Abstract
OBJECTIVE
To assess the glucagon response to hypoglycemia and identify influencing factors in patients with type 1 diabetes compared with nondiabetic control subjects.
RESEARCH DESIGN AND METHODS
Hyperinsulinemic hypoglycemic clamp studies were performed in all participants. The glucagon response to both hypoglycemia and arginine was measured, as well as epinephrine, cortisol, and growth hormone responses to hypoglycemia. Residual β-cell function was assessed using fasting and stimulated C-peptide.
RESULTS
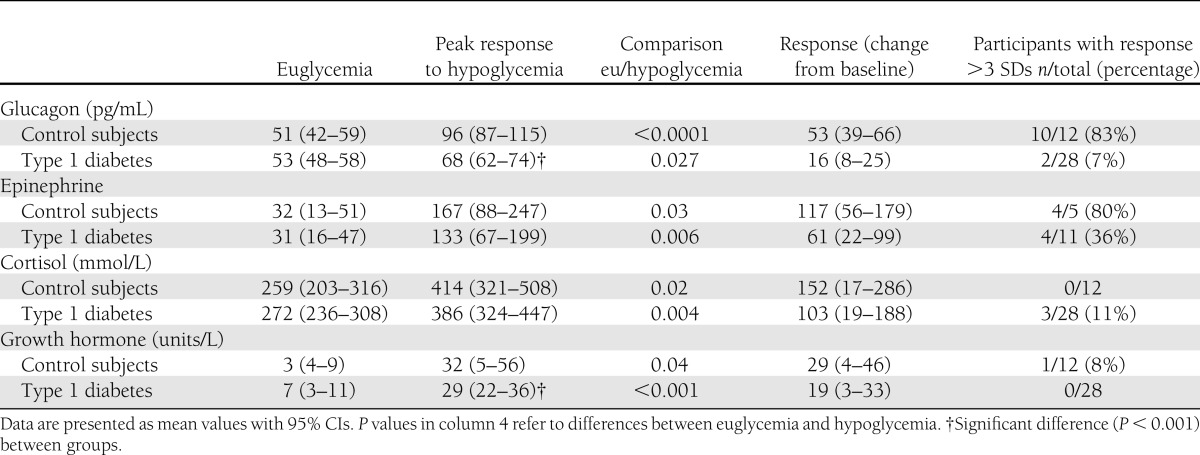
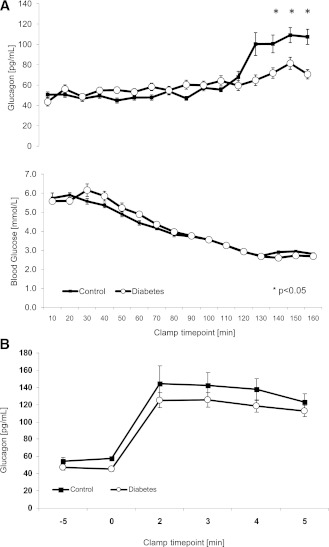
Twenty-eight nonobese adolescents with type 1 diabetes (14 female, mean age 14.9 years [range 11.2–19.8]) and 12 healthy control subjects (6 female, 15.3 years [12.8–18.7]) participated in the study. Median duration of type 1 diabetes was 0.66 years (range 0.01–9.9). The glucagon peak to arginine stimulation was similar between groups (P = 0.27). In contrast, the glucagon peak to hypoglycemia was reduced in the group with diabetes (95% CI): 68 (62–74) vs. 96 (87–115) pg/mL (P < 0.001). This response was greater than 3 SDs from baseline for only 7% of subjects with type 1 diabetes in comparison with 83% of control subjects and was lost at a median duration of diabetes of 8 months and as early as 1 month after diagnosis (R = −0.41, P < 0.01). There was no correlation in response with height, weight, BMI, and HbA1c. Epinephrine, cortisol, and growth hormone responses to hypoglycemia were present in both groups.
CONCLUSIONS
The glucagon response to hypoglycemia in adolescents with type 1 diabetes is influenced by the duration of diabetes and can be lost early in the course of the disease.
