Abstract
OBJECTIVE
Older people with type 2 diabetes are at high risk of mobility disability. We investigated the association of diabetes with lower-limb muscle mass and muscle quality to verify whether diabetes-related muscle impairments mediate the association between diabetes and low walking speed.
RESEARCH DESIGN AND METHODS
We performed a cross-sectional analysis of 835 participants (65 years old and older) enrolled in the InCHIANTI (Invecchiare in Chianti, aging in the Chianti area) population-based study. Total, muscular, and fat cross-sectional areas of the calf and relative muscle density were measured using peripheral quantitative computerized tomography. Indicators of muscle performance included knee-extension torque, ankle plantar flexion and dorsiflexion strength, lower-extremity muscle power, and ankle muscle quality (ratio of ankle strength to the muscle area [kilograms per centimeters squared]). Gait performance was assessed by 4- and 400-m walking speed. Diabetes was ascertained by standard American Diabetes Association criteria.
RESULTS
Prevalence of diabetes was 11.4%. After adjustment for age and sex, participants with diabetes had lower muscle density, knee and ankle strength, and muscle power and worse muscle quality (all P < 0.05). Diabetic participants were also slower on both 4-m (β: −0.115 ± 0.024 m/s, P < 0.001) and 400-m (β:−0.053 ± 0.023 m/s, P < 0.05) walking tests. In multivariable linear regression models, lower-limb muscle characteristics accounted for 24.3 and 15.1% of walking speed difference comparing diabetic and nondiabetic subjects in the 4- and 400-m walks, respectively.
CONCLUSIONS
In older persons, diabetes is associated with reduced muscle strength and worse muscle quality. These impairments are important contributors of walking limitations related to diabetes.
Walking performance has been shown to reflect health and functional status in older adults, and in observational studies walking speed is a powerful predictor of survival (1) and long-term risk of disability (2). Type 2 diabetes has been consistently reported to be one of the strongest correlates of the presence of poor walking performance or mobility difficulty (3), and older patients with diabetes are at high risk of future mobility disability and loss of independence (4,5). However, the mechanisms for impaired walking in diabetes have been poorly understood (6). Traditional long-term complications and diabetes-related comorbidities only partially explain the excess risk of disability associated with diabetes. For example, in the Women’s Health and Aging Study, chronic conditions including cardiovascular diseases, peripheral arterial disease, peripheral neuropathy, overweight, depression, and visual impairment explained <60% of the risk of severe walking limitation (7).
Lower-extremity muscle strength and muscle quality are strong determinants of walking performance and powerful predictors of the risk of mobility limitation (8,9). Cross-sectional and longitudinal analyses of the Health Aging and Body Composition study have demonstrated that older diabetic individuals had lower muscle strength and muscle quality compared with their nondiabetic counterparts (10,11). These analyses also demonstrated that older persons with type 2 diabetes had accelerated loss of muscle strength over time, suggesting an additional biological mechanism to explain the association between diabetes and poor physical function (12). Nevertheless, whether diabetes-related muscle impairments mediate the association between diabetes and low walking speed has never been investigated.
To address this question, we assessed walking performance and lower-extremity muscle mass, strength, and power in a sample of older Italian persons with and without diabetes enrolled in the InCHIANTI (Invecchiare in Chianti, aging in the Chianti area) study, a population-based study designed to elucidate how specific impairment in physiological subsystems may influence walking ability in older persons (13). We hypothesized that compared with persons without diabetes, those with diabetes would have poorer skeletal muscle performance and that these muscle impairments would account for at least part of the slower walking speed observed in persons with diabetes.
RESEARCH DESIGN AND METHODS
The InCHIANTI study is an epidemiological, population-based study of randomly selected older people living in the Chianti area, Tuscany, Italy. The study was designed to identify risk factors for late-life disability, as previously described (13). Briefly, participants were selected from the city registries of Greve in Chianti and Bagno a Ripoli using a multistage sampling method (13). In 1998, 1,453 persons who were randomly selected agreed to participate in the project. The Italian National Research Council on Aging Ethical Committee ratified the study protocol, and participants provided written consent to participate. Of the original 1,453 subjects, we excluded 298 participants younger than 65 years old; 303 for whom information on muscle function and calf muscle cross-sectional area was not available, either because participants refused to come to the clinic for an examination or were too sick to be evaluated; and 17 participants who did not perform the 4-m walking speed test. The final study population thus included 835 persons, 379 men and 456 women, aged 65 years and older.
Assessment of diabetes
Prevalent diabetes was defined as self-report of physician diagnosis or hypoglycemic medication use. Among undiagnosed diabetic participants, presence of diabetes was identified by fasting plasma glucose level ≥126 mg/dL, based on the American Diabetes Association 2003 criteria. Among those with previously diagnosed diabetes, duration was determined by an interviewer-administered questionnaire. Current use of hypoglycemic medications (oral agents and insulin) was determined during the baseline interview.
