A 2009 American Heart Association scientific statement titled “Dietary Sugars Intake and Cardiovascular Health” (1) concluded that current intake of added sugars among Americans greatly exceeds discretionary calorie allowances based on the 2005 U.S. Dietary Guidelines (2). For this reason, the American Heart Association Nutrition Committee recommended population-wide reductions in added sugars intake. The present statement from the American Heart Association and the American Diabetes Association addresses the potential role of nonnutritive sweeteners (NNS) in helping Americans to adhere to this recommendation in the context of current usage and health perspectives.
By definition, NNS, otherwise referred to as very low-calorie sweeteners, artificial sweeteners, noncaloric sweeteners, and intense sweeteners, have a higher intensity of sweetness per gram than caloric sweeteners such as sucrose, corn syrups, and fruit juice concentrates. As a caloric sweetener replacement, they are added in smaller quantities; hence, they provide no or few calories. In our current food supply, NNS are widely used in thousands of beverages and other food products such as diet soft drinks, yogurts, desserts, and gum. Food manufacturers often use a blend of NNS or use a blend of sugar and NNS to improve the flavor acceptability of NNS. In developing this scientific statement, the writing group reviewed issues pertaining to NNS in the context of data on consumer attitudes, consumption patterns, appetite, hunger and energy intake, body weight, and components of cardiometabolic syndrome. The objective was to review the literature to determine whether there were adequate data to provide guidance for the use of NNS.
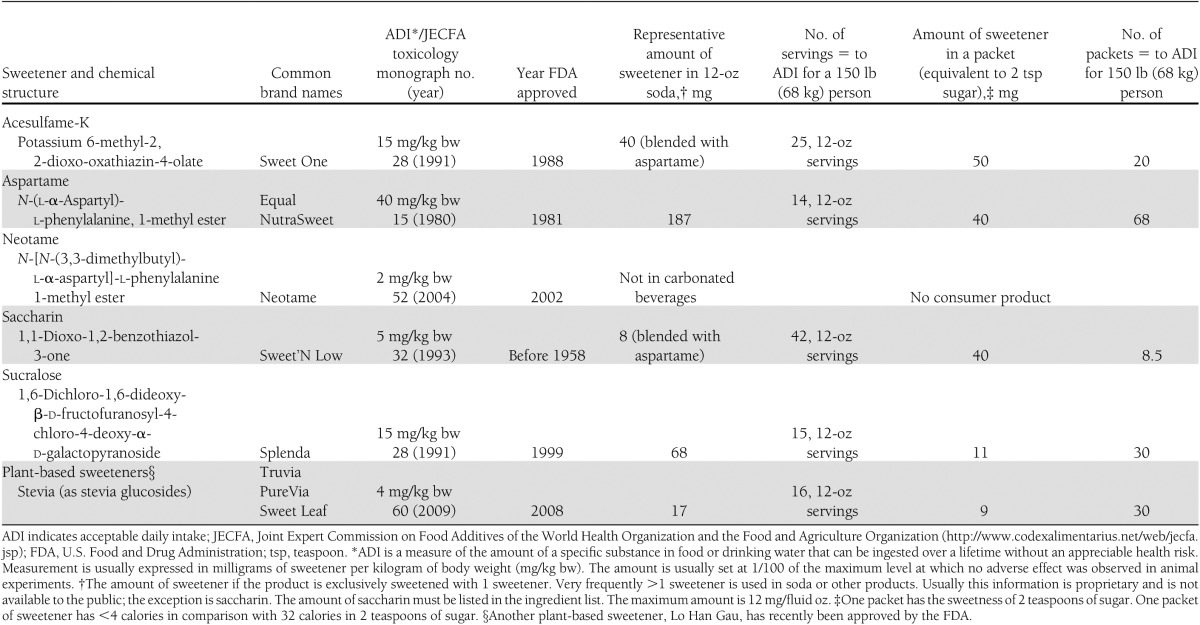
The focus of the statement is on the 6 NNS that are described in Table 1. Aspartame, acesulfame-K, neotame, saccharin, and sucralose are regulated as food additives by the U.S. Food and Drug Administration and therefore had to be approved as safe before being marketed. Regarding stevia, at this time, the U.S. Food and Drug Administration has not made a determination as to the Generally Recognized As Safe status, but has issued no objection letters for a number of Generally Recognized As Safe notifications for stevia sweeteners (http://www.fda.gov/Food/FoodIngredientsPackaging/GenerallyRecognizedasSafeGRAS/GRASNotificationProgram/default.htm). Because all 6 of these NNS have current U.S. Food and Drug Administration approval, issues related to safety of these compounds are not addressed. In addition, the review of the literature is primarily restricted to human studies in which noncaloric sweeteners are used as a replacement for caloric sweeteners.
Table 1.
Literature Search
Approach
The literature search was conducted by using the following search terms: nonnutritive sweetener(s), artificial sweetener(s), noncaloric sweetener(s), diet (beverages and soft drinks), and the names of each of the 6 NNS available for use in the United States (including their brand names). The initial search by use of PubMed was limited to original research published after 2000, studies conducted in humans (primarily restricted to controlled trials and prospective cohort studies), and systematic reviews. Additional articles were identified by use of the literature cited in the original publications and review articles, and the Evidence Analysis Library of the American Dietetic Association (http://www.adaevidencelibrary.com/), as well. A Google search was used to identify published marketing research related to consumer views of NNS.
Summary of available literature
The literature search yielded relatively few research studies that focused on the specific objective of this scientific statement, the potential role of NNS in facilitating reduction of added sugars intake in humans. This is likely attributable to inherent complexities involved in the design and implementation of these types of studies. In particular, experimental studies designed to test the effects of NNS on health outcomes in food are particularly challenging, because replacing added sugars with NNS alters dietary composition (i.e., assuming portion size is not reduced, the relative proportions of fat, protein, and carbohydrate may be changed or the nature of the carbohydrate alone may be changed) in ways that can add potential confounding to the simple comparison of added sugars versus NNS. The majority of the human data in prospective observational studies and randomized controlled trials on the use of NNS focus specifically on diet soft drinks as a replacement/displacement of regular soft drinks. Assessment in large prospective cohorts of the use of NNS from sources other than diet soft drinks has been limited methodologically by such factors as lack of specificity in intake data collected by food frequency questionnaires (FFQs) and lack of accurate NNS composition values in available databases. Much of the published literature on NNS addresses research conducted in animal models and evaluations of potential toxicity, neither of which were areas of focus for this statement. Hence, the review that follows is notably limited by the lack of an extensive evidence base.
