Abstract
Cisplatin derivatives are used as the mainline treatment of ovarian cancer, despite their severe side effects and development of resistance. We developed a novel combination therapy by combining cisplatin with withaferin A. Treatment of ovarian cancer cell lines with combination therapy acted synergistically to induce cell death, thus required a lower dose of cisplatin to achieve the same therapeutic effect. WFA and cisplatin combination induced cell death through the generation of reactive oxygen species (ROS) for WFA, while DNA damage for cisplatin, suggesting that cisplatin binds directly to DNA to form adducts while WFA indirectly damages DNA through ROS generation. Our results for the first time suggest that combining low dose of cisplatin with suboptimal dose of WFA can serve as a potential combination therapy for the treatment of ovarian cancer with the potential to minimize/eliminate the side effects associated with high doses of cisplatin.
Keywords: cisplatin, withaferin A, ovarian cancer, combination treatment, ROS, DNA damage
1. Introduction
The mainline treatment of ovarian cancer is cytoreductive surgery followed by platinum-based chemotherapy, namely carboplatin in combination with paclitaxel [1,2]. Initially, ovarian cancer responds positively in 70 to 80% of the cases [3]. However, approximately 70% of patients develop recurrent cancer and eventually succumb to their disease, which is attributed to the carcinomas having become platinum-resistant [3]. If the relapse occurs within 6 months of treatment, the carcinomas are considered platinum-resistant [3]. Despite the frequency of relapse, platinum-based chemotherapy remains the main stream for treatment of ovarian cancer [3], in which after five years only 30% of women survive [2]. The poor survival rate for women with platinum-resistant ovarian carcinomas points to an urgent need for an alternative treatment strategy.
Cis-diamminedichloroplatinum(II) (best known as cisplatin) is a platinum-based compound that has clinical activity against a wide spectrum of solid cancers including ovarian, testicular, bladder, colorectal, lung, and head and neck [4]. While cisplatin itself is inert, it spontaneously undergoes an aqueous reaction, resulting in the replacement of one or both cis-chloro groups with water leading to the generation of highly reactive mono- and bi-aquated cisplatin forms [5], which avidly bind DNA and cause formations of protein-DNA complexes and DNA-DNA inter-and intra-strand adducts [5]. In addition, aquated cisplatin interacts with cytoplasmic targets, such as reduced glutathione, to cause oxidative stress [4] in addition to generating superoxide anions and hydroxyl radicals [6]. The use of cisplatin is mainly limited by chemo-resistance [3,4], which can be intrinsic or acquired [7]. Side effects associated with cisplatin include nausea, vomiting, myelosuppression, hepatotoxicity, neurotoxicity, and ototoxicity [4,7]. However, the main limiting factor is cumulative nephrotoxicity as a result of ROS production inducing apoptosis [8,9].
Recently, to reduce the side effects and resistance caused by cisplatin-based chemotherapy a number of combinations with other compounds have been explored. Some of these include N-acetylcysteine [10], naltrexone [11], glutathione ester [12], vitamin E and losartan [13], melatonin [14], quercetin [15], metformin [16,17], and rehmannia [18]. However, none of the combinations have rendered the desired outcome of leading to clinical application.
Withaferin A (WFA) is a bioactive, cell permeable compound isolated from the plant Withania somniferia that has been a part of Indian traditional medicine for centuries and is now available as an over-the-counter dietary supplement in the US. It is being used to treat various disorders due to its anti-inflammatory, anti-bacterial, and cardio-protective properties. Recently, WFA has been suggested as a potential anti-cancer compound shown to prevent tumor growth, angiogenesis, and metastasis [19,20]. Several biological functions have been influenced by WFA including induction of apoptosis through inactivation of Akt and NF-κB [21] as well as decrease of pro-survival protein Bcl-2 [22,23], induction of Par-4 [24], inhibition of Hsp90 and Notch-1 [25], G2/M cell cycle arrest [19], FOXO3a and Bim regulation [26], generation of ROS [27,28], and down regulation of expression of HPV E6 and E7 oncoproteins [29]. However, the effect of WFA on ovarian cancer has not been studied, nor has the combined effects of WFA with cisplatin been explored.
We propose that WFA when combined with cisplatin will elicit a synergistic effect on the suppression of ovarian tumor growth, hence, will reduce the dosage requirement of cisplatin resulting in minimizing/eliminating the side effects, and induction of drug resistance associated with high doses of cisplatin. To test our hypothesis, we studied the combined effect of cisplatin and WFA on cisplatin-sensitive ovarian epithelial cancer cell line A2780, cisplatin-resistant variant A2780/CP70, and p53 mutant ovarian epithelial cell line CAOV3. Ovarian cancer cells treated with WFA (0.5 μM) in combination with low dose of cisplatin (20 μM) exhibited a synergetic effect on cell death through the generation of ROS leading to DNA damage and culminating in apoptosis.