Hypoglycemia is a complication of insulin therapy of type 1 diabetes that can cause significant morbidity and rarely, mortality; as a result, it proves a significant barrier to contemporary targets for glycemic control (1). Young people with type 1 diabetes are especially prone to hypoglycemia due to the nonphysiological nature of insulin therapy, as well as defective counterregulation (1–4). Impairment of the glucagon response to hypoglycemia is well documented in adult patients with long-standing diabetes (2,5); however, the natural history and underlying pathophysiology have not been well characterized in children and adolescents. Studies with small sample numbers suggested that the glucagon response to hypoglycemia is lost during the first months after diagnosis of type 1 diabetes (6,7), but this remains to be studied further.
The purpose of this study was to 1) assess the glucagon response to both hypoglycemia and arginine as an independent stimulus in adolescents with type 1 diabetes with a range of diabetes duration, as well as in healthy control subjects, and 2) identify clinical and demographic factors that predict the glucagon response to hypoglycemia.
RESEARCH DESIGN AND METHODS
Adolescents with type 1 diabetes were identified from the Western Australian Children’s Diabetes Database. Eligible patients aged 12–16 years attending the Diabetes Service at Princess Margaret Hospital for Children were approached to participate in the study. Exclusion criteria included a history of seizures not related to hypoglycemia, any episode of hypoglycemia (blood glucose level <3.5 mmol/L) in the 24 h preceding the study, and a history of severe hypoglycemia within the preceding 3 months. Clinical and demographic factors are prospectively recorded from diagnosis and recorded in the Western Australian Children’s Diabetes Database. These data were used to determine the factors included in the multivariate analysis. The control subjects were siblings of children with type 1 diabetes recruited from the community. The cross-sectional study design involved admission for a hypoglycemic hyperinsulinemic clamp study followed by an arginine stimulation test. The protocol was approved by the local ethics committee and signed informed consent was obtained for all subjects.
Protocol
Hyperinsulinemic hypoglycemic clamp study.
A modification of the glucose clamp technique described by Amiel et al. (8) was used. Patients were advised to fast for at least 8 h prior to admission to the research laboratory on the morning of the study. Only short-acting insulin was used in the 12 h preceding the study. Fasting samples were taken for plasma glucose, C-peptide, HbA1c, and islet cell/glutamic acid decarboxylase antibodies.
The subjects were given an intravenous infusion of insulin (Humalog; Eli Lilly) at a continuous rate of 80 mU/m2/min, with doubling of the rate for the first 5 min. Plasma glucose was measured bedside at 5-min intervals and the glucose infusion rate (20% dextrose in saline) adjusted to achieve the target plasma glucose concentration. Blood samples were taken at 10- to 20-min intervals for measurement of counterregulatory hormones (glucagon, epinephrine, cortisol, and growth hormone) and free insulin levels.
The plasma glucose was initially stabilized between 5.0 and 5.5 mmol/L (90–108 mg/dL) for 60 min (euglycemic phase) and then reduced over a period of 30–40 min to achieve a target nadir of 2.8 mmol/L (50.4 mg/dL). Hypoglycemia was maintained for 40 min (hypoglycemic phase). On completion of the hypoglycemic phase, the insulin infusion was stopped and the plasma glucose was stabilized between 5.0 and 5.5 mmol/L (90–108 mg/dL) for at least 20 min.
Arginine stimulation test
To assess glucagon release to stimuli other than hypoglycemia and residual insulin reserve, arginine was used as an alternative stimulus (9,10). Baseline samples for plasma glucagon were taken at 5 (t = −5) and 2 min (t = −2) prior to infusion of 10% arginine solution (5 g in 50 mL 0.9% sodium chloride over 30 s) (11). Stimulated plasma glucagon was measured at 2, 3, 4, and 5 min postarginine (11).
Assays
Plasma glucose was assessed at bedside by the glucose oxidase method (YSI analyzer; YSI, Yellow Springs, OH). Blood for plasma glucagon was collected in EDTA with trasylol, immediately stored on ice, separated within 4 h, and stored at −70°C until assay. Under these conditions, glucagon immunoreactivity is preserved (12). Plasma glucagon was analyzed using a radioimmunoassay (Glucagon RIA; Linco Research, Inc., St. Charles, MO). The coefficient of variation for the glucagon assay was 13.9% at 51.5 pg/mL and 10.2% at 70.1 pg/mL.