Consumer Attitudes and Rationale for Using or Avoiding NNS
Food and beverage industry publications provide some insights into consumers' rationale for NNS use. As expected, some of this use is tied to consumer efforts to decrease their intake of calories and caloric sweeteners. A 2012 survey of American adults by the International Food Information Council Foundation found that 51% of the respondents reported trying to limit sugar, 44% were trying to limit high-fructose corn syrup, and 29% were trying to limit low-calorie sweeteners (7). When asked to select ≥1 choices from a list of reasons for using NNS, 41% chose “are an option for people with diabetes,” 41% chose “can reduce the calorie content of foods,” 40% chose “can play a role in weight loss or weight management,” 30% chose “can be part of an overall healthful diet,” and 31% chose “don't know enough about them to provide an answer.” Seventy-three percent of those consuming NNS reported doing so to reduce total calories consumed.
Although consumer demand for reduced-calorie and added-sugars products has resulted in a proliferation of new NNS products, American consumers have concerns about NNS, as well. In a Mintel survey conducted in a representative sample of Americans, the vast majority of survey respondents (81%) agreed with the statement, “Sugar-free foods do not taste as good as those made with real sugar” (7a). The Mintel survey also found that 64% of respondents indicated they were concerned about the safety of “artificial” sweeteners (7a). Survey data from the Hartman Group indicated that 44% of consumers reported they “deliberately avoid/reduce intake of artificial (flavors, sweeteners, colors or dyes),” but, when asked about specific NNS, the proportion drops to 39%, 33%, and 21% for saccharin, aspartame, and sucralose, respectively. The survey did not include other NNS (8). Consumers' concerns about taste and artificial ingredients may motivate food manufacturers to promote sucralose as being made from “real” sugar and stevia-derived NNS as being “natural” and made from the leaves of the stevia plant. Consumer education will need to stress that promotion of a certain NNS as “real” or “natural” does not necessarily indicate that such products are more efficacious or safer than other NNS.
Estimates of Consumption Patterns
Information on the amounts of NNS in beverages and foods is limited and not easily accessible. Food processors and manufacturers are required to list the NNS in product ingredient lists but, with the exception of saccharin, are not required to provide the amount used in products or to release the information to federal agencies or the general public (3). Between 1999 and 2004, there was a large increase in the number of new products with NNS available in the United States, estimated to be >6,000 (9). Of the 6 available NNS, sucralose (found in 2,500 products), acesulfame-K (found in 1,103 products), and aspartame (found in 974 products) were the most popular (9). NNS are most commonly used in carbonated beverages (3). Estimates of consumption patterns are typically made by reporting amounts of beverage and food items containing NNS, rather than amounts of specific NNS consumed.
Nationwide Food Consumption Survey, National Health and Nutrition Examination Survey, andU.S. Department of Agriculture Economic Research Service Data
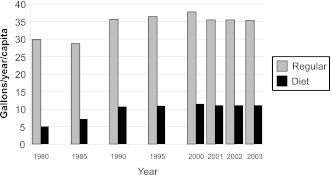
Mattes and Popkin (3) examined the trends in consumption of foods and beverages with NNS among Americans aged ≥2 years by use of the U.S. Department of Agriculture Nationwide Food Consumption Survey for 1965, 1977 to 1978, and 1989 to 1991 and the National Health and Nutrition Examination Survey (NHANES) for 1999 to 2000, 2001 to 2002, and 2003 to 2004. Consumption of NNS in both beverages and foods increased over time. The survey data indicate that, on any given day, 15% of the U.S. population consumed NNS in 2003 to 2004 in comparison with 3% in 1965. The proportion of the population choosing beverages with NNS remained relatively stable between 1989 and 2004 (10.1% and 10.8%, respectively), whereas the proportion consuming NNS in foods increased from 3.2% to 5.8% during that time period (3). Notably, the increase in NNS products was not accompanied by a corresponding decrease in reported consumption of products sweetened with added sugars, which indirectly suggests that NNS are not being used to displace products sweetened with added sugars. This same pattern of parallel increases in both NNS products and products with added sugars has been reported elsewhere (10,11) and is evident from per capita food availability data as compiled by U.S. Department of Agriculture Economic Research Service for regular versus diet soft drinks, with intake increasing primarily from 1980 to 1990 and then leveling off between 1990 and 2003 (Fig. 1).
Figure 1.
Carbonated soft drinks, gallons per year per capita. U.S. availability as determined by U.S. Department of Agriculture Economic Research Service (http://www.ers.usda.gov/Data/FoodConsumption).
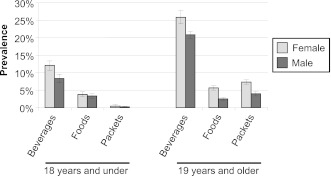
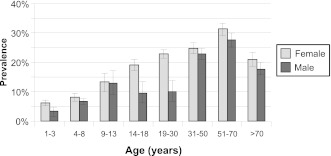
One of the authors (L.M.S.) examined the prevalence of consuming NNS foods and beverages in children and adults with the use of data from NHANES 2007–2008. Dietary intake in NHANES was assessed by a 24-hour recall in-person interview, which presents a “snapshot” of foods and beverages consumed by NHANES participants on a given day. The most frequently reported and consumed NNS product was NNS beverages in comparison with intake of NNS foods or packets of artificial sweeteners among male and female children and adults in NHANES 2007–2008 (Fig. 2; L.M. Steffen, unpublished data, 2011). After further examination of NNS beverage intake by age group, the prevalence of consumption of these beverages increased across age groups, especially among females (Fig. 3; L.M. Steffen, unpublished data, 2011). In general, more adults were NNS beverage consumers than children or adolescents.
Figure 2.
Prevalence (%, SE) of nonnutritive sweetened beverage and food intake in the U.S. population as determined by one 24-hour recall (National Health and Nutrition Examination Survey 2007–2008) among individuals. Sample sizes: for females and males aged <18 years, n = 1,781 and n = 1,911, respectively; and for females and males aged >19 years, n = 2,820 and n = 2,742, respectively.
Figure 3.
Prevalence (%, SE) of nonnutritive sweetened beverage intake among U.S. children and adults as determined by one 24-hour recall (National Health and Nutrition Examination Survey 2007–2008) according to age group. The sample size of participation by age group for females was ages 1 to 3 (n = 575), 4 to 8 (n = 435), 9 to 13 (n = 418), 14 to 18 (n = 353), 19 to 30 (n = 513), 31 to 50 (n = 950), 51 to 70 (n = 873), and >70 (n = 484). The sample size of participation by age group for males was ages 1 to 3 (n = 617), 4 to 8 (n = 502), 9 to 13 (n = 412), 14 to 18 (n = 380), 19 to 30 (n = 518), 31 to 50 (n = 889), 51 to 70 (n = 869), and >70 (n = 466).