2. Materials and Methods
2.1 Materials
RPMI, DMEM, FBS, penicillin/streptomycin, insulin, cisplatin, withaferin A, N-acetyl-L-cysteine, and DMSO were purchased from Sigma. Human epithelial ovarian tumor cisplatin-sensitive (A2780) cell line was obtained from Dr. Denise Connolly (Fox Chase Cancer Center, Philadelphia, PA). The cisplatin-resistant (A2780/CP70) cell line was obtained from Dr. Christopher States (University of Louisville, Louisville, KY). CAOV3 cell line was purchased from American Type Culture Collection (ATCC).
2.2 Cell culture and treatment with cisplatin and WFA
A2780 and A2780/CP70 cells were cultured in RPMI with 10% FBS, 1% penicillin/streptomycin, and 0.05% (v/v) Insulin. CAOV3 cells were cultured in DMEM with 10% FBS and 1% penicillin/streptomycin. Cells growing in log phase were trypsinized and seeded into 96 wells plates (approximately 5,000 cells/well). After 24 h of plating, cells were treated with various concentrations of cisplatin and WFA both alone or combination of cisplatin/WFA. Treatments of cells were performed in 5% FBS medium by adding cisplatin (final concentration of 2, 5 10, 20, 50 or 100 μM) solubilized in DMSO and/or WFA (final concentration of 0.1, 0.5, 1, 1.5, 2, 3, or 5 μM) solubilized in DMSO to a concentration of 0.2% (v/v). DMSO (0.2% v/v) was used as a vehicle control.
2.3 Cell proliferation assays
A2780, A2780/CP70, and CAOV3 cells were seeded into 96-wells plates. After 24 h of plating, cells were treated in triplicates with cisplatin and WFA alone or combination of cisplatin/WFA for 24 h, 48 h, or 72 h as described above. Twenty μl of MTT reagent from cell proliferation assay kit (Promega) was added to each well and cell proliferation was measured as described previously [30].
2.4 Isobologram analysis
A2780 cells were treated in triplicates for 48 h using 6 different concentrations of cisplatin and WFA at a constant ratio. Viable cells were quantitated with MTT assays as described above and fraction affected was calculated from percent inhibition. Fraction affected was then used in CalcuSyn software to generate an isobologram.
2.5 Measurement of cell apoptosis using flow cytometry for Annexin V
A2780 cells were treated with cisplatin and WFA both alone and in combination of cisplatin/WFA for 24 h and dissociated with versene (Invitrogen). Cells were resuspended in Annexin V binding buffer to a concentration of 1 × 106 cells/ml. Annexin V-FITC (2 μl, BD Biosciences) was incubated for 15 min in the dark in 100 μl of cells suspension. Propidium iodide (PI) was then spiked into 400 μl of Annexin V binding buffer and then was added immediately to cell suspension and used on a FACSCaliber (BD Biosciences) and analyzed with FlowJo software.
2.6 Measurement of the generation of ROS
A2780 cells (20,000/dish) were seeded on glass bottom 35 mm2 dishes overnight followed by treatment with cisplatin and WFA as described above for 24 h. Medium was replaced with fresh medium containing 2 μM H2DCFDA (Invitrogen) and incubated for 30 min at 37°C. Cells were washed with PBS, and examined under confocal microscopy [31]. Relative fluorescence (RF) of ROS positive cells was quantified at green channel using NIS-AR Elements analysis software (Nikon). RF values were measured from 8 representative fields from 2 independent experiments.
2.7 Measurement of DNA damage (TUNEL assay)
A2780 cells were plated on chamber slides and treated with cisplatin and WFA as described above. Cells were then assayed for DNA damage using Dead End Fluorometric TUNEL assay kit (Promega) according to the manufacturer's instructions. RF was quantified at green channel as described above.
2.8 Statistical analysis
Standard error of mean (SEM) and level of significance (P value) were calculated using unpaired non-parametric Mann-Whitney t-test using Graph Pad Prism software.