Peripheral quantitative computerized tomography measures
A lower-leg peripheral quantitative computerized tomography scan was performed in all participants by a recent generation device (XCT 2000; Stratec, Pforzheim, Germany) to evaluate the total, muscular, and fat cross-sectional areas of the calf. Data presented here were derived from standard 2.5-mm-thick transverse scans obtained at 66% of the tibial length starting from the tibiotarsal joint (Supplementary Fig. 1). Previous studies have demonstrated that this is the region with the largest outer calf diameter, with a small variability across individuals (9). The muscle density (milligrams per centimeter cubed), the muscle area (centimeters squared), the fat area (centimeters squared), and the total area (centimeters squared) were calculated using the BonAlyse software version 3.1 (BonAlyse, Jyvaskyla, Finland [http://www.bonalyse.com]). Different tissues in the analyses were separated according to different density thresholds, using the ‘‘soft tissue’’ algorithm: a density value of 15 mg/mm3 was used to separate fat from muscle tissue and 180 mg/mm3 to separate muscle from bone tissue (14). For the current study, the muscle area, the fat area, and the muscular density were considered in the analysis. Measurements of computed tomography calf muscle area (centimeters squared) were divided by body weight in kilograms to provide a standardized measure of skeletal muscle mass (15).
Measures of lower-extremity strength
Isometric muscle strength was assessed on eight muscle groups of the lower extremity by a handheld dynamometer (Nicholas Muscle Tester; Sammon Preston, Chicago, IL) by using a standard protocol (16). All measures of lower-extremity muscle strength were highly correlated (Pearson correlation coefficients ranging from 0.87 to 0.92). Therefore, in the analyses presented here, we used knee-extension torque to indicate lower-extremity muscle strength. Knee-extension torque was calculated according to the following formula: knee-extension torque = (knee extension × 9.81) × [(calf length − 100)/100]N × dm. Data were analyzed after standardization for kilograms of weight. Furthermore, in order to capture the detrimental effect of diabetes on the distal portion of the lower limb we analyzed ankle dorsi-flexion and plantar-flexion strength. A measure of muscle quality was created by taking the ratio of ankle strength (sum of the dorsi- and plantar-flexion values) to the muscle area (kilograms per centimeter squared) measured at 66% of the tibial length starting from the tibiotarsal joint by peripheral quantitative computerized tomography.
Lower-extremity muscle power was measured in a single-leg extension movement, according to the method described by Bassey and Short (17). The value of the best performance obtained over eight repetitions on the right side and eight repetitions on the left side was used in the analysis (9).
Measures of physical performance
Two objective performance-based tests were used to assess physical performance: 4-m walking speed at usual pace and 400-m walking speed. To measure walking speed on a 4-m course, two photocells connected with a recording chronometer were placed at the beginning and end of a 4-m course. The second objective test measured the ability to walk a standard 400-m course (20 laps of 20 m). A maximum of two standing rests was permitted for <2 min each. Assistive devices (e.g., canes, walkers) were permitted. Seven hundred and thirty-five participants had valid data on the 400-m test. Participants were excluded from this test if they had any of the following conditions: pathologic electrocardiographic abnormality, systolic blood pressure >180 mmHg, diastolic blood pressure >100 mmHg, resting heart rate <40 or >135 bpm, myocardial infarction and/or episodes of angina in the past 3 months, severe dementia, or poor visual acuity. The ability to complete the 400-m walk test has demonstrated perfect 1-week test-retest reliability (κ = 1) in older adults.
Covariates
Sociodemographic characteristics.
Cigarette-smoking behavior was assessed through survey questions. Physical activity during the year prior to the interview was assessed through an interviewer-administered questionnaire as previously described and coded as follows: 1) sedentary, completely inactive or light-intensity activity <1 h per week; 2) light physical activity, light-intensity activity 2–4 h per week; and 3) moderate–high physical activity, light activity at least 5 h/week or more or moderate activity at least 1–2 h/week.
Diabetes-related and diabetes-associated comorbidities.
The ankle-brachial index (ABI), measured using a Doppler stethoscope (Parks model 41-A; Parks Medical Electronics, Aloha, OR) as previously described (18), was used as an indicator of presence and severity of lower-extremity peripheral artery disease. Individuals were excluded if their ABI was >1.3, indicating poorly compressible leg arteries and inability to gauge arterial perfusion accurately.
The measure of nerve motor conduction velocity was performed on the right superficial peroneal nerve using standard neurophysiologic equipment (EMG Myto, EBNeuro; ESAOTE, Florence, Italy) (19). Among the several peripheral nerves involved in lower-leg control, the superficial peroneal nerve was considered the simplest nerve to be studied using standard methods in a large cohort of older persons like the InCHIANTI population. The following parameters of nerve conduction studies were measured: 1) the nerve conduction velocity (NCV), which reflects the conduction velocity of the fastest motor axons, was calculated by dividing the length of the nerve segment between the two stimulation points by the difference between the proximal and distal time latency; 2) the amplitude of the compound muscle action potential (CMAP), which is related to the number of axons that conduct impulses from the stimulus point to the muscle and the number of functioning motor end plates. It was measured from peak to peak of the action potential (20,21). Previous analysis on this sample showed that low peroneal amplitude is associated with clinical features of tendon hyporeflexia, impaired touch sensitivity, impaired vibration sense, and mild imbalance, suggesting that the reduction in CMAP was associated with a parallel reduction of sensory spinal neurons (21).