Other perspectives on NNS users
Dietary intake assessed by FFQ, as is common in observational cohort studies, provides an estimate of those who “ever” versus “never” use NNS-containing beverage or food items included in the questionnaire; this is a different perspective than that derived from 24-hour recall data as above. The prevalence of saccharin intake in 1980 (the only NNS available at that time) was assessed in a subset of the Nurses' Health Study (NHS) characterized as being free from major illnesses and other lifestyle factors that might modify weight gain. Intake was assessed by use of a 61-item FFQ completed by 31,940 women. The report indicated that the prevalence of saccharin users was 56% (12). In the Health Professionals Follow-up Study (HPFS), artificially sweetened beverage intake was assessed in 1986 and every 4 years after that during 20 years of follow-up with use of a 131-item FFQ (13). By use of a cumulative average over this time period, 54% of the men reported consuming artificially sweetened beverages at least 2 times per month. The San Antonio Heart Study enrolled a randomly selected sample from non-Hispanic white and Mexican American neighborhoods. Among 3,682 participants who were examined first between 1979 and 1988 and then again 7 to 8 years later, 48% reported some consumption of NNS in beverages that included diet soft drinks, coffee, or tea. Consumption of NNS in food items was not assessed (14).
Appetite, Hunger, and Energy Intake
For NNS-sweetened items to successfully contribute to reductions in calories from added sugars or other sources, they must also avoid causing compensatory energy intake immediately or later in the day. If choosing a diet soft drink over a regular calorie soft drink leads to a decrease of ∼100 kcal per 8-oz serving, but later in the day causes an alteration in appetite or hunger that results in an additional 50, 100, or 200 kcal of intake, the initial “calorie saving” effect would be altered or reversed. Or, if choosing a diet soft drink enables an individual to simultaneously justify eating a 150-calorie snack, the calorie savings from the NNS would also be negated.
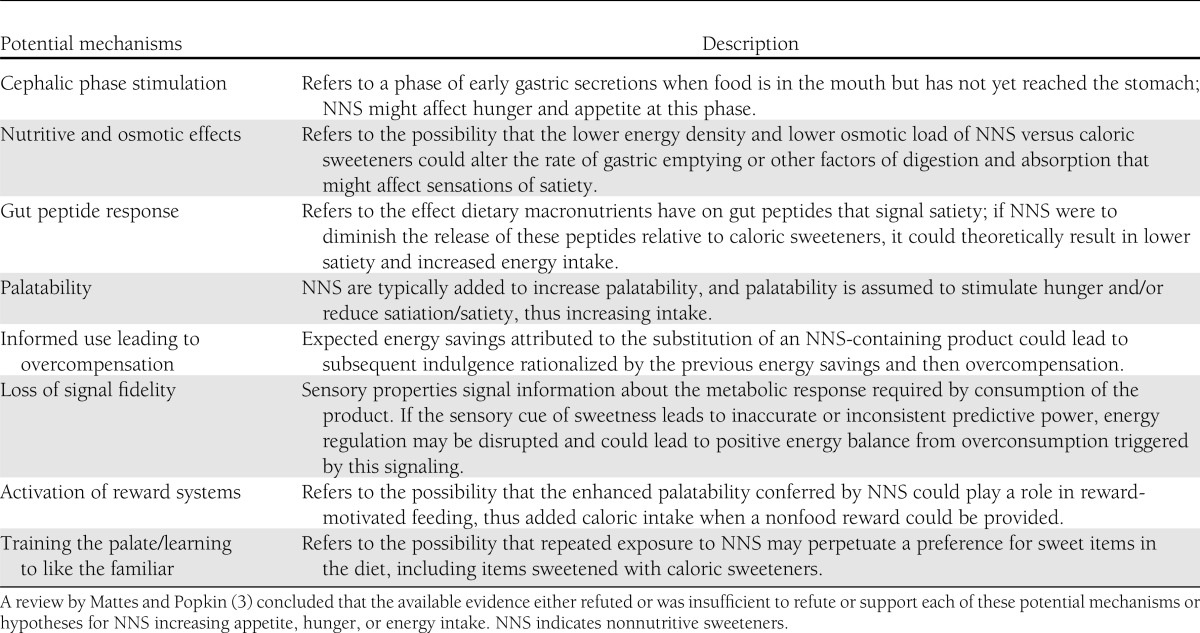
Mattes and Popkin (3) critically examined the evidence for effects of NNS on compensatory appetite and food intake. The 8 potential mechanisms they reviewed are listed and briefly described in Table 2. The review concluded that the available evidence either refuted or was insufficient to refute or support each of these potential mechanisms or hypotheses for NNS increasing appetite, hunger, or energy intake (3).
Table 2.
Potential mechanisms of effects of NNS on compensatory appetite and food intake
The complexity in attributing the extent to which NNS consumption is compensated for by intake of other foods is fraught with methodological challenges both in controlled feeding experiments and free-living trials. According to International Food Information Council Foundation research, <10% of Americans can accurately estimate the number of calories they should consume in a day. Consumer education on daily energy requirements is needed, and that products containing NNS may assist in weight control when used in place of full-calorie products, if not compensated for otherwise. Controlled feeding experiments might or might not show an impact of NNS on future caloric intake, but, by definition, these are not real-world situations. Conversely, in the real world, so many factors impact food choice that attributing caloric intake after NNS consumption uniquely to that consumption may also not be true and may be confounded particularly by reverse causality; that is, the previous NNS choice might be deliberate in anticipation of a known future exposure to higher caloric meal.
Energy intake compensation
Various studies that have examined energy intake as a primary or secondary outcome have used designs that contrast beverages and foods made with caloric sweeteners versus NNS. In a meta-analysis of weight loss studies that tested the effectiveness of aspartame in reducing energy intake, data from 12 studies were pooled to address energy intake compensation (15). The weighted average of energy intake compensation in the ∼24 hours that followed aspartame intake was 32% (i.e., 68% of the original energy deficit was uncompensated and was maintained through the subsequent period of intake). Of the 4 studies from the meta-analysis that used beverages alone, the compensation was just 15% for the subsequent 24 hours; that is, the data suggest there is less compensation in beverages than foods, resulting in a more effective net reduction in calories when replacing sweetened beverages with NNS beverages (15). The implication here is that anything <100% compensation results in a net reduction in 24-hour energy intake.