Plasma epinephrine levels were measured by ELISA (Diagnostika GmbH, Hamburg, Germany), and samples were analyzed in duplicate according to the manufacturer’s instructions (13). Blood was collected into tubes containing EDTA preservative. Samples were stored in an ice bath until they were centrifuged to separate the plasma within 2 h and then stored at −70°C prior to assay. The interassay coefficient of variation (CV) at 10 pmol/L and 5,460 pmol/L were 2 and 5.5%, respectively.
We assessed residual β-cell function based on both fasting- and arginine-stimulated C-peptide values. Cortisol, growth hormone, and C-peptide were measured by immunoassays (Immulite; Diagnostic Products Corporation, Los Angeles, CA) (14).
Free and total insulin were measured by radioimmunoassay after precipitation of endogenous antibodies with polyethylene glycol. Analytical recovery of free insulin was 99.3%, of total insulin 96.4%. For free insulin, assay precision (CV) was 4.0–13.0% (intra-assay) and 7.8–10.7% (interassay); for total insulin, 3.6–9.5 and 6.6–11.7%, respectively (15). Glycated hemoglobin was measured by agglutination inhibition immunoassay (Ames DCA 2000, non–type 1 diabetes reference interval <6.2%).
Data analysis
Demographic data were expressed as mean and range. Duration of diabetes was also expressed as median and range to allow a more detailed analysis. All other results were described as mean and 95% CIs.
For the calculation of baseline and stimulated values, we used the average of time points 0–60 min for euglycemia and 130–150 min for hypoglycemia. A positive response to hypoglycemia was defined as a stimulated value greater than 3 SDs from the respective baseline during euglycemia. This approach was used by the Diabetes in Children Network Study Group (16) (see Table 2).
Table 2.
Counterregulatory hormone response to hypoglycemia
The glucagon responses to hypoglycemia and arginine were compared between the groups for each time point of analysis and are illustrated in Fig. 1A and B.
Figure 1.
A and B: Glucagon response to hypoglycemia and arginine. The line diagrams show glucagon levels from clamp studies as mean and SEM during euglycemia and in response to hypoglycemia and arginine infusion, respectively. n = 28 for type 1 diabetes and n = 12 for control subjects. A linear mixed model was used for analysis.