3. Results
3.1 WFA synergistically enhances the antitumor effects of cisplatin
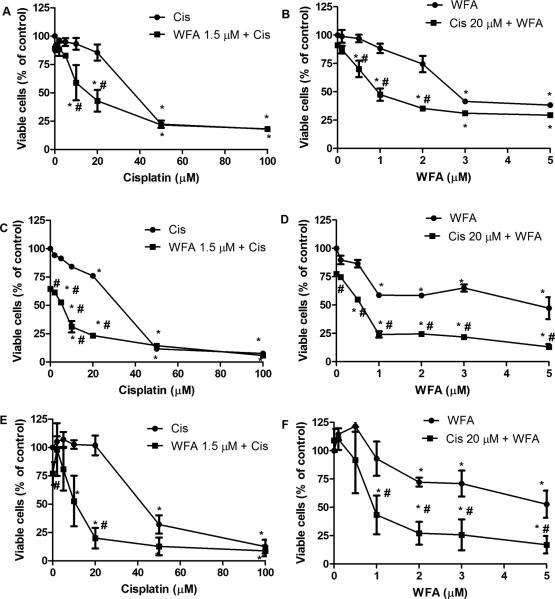
Patients treated with cisplatin-based chemotherapy present with serious side effects and eventually develop resistance to cisplatin. To overcome these problems, we combined WFA with cisplatin to minimize/eliminate the side effects associated with high doses of cisplatin. Two cisplatin-sensitive ovarian cancer cell lines A2780 and CAOV3 and one cisplatin-resistant ovarian cancer cell line A2780/CP70 were treated with various concentrations of cisplatin and WFA, both alone and in combination for 24 h, 48 h, and 72 h. Cell death induced was determined by MTT assays. Both cisplatin and WFA induced cell death in a time- and dose-dependent manner. After 48 h of treatment, IC50 values for cisplatin to inhibit cell proliferation of A2780, A2780/CP70, and CAOV3 cells were found to be 40 μM, 32 μM and 40 μM respectively (Fig. 1), which decreased significantly to 10 μM, 6 μM and 12 μM upon combination with WFA 1.5 μM (Fig. 1A, C, E). IC50 values for WFA alone was found to be 6 μM, 4.5 μM and 5 μM respectively, which decreased significantly to 0.8 μM, 0.6 μM, and 1 μM respectively upon combination with cisplatin 20 μM (Fig. 1B, D, F). These results indicate that interaction between cisplatin and WFA is synergistic in inducing cell death. Isobologram analysis using 6 different concentrations of WFA and cisplatin at a constant ratio (as little as 1:5) of WFA to cisplatin demonstrated that cisplatin and WFA acted synergistically (Fig. S1).
Figure 1.
Cell proliferation of A2780 (A-B), A2780/CP70 (C-D), and CAOV3 (E-F). Cells growing in log phase were trypsinized and plated into 96 wells plates (approximately 5000 cells/well). After 24 h of plating, cells were treated with various concentrations of cisplatin and WFA both alone or combination of cisplatin/WFA. After 48 h of treatment, cell proliferation was performed using MTT assays. Values shown are mean ± SD for three independent experiments. * p < 0.05 compared to control, # p < 0.05 compared to cisplatin (Cis) or WFA alone.
3.2 WFA enhances induction of apoptosis by cisplatin
Compared to control tumor cells (treated with vehicle; 0.2% DMSO), tumor cells treated with the cisplatin/WFA combination for 24 h, showed significant changes in morphology when visualized under a light microscope. Cells treated with cisplatin 20 μM alone or a low dose of WFA (0.5 μM) alone did not show any changes in cell morphology. Cells treated with WFA 1.5 μM began to exhibit moderate changes in morphology. However, co-treatment of cells with cisplatin 20 μM and WFA 1.5 μM resulted in a significant changes in cell morphology, showing fragmentation, rounding of cells, cellular granulation, loss of cytoplasm, chromatin condensation, and presence of apoptotic bodies similar to high concentration of cisplatin (100 μM, Fig. S2), indicating induction of cell apoptosis.
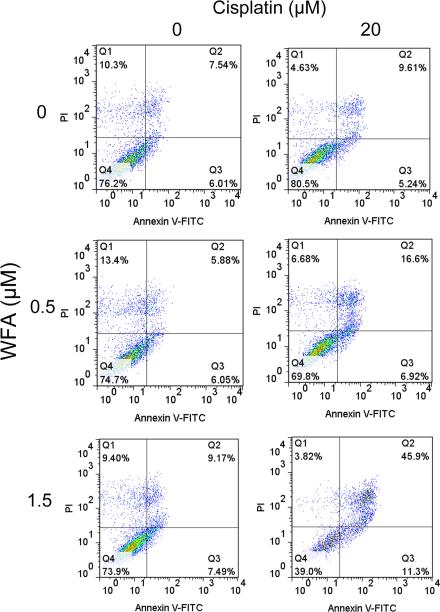
Induction of cell apoptosis upon co-treating the cells with cisplatin/WFA combination was further confirmed by Annexin V-FITC staining, analyzed by FACS analysis, which showed a significant increase of 46% apoptotic cells on co-treatment with cisplatin 20 μM with WFA 1.5 μM within 24 h of treatment as compared to DMSO treated (7.54%) or cells treated with cisplatin or WFA alone which each showed approximately 10% apoptotic cells (Fig. 2).