One of the studies included in the meta-analysis involved 24 men and women who consumed 4 different beverages, each for 4 weeks, in a crossover design. Subsequent food intake was monitored. The beverages included a full caloric beverage (sucrose) versus an NNS beverage (aspartame), provided with either an orange or raspberry flavor. Data were collected at baseline and after 4 weeks of habituation (16). The investigators observed a significant lack of energy intake compensation over the course of the day among study participants when they were consuming the full caloric beverages relative to the NNS beverages. In another study, 21 overweight adults were assigned to consume 28% of their energy intake as sucrose, mostly as beverages. After 10 weeks, there was a significant increase in their energy intake, body weight, fat mass, and blood pressure (17). These effects were not observed in a similar group of 20 overweight adults who consumed NNS in place of the sucrose, but otherwise had a similar dietary intake composition in comparison with the other group (17). In a controlled feeding experiment that involved a preload snack containing sucrose (493 kcal), aspartame (290 kcal), or stevia (290 kcal), the energy intake of subsequent meals was similar; the energy intake for the combined preload snack and later meals combined was lower for the aspartame and stevia phases versus the sucrose phase (P = 0.01) (18). All of these studies, therefore, observed a net reduction in energy intake for NNS use relative to the comparison condition with sucrose.
Not all such studies have observed a net reduction in overall energy intake when NNS were used instead of caloric sweeteners. A 4-week trial, providing either diet soft drinks (sweetened with aspartame) or regular calorie soft drinks resulted in similar total energy intake in healthy-weight adults of both sexes (19) and in overweight women as well (20). Similarly, the results of a 25-week study conducted in adolescents suggested that normal-weight youth compensated for the energy reduction when consuming diet beverages. However, as evidence of the complexity of this issue, the same study reported that the use of NNS beverages did result in a net energy deficit in those adolescents in the highest body mass index (BMI) tertiles (a conclusion that should be tempered by awareness that the intervention group was counseled to replace sugar-sweetened beverages with diet beverages and/or water, making it difficult to differentiate the effects of NNS versus water) (21).
Overall, the limited human studies literature in this area of NNS research suggests that compensation is an important factor to consider when assessing impact on energy intake over periods of ≥24 hours. Further studies are warranted to address some of the complexities inherent in this issue, including the potential impact of being informed versus naïve about the presence or absence of NNS in items being consumed. It is worth noting that, in preschool children, when energy intake is reduced by substitution of nutrient-dense, lower-calorie food choices instead of NNS (e.g., fruits and vegetables), some energy intake compensation occurs, but net energy intake remains lower over several days (22,23).
Appetite, or preference, for sweet taste
Examination of the potential effects of NNS on appetite requires consideration of genetic and environmental factors that may regulate preference for intensity of sweet taste (24–26). One focus of such research concerns the metabolic/hormonal responses to both caloric sweeteners and NNS to determine whether these can be linked to mechanisms that promote future weight gain or other adverse physiological consequences. Sweet taste is mediated largely by a single receptor composed of the 2 subunits, TAS1R2 and TAS1R3. Understanding how the polymorphism of this receptor gene affects preferences is important to understanding usage of NNS. Recent data in mice suggest that additional mechanisms may exist, but these have not been confirmed in humans (27). Newer animal research also suggests the possibility that sweet-taste receptors exist in the gut, triggering complex metabolic/hormonal responses to NNS. The impact of these receptors and their metabolic consequences on obesity and diabetes mellitus in humans is just beginning to be studied (28). Although ingestion of foods containing NNS may trigger a wide array of responses related to food absorption that may impact future food consumption (29), adverse effects related to these gut receptors have yet to be identified (30,31).
A preference for higher intensity of sweetness exists in humans in infancy, declines in adolescence in comparison with infancy, and then plateaus or slowly declines in young adulthood, and may or may not decline further in old age (32,33). There is significant interindividual variation in sweet- and bitter-taste preference, and this preference may be genetically determined (34–37). These genetic differences may impact preferences for both caloric sweeteners and NNS, which may also have a bitter-taste component. Although animal research suggests that sweet-taste preference can be conditioned through repeated exposure (38), preference for sweet taste in humans is more complex and involves many central receptor mechanisms, including but not limited to dopamine signaling (39). Critical factors such as food culture and social expectations may also have an impact (3). The presence of caloric sweeteners may also enhance taste acceptance (40).
Cardiovascular and Metabolic Health Outcomes
The potential effects of NNS on body weight, cardiometabolic variables, and diabetes mellitus/glycemic response were examined. The evidence considered was limited to prospective studies and controlled trials conducted in humans. For the controlled trials, primary emphasis was given to study designs that involved replacing caloric sweeteners with NNS. Prospective cohort studies were also considered, but cross-sectional data were not considered. Although NNS intake may precede and lead to changes in weight or metabolic variables, the opposite is also plausible (i.e., individuals who become overweight/obese may seek out NNS items after becoming overweight/obese or being informed they have type 2 diabetes mellitus). Therefore, it is not possible to distinguish the temporal relationship between these by using cross-sectional studies, and, as will be discussed, it can be difficult to distinguish this relationship even in some prospective studies.
Body weight
The vast majority of data available related to NNS consumption and body weight has focused on creating an energy deficit by substituting NNS for sugars in the beverage component of the diet, with only a few intervention studies also targeting foods. Findings in both controlled intervention trials and prospective observational studies have been inconsistent.
Controlled intervention trials have reported that use of products with NNS results in weight loss or has negligible effects on weight. In a review of 16 trials with a primary focus on aspartame and weight control, weight loss was a primary outcome in 9 of the 16 trials (15). Beyond summarizing the overall pattern of success with weight loss in these studies, the investigators attempted to quantify the magnitude of caloric substitution required for meaningful weight loss. By their calculations, a weight loss of 0.2 kg/week over 12 weeks was achieved from a net energy deficit of 220 kcal/day attributable to aspartame substitutions for caloric sweeteners. Determining the net energy deficit required consideration of energy intake compensation, which was different for foods and beverages (as described previously, see energy intake compensation). For foods it was determined that displacing 330 kcal/day of sugar (82.5 g), and assuming 32% compensation, would yield the 220-kcal deficit. For beverages, with a calculated 15% compensation, 260 kcal of caloric sweetener, or 70 g (the amount found in ∼2, 12-oz soft drinks), would need to be displaced to yield the same net 220-kcal deficit.
In a study conducted with 103 subjects who were 13 to 18 years of age and who regularly consumed sugar-sweetened beverages, BMI change was the primary outcome. The randomized trial contrasted home delivery of NNS beverages (i.e., water and diet soft drinks, iced teas, lemonade, and punches) to a control group that did not receive beverages and were instructed to continue their habitual consumption. Overall, there were no significant differences in BMI changes over the 25-week period; however, in a post hoc stratified analysis of participants in the highest tertile of baseline BMI, the BMI change was significantly greater, reflecting weight loss, in the group receiving the NNS beverages (21). Another study involved a family-based randomized trial testing the impact of promoting sucralose and sucralose-containing products as substitutes for caloric sweeteners in beverages and foods (41). This study was conducted in conjunction with instructions to add 2,000 steps/day to their habitual physical activity. One hundred families assigned to the intervention were compared with 93 families assigned to follow their habitual diet and activity patterns. After 6 months, none of the primary study outcomes (changes in BMI for age, waist circumference, or percentage of body fat) were significantly different between treatment groups, either for the children (primary target population), or their parents (secondary target population). In post hoc analyses, the investigators found a borderline significant improvement in the intervention group for the percentage of children who maintained or reduced their BMI for age.
