Abstract
OBJECTIVE
We examined the prevalence, extent, severity, and prognosis of coronary artery disease (CAD) in individuals with and without diabetes (DM) who are similar in CAD risk factors.
RESEARCH DESIGN AND METHODS
We identified 23,643 consecutive individuals without known CAD undergoing coronary computed tomography angiography. A total of 3,370 DM individuals were propensity matched in a 1-to-2 fashion to 6,740 unique non-DM individuals. CAD was defined as none, nonobstructive (1–49% stenosis), or obstructive (≥50% stenosis). All-cause mortality was assessed by risk-adjusted Cox proportional hazards models.
RESULTS
At a 2.2-year follow-up, 108 (3.2%) and 115 (1.7%) deaths occurred among DM and non-DM individuals, respectively. Compared with non-DM individuals, DM individuals possessed higher rates of obstructive CAD (37 vs. 27%) and lower rates of having normal arteries (28 vs. 36%) (P < 0.0001). CAD extent was higher for DM versus non-DM individuals for obstructive one-vessel disease (19 vs. 14%), two-vessel disease (9 vs. 7%), and three-vessel disease (9 vs. 5%) (P < 0.0001 for comparison), with higher per-segment stenosis in the proximal and mid-segments of every coronary artery (P < 0.001 for all). Compared with non-DM individuals with no CAD, risk of mortality for DM individuals was higher for those with no CAD (hazard ratio 3.63 [95% CI 1.67–7.91]; P = 0.001), nonobstructive CAD (5.25 [2.56–10.8]; P < 0.001), one-vessel disease (6.39 [2.98–13.7]; P < 0.0001), two-vessel disease (12.33 [5.622–27.1]; P < 0.0001), and three-vessel disease (13.25 [6.15–28.6]; P < 0.0001).
CONCLUSIONS
Compared with matched non-DM individuals, DM individuals possess higher prevalence, extent, and severity of CAD. At comparable levels of CAD, DM individuals experience higher risk of mortality compared with non-DM individuals.
Current American Diabetes Association guidelines endorse the widespread use of cardiovascular prevention measures for individuals with diabetes (DM) based upon an abundance of observational and population-based studies demonstrating excess cardiovascular risk and death attributable to diabetes (1). However, previous observational data examining the precise incremental risk of DM for future adverse coronary artery disease (CAD) events have demonstrated variability, and outcomes-based analyses of DM individuals generally have lacked information regarding CAD prevalence, extent, and severity (2–5).
Coronary computed tomography angiography (CCTA) is a noninvasive imaging test that demonstrates high diagnostic performance for the detection and exclusion of CAD, with recent multicenter studies demonstrating a robust prognostic utility to CCTA CAD findings for the prediction of mortality and other major adverse cardiac events (6–9). CCTA studies to date have examined CAD findings and prognosis in DM individuals but have been limited to single centers in small patient cohorts (n = 140–313) (10,11). In addition, DM and non-DM individuals within these studies demonstrated important differences in age, sex, and CAD risk factors, thus precluding the precise influence of the DM state to CAD presence and risk.
From a large international prospective multicenter observational cohort study of individuals undergoing CCTA, we thus sought to compare the prevalence, extent, severity, and prognosis of CAD for propensity-matched DM and non-DM individuals.
RESEARCH DESIGN AND METHODS
The COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter Registry (CONFIRM) is a dynamic, prospective, international multicenter registry whose rationale and design has been described previously (12). In brief, the CONFIRM enrolled consecutive adults, aged ≥18 years, between 2005 and 2009 who underwent ≥64-detector row CCTA for suspected CAD at 12 centers (Cedars Sinai Medical Center, Los Angeles, CA; Harbor University of California Los Angeles Medical Center, Los Angeles, CA; Tennessee Heart and Vascular Institute, Hendersonville, TN; Capital Cardiology Associates, Albany, NY; University of Munich, Munich, Germany; Ottawa Heart Institute, Ottawa, Ontario, Canada; Henry Ford Medical Center, Detroit, Michigan; Yonsei Medical Center, Seoul, Korea; University Hospital, Zurich, Switzerland; William Beaumont Hospital, Royal Oak, MI ; Walter Reed Armey Medical Center, Washington, DC; University Hospital of Parma, Parma, Italy) from six countries. Individuals with known CAD, as defined by prior myocardial infarction or coronary revascularization, were excluded from the current study analysis.
All patients were in normal sinus rhythm and were capable of the breath hold needed for CCTA. Patients with heart rates >70 beats per minute (bpm) were given oral or intravenous metoprolol as per local site protocol. All centers used intravenous metoprolol at the time of CCTA performance to lower heart rates to <70 bpm. If the patient’s heart rate did not drop to <70 bpm, CCTA was performed at the lowest heart rate.