Figure 2.
Annexin V-FITC flow cytometry for the measurement of apoptosis. A2780 cells were treated with cisplatin and WFA both alone or combination of cisplatin/WFA for 24 h as described in Figure 1. Cells were washed with PBS and then dissociated with versene and stained with Annexin V-FITC and PI. Samples were run on a FACSCaliber and analysis was performed using FlowJo software.
3.3 WFA enhance the effect of cisplatin in generation of ROS
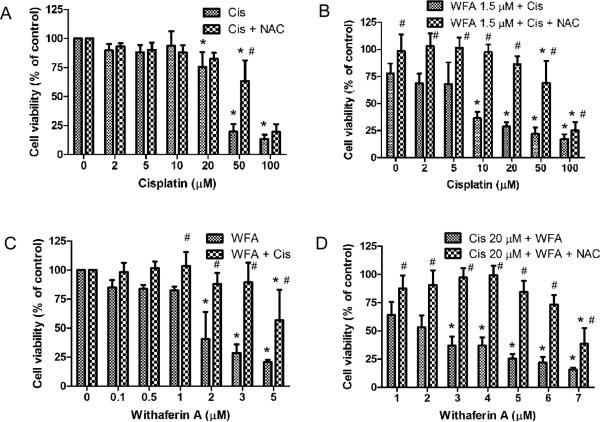
ROS is a key component of the antitumor activity of cisplatin based drugs in a variety of cancer cells. Numerous studies have suggested generation of ROS as a part of WFA's apoptotic mechanism (28,29). Therefore, we studied the combined effect of cisplatin and WFA on ROS production by using H2DCFDA as described by Das et al. [31]. Treatment of A2780 cells with cisplatin 20 μM and WFA 0.5 μM both alone resulted in a low number of positive cells with RF values of 105.89 ± 7.59 % and 132.63 ± 5.81% respectively compared to control (RF = 100%). WFA showed a dose-dependent induction of ROS as treatment with WFA 1.5 μM showed a significant increase in number of positive cells (RF = 260.70 ± 21.23%) compared to control (Fig. 3A and C). Co-treatment of cells with cisplatin 20 μM and WFA 0.5 μM or 1.5 μM showed a significant increase in ROS positive cells with RF values of 183.44 ± 4.00% and 260.00 ± 13.60% respectively (Fig. 3A and C). To test if ROS was a major mechanism of cell death induced by WFA, cisplatin, and combination of cisplatin/WFA, we pretreated A2780 cells with 5 mM N-acetyl-L-cysteine (NAC) for 90 min followed by treatment of cells with cisplatin, WFA, or cisplatin/WFA combination for 24 h. While NAC partially blocked cell death induced by cisplatin at higher concentration (Fig. 4A), it was very effective in blocking the cell death induced by WFA (Fig. 4B). Combination of WFA with cisplatin also showed a reversal of cell death by 70-80% (Fig. 4B-D), suggesting that ROS production is the major mechanism of WFA and cisplatin/WFA-induced cell death.
Figure 3.
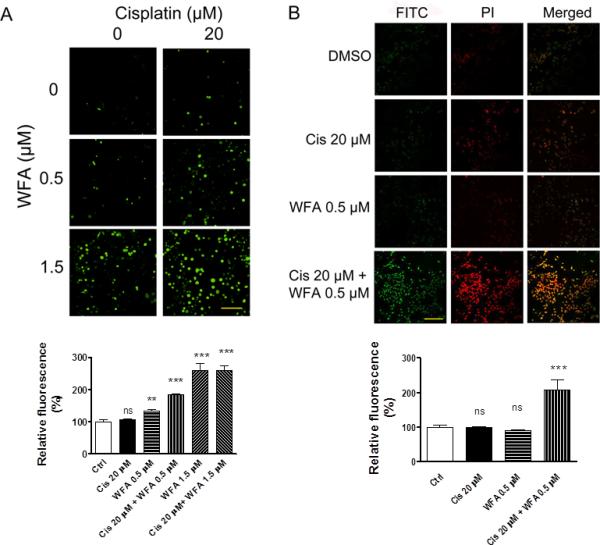
Effect of WFA and cisplatin on ROS production and DNA damage. A) Measurement of ROS in A2780 cells. A2780 cells were treated with cisplatin and WFA alone or combination of cisplatin/WFA for 24 h as described in Figure 1. The detection agent H2DCFDA was incubated for 30 min in medium. Cells were then washed with PBS and examined under a confocal microscope at 20X magnification. The data shown is representative of two independent experiments. B) Measurement of DNA damage using TUNEL assays. A2780 cells were treated with cisplatin and WFA both alone or combination of Cis/WFA for 24 h. Cells were washed with PBS and processed for TUNNEL assays. Images were obtained using confocal microscopy at 20X magnification. C) RF for ROS positive cells. D) RF for DNA damage cells. RF values for controls were taken as 100%. Scale bar indicates 100 μM. Values shown are mean ± SEM. Ns = no significance, ** = p<0.01, *** = p<0.0001.
