Abstract
Mechanistic proposals for the carbocation cas cade reaction leading to the tricyclic sesquiterpene pentalenene are assessed in light of the results of isotopically sensitive branching experiments with the H309A mutant of pentalenene synthase. These experimental results support a mechanism for pentalenene formation involving a 7-protoilludyl cation intermediate that was first predicted using quantum chemical calculations.
Keywords: terpene, kinetic isotope effect, quantum chemical calculations, carbocation, reaction mechanism, natural product biosynthesis
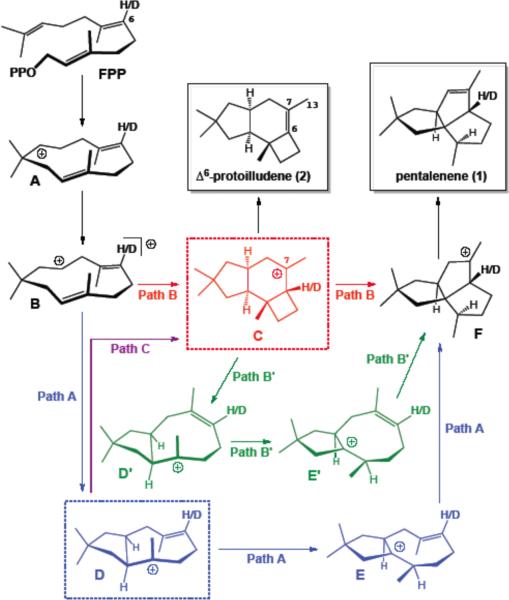
Pentalenene (1, Scheme 1) is a tricyclic sesquiterpene,1,2 produced in Nature from farnesyl diphosphate (FPP) through a cationic cascade reaction promoted by the enzyme pentalenene synthase.3 The mechanism of this transformation is one of the most highly studied terpene-forming reactions,3,4,5 in part due to the efficient generation of complexity that accompanies conversion of FPP — the universal acyclic, achiral precursor of all sesquiterpenes — into pentalenene—a tricyclic, chiral, stereodense product.
Scheme 1.
Three mechanisms proposed for the formation of pentalenene (1) and Δ6-protoilludene (2) from (E,E)-farnesyl diphosphate.
At least two mechanisms have been suggested for the formation of pentalenene from FPP. Path A in Scheme 1 (A→B→D→E→F) represents the earliest and until recently the most commonly accepted mechanistic proposal, involving conversion of the humulenyl cations A and B to a secoillud-6-en-3-yl cation (D) that then undergoes a 1,2-hydride shift and subsequent cyclization to produce the penultimate intermediate, the pentalenyl cation (F).3,4 The basic details of this mechanism have been supported by a wide range of experiments with stereospecifically labeled FPP and determination of the precise position and stereochemistry of isotopic labeling in the enzymatically derived pentalenene product.3,4 In 2006, Guttaand Tantillo proposed an alternative cyclization mechanism leading from B to F, based on quantum chemical calculations (mPW1PW91/6-31+G(d,p)//B3LYP/6-31+G(d,p) in the absence of the enzyme active site).2d,5 In this mechanism, the 7-protoilludyl cation (C), formed directly from B, would be a mandatory intermediate along the pathway to pentalenene (Path B, Scheme 1; A→B→C→F).5 Although this mechanism invokes an unexpected intermediate (C) followed by an unusual dyotropic rearrangement (C→F),6,7 it is completely consistent with all reported mechanistic and stereochemical results on the pentalenene synthase reaction.3,4 Moreover, the predicted intermediacy of the protoilludyl cation C is also consistent with the previously reported formation of the corresponding deprotonation product, Δ6-protoilludene (2), as a minor (10–13%) coproduct of pentalenene resulting from the cyclization of FPP by the four pentalenene synthase active site mutants H309A, H309C, H309S, and H309F.4d It is also noteworthy that refluxing Δ7,13-protoilludene or either epimer of the 7-protoilludyl alcohol in formic acid gives pentalenene in up to 28% yield, consistent with the intermediacy of a species such as cation C.8 Although the enzymatic generation of cation C by the pentalenene synthase mutants had previously been thought to result from diversion (Path C) of the natural cyclization