As would be expected, controlled intervention trials involving dietary manipulations, as described above, are generally limited to relatively short durations of ≤6 months (1 exception being a 16-week intervention with aspartame-sweetened foods and beverages with a 1-year maintenance period and 2 years of follow-up; this study was included in the review of 16 trials previously described) (15,42). Longer-term perspectives are available from prospective observational studies, with the important limitation that these studies can address only associations, and not causal effects.
A limited number of prospective observational studies have addressed NNS and weight, most of which assessed NNS intake by FFQ. None of the studies were designed specifically to assess associations between NNS and weight. Two studies published >20 years ago reported a positive association between NNS use and weight gain (12,43). However, these findings were observed by the use of limited dietary assessment methods, and in selected (i.e., not representative) study populations. A 2008 study examining the San Antonio Heart Study population reported a positive dose-response association between artificially sweetened beverage consumption (i.e., diet soft drinks, and coffee and tea with added NNS) and incidence of overweight/obesity during a 6- to 7-year follow-up period of 5,158 adults. The association was consistent across sex and ethnicity; overall, the change in BMI was 47% greater among NNS users than nonusers after adjusting for baseline BMI and demographic and behavioral characteristics (14). A 2011 study reported findings on associations of a broad range of diet and lifestyle variables with 12- to 20-year weight change observed in 3 separate cohorts that included 120,877 U.S. women and men from the NHS, NHS II, and the HPFS. For a given unit of 1 diet soft drink serving/day, the association with weight change was not statistically significant in the NHS, whereas there was a statistically significant but modest association with weight loss (<0.25 lb/4 years) observed in NHS II and HPFS after adjusting for a broad range of demographic and lifestyle variables (44).
A separate publication based on only the HPFS population focused specifically on sugar-sweetened beverage versus artificially sweetened beverage (ASB) intake and provides important insights into the opportunities and limitations of the perspective offered by prospective observational studies. Cumulative averages of intake were determined by FFQs administered every 4 years for 20 years, starting in 1986, and the main outcome of interest in this publication was incidence of type 2 diabetes mellitus. At the start of the study, in 1986, participants were asked to report any weight change in the preceding 5 years, between 1981 and 1986. The weight change data were presented separately for those who reported losing weight (14%–18% of the population, depending on the specific quartile of ASB intake [L. de Koning, personal communication, 2011]) and those who reported gaining weight (45%–51% of the population, depending on the specific quartile of ASB intake) in that time frame; the remainder reported no weight change (13). Among those who lost weight, there was a significant trend of greater weight lost for higher versus lower ASB intake quartiles (0.9 ± 0.2 versus 0.5 ± 0.5 kg, means ± SD, respectively, for the highest versus lowest quartile). Among those who gained weight, there was a significant trend of greater weight gained for higher versus lower ASB intake quartiles (2.6 ± 4.6 versus 1.8 ± 3.4 kg, respectively). This may appear contradictory; how could greater intake be associated with both greater weight loss and greater weight gain? However, this suggests there may be importantly different subsets of the population: one that successfully uses ASB for weight control and another that does not. On the other hand, the different time frames of the weight change data versus ASB intake data must also be considered; the 1981 to 1986 weight change preceded the 1986 to 2006 cumulative average ASB intake reported in the article; from a chronological perspective it is the weight change that is predicting ASB consumption, rather than vice versa (i.e., weight gain may predict subsequent increased ASB consumption). Three additional findings presented in this study further highlight the complexities involved: the following trends for baseline characteristics for the full study population, regardless of 1981 to 1986 weight change, were all statistically significantly related to ASB intake; diet quality as assessed by the Alternative Healthy Eating Index was higher, total energy intake was lower, and BMI was higher for those consuming greater amounts of ASB. Overall, the data from this study suggest there may be important subsets of the population that incorporate NNS products into their diet with different objectives or with differential success, and that there is likely substantial confounding in these types of analyses given the many dietary and health variables that correlate with NNS intake (12,43).
Assimilating results from intervention and observational studies.
The 2 sets of studies reviewed above help to demonstrate what have been and will continue to be challenges in reaching definitive conclusions about the effectiveness of NNS in supporting energy intake restriction and promoting weight loss and weight control. The short-term nature of intervention studies limits their ability to observe long-term weight change, and the typical personal/individual support from study staff and related heightened awareness of NNS intake among study participants limit the generalizability of the findings. On the other hand, among the observational studies, the challenges of accurately assessing NNS intake, the many potential confounders, and the difficulty in determining directionality (e.g., possible reverse causality) represent important limitations. Nonetheless, the strength of intervention trials in establishing causality and the opportunity in observational studies to address long-term changes in health outcomes suggest that both approaches will continue to be useful and complementary in providing guidance regarding NNS use. Currently, available data provide, at best, modest support for a modest effect on weight with NNS use, with many important caveats to the available published findings and ample opportunity for future studies to build on past studies to more effectively address this issue.
Cardiovascular disease risk factors, cardiometabolic syndrome, and coronary heart disease
Several prospective studies have been conducted to examine associations of NNS soft drink (i.e., diet soft drink) intake with chronic disease, including coronary heart disease (CHD), chronic kidney disease, and cardiometabolic syndrome and its individual components. In the NHS, consuming ≥2 servings of diet soft drinks per day was associated with an increased risk of CHD and chronic kidney disease in comparison with consuming <1 serving of diet soft drinks per month over 11 to 12 years of follow-up (45,46). Middle-aged men and women consuming >1 servings of diet soft drinks per day had >30% higher risk of developing metabolic syndrome over 9 years of follow-up (47). Similar results were also observed for the components of metabolic syndrome (47). In the Northern Manhattan Study of >2,500 multiethnic adults with 10 years of follow-up and a cardiovascular event incidence of ∼2%, daily diet soft drink intake was associated with an increased risk of events; regular soft drink intake was not associated with events. However, the small numbers of events, the unusually small numbers of daily consumers of diet soft drinks in this population, and the plausibility of reverse causality led the authors to caution against possible misinterpretation of the findings (48). In contrast, a recent report from the HPFS examining CHD incidence over 22 years in almost 43,000 men with 3,683 CHD events, observed a positive association between sugar-sweetened beverage intake and CHD risk, but no association with ASB (49). The authors specifically noted in their discussion that ASB consumption was probably, at least in part, attributable to response to the diagnosis of a chronic condition. Finally, in a recent study of the joint associations of consuming a prudent (healthy) or Western diet pattern with or without diet beverage consumption among young adults with >20 years of follow-up (CARDIA [Coronary Artery Risk Development In Young Adults] study), consumers of both a prudent (healthy) diet pattern and diet beverages had a lower risk of having elevated glucose or low high-density lipoprotein-cholesterol, but not the metabolic syndrome, than nonconsumers following a prudent diet pattern or consumers and nonconsumers following a Western diet pattern (50). In general, the literature in this area is sparse, limited to prospective observational studies, and examines NNS intake from beverages, but not foods. In addition, these observational prospective studies can only identify associations, not causal determinants, and there is often a strong plausibility of reverse causality for some of the significant associations observed.