In order to assess lower-limb somatosensory function, the Cumulative Somatosensory Impairment Index (CSII) was used. This index was previously derived and validated from the InCHIANTI dataset, using clinical tests of pressure and vibration sensitivity, ankle proprioception, and graphesthesia as previously described (22). Briefly, the CSII score ranged from 0 to 8, with 8 being the worst. CSII score is significantly higher in persons with diabetes without overt neuropathy or peripheral arterial disease (22).
The baseline prevalence of specific medical conditions was established using standardized criteria that combined information from self-reported history, medical records, and a clinical medical examination. Diagnostic algorithms were modified versions of those created for the Women’s Health and Aging Study (23). The following diseases were assessed: coronary artery disease (angina and acute myocardial infarction), stroke (and/or transient ischemic attack), hypertension, congestive heart failure (CHF), and hip and knee osteoarthritis. Depressive symptoms were assessed by means of the standard Center for Epidemiologic Studies Depression Scale (score >16) (24). Cognitive impairment was defined as a Mini-Mental State Exam (MMSE) score <24; executive function, a cognitive domain strongly related to gait performance in older persons (25), was assessed by means of the Trail-Making tests (TMTs) A and B. To remove the upper-extremity motor speed element from the test evaluation, a difference score (with ΔTMT equal to time on part B minus time on part A) was calculated. Study participants were grouped according to ΔTMT performance in four categories according to tertile of performance (good, intermediate, and poor), and participants who were not able to complete the test in the time allowed (300 s) were included in the forth category. Weight and height were measured using objective standard techniques and used to calculate BMI (measured as kilograms divided by the square of height in meters).
Inflammatory markers.
Serum interleukin (IL)-6 and tumor necrosis factor-α (TNF-α) were measured in duplicate by high-sensitivity enzyme-linked immunoabsorbent assays (ELISAs) (BIOSOURCE, Camarillo, CA). Sensitivity was 0.1 pg/mL for IL-6 and 0.09 pg/mL for TNF-α, and the coefficient of variation was <7% for both tests. C-reactive protein (CRP) was measured in duplicate using an ELISA and colorimetric competitive immunoassay (sensitivity 0.03 mg/L and interassay coefficient of variation <5%).
Statistical analysis
