;50:433–440. doi: 10.1016/j.visres.2009.09.025. [DOI] [PubMed] [Google Scholar]
- Laukka P. Categorical perception of vocal emotional expressions. Emotion. 2005;5(3):277–295. doi: 10.1037/1528-3542.5.3.277. [DOI] [PubMed] [Google Scholar]
- Liberman AM, Harris KS, Hoffman HS, Griffith BC. The discrimination of speech sounds within and across phoneme boundaries. Journal of Experimental Psychology. 1957;54(5):358–368. doi: 10.1037/h0044417. [DOI] [PubMed] [Google Scholar]
- Losada MA, Mullen KT. Color and luminance spatial tuning estimated by noise masking in the absence of off-frequency looking. Journal of the Optical Society of America. A, Optics, Image Science, and Vision. 1995;12:250–260. doi: 10.1364/josaa.12.000250. [DOI] [PubMed] [Google Scholar]
- Low DJ. Statistical physics. Following the crowd. Nature. 2000;407(6803):465–466. doi: 10.1038/35035192. [DOI] [PubMed] [Google Scholar]
- Magnussen S, Kurtenbach W. Linear summation of tilt illusion and tilt aftereffect. Vision Research. 1980;20(1):39–42. doi: 10.1016/0042-6989(80)90139-x. [DOI] [PubMed] [Google Scholar]
- Mareschal I, Morgan MJ, Solomon JA. Contextual effects on decision templates for parafoveal orientation identification. Vision Research. 2008;48:2689–2695. doi: 10.1016/j.visres.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Matthews N, Welch L. Velocity-dependent improvements in single-dot direction discrimination. Perception & Psychophysics. 1997;59(1):60–72. doi: 10.3758/bf03206848. [DOI] [PubMed] [Google Scholar]
- McKone E, Martini P, Nakayama K. Categorical perception of face identity in noise isolates configural processing. Journal of Experimental Psychology: Human Perception and Performance. 2001;27(3):573–599. doi: 10.1037//0096-1523.27.3.573. [DOI] [PubMed] [Google Scholar]
- Neri P, Morrone MC, Burr DC. Seeing biological motion. Nature. 1998;395(6705):894–896. doi: 10.1038/27661. [DOI] [PubMed] [Google Scholar]
- Newell FN, Bülthoff HH. Categorical perception of familiar objects. Cognition. 2002;85:113–143. doi: 10.1016/s0010-0277(02)00104-x. [DOI] [PubMed] [Google Scholar]
- Oram MW, Perrett DI. Responses of Anterior Superior Temporal (STPa) Neurons to “Biological Motion” Stimuli. Journal of Cognitive Neuroscience. 1994;6(2):99–116. doi: 10.1162/jocn.1994.6.2.99. [DOI] [PubMed] [Google Scholar]
- Parkes L, Lund J, Angelucci A, Solomon JA, Morgan M. Compulsory averaging of crowded orientation signals in human vision. Nature Neuroscience. 2001;4(7):739–744. doi: 10.1038/89532. [DOI] [PubMed] [Google Scholar]
- Perkins ME, Landy MS. Nonadditivity of masking by narrow-banned noises. Vision Research. 1991;31:1053–1065. doi: 10.1016/0042-6989(91)90209-n. [DOI] [PubMed] [Google Scholar]
- Peuskens H, Vanrie J, Verfaillie K, Orban GA. Specificity of regions processing biological motion. The European Journal of Neuroscience. 2005;21(10):2864–2875. doi: 10.1111/j.1460-9568.2005.04106.x. [DOI] [PubMed] [Google Scholar]
- Rauber HJ, Treue S. Reference repulsion when judging the direction of visual motion. Perception. 1998;27(4):393–402. doi: 10.1068/p270393. [DOI] [PubMed] [Google Scholar]
- Rossion B, Schiltz C, Robaye L, Pirenne D, Crommelinck How does the brain dissociate familiar and unfamiliar faces? A PET study of face categorical perception. Journal of Cognitive Neuroscience. 2001;13(7):1019–1034. doi: 10.1162/089892901753165917. [DOI] [PubMed] [Google Scholar]
- Schouten B, Troje NF, Verfaillie K. The facing bias in biological motion perception: structure, kinematics, and body parts. Attention, Perception & Psychophysics. 2011;73(1):130–143. doi: 10.3758/s13414-010-0018-1. [DOI] [PubMed] [Google Scholar]