Diabetes mellitus and glycemic response
A recent publication of 20-year follow-up data from healthy men in the HPFS addressed the relationship between intake of sugar-sweetened beverages and ASB and the development of type 2 diabetes mellitus (13). For sugar-sweetened beverages, the association with developing diabetes mellitus remained significant after adjusting for potential confounders. ASB intake was significantly associated with developing type 2 diabetes mellitus in an age-adjusted analysis. However, the association was no longer significant in subsequent multivariate-adjusted analysis; the association between ASB and diabetes mellitus was largely explained by health status/family history related to diabetes mellitus risk, preenrollment weight change, dieting, and BMI. These findings suggest that the men who consumed ABS may have done so to address a weight gain and health conditions associated with increased risk of developing diabetes mellitus.
With regard to NNS and glycemic response, 4 randomized trials that varied from 1 to 16 weeks in duration found no significant difference between the effects of NNS and various comparisons (sucrose, starch, or placebo) on standard measures of glycemic response (i.e., plasma glucose and insulin, HbA1c, C-peptide) and, in general, did not detect clinically relevant effects (51–54). One study that involved contrasting treatment arms assigned participants to either 1) a diet consistent with American Diabetes Association guidelines (control arm) or 2) a lower calorie experimental diet containing sucralose. The result was greater reductions in HbA1c, weight, and BMI for the experimental diet group (55). The experimental diet, however, differed from the control diet in fat replacer and fructose content, and sucralose content, as well, and therefore it is not possible to determine the separate contribution of the sucralose content to the study effects. The submission to the U.S. Food and Drug Administration to designate stevia extract, rebaudioside A, also known as rebiana, with Generally Recognized As Safe status noted the lack of effects on blood glucose. Information about a lack of blood glucose effects was presented to address claims that some stevia-derived compounds had a therapeutic blood glucose–lowering benefit (56). Potential effects of beverages or foods containing NNS on glycemic response are likely attributable to the replacement of carbohydrate by NNS rather than any direct effect. The American Dietetic Association (renamed the Academy of Nutrition and Dietetics in 2012) concluded in a 2010 review that, in general, NNS do not affect glycemic response (do not raise postmeal glucose response or have a negligible inherent effect on glycemic response) in people with diabetes mellitus. Although the intrinsic glycemic effects of NNS are similar to other calorie-free/carbohydrate-free products, some NNS-containing products may contain energy and carbohydrate from other sources that need to be considered (57).
Needs for Further Research
Because of the paucity of well-designed human studies that address the specific, practical, public health issues, as presented above, further research is warranted in several areas.
NNS and energy compensation
The most obvious potential benefit of NNS is the substitution of energy from added sugars, thereby facilitating both energy and carbohydrate intake reductions (with potential glycemic control benefits). However, what would appear to be a potential benefit is complicated by compensation; that is, the extent to which an immediate energy and carbohydrate reduction through NNS use is followed by a subsequent increase in energy intake (regardless of macronutrient type) beyond what would have been consumed in the absence of the use of NNS. Existing data suggest that there is partial compensation with differences in intake after consuming food and beverages containing NNS. Energy intake compensation appears to be greater after consuming foods containing NNS than that for beverages containing NNS. Future work should test and compare different food and beverage vehicles of the NNS, and compare the different NNS themselves and NNS combinations as well, in an effort to maximize potential impact.
NNS mechanisms of action and pathophysiology
Research in the area of energy compensation would be facilitated and complemented by continued research into the mechanisms of potential NNS effects. This would include, but not be limited to, increasing the body of knowledge of oral, gut, and neural receptors, and hormonal responses that contribute to alterations in food preferences, appetite regulation, and satiety that are elicited by NNS. This research should inform the selection of foods, beverages, specific NNS (or combinations), and perhaps specific population subsets for studies of compensation effects.
Randomized controlled trials using NNS to impact body weight or glycemic control
Well-designed and controlled designed clinical trials are needed to determine the long-term effect of NNS on body weight regulation and glycemic control at different points in the life cycle. Weight control studies (e.g., weight loss) should be minimally 1 year in duration or longer, whereas glycemic control studies could be shorter in duration. Studies of both end points would benefit from better informed selections of the types, amounts, combinations, and vehicles of NNS delivery, as described in the previous 2 areas of suggested further research.
Assessment of NNS intake
Immediate needs include updating of current food databases with accurate data for the NNS content of foods and revision of instruments used to estimate food intake to accurately capture NNS content.
Conclusions and Recommendations
At this time, there are insufficient data to determine conclusively whether the use of NNS to displace caloric sweeteners in beverages and foods reduces added sugars or carbohydrate intakes, or benefits appetite, energy balance, body weight, or cardiometabolic risk factors. Limiting added sugars is an important strategy for supporting optimal nutrition and healthy weights, as concluded in the 2009 American Heart Association scientific statement “Dietary Sugars Intake and Cardiovascular Health” (1). Monitoring carbohydrate intake, which includes limiting added sugars, is also a key strategy to achieve glycemic control as published in the American Diabetes Association clinical practice recommendations (58). There are some data to suggest that NNS may be used in a structured diet to replace sources of added sugars and that this substitution may result in modest energy intake reductions and weight loss. Successful reduction in energy intake requires that there is incomplete compensation of energy reduction from the use of NNS-containing beverages and/or foods. The impact of incorporating NNS and NNS-containing beverages and foods on overall diet quality should be included in assessing the overall balance of benefits and risks. Apparent from the available literature is the paucity of data from well-designed human trials exploring the potential role of NNS in achieving and maintaining a healthy body weight and minimizing cardiometabolic risk factors.
The evidence reviewed suggests that when used judiciously, NNS could facilitate reductions in added sugars intake, thereby resulting in decreased total energy and weight loss/weight control, and promoting beneficial effects on related metabolic parameters. However, these potential benefits will not be fully realized if there is a compensatory increase in energy intake from other sources.