Path A,4d,e the quantum mechanical calculations would place the protoilludyl cation C directly on the natural cyclization Path B. More recently, further quantum mechanical calculations on other possible conformations of intermediate C have revealed that C can be converted to F by an alternative stepwise rearrangement, illustrated as Path B' in Scheme 1 (A→B→C→DE'→EE'→F), in which DE' and EE' are geometric isomers of D and E, having Z rather than E C=C double bonds. In fact, Path B' is predicted to have a barrier of only approximately 6 kcal/mol for the conversion of C to F from the lowest energy conformer of C (at the mPW1PW91/6-31+G(d,p)//B3LYP/6-31+G(d,p) level),9 considerably lower than the barrier of nearly 20 kcal/mol for the direct dyotropic reaction.5,7
While these experimental observations and calculations are consistent with a 7-protoilludyl cation intermediate, they do not provide conclusive evidence as to whether such an intermediate is on the direct pathway to pentalenene (Paths B/B') or instead represents a derailment of the pentalenene pathway (Path C). The production of both 1 and 2 by H309A pentalenene synthase does, however, provide us with an opportunity to distinguish Path A from Paths B/B' using the well-established method of the isotopically sensitive branching experiment.10,11 A key difference between these two mechanistic scenarios is the point at which each pathway diverges toward pentalenene and Δ6-protoilludene. For the mechanism of Path A, the branch point for commitment to the formation of either the natural product pentalenene or the derailment product Δ6-protoilludene would be cation D. By contrast, for both Paths B and B', the branch point is the protoilludyl cation itself (C). We therefore envisaged that substitution of the C-6 proton of FPP by deuterium in [6-2H]-FPP should have essentially no effect on the ratio of 1 to 2 if the cyclization mechanism proceeds through intermediate cation D via Path A, but will result in an increase in the ratio of 1:2 if either Path B or B' is followed, due to a primary deuterium kinetic isotope effect (KIE) on the deprotonation of C to give 2. The KIE that suppresses the formation of Δ6-protoilludene when using [6-2H]-FPP as substrate will result in a diversion of the common cation C intermediate toward pentalenene, with Path B or B' resulting in a net increase in the ratio of the final products 1 and 2.
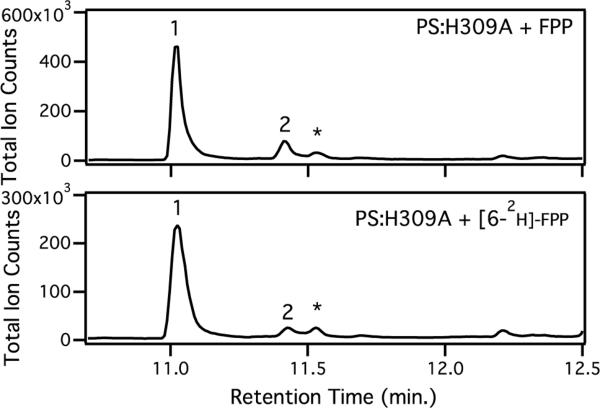
Incubation of FPP with the purified recombinant pentalenene synthase mutant H309A gave a 6.0:1 mixture of pentalenene (1, 81%) and the derailment product Δ6-protoilludene (2, 13.4 ± 0.3%), accompanied by minor (<6%) quantities of germacrene A, detected as the derived Cope rearrangement product, β-elemene, as previously described, consistent with the results of earlier reported incubations with H309 mutants.4d–e The assays were carried out in triplicate and analyzed by capillary GC-MS (Figure 1, top) with the identity of each product confirmed by comparison of both EI mass spectrum and retention index with standards in the MassFinder 4.0 database.12 When using [6-2H]-FPP as substrate,13 the distribution of sesquiterpene products was significantly shifted, with the intensity of the protoilludene peak being reduced to only 7.5 ± 0.4% of the total products while the