A linear mixed model was used for analysis of the glucagon responses. Stepwise linear regression was used in the subjects with type 1 diabetes to identify influencing factors. Statistical significance was set at P < 0.05 (Stata Statistical Software, release 12; StataCorp, College, Station, TX).
RESULTS
Subjects
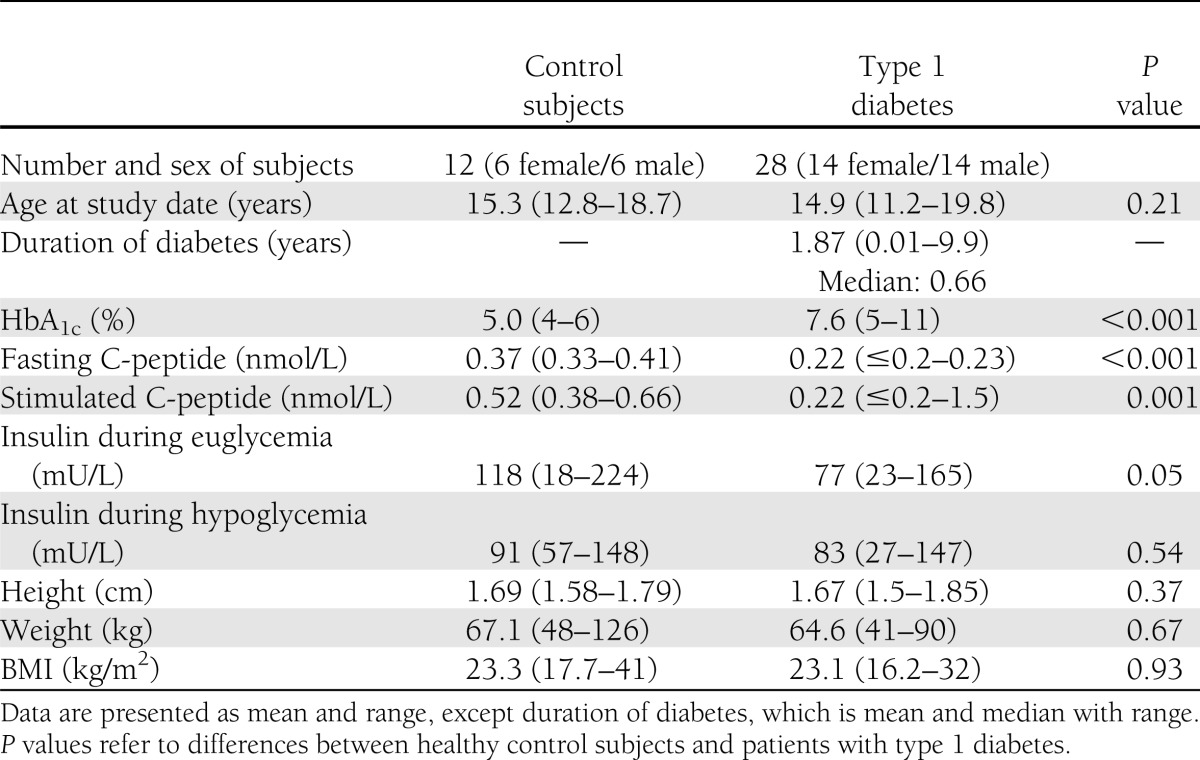
A total of 28 individuals with type 1 diabetes (14 females and 14 males) and 12 healthy volunteers (6 females and 6 males) were studied. Clinical characteristics are summarized in Table 1. All patients with type 1 diabetes were islet cell and/or glutamic acid decarboxylase antibody positive and on regular insulin therapy: short-acting insulin via insulin pump (n = 6) and combinations of long- and short-acting insulin using two (n = 7), three (n = 6), or four (n = 9) injections per day. One subject was taking thyroxine for autoimmune hypothyroidism. Median duration of type 1 diabetes was 0.66 years (range 0.01–9.9).
Table 1.
Clinical characteristics
Counterregulatory response
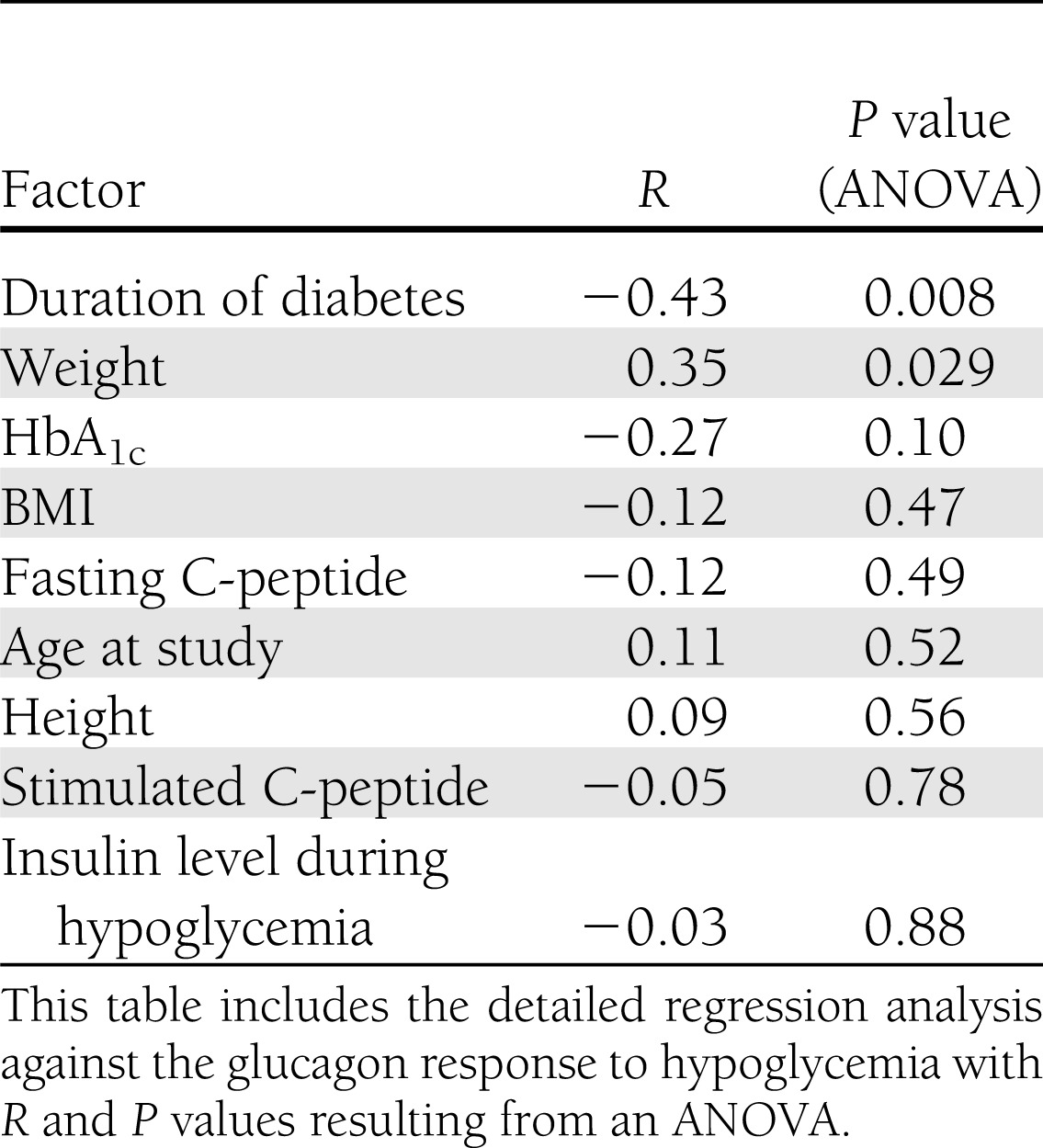
The glucagon peak to arginine stimulation was similar between groups (P = 0.27) (Fig. 1B). In contrast, the glucagon peak to hypoglycemia was reduced in the group with diabetes (95% CI): 68 (62–74) vs. 96 (87–115) pg/mL (P < 0.001) (Fig. 1A). A positive response was defined as greater than 3 SDs from baseline (25 pg/mL for control subjects and 48 pg/mL for the group with type 1 diabetes). Based on these values, only 7% of subjects with type 1 diabetes demonstrated a positive response in comparison with 83% of control subjects (Table 2). The response was lost at a median duration of diabetes of 8 months and as early as 1 month after diagnosis (R = −0.43, P < 0.01) (Table 3). Fasting and stimulated C-peptide were higher in the control group (Table 1).
Table 3.
Regression analysis
Epinephrine, cortisol, and growth hormone responses to hypoglycemia were present, consistent with the generation of an adequate hypoglycemic stimulus (P < 0.05) (Table 2).
Regression analysis