Disclosures
Writing group disclosures
Reviewer disclosures
Acknowledgments
For potential conflicts of interest, please see DISCLOSURES.
The authors thank Xia Zhou, MS, for programming, data presented in Figs. 2 and 3, and working with Dr. Steffen.
References
- 1.Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, Sacks F, Steffen LM, Wylie-Rosett J; on behalf of the American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism and the Council on Epidemiology and Prevention. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2009;120:1011–1020 [DOI] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services and U S. Department of Agriculture. Dietary Guidelines for Americans, 2005. 6th ed. Washington, DC: U.S. Government Printing Office; 2005 [Google Scholar]
- 3.Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr. 2009;89:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duffy FB, Sigman-Grant M. Position of the American Dietetic Association: Use of Nutritive and Nonnutritive Sweeteners. J Am Diet Assoc. 2004;104:255–275 [DOI] [PubMed] [Google Scholar]
- 5.The Joint FAO/WHO Expert Committee on Food Additives. July 29, 2011. http://www.codexalimentarius.net/web/jecfa.jsp Accessed .
- 6.The lowdown on low-calorie sweeteners The Beverage Institute for Health and Wellness. http://www.beverageinstitute.org/shared/downloads/low-calorie-sweeteners.pdf Accessed November 5, 2010
- 7.International Food Information Council Foundation 2012 Food & Health Survey: Consumer Attitudes toward Food Safety, Nutrition and Health. http://www.foodinsight.org/Resources/Detail.aspx?topic=2012_Food_Health_Survey_Consumer_Attitudes_toward_Food_Safety_Nutrition_and_Health Accessed June 11, 2012
- 7a.Pereira M. Acting sweet: drivers, trends and forecasts in the sugar-free food and beverage market are explored. CBS Interactive. http://findarticles.com/p/articles/mi_m3289/is_1_175/ai_n26689456/ Accessed June 5, 2012
- 8.Wellness Lifestyle Insights 2007: Emerging Trends to Shape the Future Marketplace. Bellevue, WA: The Hartman Group; 2007 [Google Scholar]
- 9.Yang Q. Gain weight by “going diet?” Artificial sweeteners and the neurobiology of sugar cravings: neuroscience 2010. Yale J Biol Med. 2010;83:101–108 [PMC free article] [PubMed] [Google Scholar]
- 10.Duffey KJ, Popkin BM. High-fructose corn syrup: is this what's for dinner? Am J Clin Nutr. 2008;88:1722S–1732S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popkin BM, Nielsen SJ. The sweetening of the world's diet. Obesity Res. 2003;11:1325–1332 [DOI] [PubMed] [Google Scholar]
- 12.Colditz GA, Willett WC, Stampfer MJ, London SJ, Segal MR, Speizer FE. Patterns of weight change and their relation to diet in a cohort of healthy women. Am J Clinl Nutr. 1990;51:1100–1105 [DOI] [PubMed] [Google Scholar]
- 13.de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93:1321–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fowler SP, Williams K, Resendez RG, Hunt KJ, Hazuda HP, Stern MP. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity (Silver Spring). 2008;16:1894–1900 [DOI] [PubMed] [Google Scholar]
- 15.de la Hunty A, Gibson S, Ashwell M. A review of the effectiveness of aspartame in helping with weight control. Nutr Bull. 2006;31:115–128 [Google Scholar]
- 16.Van Wymelbeke V, Beridot-Therond ME, de La Gueronniere V, Fantino M. Influence of repeated consumption of beverages containing sucrose or intense sweeteners on food intake. Eur J Clin Nutr. 2004;58:154–161 [DOI] [PubMed] [Google Scholar]
- 17.Raben A, Vasilaras TH, Møller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76:721–729 [DOI] [PubMed] [Google Scholar]
- 18.Anton SD, Martin CK, Han H, Coulon S, Cefalu WT, Geiselman P, Williamson DA. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite. 2010;55:37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reid M, Hammersley R, Hill AJ, Skidmore P. Long-term dietary compensation for added sugar: effects of supplementary sucrose drinks over a 4-week period. Br J Nutr. 2007;97:193–203 [DOI] [PubMed] [Google Scholar]
- 20.Reid M, Hammersley R, Duffy M. Effects of sucrose drinks on macronutrient intake, body weight, and mood state in overweight women over 4 weeks. Appetite. 2010;55:130–136 [DOI] [PubMed] [Google Scholar]
- 21.Ebbeling CB, Feldman HA, Osganian SK, Chomitz VR, Ellenbogen SJ, Ludwig DS. Effects of decreasing sugar-sweetened beverage consumption on body weight in adolescents: a randomized, controlled pilot study. Pediatrics. 2006;117:673–680 [DOI] [PubMed] [Google Scholar]
- 22.Leahy KE, Birch LL, Rolls BJ. Reducing the energy density of multiple meals decreases the energy intake of preschool-age children. Am J Clin Nutr. 2008;88:1459–1468 [DOI] [PubMed] [Google Scholar]
- 23.Spill MK, Birch LL, Roe LS, Rolls BJ. Serving large portions of vegetable soup at the start of a meal affected children's energy and vegetable intake. Appetite. 2011;57:213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duffy VB, Hayes JE, Sullivan BS, Faghri P. Surveying food and beverage liking: a tool for epidemiological studies to connect chemosensation with health outcomes. Ann N Y Acad Sci. 2009;1170:558–568 [DOI] [PubMed] [Google Scholar]
- 25.Mainland JD, Matsunami H. Taste perception: how sweet it is (to be transcribed by you). Curr Biol. 2009;19:R655–R656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drewnowski A, Kristal A, Cohen J. Genetic taste responses to 6-n-propylthiouracil among adults: a screening tool for epidemiological studies. Chem Senses. 2001;26:483–489 [DOI] [PubMed] [Google Scholar]
- 27.Yee KK, Sukumaran SK, Kotha R, Gilbertson TA, Margolskee RF. Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc Natl Acad Sci U S A. 2011;108:5431–5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pepino MY, Bourne C. Non-nutritive sweeteners, energy balance, and glucose homeostasis. Curr Opin Clin Nutr Metab Care. 2011;14:391–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delzenne N, Blundell J, Brouns F, Cunningham K, De Graaf K, Erkner A, Lluch A, Mars M, Peters HP, Westerterp-Plantenga M. Gastrointestinal targets of appetite regulation in humans. Obes Rev. 2010;11:234–250 [DOI] [PubMed] [Google Scholar]