intensity of the pentalenene peak increased to 87% (Figure 1, bottom). This nearly 2-fold increase in the ratio of 1:2 (11.6 vs. 6.1) as a result of isotopically sensitive branching establishes that the protoilludyl cation C is a common intermediate of the pathways for formation of both 1 and 2, as required by either Path B or B', but inconsistent with formation of cation C as a diversion product of Path A to pentalenene (assuming that C and D do not rapidly interconvert, i.e., for Path A, conversion of D to C is effectively irreversible). The observed increase in the ratio of 1:2 corresponds to a primary kH/kD isotope effect of 1.9 on the deprotonation of cation C to yield 2, consistent with previously measured kH/kD values for deprotonation of tertiary carbocations in terpene synthase-promoted reactions (typically ranging from ~2–6).11 Quantum chemical calculations using H2PO4− as a model base predict a kH/kD in the range of 1.6–1.8.14,15 The conversion of C→F, whether by Path B or B', is expected to be subject to at most a small normal secondary KIE as C6 changes from sp3 toward sp2 hybridization in the transition state structures for the C→F5 or C→D' reactions (these assumptions are supported by our quantum chemical calculations14). By contrast, the diversion of cation D, formed by the previously postulated Path A, to the protoilludyl cation C,16 is expected to be subject to only a minor secondary KIE (kH/kD slightly less than 1), since C6 would be changing from sp2 toward sp3 hybridization. Similarly, conversion of D to E along Path A should have no KIE, since H6 is not directly involved in this step. Path A for the cyclization of FPP to pentalenene by way of cation D is therefore excluded by the observation of a decrease in the proportion of 2 due to isotopically sensitive branching of the common intermediate C, whether it is further converted to pentalenene (1) by Path B or Path B'. The mechanisms proposed on the basis of results from quantum chemical calculations on the enzyme-free reaction mechanism are therefore fully consistent with the experimental results described herein. Further experimentation will be necessary to distinguish between the downstream Paths B and B'.16
Figure 1.
Effect of deuteration at C-6 of FPP on the ratio of products generated by H308A pentalenene synthase. GC-MS chromatograms of reaction mixture from either unlabeled FPP (top) or [6-2H]-FPP (bottom); * indicates β-elemene from Cope rearrangement of germacrene A.
Supplementary Material
ACKNOWLEDGMENT
D.J.T., L.Z. and M.W.L. gratefully acknowledge the National Science Foundation (grants CHE-0957416, CHE-0449845 and CHE-030089 with the Pittsburgh Supercomputer Center) for support. D.E.C. is supported by NIH grant GM30301. M.X. and R.J.P. are supported by NIH grant GM076324. We acknowledge Dr. Wayne K. W. Chou for performing the analysis with the MassFinder 4.0 database, Ms. Taylor Chesnut for assistance with the enzyme assays, and Drs. Pradeep Gutta and Dan Willenbring for their work on preliminary quantum chemical calculations.
Footnotes
Supporting Information. Additional details on computations, including full Gaussian citation. Description of enzyme assays and product identification. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions L.Z., M.X. and M.W.L. contributed equally to this work.