Figure 4.
Effect of non-enzymatic ROS antioxidant NAC on A2780 cell proliferation. A2780 cells were pretreated with NAC for 90 min. The medium was changed and cells were treated with cisplatin and WFA alone or combination of Cis/WFA for 24 h as described in Figure 1. Cell proliferation was then determined using MTT assays. Values shown are mean ± SD for three independent experiments. * p < 0.05 compared to control, # p < 0.05 compared to no NAC and NAC.
3.4 WFA enhances DNA damage induced by cisplatin
To determine if combination of WFA with cisplatin enhances DNA damage, we performed TUNEL assays and counterstained the nuclei with PI. Untreated cells, cisplatin 20 μM, and WFA 0.5 μM treated cells resulted in a few positive cells with RF values of 98.32 ± 3.64% and 89.79 ± 2.70% respectively compared to control (RF = 100%). However, combination of cisplatin 20 μM with WFA 0.5 μM resulted in intense DNA damage in nearly every cell (Fig. 3B and D) with RF value of 208.14 ± 27.40%, showing a synergistic enhancement of DNA damage on treatment of cells with combination of cisplatin/WFA, indicating that combining WFA with cisplatin elicits synergistic effect on induction of DNA damage leading to cell death.
4. Discussion
Cisplatin has been used in combination with several compounds to reduce its associated side effects, such as cardiotoxicity which was reduced in rats pre-treated with either acetyl-L-carnitine, DL-α-lipoic acid, or silymarin [7], however, the effect of these combinations was not tested in tumor-bearing rats, and as such, the effect of these compounds on tumor growth is unknown. All three compounds inhibited nuclear and mitochondrial DNA fragmentation. Therefore, it is possible that they could antagonize the therapeutic effect of cisplatin [7]. WFA has been shown to have cardiotonic activity and provide a beneficial effect in chronic heart failure [32], and therefore, may be a potential protective agent against chemotherapy-induced cardiotoxicity.
Several compounds have been shown to ameliorate cisplatin-induced nephrotoxicity including proteasome inhibitors by preventing mitochondrial release of apoptosis-inducing factor [33] and knockdown of death-associated protein 5 (DAP5) through the translational regulation of Bcl-2 [34]. Bcl-2 overexpression in conjunction with p53 has been found in fresh ovarian tissue biopsies [35]. In addition Bcl-2 may play a role in cisplatin-resistance as chemo-resistant cell lines overexpress Bcl-2 and/or p53 [38]. WFA has been shown to down-regulate Bcl-2 expression [22], and therefore, may be a valuable compound in treating cisplatin-resistant carcinomas as well as reducing nephrotoxicity.
There has been increasing support for natural compounds in the treatment of cancer that can enhance the therapeutic effect of an anti-neoplastic agent so that a lower dose can be used to achieve the same anti-neoplastic effect while simultaneously avoiding or minimizing the side effects associated with high doses. In the present study, we showed that WFA was able to synergistically enhance the antitumor function of cisplatin in a time- and dose-dependent manner (Fig. 1, S1) while working through different primary mechanisms. WFA and cisplatin are known to produce ROS as part of their mechanism [8,9,27,28]. We showed that WFA induced ROS generation in a dose-dependent manner but was enhanced by the addition of cisplatin (Fig. 3A and C), suggesting that WFA and cisplatin are using different mechanisms to generate ROS. Blocking of ROS with NAC resulted in a complete remission of cell death in WFA treated cells but partially blocked cisplatin treated cells at higher concentration (Fig. 4), further confirming that ROS production is the main mechanism for WFA but not for cisplatin. ROS damage the mitochondrial membrane and result in leakage of ROS to the cytosol where they can damage other organelles in addition to causing DNA damage [36,37]. To measure DNA damage induced by ROS generation, we performed TUNEL assays and found a synergistic enhancement of DNA damage in cells when treated with cisplatin/WFA combination (Fig. 3B and D), suggesting that WFA produces ROS to cause DNA damage while cisplatin induces DNA damage mainly through direct binding of DNA and causing the formation of DNA adducts [5]. As a result, we observed a synergistic effect in induction of cell apoptosis analyzed by morphological changes (Fig. S2) and Annexin V-FITC flow cytometry (Fig. 2).