Prior to the initiation of the scan, we prospectively collected information on the presence of categorical cardiac risk factors in each individual. Systemic arterial hypertension was defined as a documented history of high blood pressure or treatment with antihypertensive medications. DM was defined by a previous diagnosis of DM made by a physician (using a fasting glucose threshold of ≥126 mg/dL) and/or use of insulin or oral hypoglycemic agents. Dyslipidemia was defined as known but untreated dyslipidemia or current treatment with lipid-lowering medications. A positive smoking history was defined as current smoking or cessation of smoking within 3 months of testing. Family history of premature coronary heart disease was determined by patient query. Symptom presentation was classified into one of four categories: typical angina, atypical angina, noncardiac pain, or dyspnea. Age, sex, and angina typicality for each patient were used to determine the expected pretest probability of CAD with >50% luminal diameter stenosis according to Diamond and Forrester criteria (13).
Scan protocol and image reconstruction
CCTA scans were performed on multiple scanner platforms (Light speed VCT, GE Healthcare, Milwaukee, WI; Somatom Definition CT, Siemens, Ehrlangen, Germany; Somatom Definition Flash CT, Siemens, Ehrlangen, Germany). During the CCTA acquisition, 80–140 mL iodinated contrast (Isovue 370, Bracco Diagnostics, Princeton, NJ; Omnipaque, GE Healthcare, Princeton, NJ; Visipaque, GE Healthcare, Princeton, NJ; Imeron 350, Bracco Atlana Pharma, Konstanz, Germany) was injected. Contrast timing was performed to optimize uniform contrast enhancement of the coronary arteries. The scan parameters were 64 × 0.625/0.750 mm collimation and tube voltage 100 or 120 kVp, and the tube current was assigned based on body size and scanner platform.
Dose-reduction strategies, including electrocardiogram-gated tube current modulation, reduced tube voltage, and prospective axial triggering, were used whenever feasible. Estimated radiation doses ranged from 3 to 18 mSv.
Helical or axial scan data were obtained with retrospective or prospective electrocardiogram gating, respectively. Images were reconstructed immediately after completion of the scan to identify motion-free coronary artery images. Optimal phase reconstruction was assessed by comparison of different phases, if available, and the phase with the least amount of coronary artery motion was chosen for analysis. Multiple phases were used for image interpretation if minimal coronary artery motion was different for different arteries. CCTAs were evaluated by an array of postprocessing imaging techniques, including axial, multiplanar reformat, maximum intensity projection, and short-axis cross-sectional views. In all individuals, irrespective of image quality, every arterial segment was scored in an intent-to-diagnose fashion. If a coronary artery segment was uninterpretable despite these multiple techniques, the nonevaluable segment was scored similarly to the most proximal segment that was evaluable.
Noninvasive coronary artery analysis by CCTA
All scans were analyzed by level III–certified cardiologists with experience interpreting several thousand CCTA scans. In direct accordance with the Society of Cardiovascular Computed Tomography guidelines, CCTA interpretation was uniform across all study sites, with coronary segments visually scored for the presence of coronary plaque using a 16-segment coronary artery model in an intent-to-diagnose fashion (14). In each coronary artery segment, coronary atherosclerosis was defined as tissue structures >1 mm2 that existed either within the coronary artery lumen or adjacent to the coronary artery lumen that could be discriminated from surrounding pericardial tissue, epicardial fat, or the vessel lumen itself. Coronary atherosclerotic lesions were quantified for stenosis by visual estimation. Luminal diameter stenosis severity was scored as none (0% luminal stenosis), nonobstructive (1–49% luminal stenosis), or obstructive (≥50% luminal stenosis). Percent obstruction of coronary artery lumen was based on a comparison of the luminal diameter of the segment exhibiting obstruction to the luminal diameter of the most normal-appearing site immediately proximal to the plaque. In instances in which plaque was highly calcified, two-dimensional oblique images also were visualized without maximal intensity projection (i.e., 0.625–0.75 mm isotropic voxel resolution) or multiplanar reformats with cross-sectional views to minimize partial volume averaging artifact of calcium.
Plaque severity was graded on a per-patient, per-vessel, and per-segment level. Per-patient maximal stenosis severity was defined by the maximum intraluminal stenosis in any of the coronary segments at the ≥50% stenosis threshold. For purposes of classification for per-vessel analyses, we considered four arterial territories: left main artery, left anterior descending (LAD) artery, left circumflex (LCx) artery, and right coronary artery (RCA). Obstructive CAD in the diagonal branches, obtuse marginal branches, and posterolateral branches was considered to be part of the LAD, LCx, and RCA system, respectively. The posterior descending artery was considered as part of the RCA or LCx system, dependent upon the coronary artery dominance. A ≥50% stenosis in the left main artery was considered obstructive in all models. Per-vessel CAD severity was defined by ≥50% stenosis in zero, one, two, or three coronary artery vessels.