REFERENCES
- 1.(a) Seto H, Yonehara H. J. Antibiot. 1980;33:92–93. doi: 10.7164/antibiotics.33.92. [DOI] [PubMed] [Google Scholar]; (b) Cane DE, Sohng JK, Williard PG. J. Org. Chem. 1992;57:844–852. [Google Scholar]
- 2.(a) Cane DE. Compr. Nat. Prod. Chem. 1999;2:155–200. [Google Scholar]; (b) Cane DE. Chem. Rev. 1990;90:1089–1103. [Google Scholar]; (c) Davis EM, Croteau R. Top. Curr. Chem. 2000;209:53–95. [Google Scholar]; (d) Tantillo DJ. Nat. Prod. Rep. 2011;28:1035–1053. doi: 10.1039/c1np00006c. [DOI] [PubMed] [Google Scholar]
- 3.(a) Cane DE, Tillman AM. J. Am. Chem. Soc. 1983;105:122–124. [Google Scholar]; (b) Cane DE, Sohng J-K, Lamberson CR, Rudnicki SM, Wu Z, Lloyd MD, Oliver JS, Hubbard BR. Biochemistry. 1994;33:5846–5857. doi: 10.1021/bi00185a024. [DOI] [PubMed] [Google Scholar]; (c) Miller DJ, Allemann RK. Nat. Prod. Rep. 2012;29:60–71. doi: 10.1039/c1np00060h. [DOI] [PubMed] [Google Scholar]
- 4.(a) Cane DE, Abell C, Lattman R, Kane CT, Hubbard BR, Harrison PHM. J. Am. Chem. Soc. 1988;110:4081–4082. [Google Scholar]; (b) Cane DE, Oliver JS, Harrison PHM, Abell C, Hubbard BR, Kane CT, Lattman R. J. Am. Chem. Soc. 1990;112:4513–4524. [Google Scholar]; (c) Harrison PHM, Oliver JS, Cane DE. J. Am. Chem. Soc. 1988;110:5922–5923. [Google Scholar]; (d) Seemann M, Zhai G, Umezawa K, Cane DE. J. Am. Chem. Soc. 1999;121:591–592. [Google Scholar]; (e) Seemann M, Zhai G, de Kraker J-W, Paschall CM, Christianson DW, Cane DE. J. Am. Chem. Soc. 2002;124:7681–7689. doi: 10.1021/ja026058q. [DOI] [PubMed] [Google Scholar]; (f) Lesburg CA, Zhai G, Cane DE, Christianson DW. Science. 1997;277:1820–1824. doi: 10.1126/science.277.5333.1820. [DOI] [PubMed] [Google Scholar]
- 5.Gutta P, Tantillo DJ. J. Am. Chem. Soc. 2006;128:6172–6179. doi: 10.1021/ja058031n. [DOI] [PubMed] [Google Scholar]
- 6.Reetz MT. Angew. Chem., Int. Ed. Engl. 1972;11:129–130. [Google Scholar]; (b) Reetz MT. Angew. Chem., Int. Ed. Engl. 1972;11:130–131. [Google Scholar]; (c) Reetz MT. Tetrahedron. 1973;29:2189–2194. [Google Scholar]; (d) Reetz MT. Adv. Organomet. Chem. 1977;16:33–65. [Google Scholar]; (e) Hoffmann R, Williams JE., Jr. Helv. Chim. Acta. 1972;55:67–75. [Google Scholar]; (f) Fernández I, Cossío FP, Sierra MA. Chem. Rev. 2009;109:6687–6711. doi: 10.1021/cr900209c. [DOI] [PubMed] [Google Scholar]
- 7.We have recently found that dyotropic rearrangement via a different conformer of C than described in ref. 5 can occur through a transition state structure that is ~12 kcal/mol lower in energy than the one described in ref. 5, i.e., corresponding to a barrier for the dyotropic rearrangement step of slightly less than 20 kcal/mol; a full account of this work will be reported in due course.
- 8.(a) Ohfune Y, Shirahama H, Matsumoto T. Tetrahedron Lett. 1976:2869–2872. [Google Scholar]; (b) Misumi S, Ohtsuka T, Ohfune Y, Sugita K, Shirahama H, Matsumoto T. Tetrahedron Lett. 1979:31–34. [Google Scholar]; (c) Pattenden G, Teague SJ. Tetrahedron. 1987;43:5637–5652. [Google Scholar]
- 9.(a) A full account of the conformational potential energy surface for C and D' will be published in due course. (b) Note that no minimum corresponding to D has yet been located, likely due to the proximity of the carbocation center and π-bond in such structures.