Stepwise linear regression revealed a significant correlation between glucagon response, duration of diabetes, and weight. There was no significant correlation with HbA1c, BMI, fasting and stimulated C-peptide, height, and insulin level during hypoglycemia (Table 3).
CONCLUSIONS
This study demonstrates that in adolescents with type 1 diabetes, the glucagon response to an arginine stimulus is similar to healthy control subjects. However, adolescents with type 1 diabetes have a blunted glucagon response after hypoglycemia. Although this finding is not novel, this, to our knowledge, is the first study to show that the glucagon response to hypoglycemia was lost as early as 1 month after diagnosis and at a median duration of diabetes of 8 months. The glucagon response to hypoglycemia decreased with increasing duration of diabetes.
We assessed the counterregulatory response to hypoglycemia using clamp studies in adolescents with type 1 diabetes early in the course of the disease. Hoffman et al. (6) and Singer-Granick et al. (7) also demonstrated an impaired glucagon response to hypoglycemia in adolescents with type 1 diabetes. To further analyze and specify the participants’ response, Hoffman et al. (17) also included the pancreatic polypeptide release after hypoglycemia and the glucagon response to a mixed-meal stimulus. The interpretation of these studies, however, is limited by the methodology that used an insulin bolus to induce hypoglycemia. Compared with using clamp studies, this does not produce a reproducible hypoglycemic stimulus, resulted in a wide range of insulin levels, and is a technique in which the response to hypoglycemia cannot be compensated. This is why the stimulus may be modified. The Diabetes Research in Children Network Study Group demonstrated that the counterregulatory response can be lost in children aged 4–7 years with a diabetes duration of 3.3 ± 1.1 years (16). Hypoglycemia was induced using a subcutaneous insulin infusion protocol. In contrast to our study, that group focused on hypoglycemia-associated autonomic failure. Data on the glucagon release suggest a blunted response to hypoglycemia similar to our findings. However, in contrast to our study, this was not compared with a control group of healthy individuals (16).