Per-segment analysis was judged for individual coronary artery segments that included the left main artery; proximal, mid-, and distal LAD; first and second diagonal branch; proximal and distal LCx; first and second obtuse marginal branch; proximal, mid-, and distal RCA; left and right posterolateral artery; and posterior descending artery. Clinical coronary artery plaque scores were calculated, as we have previously described (15). A segment involvement score (SIS) reflected CAD distribution and was calculated as the total number of coronary artery segments exhibiting plaque, irrespective of the degree of luminal stenosis within each segment (minimum = 0; maximum = 16). A segment stenosis score (SSS) was used as a measure of overall coronary artery plaque burden. Each individual coronary segment was graded as having no to severe plaque (i.e., scores from 0 to 3) based on the extent of the obstruction of the coronary luminal diameter. Then, the extent scores of all 16 individual segments were summed to yield a total score ranging from 0 to 48.
Follow-up
The primary end point was time to death from all causes. Follow-up procedures were approved by all study centers’ institutional review boards. Death status for non-U.S. centers was gathered by clinical visits, telephone contacts, and questionnaires sent by mail, with verification of all reported events by hospital records or direct contact with a patient’s attending physician. Death status for U.S. centers was ascertained either by query of the Social Security Death Index or by direct physician and/or patient contact.
Statistical analysis
SPSS version 12.0 (SPSS, Chicago, IL) and SAS version 9.2 (SAS, Cary, NC) were used for all statistical analyses. Categorical variables are presented as frequencies and continuous variables as means ± 1 SD. Variables were compared with the χ2 statistic for categorical variables and by Student unpaired t tests for continuous variables. Time to death from all causes and death rates were calculated using univariable Cox proportional hazards models. In each case, the proportional hazards assumption was met. Adjusted models also were devised, including multivariable stepwise models adjusting for baseline demographics, CAD risk factors, angina typicality, and pretest likelihood of obstructive CAD. Adjusted models were also developed to test first-order interactions related to age, sex, and study site. A two-tailed P value <0.05 was considered statistically significant.