- 30.Ford HE, Peters V, Martin NM, Sleeth ML, Ghatei MA, Frost GS, Bloom SR. Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. Eur J Clin Nutr. 2011;65:508–513 [DOI] [PubMed] [Google Scholar]
- 31.Steinert RE, Frey F, Topfer A, Drewe J, Beglinger C. Effects of carbohydrate sugars and artificial sweeteners on appetite and the secretion of gastrointestinal satiety peptides. Br J Nutr. 2011;105:1320–1328 [DOI] [PubMed] [Google Scholar]
- 32.Beauchamp GK, Mennella JA. Early flavor learning and its impact on later feeding behavior. J Pediatr Gastroenterol Nutr. 2009;48(Suppl. 1):S25–S30 [DOI] [PubMed] [Google Scholar]
- 33.Desor JA, Beauchamp GK. Longitudinal changes in sweet preferences in humans. Physiol Behav. 1987;39:639–641 [DOI] [PubMed] [Google Scholar]
- 34.Meyers B, Brewer MS. Sweet taste in man: a review. J Food Sci. 2008;73:R81–R90 [DOI] [PubMed] [Google Scholar]
- 35.Calo C, Padiglia A, Zonza A, Corrias L, Contu P, Tepper BJ, Barbarossa IT. Polymorphisms in TAS2R38 and the taste bud trophic factor, gustin gene co-operate in modulating PROP taste phenotype. Physiol Behav. 2011;104:1065–1071 [DOI] [PubMed] [Google Scholar]
- 36.Reed DR, Margolskee RF. Gustation genetics: sweet gustducin! Chem Senses. 2010;35:549–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riera CE, Vogel H, Simon SA, le Coutre J. Artificial sweeteners and salts producing a metallic taste sensation activate trpv1 receptors. Am J Physiol. 2007;293:R626–R634 [DOI] [PubMed] [Google Scholar]
- 38.Swithers SE, Martin AA, Davidson TL. High-intensity sweeteners and energy balance. Physiol Behav. 2010;100:55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Touzani K, Bodnar RJ, Sclafani A. Neuropharmacology of learned flavor preferences. Pharmacol Biochem Behav. 2010;97:55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeomans MR, Leitch M, Gould NJ, Mobini S. Differential hedonic, sensory and behavioral changes associated with flavor-nutrient and flavor-flavor learning. Physiol Behav. 2008;93:798–806 [DOI] [PubMed] [Google Scholar]
- 41.Rodearmel SJ, Wyatt HR, Stroebele N, Smith SM, Ogden LG, Hill JO. Small changes in dietary sugar and physical activity as an approach to preventing excessive weight gain: the America on the Move family study. Pediatrics. 2007;120:e869–e879 [DOI] [PubMed] [Google Scholar]
- 42.Blackburn GL, Kanders BS, Lavin PT, Keller SD, Whatley J. The effect of aspartame as part of a multidisciplinary weight-control program on short- and long-term control of body weight. Am J Clin Nutr. 1997;65:409–418 [DOI] [PubMed] [Google Scholar]
- 43.Stellman SD, Garfinkel L. Patterns of artificial sweetener use and weight change in an American Cancer Society prospective study. Appetite. 1988;11(Suppl. 1):85–91 [PubMed] [Google Scholar]
- 44.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009;89:1037–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin J, Curhan GC. Associations of sugar and artificially sweetened soda with albuminuria and kidney function decline in women. Clin J Am Soc Nephrol. 2011;6:160–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008;117:754–761 [DOI] [PubMed] [Google Scholar]
- 48.Gardener H, Rundek T, Markert M, Wright CB, Elkind MS, Sacco RL. Diet soft drink consumption is associated with an increased risk of vascular events in the Northern Manhattan Study. J Gen Intern Med. January 27, 2012, Epub ahead of print. 10.1007/s11606-011-1968-2 http://www.springerlink.com Accessed March 30, 2012 [DOI] [PMC free article] [PubMed]
- 49.de Koning L, Malik VS, Kellogg MD, Rimm EB, Willett WC, Hu FB. Sweetened beverage consumption, incident coronary heart disease and biomarkers of risk in men. Circulation. 2012;125:1735–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duffey KJ, Steffen LM, Van Horn L, Jacobs DR, Jr, Popkin BM. Dietary patterns matter: diet beverages and cardiometabolic risks in the longitudinal Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Clin Nutr. 2012;95:909–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barriocanal LA, Palacios M, Benitez G, Benitez S, Jimenez JT, Jimenez N, Rojas V. Apparent lack of pharmacological effect of steviol glycosides used as sweeteners in humans. A pilot study of repeated exposures in some normotensive and hypotensive individuals and in Type 1 and Type 2 diabetics. Regul Toxicol Pharmacol. 2008;51:37–41 [DOI] [PubMed] [Google Scholar]
- 52.Grotz VL, Henry RR, McGill JB, Prince MJ, Shamoon H, Trout JR, Pi-Sunyer FX. Lack of effect of sucralose on glucose homeostasis in subjects with type 2 diabetes. J Am Diet Assoc. 2003;103:1607–1612 [DOI] [PubMed] [Google Scholar]
- 53.Maki KC, Curry LL, Reeves MS, Toth PD, McKenney JM, Farmer MV, Schwartz SL, Lubin BC, Boileau AC, Dicklin MR, Carakostas MC, Tarka SM. Chronic consumption of rebaudioside a, a steviol glycoside, in men and women with type 2 diabetes mellitus. Food Chem Toxicol. 2008;46(Suppl. 7):S47–S53 [DOI] [PubMed] [Google Scholar]
- 54.Cooper PL, Wahlqvist ML, Simpson RW. Sucrose versus saccharin as an added sweetener in non-insulin-dependent diabetes: short- and medium-term metabolic effects. Diabet Med. 1988;5:676–680 [DOI] [PubMed] [Google Scholar]
- 55.Reyna NY, Cano C, Bermudez VJ, Medina MT, Souki AJ, Ambard M, Nunez M, Ferrer MA, Inglett GE. Sweeteners and beta-glucans improve metabolic and anthropometrics variables in well controlled type 2 diabetic patients. Am J Ther. 2003;10:438–443 [DOI] [PubMed] [Google Scholar]
- 56.Curry LL. GRAS exemption claim for rebiana (rebaudioside a): summary of data concerning the safety and GRAS determination of rebiana (rebaudioside a) for use as a general purpose sweetener. 2008 http://www.accessdata.fda.gov/scripts/fcn/gras_notices/grn000253.pdf Accessed May 17, 2012 [Google Scholar]
- 57.Franz MJ, Powers MA, Leontos C, Holzmeister LA, Kulkarni K, Monk A, Wedel N, Gradwell E. The evidence for medical nutrition therapy for type 1 and type 2 diabetes in adults. J Am Diet Assoc. 2010;110:1852–1889 [DOI] [PubMed] [Google Scholar]
- 58.American Diabetes Association Standards of medical care in diabetes–2012. Diabetes Care. 2012;35(Suppl. 1):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]