As has been shown repeatedly in humans (5) and animal models (18), the preservation of the release of glucagon after arginine stimulation in all participants demonstrates that α-cells of patients with type 1 diabetes maintain their capability to release glucagon.
Importantly, the insulin infusion during clamp studies in itself can negatively impact the response to both hypoglycemia and arginine (19). Liu et al. (20) described that the glucagon response is suppressed by insulin levels of 125 mU/L. In a study by Diamond et al. (21), the glucagon response was negatively affected at insulin levels >279 mU/L. Mean insulin levels in this study were 91 and 83 mU/L in the control subjects and the type 1 patients with diabetes, respectively, and glucagon responses in the control group were greater after arginine than the hypoglycemic stimulus, suggesting that α-cell function was not significantly blunted by circulating insulin. Prior to the arginine stimulation, insulin was stopped and normoglycemia established.
The exact mechanisms regulating glucagon secretion in vivo remain to be identified. Several hypotheses have been proposed to explain the blunted response of glucagon release to hypoglycemia in type 1 diabetes patients. Changes to the intraislet insulin concentration as the result of β-cell loss is the most favored concept (22–24). Recent studies focused on the importance of zinc as a cofactor; however, this remains controversial (25,26). Looking at the central detection of hypoglycemia to induce and regulate counterregulatory responses, it appears from rat models that signal transduction in the ventromedial hypothalamus plays an important role. Proposed transmitters include glutamate, γ-aminobutyric acid, and catecholamines (27). They can be directly influenced by local glucose and insulin concentrations (28,29). In addition, the hypothalamus and brainstem might be involved as well (27). Other areas of research include the transcriptional control of pancreatic cell development and of the glucagon gene as well as proglucagon processing (30).
With respect to alternative stimuli of glucagon release, Pörksen et al. (31) conducted a large prospective study on the effect of a mixed-meal stimulus on glucagon release in adolescents with type 1 diabetes 1, 6, and 12 months after diagnosis. No association with residual β-cell function, age, and sex was noted, but blood glucose and glucagon-like peptide-1 were observed to be influencing factors. Similarly, Brown et al. (32) analyzed the change in glucagon response to a mixed-meal stimulus in young patients on five occasions during the first year after the diagnosis of diabetes. They observed a decline in C-peptide secretion paralleled by an increase in glucagon response over time, also suggesting that the α-cell secretory reserve had not been affected by the autoimmune process in type 1 diabetes. Neither study included an analysis of the response to hypoglycemia.
Several demographic and clinical factors were identified as predictors of the counterregulatory response to hypoglycemia. Consistent with our findings, the impact of duration of diabetes was reported previously. Most studies focused on adults (5,33,34). Results for children and adolescents vary; a loss of the counterregulatory response was detected as early as at the diagnosis of type 1 diabetes and shortly thereafter (6,35). Singer-Granick et al. (7) demonstrated a positive correlation with age but did not find a correlation between glucagon response and duration of type 1 diabetes in children and adolescents. This may be explained by a significant number of prepubertal participants, indicating a possible effect of puberty that could not be replicated in our group. Unlike Ross et al. (36) and Singer-Granick et al. (7), we did not find a relationship between glycemic control and the preservation of counterregulation in children and adolescents.
We conclude that adolescents with type 1 diabetes have impaired glucagon responses to hypoglycemia within 12 months of diagnosis. Prospective studies in children and adolescents starting from the onset of type 1 diabetes are needed to further characterize the mechanisms influencing the change over time in the glucagon response to hypoglycemia.
Supplementary Material
Acknowledgments
This study was funded by the Juvenile Diabetes Research Foundation.
No potential conflicts of interest relevant to this article were reported.
A.S., T.W.J., and E.A.D. developed the study protocol, researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript. R.J.J. researched data, contributed to discussion, and reviewed and edited the manuscript. M.K.B. contributed to discussion and reviewed and edited the manuscript. P.O. researched data and reviewed and edited the manuscript. E.A.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Leanne Youngs (Princess Margaret Hospital for Children) for her help with the clamp studies and work on the assays, and the diabetes clinical team at Princess Margaret Hospital for Children for their help with the recruitment of subjects. The authors also acknowledge the subjects and their families and thank them for their time and enthusiasm for this study.
Footnotes
A slide set summarizing this article is available online.