To compare the effects of DM status on CAD prevalence, extent, severity, and prognosis, we constructed a propensity score for DM by multivariate logistic regression for age, sex, dyslipidemia, hypertension, smoking, and family history of premature CAD. A total of 3,370 DM patients were propensity matched in a 1-to-2 fashion by age, sex, and CAD risk factors to 6,740 unique non-DM patients up to eight decimal places. In all matched patients, the balancing property was achieved. Propensity-matched groups then were compared using the Wilcoxon rank sum tests for continuous variables and the McNemar test for categorical variables in univariable analysis, with Cox proportional hazards regression performed comparing the overall propensity matched DM and non-DM groups for presence, extent, severity, and prognosis related to CAD findings by CCTA.
RESULTS
Clinical characteristics of the study group
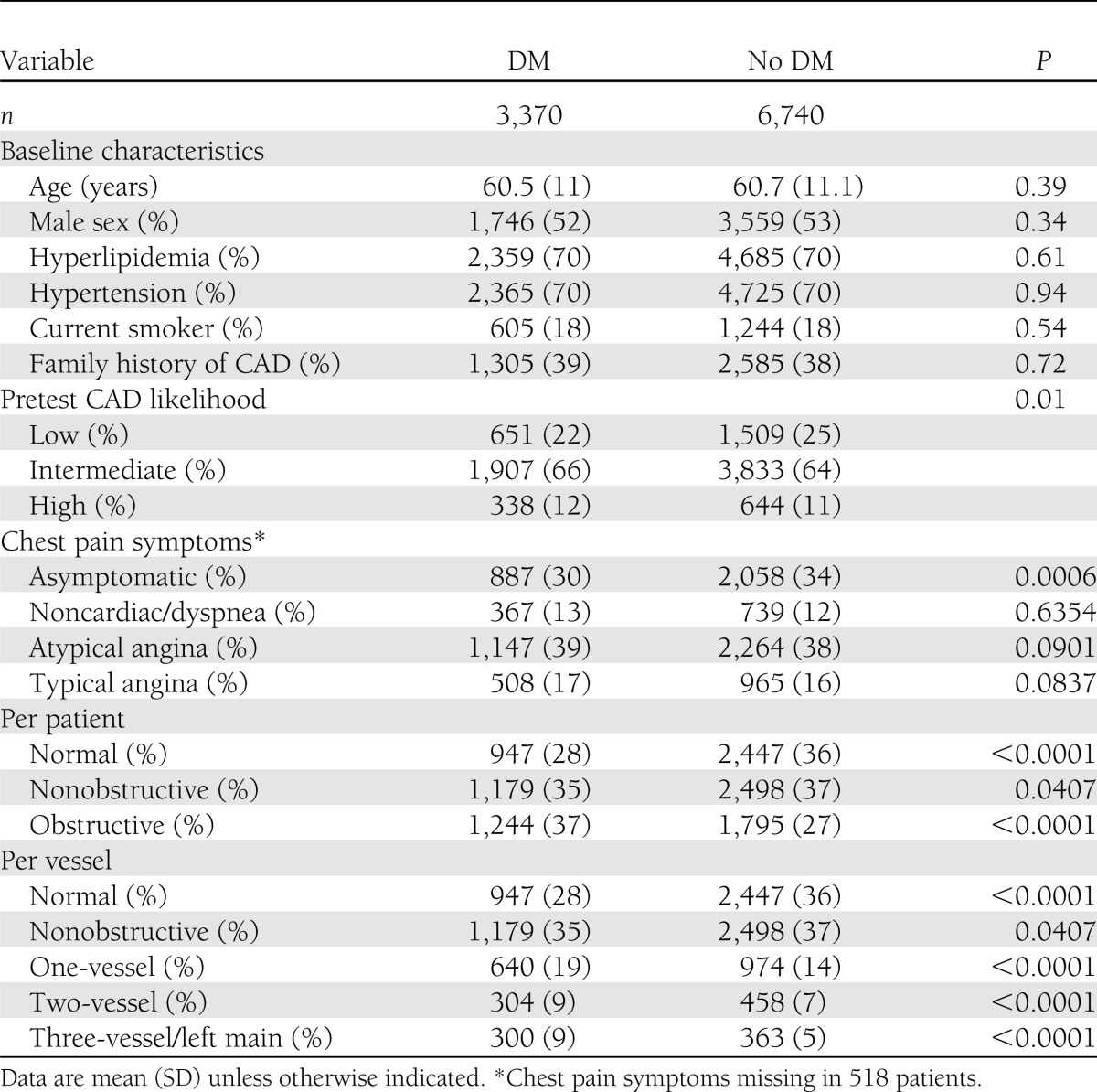
After propensity matching, the DM group consisted of 3,370 individuals and the non-DM group consisted of 6,740 individuals (Table 1). Propensity-matched DM and non-DM groups were similar for age, sex, and CAD risk factors, with differences in pretest likelihood of obstructive CAD and prevalence of asymptomatic individuals.
Table 1.
Baseline characteristics and coronary artery findings of matched DM and non-DM individuals
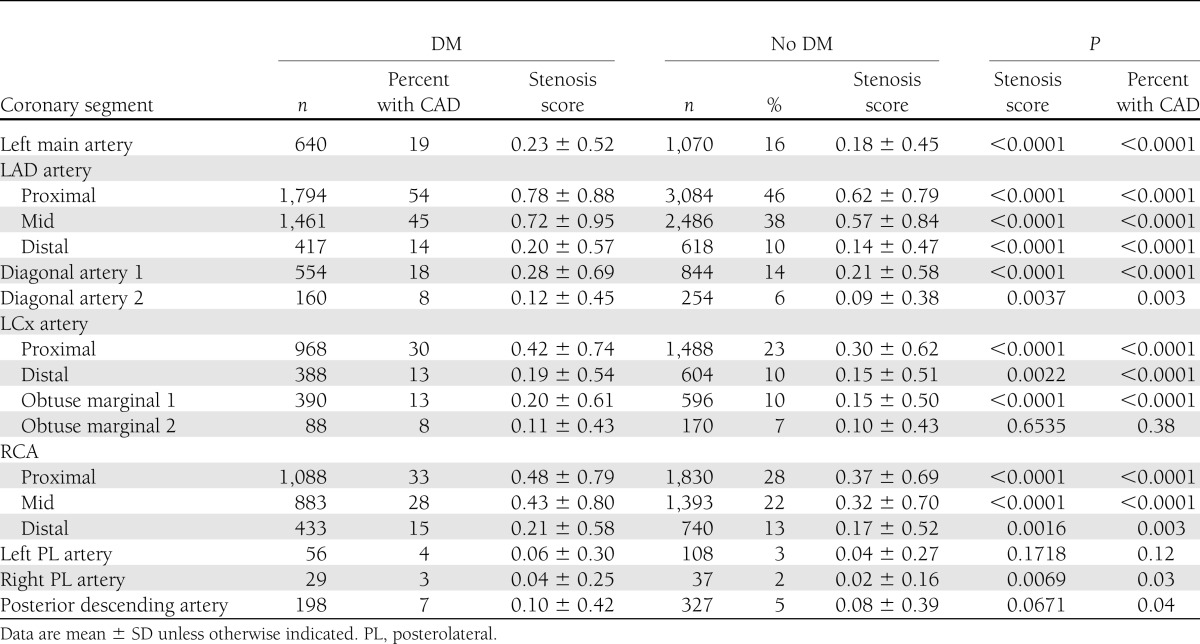
Among matched DM and non-DM individuals, CAD prevalence, extent, and severity differed (Table 1). On a per-patient basis, as compared with non-DM individuals, DM individuals exhibited lower prevalence of normal coronary arteries and higher rates of obstructive CAD. On a per-vessel basis, higher rates of obstructive one-vessel, two-vessel, and three-vessel disease or left main disease were noted for DM individuals compared with matched non-DM individuals. Both SSS and SIS for overall population were similar (median of 2 [interquartile ranges 0–5 and 0–4], respectively). Overall, SSS for DM individuals was higher than non-DM individuals (3 vs. 2, P < 0.0001). Likewise, overall SIS for DM individuals was also higher than non-DM individuals (2 vs. 1, P < 0.0001). On a per-segment basis, DM individuals possessed higher segmental stenosis scores compared with non-DM individuals for nearly every coronary segment and for all proximal and mid-coronary segments (Table 2).