For descriptive purpose, baseline characteristics of the study population were compared according to presence or absence of diabetes and by diabetes therapy, using a χ2 test and ANOVA model for categorical and continuous variables, respectively. We used age- and sex-adjusted linear regression analysis to study the association between diabetes and anthropometric and functional muscle characteristics. Multivariate linear regression was also used to estimate the association between diabetes status and walking speed according to diabetes status. Models were adjusted for age and sex. Muscle characteristics hypothesized to be potential mediators of the association between diabetes and walking performance were progressively added to the models. Finally, to estimate the contribution of each diabetes-related condition to the association between diabetes and walking speed, we compared the point estimate measure of the association for diabetes in the basic model with the point estimates for diabetes in models including each condition individually; percent change in the β coefficient was calculated as follows: [1 − (β adjusted/β basic)]. All analyses were performed using Stata 11.0 for Windows (StataCorp, College Station, TX).
RESULTS
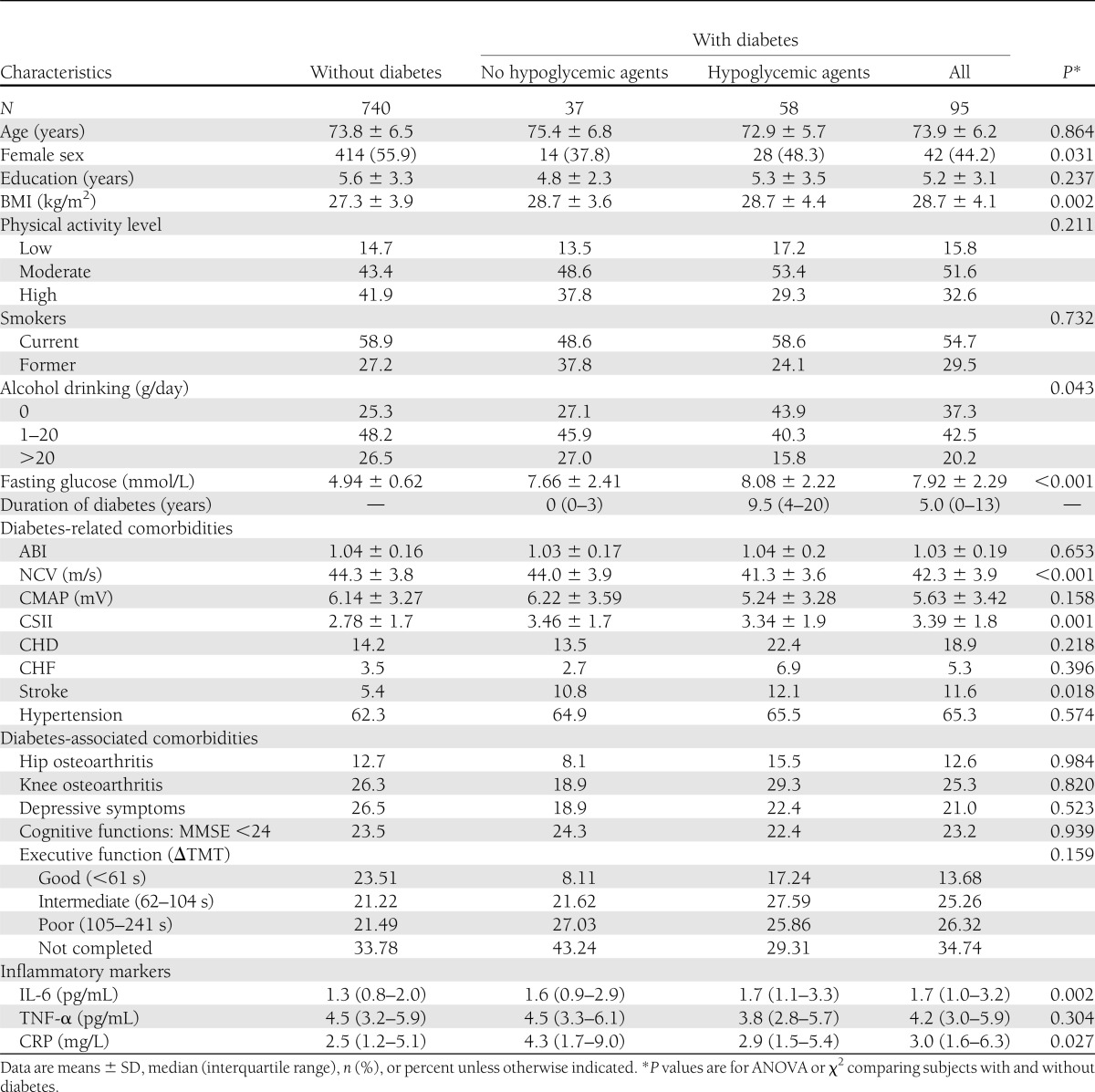
Of 835 InCHIANTI participants enrolled into the current study, 95 (11.4%) had an adjudicated diagnosis of diabetes at baseline, with an average self-reported duration of disease of 8.6 years (median 5.0). The mean age of the study population was 73.8 years. General characteristics of the sample are presented in Table 1 according to diabetes status and type of current therapy. Among subjects with diabetes, almost 60% were taking at least one hypoglycemic medication. Persons with diabetes were more likely to be men and had a lower level of education, higher BMI, lower NCV, greater somatosensory impairment, greater stroke prevalence, and higher levels of IL-6 and CRP. They also tended to have greater prevalence of coronary heart disease (CHD) and CHF, but these associations were not statistically significant.
Table 1.
Characteristics of the study population by diabetes status and type of therapy
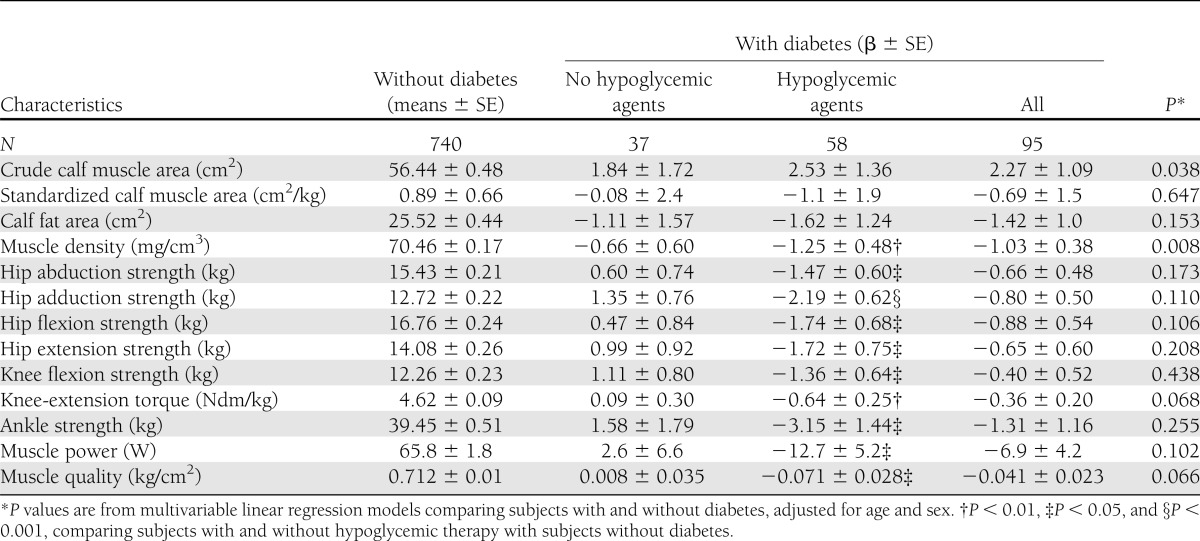
Table 2 shows sex- and age-adjusted difference in anthropometric and functional muscle characteristics according to diabetes therapy. Participants with diabetes had larger crude calf muscle area (β: 2.27 ± 1.09 cm2, P = 0.038) but similar calf muscle area when standardized by body weight. They also had lower muscle density (P = 0.008) and tended to have lower knee-extension torque, ankle strength, and muscle power and worse muscle quality. No significant interaction between diabetes status and sex was found. When the analysis was limited to patients on pharmacological therapy (oral agents or/and insulin), patients with diabetes had statistically significant lower muscle density, lower-limb muscle strength, muscle power, and muscle quality compared with the nondiabetic counterparts (all P values <0.05). Additional adjustment for NCV, CMAP, and ABI did not reduced the strength of these associations, whereas further controlling for BMI determined a more important attenuation of the difference in muscle density (β: −0.57 ± 0.45, P = 0.210), knee-extension torque (β: −0.44 ± 2.3, P = 0.063), muscle power (β: −9.2 ± 5.3, P = 0.078), and ankle muscle quality (β: −0.06 ± 0.27, P < 0.02).