- Schouten B, Troje NF, Vroomen J, Verfaillie K. The effect of looming and receding sounds on the perceived in-depth orientation of depth-ambiguous biological motion figures. PLoS One. 2011;6(2):e14725. doi: 10.1371/journal.pone.0014725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon JA. Channel selection with non-white-noise masks. Journal of the Optical Society of America. A, Optics, Image Science, and Vision. 2000;17:986–993. doi: 10.1364/josaa.17.000986. [DOI] [PubMed] [Google Scholar]
- Solomon JA. Noise reveals visual mechanisms of detection an discrimination. Journal of Vision. 2002;2:105–120. doi: 10.1167/2.1.7. [DOI] [PubMed] [Google Scholar]
- Sumpter DJ. The principles of collective animal behaviour. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2006;361(1465):5–22. doi: 10.1098/rstb.2005.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S. High-level pattern coding revealed by brief shape aftereffects. In: Clifford C, Rhodes G, editors. Fitting the mind to the world: Adaptation and aftereffects in high-level vision. Advances in visual cognition series. Vol. 2. Oxford University Press; 2005. pp. 135–172. [Google Scholar]
- Suzuki S, Cavanagh P. Focused attention distorts visual space: an attentional repulsion effect. Journal of Experimental Psychology. Human Perception and Performance. 1997;23(2):443–463. doi: 10.1037//0096-1523.23.2.443. [DOI] [PubMed] [Google Scholar]
- Sweeny TD, Haroz S, Whitney D. Perceiving group behavior: sensitive ensemble coding mechanisms for biological motion of human crowds. Journal of Experimental Psychology: Human Perception and Performance. doi: 10.1037/a0028712. In Press. [DOI] [PubMed] [Google Scholar]
- Sweeny TD, Grabowecky M, Kim YJ, Suzuki S. Internal curvature signal and noise in low- and high-level vision. Journal of neurophysiology. 2011;105(3):1236–1257. doi: 10.1152/jn.00061.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeny TD, Grabowecky M, Suzuki S. Simultaneous shape repulsion and global assimilation in the perception of aspect ratio. Journal of vision. 2011;11(1):16. doi: 10.1167/11.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman SM, Grossman ED. Temporal “Bubbles” reveal key features for point-light biological motion perception. Journal of Vision. 2008;8(3):28, 21–11. doi: 10.1167/8.3.28. [DOI] [PubMed] [Google Scholar]
- Troje NF, Sadr J, Geyer H, Nakayama K. Adaptation aftereffects in the perception of gender from biological motion. Journal of Vision. 2006;6(8):850–857. doi: 10.1167/6.8.7. [DOI] [PubMed] [Google Scholar]
- Troje NF, Westhoff C. The inversion effect in biological motion perception: evidence for a “life detector”? Current Biology. 2006;16(8):821–824. doi: 10.1016/j.cub.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Vaina LM, Lemay M, Bienfang DC, Choi AY, Nakayama K. Intact “biological motion” and “structure from motion” perception in a patient with impaired motion mechanisms: a case study. Visual Neuroscience. 1990;5(4):353–369. doi: 10.1017/s0952523800000444. [DOI] [PubMed] [Google Scholar]
- Vanrie J, Dekeyser M, Verfaillie K. Bistability and biasing effects in the perception of ambiguous point-light walkers. Perception. 2004;33(5):547–560. doi: 10.1068/p5004. [DOI] [PubMed] [Google Scholar]
- Vanrie J, Verfaillie K. Perception of biological motion: a stimulus set of human point-light actions. Behavior research methods, instruments, & computers. 2004;36(4):625–629. doi: 10.3758/bf03206542. [DOI] [PubMed] [Google Scholar]
- Wuerger SM, Crocker-Buque A, Meyer GF. Evidence for auditory-visual processing specific to biological motion. Seeing and perceiving. 2012;25(1):15–28. doi: 10.1163/187847611X620892. Wuerger, S. M., Parkes, L., Lewis, P. A., Crocker-Buque, A., Rutschmann, R., & Meyer, G. F. (2012). Premotor cortex is sensitive to auditory-visual congruence for biological motion. Journal of Cognitive Neuroscience, 24(3), 575-587. [DOI] [PubMed] [Google Scholar]