Table 2.
SSS for matched DM and non-DM individuals
Clinical factors and risk of mortality in matched DM and non-DM individuals
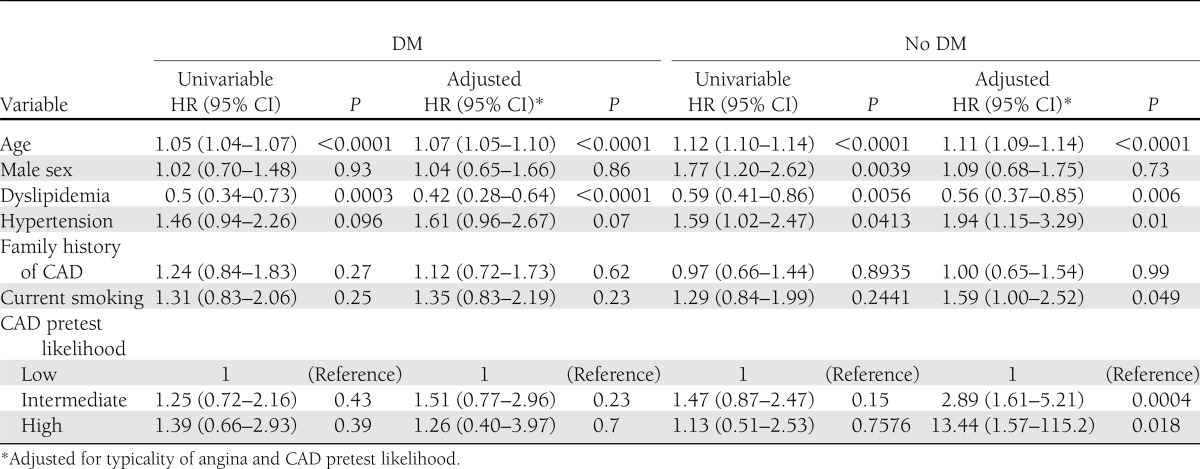
Survival was examined after a median follow-up of 2.2 years (interquartile range 1.5–3.1). DM individuals experienced a significantly higher rate of death (n = 108 [3.2%]) compared with non-DM individuals (n = 115 [1.7%]) (P < 0.0001). Among DM individuals, age and dyslipidemia were associated with increased risk of mortality (P < 0.0001 for both), whereas male sex, hypertension, family history of CAD, current smoking, and pretest CAD likelihood was not (P > 0.05 for all) (Table 3). Clinical factors associated with death in non-DM individuals also included age (P < 0.001) and dyslipidemia (P = 0.006), as well as hypertension (P = 0.01). In non-DM individuals, low pretest likelihood of CAD was associated with a significantly lower risk of death, a finding not observed for DM individuals.
Table 3.
Univariable and multivariable HRs for all-cause mortality for clinical variables in matched DM and non-DM individuals
CAD findings and risk of mortality in matched DM and non-DM individuals
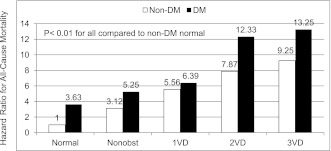
On a per-patient basis, when using a non-DM individual without CAD as a reference, both DM state and increasing severity of CAD were associated with increased risk of mortality. In multivariable analyses, as compared with a non-DM individual with no CAD, risk of mortality increased for non-DM individuals with nonobstructive CAD (hazard ratio [HR] 3.12 [95% CI 1.55–6.21], P = 0.0013), DM individuals with no CAD (3.63 [1.67–7.92], P = 0.001), DM individuals with nonobstructive CAD (5.25 [2.56–10.8], P < 0.0001), non-DM individuals with obstructive CAD (6.8 [3.52–13.1], P < 0.0001), and DM individuals with obstructive CAD (9.39 [4.85–18.2], P < 0.0001). On a per-vessel basis, multivariable risk-adjusted analyses similarly demonstrated an increased risk for DM individuals for nonobstructive CAD; one-vessel, two-vessel, and three-vessel disease; or left main disease (Fig. 1).
Figure 1.
Multivariable risk-adjusted HR for CAD extent and severity on a per-vessel basis for matched individuals with and without DM. 1VD, one-vessel obstructive CAD >50% stenosis; 2VD, two-vessel obstructive CAD >50% stenosis; 3VD, three-vessel obstructive CAD >50% stenosis; Nonobst, nonobstructive CAD <50% maximal per-patient stenosis.
CONCLUSIONS
These present results of the CONFIRM represent the first prospective multicenter data to relate per-patient, per-vessel, and per-segment prevalence, extent, and severity of CAD findings by CCTA for DM and non-DM individuals who are similar to each other in age, sex, and CAD risk factors. Given the study size, high number of sites, and international nature of the CONFIRM, our findings enable quantification of excess CAD among DM individuals compared with non-DM individuals with high precision and can be considered widely generalizable. Of interest, increased prevalence, extent, and severity of CAD for DM individuals were remarkably consistent across patient-, vessel-, and segment-based comparisons. Individuals with DM possessed a 37% higher prevalence of obstructive CAD at a per-patient level, which was consistent with the 36 and 29% increase in prevalence of obstructive one-vessel and two-vessel disease, respectively, at the per-vessel level. Among segmental analyses for proximal and mid-portions of coronary arteries, stenosis scores ranged between 26 and 40% higher for DM individuals compared with matched non-DM individuals.