Table 2.
Age- and sex-adjusted difference in anthropometric and functional muscle characteristics according to diabetes status and type of therapy
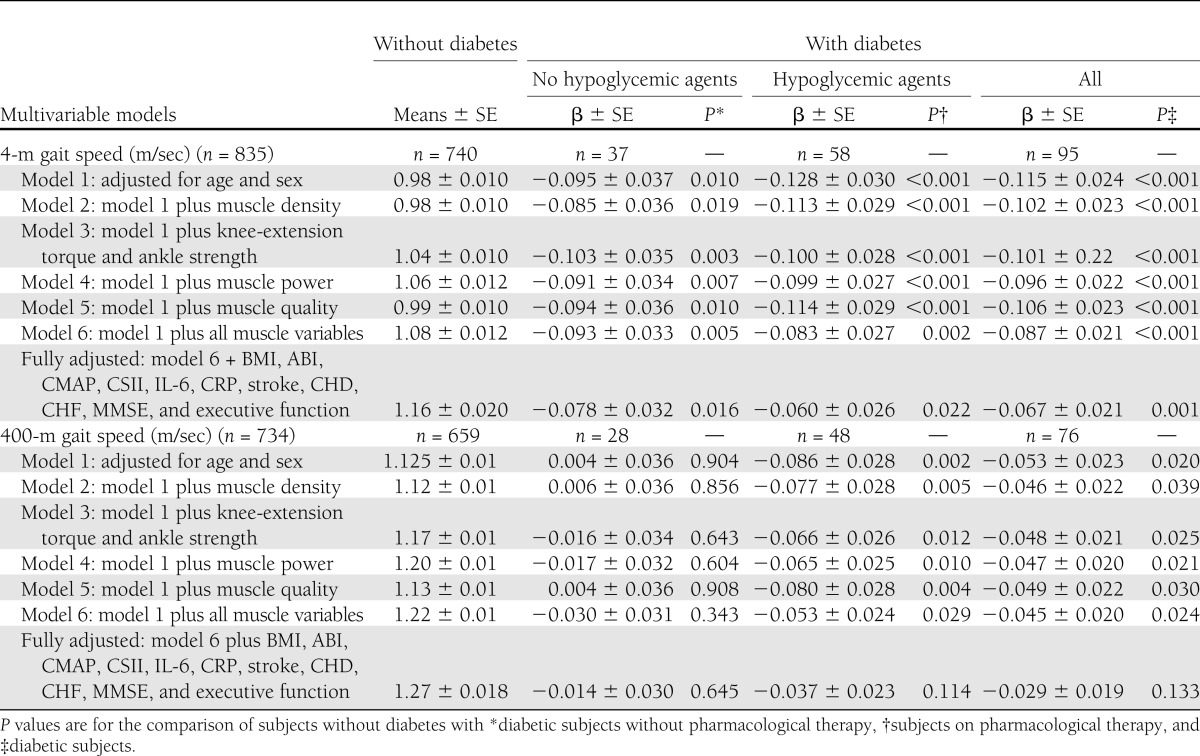
Table 3 shows differences in walking speed according to diabetes status. In model 1, adjusted for age and sex, and compared with persons without diabetes, diabetic patients on pharmacological therapy were significantly slower on both the 4-m (β: −0.128 ± 0.03 m/s, P < 0.001) and 400-m (β: −0.086 ± 0.028 m/s, P = 0.002) walking test, whereas patients on dietetic treatment had a poorer walking performance only in the 4-m walking test (β: −0.09 ± 0.037 m/s, P = 0.01). Other models display results after adjustment for anthropometric and functional muscle characteristics and other potential mediators or confounders. In the 4-m walking test, patients with diabetes had slower waking speed compared with the nondiabetic counterparts; difference remained statistically significant after controlling for different indicators of muscle characteristics. Both groups of diabetic patients (pharmacological therapy or not) were slower compared with subjects without diabetes, although the strength of the association was progressively attenuated including additional mediators or confounders to the statistical model (model 6 and fully adjusted model). Seven hundred and thirty-five subjects completed the 400-m walking test. After adjustment for age and sex, patients with diabetes had 2.6-fold (95% CI 1.4–4.9) likelihood of not being able to attempt or complete the walking test. Among those who were able to complete the test, only patients using oral agents and/or insulin therapy had a worse walking performance compared with persons free of diabetes. Again, the strength of the association was progressively attenuated including additional mediators or confounders to the statistical model and was no longer statistically significant after controlling for BMI, ABI, CMAP, CSII, CHD, CHF, cognitive functions (MMSE <24 and executive function), prevalent stroke, and inflammatory markers.