References
- 1.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.Cryer PE, Gerich JE. Glucose counterregulation, hypoglycemia, and intensive insulin therapy in diabetes mellitus. N Engl J Med 1985;313:232–241 [DOI] [PubMed] [Google Scholar]
- 3.Cryer PE, White NH, Santiago JV. The relevance of glucose counterregulatory systems to patients with insulin-dependent diabetes mellitus. Endocr Rev 1986;7:131–139 [DOI] [PubMed] [Google Scholar]
- 4.Bolli GB, Tsalikian E, Haymond MW, Cryer PE, Gerich JE. Defective glucose counterregulation after subcutaneous insulin in noninsulin-dependent diabetes mellitus. Paradoxical suppression of glucose utilization and lack of compensatory increase in glucose production, roles of insulin resistance, abnormal neuroendocrine responses, and islet paracrine interactions. J Clin Invest 1984;73:1532–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science 1973;182:171–173 [DOI] [PubMed] [Google Scholar]
- 6.Hoffman RP, Arslanian S, Drash AL, Becker DJ. Impaired counterregulatory hormone responses to hypoglycemia in children and adolescents with new onset IDDM. J Pediatr Endocrinol Metab 1994;7:235–244 [DOI] [PubMed] [Google Scholar]
- 7.Singer-Granick C, Hoffman RP, Kerensky K, Drash AL, Becker DJ. Glucagon responses to hypoglycemia in children and adolescents with IDDM. Diabetes Care 1988;11:643–649 [DOI] [PubMed] [Google Scholar]
- 8.Amiel SA, Sherwin RS, Simonson DC, Tamborlane WV. Effect of intensive insulin therapy on glycemic thresholds for counterregulatory hormone release. Diabetes 1988;37:901–907 [DOI] [PubMed] [Google Scholar]
- 9.Larsson H, Ahrén B. Glucose-dependent arginine stimulation test for characterization of islet function: studies on reproducibility and priming effect of arginine. Diabetologia 1998;41:772–777 [DOI] [PubMed] [Google Scholar]
- 10.Lorenzi M, Bohannon N, Tsalikian E, Karam JH. Duration of type I diabetes affects glucagon and glucose responses to insulin-induced hypoglycemia. West J Med 1984;141:467–471 [PMC free article] [PubMed] [Google Scholar]
- 11.Palmer JP, Benson JW, Walter RM, Ensinck JW. Arginine-stimulated acute phase of insulin and glucagon secretion in diabetic subjects. J Clin Invest 1976;58:565–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hendriks T, Benraad TJ. On the stability of immunoreactive glucagon in plasma samples. Diabetologia 1981;20:553–557 [DOI] [PubMed] [Google Scholar]
- 13.Westermann J, Hubl W, Kaiser N, Salewski L. Simple, rapid and sensitive determination of epinephrine and norepinephrine in urine and plasma by non-competitive enzyme immunoassay, compared with HPLC method. Clin Lab 2002;48:61–71 [PubMed] [Google Scholar]
- 14.Jones TW, Porter P, Sherwin RS, et al. Decreased epinephrine responses to hypoglycemia during sleep. N Engl J Med 1998;338:1657–1662 [DOI] [PubMed] [Google Scholar]
- 15.Arnqvist H, Olsson PO, von Schenck H. Free and total insulin as determined after precipitation with polyethylene glycol: analytical characteristics and effects of sample handling and storage. Clin Chem 1987;33:93–96 [PubMed] [Google Scholar]
- 16.Tsalikian E, Tamborlane W, Xing D, et al. Diabetes Research in Children Network (DirecNet) Study Group Blunted counterregulatory hormone responses to hypoglycemia in young children and adolescents with well-controlled type 1 diabetes. Diabetes Care 2009;32:1954–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman RP, Singer-Granick C, Drash AL, Becker DJ. Abnormal alpha cell hypoglycemic recognition in children with insulin dependent diabetes mellitus (IDDM). J Pediatr Endocrinol Metab 1994;7:225–234 [DOI] [PubMed] [Google Scholar]
- 18.Lykkelund C, Lund ED. Plasma glucagon responses to insulin-induced hypoglycaemia and arginine in normal and alloxan diabetic rats. Scand J Clin Lab Invest 1979;39:151–157 [DOI] [PubMed] [Google Scholar]