In addition, to our knowledge, our findings represent the first multicenter data to associate CCTA CAD findings to incident mortality for matched DM and non-DM individuals. For individuals with no, nonobstructive, and obstructive CAD, increased risk of mortality was evident for DM compared with non-DM individuals. As compared with non-DM individuals without CAD, the risk of mortality was more than fivefold higher for DM individuals with nonobstructive CAD and almost 10-fold higher for DM individuals with obstructive CAD. When CAD extent and severity was stratified on a per-vessel analysis as no CAD, nonobstructive CAD, or obstructive one-vessel, obstructive two-vessel, or obstructive three-vessel disease, the risk of mortality associated with the DM state resulted in the “step up” of a CAD risk category for non-DM individuals. As an example, the hazard for mortality for a DM individual with no CAD by CCTA was 3.63, whereas the hazard for a non-DM individual with nonobstructive CAD by CCTA was 3.12. Likewise, the hazard for death for a DM individual with nonobstructive CAD was 5.25, which was comparable to the 5.56 noted for non-DM individuals with obstructive one-vessel disease (Fig. 1).
Previous studies examining the prognostic value of CT for DM individuals have been limited to smaller study sizes and to single centers. Hadamitzky et al. (10) studied 140 DM individuals compared with 1,782 non-DM individuals for measures of incident cardiac events, as defined by all-cause mortality, nonfatal myocardial infarction, or unstable angina requiring hospitalization. In this study, a positive relationship was noted toward an adverse prognosis for DM individuals for the number of coronary plaques using an atherosclerotic burden score, which was a measure of total number of visualized coronary plaques. Higher plaque number conferred a 1.3 increased hazards (95% CI 1.1–1.7) for each additional lesion noted (P = 0.005). van Werkhoven et al. (11) compared 313 DM individuals to 303 non-DM individuals for CAD prevalence, distribution and a composite end point of cardiac death, nonfatal myocardial infarction, and unstable angina requiring hospitalization; DM individuals with obstructive CAD by CCTA experienced a 47% event rate, as compared with a 36% event rate for non-DM individuals with obstructive CAD. This study also observed no adverse cardiac events at the 20-month follow-up for both DM and non-DM individuals without evident CAD by CCTA. Our study extends these previous study findings by not only examining differences in CAD prevalence, extent, and severity but also prognosis in DM and non-DM individuals matched for age, sex, and other traditional CAD risk factors. In addition, we examined these differences between DM and non-DM individuals on a per-patient, per-vessel, and per-segment basis.
In direct contrast to the data reported by van Werkhoven, our study observed a higher risk of mortality for DM individuals without CAD when compared with non-DM individuals without CAD. These data are in direct accordance with the recent report from the Emerging Risk Factors Collaboration (16), which identified hazards for mortality from individual-participant data for 123,205 deaths among 820,900 people in 97 prospective studies. In this study, risk of mortality for DM individuals was 1.80-fold higher (95% CI 1.71–1.90) than for non-DM individuals for all-cause mortality. Cause-specific death analysis revealed that the elevated risk of death was not only associated with cardiovascular but also noncardiovascular causes, including death from cancer (HR 1.25 [95% CI 1.19–1.31]), death from vascular causes (2.32 [2.11–2.56]), and death from other causes (1.73 [1.62–1.85]); our data are consistent with these findings. In the current study, among 18 deaths that occurred for DM individuals with no evident CAD by CCTA, exploration of medical records revealed the causes of death to be related to complications of noncardiovascular diseases, such as pneumonia, liver disease, or renal failure. Of interest, although cause-specific death could be ascertained only for 12 of 18 DM patients without CCTA evidence of CAD, no cases of sudden cardiac death or myocardial infarction–related death were observed.
CCTA is a novel noninvasive imaging modality, which extends visualization of coronary atherosclerosis beyond that which is capable by coronary artery calcium scoring (CACS). By contrast-enhanced methods, CCTA offers the added diagnostic ability to visualize noncalcified components of atherosclerotic plaque, along with coronary artery remodeling and can provide measurement of plaque volumes (17,18). Use of CCTA generally has been endorsed for low-to-intermediate risk individuals by professional societal appropriate-use criteria (19), whereas the use of CACS has been advocated for asymptomatic individuals (20). In this regard, CACS has been associated with a heightened rate of mortality with escalating CACS score in individuals with diabetes (21), but previous direct comparative studies evaluating the role of CACS versus CCTA in patients with diabetes is lacking. Stress myocardial perfusion imaging (MPI) represents another alternative to CACS or CCTA for risk stratification of patients with diabetes, but previous studies have demonstrated a nonnegligible false-negative rate for diabetic individuals undergoing MPI (22). Future studies examining the differential clinical utility of CCTA, CACS, and MPI in diabetic populations now seem warranted.