Table 3.
Multivariable linear regression analyses for the association of diabetes with 4-m and 400-m gait speed
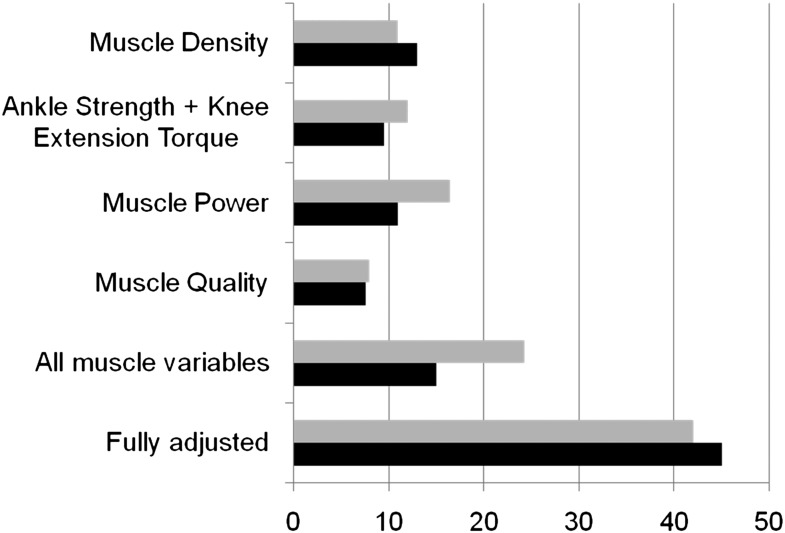
To estimate the individual contribution of lower-limb muscle impairment and other diabetes-related characteristics to the association between diabetes and walking speed, we compared the β coefficient for mean walking speed from the basic linear regression model (i.e., model adjusted for age and sex) with the β coefficient of models including each condition individually. The results of this analysis are depicted in Fig. 1. Individually, anthropometric and functional muscle characteristics explained 10–20% of the difference in the 4-m walking-speed performance associated with diabetes. When all muscle characteristics were simultaneously included in the multivariable regression model, they accounted for almost 25% of the association between diabetes and walking speed in the 4-m test and almost 15% in the 400-m test. Adjustment for other potential confounders and mediators (listed in Table 3) explained an additional 18 and 30% of the association between diabetes in the 4- and 400-m tests, respectively.
Figure 1.
Percent reduction in the association between diabetes and gait speed after adjustment for all potential mediators of the association (fully adjusted), for all muscular mediators, and for individual covariates. Bars show the reduction in β coefficient (from linear regression models) for diabetes compared with basic model (age and sex): [1 − (β adjusted/β basic)]. Gray bars, 4-m gait speed; black bars, 400-m gait speed.
CONCLUSIONS
In this study of randomly selected older individuals, persons with diabetes had slower gait speed and, despite having a larger muscle calf area, had lower muscle strength, muscle power, and muscle quality than persons without diabetes. In multivariable analyses adjusted for muscle density, muscle strength, muscle power, and muscle quality, the difference in gait speed between persons with and without diabetes was attenuated up to 25 and 15% in the 4- and 400-m tests, respectively, suggesting that impaired lower-limb skeletal muscle performance is an important contributor to mobility limitation in older patients with type 2 diabetes.
Our findings extend the results of previous studies, providing new insight into the complex and multifactorial biological pathways linking diabetes and walking impairment in older persons. Type 2 diabetes has been associated with muscle atrophy and weakness in different clinical (26) and population-based samples (10), and diabetic neuropathy involving motor neurons has been suggested to be an important determinant of accelerated muscle atrophy and loss of muscle strength in patients with diabetes. Furthermore, peripheral arterial disease, a common long-term diabetes complication, has been related to reduced motor NCV and muscle power and impaired lower-extremity performance in older persons (27). In our study, differences in walking speed, muscle strength, power, and muscle quality between persons with and without diabetes were independent of coexisting peripheral motor neuropathy or peripheral arterial disease of the lower limbs, suggesting a direct effect of diabetes on muscle characteristics and muscle performance.
Overweight and obesity are common among older persons with type 2 diabetes, and elevated BMI has been related to increased fat infiltration into the skeletal muscle. Increased muscle fat infiltration has been associated with reduced oxidative activity and reduced maximal aerobic capacity and, in epidemiological studies of older persons, fat infiltration predicted the risk of mobility disability over time (28). In our sample, muscle density, an indirect measure of muscle fat infiltration, lower-limb strength, and power were significantly lower in patients with diabetes but the differences were largely attenuated after adjustment for BMI. Although muscle quality was still significantly lower in patients with diabetes, these findings suggest that higher body fat might, at least partially, explain the impaired muscle performance observed in patients with diabetes.