Furthermore, this combination strategy was just as effective with cisplatin-resistant cell line A2780/CP70, therefore this combination therapy has the potential to treat both cisplatin-sensitive and - resistant ovarian cancers (Fig. 1).
From our results we conclude that WFA when combined with cisplatin synergistically enhances the antitumor effects of cisplatin through the inhibition of cell proliferation, generation of ROS, and DNA damage cumulating in apoptosis (Fig. S3). Since combining WFA with cisplatin reduces the dosage requirement of cisplatin to achieve same level of therapeutic effects, this combination therapy has a great potential to serve as a treatment regimen for both cisplatin-sensitive and cisplatin-resistant ovarian cancers with minimal or no side effects associated with high doses of cisplatin.
Supplementary Material
Supplementary Figure Legends
Figure S1. Isobologram analysis of A2780 cells (n = 3) using 6 doses of cisplatin and WFA maintained at a constant ratio. MTT assays results were analyzed with CalcuSyn software.
Figure S2. Morphological analysis of A2780 cells upon treatment with cisplatin and WFA both alone and in combination of cisplatin/WFA. A2780 cells were treated with cisplatin and WFA both alone and combination of cisplatin/WFA for 24 h. Cells were examined under confocal microscope and photographed. The data shown is representative of three independent experiments.
Figure S2. Schematic diagram of the mechanisms of cisplatin combined with WFA.
Highlights.
Novel combination therapy using withaferin A (WFA) and cisplatin
Combination of WFA with cisplatin elicits synergistic cytotoxic effects
Combination of WFA with cisplatin significantly reduces the cisplatin dosage requirement
WFA induces ROS generation, whereas cisplatin induces DNA damage in combination treatment
Lower dose of cisplatin in combination therapy will minimize/eliminate side effects of cisplatin
Acknowledgements
The authors would like to thank Dr. Jorge Gomez Gutierrez for his technical assistance with FACS analysis, and Dr. Denise Connolly (Fox Chase Cancer Center, Philadelphia, PA) and Dr. Christopher States (University of Louisville, Louisville, KY) for providing cell lines. This work was supported by a research grant from NCI 124630 and Brown Cancer Center, University of Louisville (SSK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pfisterer J, Ledermann JA. Management of platinum-sensitive recurrent ovarian cancer. Semin Oncol. 2006;33:S12–16. doi: 10.1053/j.seminoncol.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Mei L, Chen H, Wei DM, Fang F, Liu GJ, Xie HY, Wang X, Zou J, Han X, Feng D. Maintenance chemotherapy for ovarian cancer. Cochrane Database Syst Rev. 2010:CD007414. doi: 10.1002/14651858.CD007414.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Matsuo K, Lin YG, Roman LD, Sood AK. Overcoming platinum resistance in ovarian carcinoma. Expert Opin Investig Drugs. 2010;19:1339–1354. doi: 10.1517/13543784.2010.515585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G. Molecular mechanisms of cisplatin resistance. Oncogene. 2011 doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 5.Eastman A. The formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. Pharmacol Ther. 1987;34:155–166. doi: 10.1016/0163-7258(87)90009-x. [DOI] [PubMed] [Google Scholar]
- 6.Wozniak K, Czechowska A, Blasiak J. Cisplatin-evoked DNA fragmentation in normal and cancer cells and its modulation by free radical scavengers and the tyrosine kinase inhibitor STI571. Chem Biol Interact. 2004;147:309–318. doi: 10.1016/j.cbi.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 7.El-Awady el SE, Moustafa YM, Abo-Elmatty DM, Radwan A. Cisplatin-induced cardiotoxicity: Mechanisms and cardioprotective strategies. Eur J Pharmacol. 2011;650:335–341. doi: 10.1016/j.ejphar.2010.09.085. [DOI] [PubMed] [Google Scholar]
- 8.Kintzel PE. Anticancer drug-induced kidney disorders. Drug Saf. 2001;24:19–38. doi: 10.2165/00002018-200124010-00003. [DOI] [PubMed] [Google Scholar]