Although this study addresses several of the shortcomings of previous studies that have examined CCTA findings of CAD in DM and non-DM individuals, it is nevertheless not without limitations. Given the observational nature of the study design, this prospective international multicenter study is subject to potential biases related to referral and ascertainment. Given the latter, we chose all-cause mortality as an end point that, although not including other “softer” end points such as nonfatal myocardial infarction and unstable angina, mitigates ascertainment bias and allows for assessment of an incontrovertible end point of vital clinical importance (23). We performed propensity matching to identify comparator DM and non-DM groups that were similar in age, sex, and CAD risk factors but, in doing so, altered the makeup of the initial overall study group. In particular, the prevalence of dyslipidemia and hypertension were ostensibly higher in the non-DM matched group compared with the initial study population. However, our goal for this study was to assess the excess risk of the DM state for prevalence, extent, severity, and prognosis of CAD findings by CCTA; for this purpose, comparator groups require similarity and previous methods have been well described for application of multivariable methods applied to observational data to mitigate confounding (24). Classifications of type 1 and 2 DM, time of onset of diabetes to CCTA, medications, and glycemic control were not uniformly available for patients in our database. Longer-term follow-up of individuals enrolled into the CONFIRM is ongoing, and we hope to obtain this information in the future. Treatment effects based upon CCTA CAD findings may have precipitated salutatory therapies that positively altered outcome. Thus, whether percutaneous or surgical coronary revascularization, enhanced medical therapy, or lifestyle modifications altered prognosis in this open-label multicenter registry remains unknown. In addition, CCTA still is a relatively novel imaging modality that is capable of additional measures of CAD beyond stenosis, including atherosclerotic plaque features (e.g., composition, distribution) and arterial wall remodeling (25,26). For a minority of asymptomatic individuals with suspected CAD, CCTA was performed for an array of clinical indications, including preoperative risk assessment, family history of premature CAD, new-onset left ventricular systolic dysfunction, and others, during a period early after the introduction of CCTA. Since then, appropriate use criteria have discouraged the use of CCTA in asymptomatic individuals, and present study findings should thus not be taken as a license to perform routine CCTA assessment in such patients. Finally, current-generation CT scanners are limited by isotropic spatial resolution to 500–750 microns. As such, partial volume artifacts may have occurred, particularly with calcified plaques (27), and these artifacts may have resulted in an overestimation of stenosis severity in the current study and a potential inability to accurately assess atherosclerosis in smaller side branches of major epicardial arteries. The latter of these findings may be more relevant to a DM population, who may be more prone to diffuse patterns of atherosclerosis. Improvements in CT technology (28,29) that have occurred since the initiation of our study, including scanners with higher spatial resolution that allow for CCTA performance at lower radiation dose, may enhance the current study findings.
In the prospective, international, multicenter CONFIRM registry of DM and non-DM individuals similar in age, sex, and CAD risk factors, the presence of DM is associated with increased prevalence, extent, and severity of CAD. Assessment of these factors confers measurable increases in risk of all-cause mortality for DM and non-DM individuals.
Acknowledgments
This research was conducted without dedicated funding.
No potential conflicts of interest relevant to this article were reported.
J.S.R., A.D., T.M.L., F.Y.L., L.J.S., D.S.B., and J.K.M. contributed to the design, analyses, data collection, and writing of the manuscript. S.A., M.A.-M., M.J.B., F.C., T.Q.C., H.-J.C., V.Y.C., K.C., B.J.W.C., R.C., A.D., G.F., M.H., J.H., P.K., R.P.K., Y.-J.K., J.L., E.M., G.R., and T.C.V. contributed to the data collection, data interpretation, and writing of the manuscript. J.K.M. was involved in all steps of this study and is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Buse JB, Ginsberg HN, Bakris GL, et al. American Heart Association. American Diabetes Association Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2007;30:162–172 [DOI] [PubMed] [Google Scholar]
- 2.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–234 [DOI] [PubMed] [Google Scholar]
- 3.Whiteley L, Padmanabhan S, Hole D, Isles C. Should diabetes be considered a coronary heart disease risk equivalent?: results from 25 years of follow-up in the Renfrew and Paisley survey. Diabetes Care 2005;28:1588–1593 [DOI] [PubMed] [Google Scholar]