The association of diabetes with gait speed and muscle strength and quality was stronger for patients on pharmacological therapy compared with those on diet therapy. Patients treated with hypoglycemic agents had higher fasting glucose levels and longer duration of disease compared with both individuals on dietetic therapy and those without diabetes, suggesting that severity and duration of diabetes and degree of metabolic control might play an important role in the disablement process of older persons with diabetes (29). Long-lasting suboptimal glycemic control is associated with protein catabolism in skeletal muscle that may lead to sarcopenia, muscle fat infiltration, and in turn loss of muscle strength and functional capacity (30). Overall, our and previous observational studies would support the hypothesis that improvement in glycemic control might prevent muscle and functional impairment in older diabetic patients, although this hypothesis needs to be formally tested in randomized clinical trials.
Our findings were consistent using two different walking tests. Both short- and long-distance walk performance reflect overall health and functional status of older adults and have been shown to predict survival, disability, and other important health outcomes in different clinical settings (31–33). Walking is a highly integrated function that requires the coordinated contribution of multiple physiological subsystems (13). On the other hand, diabetes is a chronic condition characterized by a progressive and simultaneous impairment of multiple organ subsystems that play a crucial role in walking physiology (7). Our study, in agreement with the work of other groups, demonstrates that in older persons, decreased muscle quality and accelerated loss of skeletal muscle should be considered important long-term diabetes complications. We found that the association between diabetes and walking performance was stronger for the 4-m than for the 400-m test. There are multiple potential explanations for this finding. First, only a subset of participants completed the 400-m test because of exclusion criteria and health problems; since in our sample prevalent diabetes was strongly associated with the risk of not being able to attempt or complete the test, it is likely that this selection might have diluted the observed association between diabetes and 400-m performance. Second, although correlated with each other, the 4- and the 400-m tests may assess different physiological functions. Among well-functioning older persons, the performance in the 400-m test is highly affected by aerobic fitness and exercise capacity, which in turn may be less strongly related to diabetes than the functional limitations related to a short walk. This difference might also explain why lower-limb skeletal muscle characteristics and quality accounted for a greater proportion of walking-speed differences between individuals with and without diabetes in the 4-m than in the 400-m walking test.
In interpreting these findings, some limitations should be considered. The cross-sectional and observational design of the study did not allow us to determine a temporal or causal relationship; although there are several clinical and biological explanations supporting a detrimental effect of diabetes on muscle physiology and walking performance, longitudinal data are needed to confirm our findings. A second limitation of the current study is lack of a direct marker of glycemic control. The assessment of glycated hemoglobin would have allowed for a better investigation of the potential association of glycemic control and the presence of muscle impairment and walking performance. Third, because of the limited number of persons with diabetes, we were not able to investigate the relationship of diabetes with muscle function and gait speed in women and men separately. Finally, neurophysiologic study was conducted on the superficial peroneal nerve that is not directly involved in ankle dorsiflexion control.
In summary, in community-dwelling older adults type 2 diabetes is associated with slower walking speed and reduced lower-limb muscle density, strength, and quality. These diabetes-related skeletal muscle impairments accounted for an important portion of the walking limitation observed in patients with diabetes. Our findings suggest that standardized assessment of muscle characteristics and lower-extremity function, with early detection of sarcopenia and impaired muscle quality, might prompt appropriate lifestyle or medical intervention to postpone functional decline, prevent disability, and preserve independence and quality of life of older persons with diabetes.
Supplementary Material
Acknowledgments
The InCHIANTI study baseline (1998–2000) was supported as a “targeted project” (ICS110.1/RF97.71) by the Italian Ministry of Health and in part by the U.S. National Institute on Aging (263 MD 9164 and 263 MD 821336).
No potential conflicts of interest relevant to this article were reported.
S.V. and L.B. researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript. Fu.L., J.M.G., and L.F. researched data, contributed to discussion, and reviewed and edited the manuscript. Fa.L. researched data. S.B. researched data and reviewed and edited the manuscript. G.Z. contributed to discussion and reviewed and edited the manuscript. S.V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 62nd Annual Meeting of the Gerontological Society of America, Boston, Massachusetts, 18–22 November 2011.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-2202/-/DC1.