- 9.Lieberthal W, Triaca V, Levine J. Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: apoptosis vs. necrosis. Am J Physiol. 1996;270:F700–708. doi: 10.1152/ajprenal.1996.270.4.F700. [DOI] [PubMed] [Google Scholar]
- 10.Lorito G, Hatzopoulos S, Laurell G, Campbell KC, Petruccelli J, Giordano P, Kochanek K, Sliwa L, Martini A, Skarzynski H. Dose-dependent protection on cisplatin-induced ototoxicity - an electrophysiological study on the effect of three antioxidants in the Sprague-Dawley rat animal model. Med Sci Monit. 2011;17:BR179–186. doi: 10.12659/MSM.881894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donahue RN, McLaughlin PJ, Zagon IS. Low-dose naltrexone suppresses ovarian cancer and exhibits enhanced inhibition in combination with cisplatin. Exp Biol Med (Maywood) 2011;236:883–895. doi: 10.1258/ebm.2011.011096. [DOI] [PubMed] [Google Scholar]
- 12.Campbell KC, Larsen DL, Meech RP, Rybak LP, Hughes LF. Glutathione ester but not glutathione protects against cisplatin-induced ototoxicity in a rat model. J Am Acad Audiol. 2003;14:124–133. [PubMed] [Google Scholar]
- 13.Nematbakhsh M, Ashrafi F, Safari T, Talebi A, Nasri H, Mortazavi M, Khazaei M, Baradaran-Mahdavi MM. Administration of vitamin E and losartan as prophylaxes in cisplatin-induced nephrotoxicity model in rats. J. Nephrol. 2012;25:410–417. doi: 10.5301/jn.5000018. [DOI] [PubMed] [Google Scholar]
- 14.Tuncer S, Dalkilic N, Akif Dunbar M, Keles B. Comparative effects of alpha lipoic acid and melatonin on cisplatin-induced neurotoxicity. Int J Neurosci. 2010;120:655–663. doi: 10.3109/00207454.2010.510916. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez-Gonzalez PD, Lopez-Hernandez FJ, Perez-Barriocanal F, Morales AI, Lopez-Novoa JM. Quercetin reduces cisplatin nephrotoxicity in rats without compromising its anti-tumour activity. Nephrol Dial Transplant. 2011;26:3484–3495. doi: 10.1093/ndt/gfr195. [DOI] [PubMed] [Google Scholar]
- 16.Yasmeen A, Beauchamp MC, Piura E, Segal E, Pollak M, Gotlieb WH. Induction of apoptosis by metformin in epithelial ovarian cancer: involvement of the Bcl-2 family proteins. Gynecol Oncol. 2011;121:492–498. doi: 10.1016/j.ygyno.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 17.Rattan R, Graham RP, Maguire JL, Giri S, Shridhar V. Metformin suppresses ovarian cancer growth and metastasis with enhancement of cisplatin cytotoxicity in vivo. Neoplasia. 2011;13:483–491. doi: 10.1593/neo.11148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu HH, Seo SJ, Kim YH, Lee HY, Park RK, So HS, Jang SL, You YO. Protective effect of Rehmannia glutinosa on the cisplatin-induced damage of HEI-OC1 auditory cells through scavenging free radicals. J Ethnopharmacol. 2006;107:383–388. doi: 10.1016/j.jep.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 19.Stan SD, Zeng Y, Singh SV. Ayurvedic medicine constituent withaferin a causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutr Cancer. 2008;60(Suppl 1):51–60. doi: 10.1080/01635580802381477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohan R, Hammers HJ, Bargagna-Mohan P, Zhan XH, Herbstritt CJ, Ruiz A, Zhang L, Hanson AD, Conner BP, Rougas J, Pribluda VS. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis. 2004;7:115–122. doi: 10.1007/s10456-004-1026-3. [DOI] [PubMed] [Google Scholar]
- 21.Oh JH, Kwon TK. Withaferin A inhibits tumor necrosis factor-alpha-induced expression of cell adhesion molecules by inactivation of Akt and NF-kappaB in human pulmonary epithelial cells. Int Immunopharmacol. 2009;9:614–619. doi: 10.1016/j.intimp.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Mayola E, Gallerne C, Esposti DD, Martel C, Pervaiz S, Larue L, Debuire B, Lemoine A, Brenner C, Lemaire C. Withaferin A induces apoptosis in human melanoma cells through generation of reactive oxygen species and down-regulation of Bcl-2. Apoptosis. 2011;16:1014–1027. doi: 10.1007/s10495-011-0625-x. [DOI] [PubMed] [Google Scholar]