- 4.Howard BV, Best LG, Galloway JM, et al. Coronary heart disease risk equivalence in diabetes depends on concomitant risk factors. Diabetes Care 2006;29:391–397 [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM. Diabetes and coronary risk equivalency: what does it mean? Diabetes Care 2006;29:457–460 [DOI] [PubMed] [Google Scholar]
- 6.Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 2008;52:1724–1732 [DOI] [PubMed] [Google Scholar]
- 7.Meijboom WB, Meijs MF, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 2008;52:2135–2144 [DOI] [PubMed] [Google Scholar]
- 8.Min JK, Dunning A, Lin FY, et al. CONFIRM Investigators Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol 2011;58:849–860 [DOI] [PubMed] [Google Scholar]
- 9.Chow BJ, Small G, Yam Y, et al. CONFIRM Investigators Incremental prognostic value of cardiac computed tomography in coronary artery disease using CONFIRM: COroNary Computed Tomography Angiography Evaluation for Clinical Outcomes: an InteRnational Multicenter registry. Circ Cardiovasc Imaging 2011;4:463–472 [DOI] [PubMed] [Google Scholar]
- 10.Hadamitzky M, Hein F, Meyer T, et al. Prognostic value of coronary computed tomographic angiography in diabetic patients without known coronary artery disease. Diabetes Care 2010;33:1358–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Werkhoven JM, Cademartiri F, Seitun S, et al. Diabetes: prognostic value of CT coronary angiography—comparison with a nondiabetic population. Radiology 2010;256:83–92 [DOI] [PubMed] [Google Scholar]
- 12.Min JK, Dunning A, Lin FY, et al. Rationale and design of the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter) registry. J Cardiovasc Comput Tomogr 2011;5:84–92 [DOI] [PubMed] [Google Scholar]
- 13.Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med 1979;300:1350–1358 [DOI] [PubMed] [Google Scholar]
- 14.Raff GL, Abidov A, Achenbach S, et al. Society of Cardiovascular Computed Tomography SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr 2009;3:122–136 [DOI] [PubMed] [Google Scholar]
- 15.Min JK, Shaw LJ, Devereux RB, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol 2007;50:1161–1170 [DOI] [PubMed] [Google Scholar]
- 16.Seshasai SR, Kaptoge S, Thompson A, et al. Emerging Risk Factors Collaboration Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Achenbach S, Raggi P. Imaging of coronary atherosclerosis by computed tomography. Eur Heart J 2010;31:1442–1448 [DOI] [PubMed] [Google Scholar]
- 18.Cheng VY, Nakazato R, Dey D, et al. Reproducibility of coronary artery plaque volume and composition quantification by 64-detector row coronary computed tomographic angiography: an intraobserver, interobserver, and interscan variability study. J Cardiovasc Comput Tomogr 2009;3:312–320 [DOI] [PubMed] [Google Scholar]
- 19.Taylor AJ, Cerqueira M, Hodgson JM, et al. American College of Cardiology Foundation Appropriate Use Criteria Task Force. Society of Cardiovascular Computed Tomography. American College of Radiology. American Heart Association. American Society of Echocardiography. American Society of Nuclear Cardiology. North American Society for Cardiovascular Imaging. Society for Cardiovascular Angiography and Interventions. Society for Cardiovascular Magnetic Resonance ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol 2010;56:1864–1894 [DOI] [PubMed] [Google Scholar]
- 20.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010;56:e50–e103 [DOI] [PubMed] [Google Scholar]
- 21.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol 2004;43:1663–1669 [DOI] [PubMed] [Google Scholar]
- 22.Bourque JM, Beller GA. Stress myocardial perfusion imaging for assessing prognosis: an update. JACC Cardiovasc Imaging 2011;4:1305–1319 [DOI] [PubMed] [Google Scholar]
- 23.Lauer MS, Blackstone EH, Young JB, Topol EJ. Cause of death in clinical research: time for a reassessment? J Am Coll Cardiol 1999;34:618–620 [DOI] [PubMed] [Google Scholar]
- 24.Hachamovitch R, Di Carli MF. Methods and limitations of assessing new noninvasive tests: part II: outcomes-based validation and reliability assessment of noninvasive testing. Circulation 2008;117:2793–2801 [DOI] [PubMed] [Google Scholar]
- 25.Voros S, Rinehart S, Qian Z, et al. Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta-analysis. JACC Cardiovasc Imaging 2011;4:537–548 [DOI] [PubMed] [Google Scholar]
- 26.Gauss S, Achenbach S, Pflederer T, Schuhbäck A, Daniel WG, Marwan M. Assessment of coronary artery remodelling by dual-source CT: a head-to-head comparison with intravascular ultrasound. Heart 2011;97:991–997 [DOI] [PubMed] [Google Scholar]
- 27.Sarwar A, Rieber J, Mooyaart EA, et al. Calcified plaque: measurement of area at thin-section flat-panel CT and 64-section multidetector CT and comparison with histopathologic findings. Radiology 2008;249:301–306 [DOI] [PubMed] [Google Scholar]
- 28.Alkadhi H, Stolzmann P, Desbiolles L, et al. Low-dose, 128-slice, dual-source CT coronary angiography: accuracy and radiation dose of the high-pitch and the step-and-shoot mode. Heart 2010;96:933–938 [DOI] [PubMed] [Google Scholar]
- 29.Halliburton SS, Abbara S, Chen MY, et al. Society of Cardiovascular Computed Tomography SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr 2011;5:198–224 [DOI] [PMC free article] [PubMed] [Google Scholar]