References
- 1.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011;305:50–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332:556–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volpato S, Maraldi C, Fellin R. Type 2 diabetes and risk for functional decline and disability in older persons. Curr Diabetes Rev 2010;6:134–143 [DOI] [PubMed] [Google Scholar]
- 4.Volpato S, Ferrucci L, Blaum C, et al. Progression of lower-extremity disability in older women with diabetes: the Women’s Health and Aging Study. Diabetes Care 2003;26:70–75 [DOI] [PubMed] [Google Scholar]
- 5.Gregg EW, Mangione CM, Cauley JA, et al. Study of Osteoporotic Fractures Research Group Diabetes and incidence of functional disability in older women. Diabetes Care 2002;25:61–67 [DOI] [PubMed] [Google Scholar]
- 6.Gregg EW, Beckles GL, Williamson DF, et al. Diabetes and physical disability among older U.S. adults. Diabetes Care 2000;23:1272–1277 [DOI] [PubMed] [Google Scholar]
- 7.Volpato S, Blaum C, Resnick H, Ferrucci L, Fried LP, Guralnik JM, Women’s Health and Aging Study Comorbidities and impairments explaining the association between diabetes and lower extremity disability: the Women’s Health and Aging Study. Diabetes Care 2002;25:678–683 [DOI] [PubMed] [Google Scholar]
- 8.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc 2002;50:897–904 [DOI] [PubMed] [Google Scholar]
- 9.Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol 2003;95:1851–1860 [DOI] [PubMed] [Google Scholar]
- 10.Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes 2006;55:1813–1818 [DOI] [PubMed] [Google Scholar]
- 11.Park SW, Goodpaster BH, Strotmeyer ES, et al. Health, Aging, and Body Composition Study Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care 2007;30:1507–1512 [DOI] [PubMed] [Google Scholar]
- 12.Park SW, Goodpaster BH, Lee JS, et al. Health, Aging, and Body Composition Study Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 2009;32:1993–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc 2000;48:1618–1625 [DOI] [PubMed] [Google Scholar]
- 14.Cesari M, Penninx BW, Lauretani F, et al. Hemoglobin levels and skeletal muscle: results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2004;59:249–254 [DOI] [PubMed] [Google Scholar]
- 15.Kalyani RR, Metter EJ, Ramachandran R, Chia CW, Saudek CD, Ferrucci L. Glucose and insulin measurements from the oral glucose tolerance test and relationship to muscle mass. J Gerontol A Biol Sci Med Sci 2012;67:74–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bandinelli S, Benvenuti E, Del Lungo I, et al. Measuring muscular strength of the lower limbs by hand-held dynamometer: a standard protocol. Aging (Milano) 1999;11:287–293 [DOI] [PubMed] [Google Scholar]
- 17.Bassey EJ, Short AH. A new method for measuring power output in a single leg extension: feasibility, reliability and validity. Eur J Appl Physiol Occup Physiol 1990;60:385–390 [DOI] [PubMed] [Google Scholar]
- 18.Volpato S, Vigna GB, McDermott MM, et al. Lipoprotein(a), inflammation, and peripheral arterial disease in a community-based sample of older men and women (the InCHIANTI study). Am J Cardiol 2010;105:1825–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Iorio A, Cherubini A, Volpato S, et al. Markers of inflammation, vitamin E and peripheral nervous system function: the InCHIANTI study. Neurobiol Aging 2006;27:1280–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stålberg E, Fuglsang-Frederiksen A, Bischoff C. Quantitation and standardization in EMG and neurography. Suppl Clin Neurophysiol 2000;53:101–111 [DOI] [PubMed] [Google Scholar]
- 21.Lauretani F, Bandinelli S, Bartali B, et al. Axonal degeneration affects muscle density in older men and women. Neurobiol Aging 2006;27:1145–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deshpande N, Metter EJ, Ferrucci L. Validity of clinically derived cumulative somatosensory impairment index. Arch Phys Med Rehabil 2010;91:226–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME (Eds.). The Women's Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, MD, National Institute on Aging, 1995 (NIH publ. no. 95-4009) [Google Scholar]
- 24.Maraldi C, Volpato S, Penninx BW, et al. Diabetes mellitus, glycemic control, and incident depressive symptoms among 70- to 79-year-old persons: the health, aging, and body composition study. Arch Intern Med 2007;167:1137–1144 [DOI] [PubMed] [Google Scholar]
- 25.Ble A, Volpato S, Zuliani G, et al. Executive function correlates with walking speed in older persons: the InCHIANTI study. J Am Geriatr Soc 2005;53:410–415 [DOI] [PubMed] [Google Scholar]
- 26.Andersen H, Nielsen S, Mogensen CE, Jakobsen J. Muscle strength in type 2 diabetes. Diabetes 2004;53:1543–1548 [DOI] [PubMed] [Google Scholar]
- 27.McDermott MM, Guralnik JM, Albay M, Bandinelli S, Miniati B, Ferrucci L. Impairments of muscles and nerves associated with peripheral arterial disease and their relationship with lower extremity functioning: the InCHIANTI Study. J Am Geriatr Soc 2004;52:405–410 [DOI] [PubMed] [Google Scholar]
- 28.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci 2005;60:324–333 [DOI] [PubMed] [Google Scholar]
- 29.De Rekeneire N, Resnick HE, Schwartz AV, et al. Health, Aging, and Body Composition study Diabetes is associated with subclinical functional limitation in nondisabled older individuals: the Health, Aging, and Body Composition study. Diabetes Care 2003;26:3257–3263 [DOI] [PubMed] [Google Scholar]
- 30.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 2003;26:372–379 [DOI] [PubMed] [Google Scholar]
- 31.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000;55:M221–M231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA 2006;295:2018–2026 [DOI] [PubMed] [Google Scholar]
- 33.Volpato S, Cavalieri M, Sioulis F, et al. Predictive value of the Short Physical Performance Battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci 2011;66:89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.