- 10.Jones JP, Korzekwa KR, Rettie AE, Trager WF. J. Am. Chem. Soc. 1986;108:7074–7078. [Google Scholar]
- 11.(a) Croteau RB, Wheeler CJ, Cane DE, Ebert R, Ha H-J. Biochemistry. 1987;26:5383–5389. doi: 10.1021/bi00391a025. [DOI] [PubMed] [Google Scholar]; (b) Wagschal KC, Pyun H-J, Coates RM, Croteau R. Arch. Biochem. Biophys. 1994;308:477–487. doi: 10.1006/abbi.1994.1068. [DOI] [PubMed] [Google Scholar]; (c) Nes WD, McCourt BS, Marshall JA, Ma J, Dennis AL, Lopez M, Li H, He L. J. Org. Chem. 1999;64:1535–1542. doi: 10.1021/jo9819943. [DOI] [PubMed] [Google Scholar]; (d) Schenk DJ, Starks CM, Manna KR, Chappell J, Noel JP, Coates RM. Arch. Biochem. Biophys. 2006;448:31–44. doi: 10.1016/j.abb.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) He X, Cane DE. J. Am. Chem. Soc. 2004;126:2678–2679. doi: 10.1021/ja039929k. [DOI] [PubMed] [Google Scholar]; (f) Wagschal K, Savage TJ, Croteau R. Tetrahedron. 1991;47:5933–5944. [Google Scholar]; (g) Pyun HJ, Coates RM, Wagschal KC, McGeady P, Croteau RB. J. Org. Chem. 1993;58:3998–4009. [Google Scholar]
- 12. http://www.massfinder.com.
- 13.(a) [6-2H]-FPP was synthesized using a slightly modified version of the reported procedure (see Supporting Information for details). Cane DE, Tandon M. Tetrahedron Lett. 1994;35:5355–5358.
- 14.Predicted kH/kD values were computed using the Bigeleisen and Mayer method, as implemented in the program Quiver (Bigeleisen J, Mayer MG. J. Chem. Phys. 1947;15:261–267.; Saunders M, Laidig KE, Wolfsberg M. J. Am. Chem. Soc. 1989;111:8989–8994.; A modified version of Quiver provided by Prof. Daniel Singleton (Texas A&M) was utilized. The range for kH/kD reported in the text, reflects deprotonation from different conformers of C. See Supporting Information for additional details. This is part 9 of our series on sesquiterpene-related calculations; part 8: Hong YJ, Tantillo DJ. Chem. Commun. 2012;48:1571–1573. doi: 10.1039/c1cc14414f.
- 15.The active site histidine is not the base that performs deprotonation, since the H309A and related mutants all retain significant pentalenene synthase activity.4 A plausible candidate for the Brønsted base is the inorganic pyrophosphate originally released by the pentalenene synthase-catalyzed ionization of FPP, thereby prompting us to use H2PO4− as the model base in our calculations. Reports in which pyrophosphate has been proposed to be the base that carries out the final deprotonation to terminate terpene-forming reactions include: Roy A, Roberts FG, Wilderman PR, Zhou K, Peters RJ, Coates RM. J. Am. Chem. Soc. 2007;129:12453–12460. doi: 10.1021/ja072447e. Green-hagen BT, O'Maille PE, Noel JP, Chappell J. Proc. Natl. Acad. Sci.U. S. A. 2006;103:9826–9831. doi: 10.1073/pnas.0601605103. Shishova EY, Di Costanzo L, Cane DE, Christianson DW. Biochemistry. 2007;46:1941–1951. doi: 10.1021/bi0622524. Peters RJ, Croteau RB. Arch. Biochem. Biophys. 2003;417:203–211. doi: 10.1016/s0003-9861(03)00347-3. Hong YJ, Tantillo DJ. Org. Biomol. Chem. 2010;8:4589–4600. doi: 10.1039/c0ob00167h. Garms S, Chen F, Boland W, Gershenzon J, Köllner TG. Phytochem. 2012;75:6–13. doi: 10.1016/j.phytochem.2011.12.009. Zhou K, Peters RJ. Chem. Commun. 2011;47:4074–4080. doi: 10.1039/c0cc02960b. (h) See also ref. 3c.
- 16.Note, however, that (6Z)-Asterisca-3(15),6-diene, which would arise from direct deprotonation of D' is a known natural product. See: Mehta G, Umarye JD. Tetrahedron Lett. 2001;42:8101–8104. Fricke C, Hardt IH, König WA, Joulain D, Zygadlo JA, Guzman CA. J. Nat. Prod. 1999;62:694–696. doi: 10.1021/np980424v.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.