- 19.Oskarsson PR, Lins PE, Ahré B, Adamson UC. Circulating insulin inhibits glucagon secretion induced by arginine in type 1 diabetes. Eur J Endocrinol 2000;142:30–34 [DOI] [PubMed] [Google Scholar]
- 20.Liu D, Moberg E, Kollind M, Lins PE, Adamson U. A high concentration of circulating insulin suppresses the glucagon response to hypoglycemia in normal man. J Clin Endocrinol Metab 1991;73:1123–1128 [DOI] [PubMed] [Google Scholar]
- 21.Diamond MP, Hallarman L, Starick-Zych K, et al. Suppression of counterregulatory hormone response to hypoglycemia by insulin per se. J Clin Endocrinol Metab 1991;72:1388–1390 [DOI] [PubMed] [Google Scholar]
- 22.Asplin CM, Paquette TL, Palmer JP. In vivo inhibition of glucagon secretion by paracrine beta cell activity in man. J Clin Invest 1981;68:314–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergenstal RM, Polonsky KS, Pons G, Jaspan JB, Rubenstein AH. Lack of glucagon response to hypoglycemia in type I diabetics after long-term optimal therapy with a continuous subcutaneous insulin infusion pump. Diabetes 1983;32:398–402 [DOI] [PubMed] [Google Scholar]
- 24.Taborsky GJ, Jr, Ahren B, Mundinger TO, Mei Q, Havel PJ. Autonomic mechanism and defects in the glucagon response to insulin-induced hypoglycaemia. Diabetes Nutr Metab 2002;15:318–322; discussion 322–323 [PubMed] [Google Scholar]
- 25.Cooperberg BA, Cryer PE. Insulin reciprocally regulates glucagon secretion in humans. Diabetes 2010;59:2936–2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slucca M, Harmon JS, Oseid EA, Bryan J, Robertson RP. ATP-sensitive K+ channel mediates the zinc switch-off signal for glucagon response during glucose deprivation. Diabetes 2010;59:128–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szepietowska B, Zhu W, Chan O, Horblitt A, Dziura J, Sherwin RS. Modulation of β-adrenergic receptors in the ventromedial hypothalamus influences counterregulatory responses to hypoglycemia. Diabetes 2011;60:3154–3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu W, Czyzyk D, Paranjape SA, et al. Glucose prevents the fall in ventromedial hypothalamic GABA that is required for full activation of glucose counterregulatory responses during hypoglycemia. Am J Physiol Endocrinol Metab 2010;298:E971–E977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paranjape SA, Chan O, Zhu W, et al. Influence of insulin in the ventromedial hypothalamus on pancreatic glucagon secretion in vivo. Diabetes 2010;59:1521–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 2007;28:84–116 [DOI] [PubMed] [Google Scholar]
- 31.Pörksen S, Nielsen LB, Kaas A, et al. Hvidøre Study Group on Childhood Diabetes Meal-stimulated glucagon release is associated with postprandial blood glucose level and does not interfere with glycemic control in children and adolescents with new-onset type 1 diabetes. J Clin Endocrinol Metab 2007;92:2910–2916 [DOI] [PubMed] [Google Scholar]
- 32.Brown RJ, Sinaii N, Rother KI. Too much glucagon, too little insulin: time course of pancreatic islet dysfunction in new-onset type 1 diabetes. Diabetes Care 2008;31:1403–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolli G, de Feo P, Compagnucci P, et al. Abnormal glucose counterregulation in insulin-dependent diabetes mellitus. Interaction of anti-insulin antibodies and impaired glucagon and epinephrine secretion. Diabetes 1983;32:134–141 [DOI] [PubMed] [Google Scholar]
- 34.Sjöberg S, Ahrén B, Bolinder J. Residual insulin secretion is not coupled to a maintained glucagon response to hypoglycaemia in long-term type 1 diabetes. J Intern Med 2002;252:342–351 [DOI] [PubMed] [Google Scholar]
- 35.Brown RJ, Rother KI. Effects of beta-cell rest on beta-cell function: a review of clinical and preclinical data. Pediatr Diabetes 2008;9:14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ross LA, Warren RE, Kelnar CJ, Frier BM. Pubertal stage and hypoglycaemia counterregulation in type 1 diabetes. Arch Dis Child 2005;90:190–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.