- 23.Koduru S, Kumar R, Srinivasan S, Evers MB, Damodaran C. Notch-1 inhibition by Withaferin-A: a therapeutic target against colon carcinogenesis. Mol. Cancer Ther. 2010;9:202–210. doi: 10.1158/1535-7163.MCT-09-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srinivasan S, Ranga RS, Burikhanov R, Han SS, Chendil D. Par-4-dependent apoptosis by the dietary compound withaferin A in prostate cancer cells. Cancer Res. 2007;67:246–253. doi: 10.1158/0008-5472.CAN-06-2430. [DOI] [PubMed] [Google Scholar]
- 25.Yu Y, Hamza A, Zhang T, Gu M, Zou P, Newman B, Li Y, Gunatilaka AA, Zhan CG, Sun D. Withaferin A targets heat shock protein 90 in pancreatic cancer cells. Biochem Pharmacol. 2010;79:542–551. doi: 10.1016/j.bcp.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stan SD, Hahm ER, Warin R, Singh SV. Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res. 2008;68:7661–7669. doi: 10.1158/0008-5472.CAN-08-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malik F, Kumar A, Bhushan S, Khan S, Bhatia A, Suri KA, Qazi GN, Singh J. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic cell death of human myeloid leukemia HL-60 cells by a dietary compound withaferin A with concomitant protection by N-acetyl cysteine. Apoptosis. 2007;12:2115–2133. doi: 10.1007/s10495-007-0129-x. [DOI] [PubMed] [Google Scholar]
- 28.Lee TJ, Um HJ, Min do S, Park JW, Choi KS, Kwon TK. Withaferin A sensitizes TRAIL-induced apoptosis through reactive oxygen species-mediated up-regulation of death receptor 5 and down-regulation of c-FLIP. Free Radic Biol Med. 2009;46:1639–1649. doi: 10.1016/j.freeradbiomed.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 29.Munagala R, Kausar H, Munjal C, Gupta RC. Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells. Carcinogenesis. 2011;32:1697–1705. doi: 10.1093/carcin/bgr192. [DOI] [PubMed] [Google Scholar]
- 30.Hamid T, Malik MT, Kakar SS. Ectopic expression of PTTG1/securin promotes tumorigenesis in human embryonic kidney cells. Mol Cancer. 2005;4:3. doi: 10.1186/1476-4598-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das A, Durrant D, Mitchell C, Mayton E, Hoke NN, Salloum FN, Park MA, Qureshi I, Lee R, Dent P, Kukreja RC. Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction. Proc Natl Acad Sci U S A. 2010;107:18202–18207. doi: 10.1073/pnas.1006965107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Das PK, Malhotra CL, Prasad K. CARDIOTONIC ACTIVITY OF ASHWAGANDHINE AND ASHWAGANDHININE, TWO ALKALOIDS FROM WITHANIA ASHWAGANDHA, KAUL. Arch Int Pharmacodyn Ther. 1964;150:356–362. [PubMed] [Google Scholar]
- 33.Liu L, Yang C, Herzog C, Seth R, Kaushal GP. Proteasome inhibitors prevent cisplatin-induced mitochondrial release of apoptosis-inducing factor and markedly ameliorate cisplatin nephrotoxicity. Biochem Pharmacol. 2010;79:137–146. doi: 10.1016/j.bcp.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Gao JJ, Cai GY, Ning YC, Liu L, Yang JR, Dong D, Fu B, Lu Y, Cui SY, Chen XM. DAP5 Ameliorates Cisplatin-Induced Apoptosis of Renal Tubular Cells. Am J Nephrol. 2012;35:456–465. doi: 10.1159/000338302. [DOI] [PubMed] [Google Scholar]
- 35.Eliopoulos AG, Kerr DJ, Herod J, Hodgkins L, Krajewski S, Reed JC, Young LS. The control of apoptosis and drug resistance in ovarian cancer: influence of p53 and Bcl-2. Oncogene. 1995;11:1217–1228. [PubMed] [Google Scholar]
- 36.Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 37.Mazure NM, Pouyssegur J. Hypoxia-induced autophagy: cell death or cell survival? Curr Opin Cell Biol. 2010;22:177–180. doi: 10.1016/j.ceb.2009.11.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure Legends
Figure S1. Isobologram analysis of A2780 cells (n = 3) using 6 doses of cisplatin and WFA maintained at a constant ratio. MTT assays results were analyzed with CalcuSyn software.
Figure S2. Morphological analysis of A2780 cells upon treatment with cisplatin and WFA both alone and in combination of cisplatin/WFA. A2780 cells were treated with cisplatin and WFA both alone and combination of cisplatin/WFA for 24 h. Cells were examined under confocal microscope and photographed. The data shown is representative of three independent experiments.
Figure S2. Schematic diagram of the mechanisms of cisplatin combined with WFA.