Abstract
OBJECTIVE
In the U.K., people with diabetes are typically screened for retinopathy annually. However, diabetic retinopathy sometimes has a slow progression rate. We developed a simulation model to predict the likely impact of screening patients with type 2 diabetes, who have not been diagnosed with diabetic retinopathy, every 2 years rather than annually. We aimed to assess whether or not such a policy would increase the proportion of patients who developed retinopathy-mediated vision loss compared with the current policy, along with the potential cost savings that could be achieved.
RESEARCH DESIGN AND METHODS
We developed a model that simulates the progression of retinopathy in type 2 diabetic patients, and the screening of these patients, to predict rates of retinopathy-mediated vision loss. We populated the model with data obtained from a National Health Service Foundation Trust. We generated comparative 15-year forecasts to assess the differences between the current and proposed screening policies.
RESULTS
The simulation model predicts that implementing a 2-year screening interval for type 2 diabetic patients without evidence of diabetic retinopathy does not increase their risk of vision loss. Furthermore, we predict that this policy could reduce screening costs by ∼25%.
CONCLUSIONS
Screening people with type 2 diabetes, who have not yet developed retinopathy, every 2 years, rather than annually, is a safe and cost-effective strategy. Our findings support those of other studies, and we therefore recommend a review of the current National Institute for Health and Clinical Excellence (NICE) guidelines for diabetic retinopathy screening implemented in the U.K.
Diabetic retinopathy is a serious complication for people with diabetes that can lead to blindness or severe vision loss (1). Although retinopathy cannot be cured, its progression can be slowed or halted, and laser treatment can prevent visual loss if offered promptly at the proliferative stage (2). Diabetic digital retinal photography is an effective method of detecting the onset of treatable retinopathy (3). National Institute for Health and Clinical Excellence (NICE) guidelines in the U.K. currently recommend that people with diabetes are screened for retinopathy annually, or every 3–6 months for those patients who have developed beyond mild background retinopathy or who are at higher risk of progression (4). This also forms part of the National Screening Committee’s policy of a national screening program for diabetes in the U.K. (5).
The development of retinopathy may take decades (6), and the annual screening of all diabetic patients may therefore incur considerable cumulative health service cost and patient inconvenience that, for some, may be unjustified (7). With a significant increase in diabetes incidence forecast in the U.K. (8), it is imperative that screening policies for diabetes complications are cost-effective and practicable. It is therefore prudent to ask whether it would be cost-effective and safe to screen diabetic patients for retinopathy less frequently (7), particularly in light of more recent evidence that suggests an overall decline in the rates at which people with diabetes are developing vision-threatening retinopathy (9,10).
We undertook a collaborative project with the Royal Devon and Exeter National Health Service (NHS) Foundation Trust (henceforth referred to simply as Royal Devon and Exeter), which carries out annual retinopathy screening for a population of ∼20,000 patients across Devon. Using simulation modeling, we assessed the potential impact of implementing a 2-year retinopathy screening interval for those patients without retinopathy and who have type 2 diabetes. Diabetic retinopathy may progress more quickly in patients with type 1 diabetes (1,11–13), and although it has been shown that less frequent screening may be feasible for type 1 patients (14), we focus on the lower-risk type 2 diabetic patient group in this study. Previous studies have looked at the cost-effectiveness of longer screening intervals for diabetic retinopathy screening across all patients in a population (7,15,16), but we assessed an increased screening interval solely for those patients who have not yet been diagnosed with diabetic retinopathy. These patients represent ∼40% of the type 1 and type 2 diabetic population screened by Royal Devon and Exeter, and reductions in the frequency with which they are screened could therefore lead to large potential cost and resource savings.
RESEARCH DESIGN AND METHODS
The retinopathy screening model
Simulation modeling is an operational research technique that can be used to predict the impact of specified changes to a defined pathway, such as changes to the screening policies for diabetic retinopathy in this instance. By using existing data, such as the incidence and progression rates of diabetic retinopathy, we can construct a model that simulates the progression of the disease and patient screening, thereby allowing us to alter parameters to generate predictions on the impact of certain changes.
The patient-oriented simulation technique (POST) is a well-regarded example of a simulation model that has been used to evaluate screening strategies for diabetic retinopathy (1,11). POST simulates the retinopathy progression and screening of individual diabetic patients throughout their lifetimes. We developed a new model, retinopathy screening simulation (ReSS), that builds upon POST, by placing it within a modeling framework that explicitly models each patient separately, allowing us to more easily model screening and retinopathy progression. In ReSS, each patient has a “state” that specifies how far their retinopathy has progressed, whether they have lost their vision, or whether they have died. The progression of their retinopathy is described using the U.K. National Screening Committee grading system (5): R0 indicates no retinopathy, and R1, R2, and R3 indicate background diabetic retinopathy (BDR), preproliferative diabetic retinopathy (pre-PDR), and PDR, respectively. We assume that a patient’s grade is determined by their “worst” eye (the one that has the most advanced development of retinopathy). A patient who has lost their vision in at least one eye has a state called “vision loss,” and a patient who has died has a state called “death.”
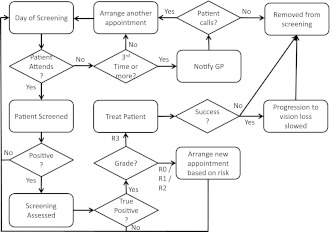
In our model, patients are given a baseline grade of retinopathy and an age. No patients have lost their vision at the start of the model. Time advances in increments of 1 day. Figure 1 summarizes the patient-level processes in the model.
Figure 1.
Patient-level processes in ReSS model. This flowchart summarizes the process steps that patients in the model follow.
For every day that elapses in the model, a patient in the model may die (which is dependent upon their age and progression of their retinopathy) or may progress to the next stage of retinopathy. In addition, a patient may have a screening or treatment appointment scheduled on that day.
If a patient in the model is due to be screened, they may or may not attend, depending on their compliance. If they do not attend, another appointment is arranged. Patients who fail to attend three consecutive appointments are contacted by their general practitioner. If they do not arrange another appointment after this, they are removed from the model. Patients in the model who attend a screening appointment are screened for retinopathy using digital retinal photography (3), which is carried out by retinal screening graders. The probability that the test provides a true positive or a true negative is given by the test’s sensitivity and specificity, respectively. If a patient receives a negative result, no further action is taken, and their next screening appointment is arranged according to their risk. R0 and R1 patients are screened annually (except in the proposed new policy, where R0 patients are screened every 2 years) and R2 patients are screened every 6 months, in line with the minimum standards derived from the NICE guidelines (4).
If a patient receives a positive result, this is assessed by a secondary retinal grader, such as the retinal screening service manager, who checks the results of the primary grader to maximize diagnostic accuracy. If the secondary grader identifies that the patient does not have retinopathy, or they have stage R1 or R2 retinopathy, the patient’s next screening appointment is arranged according to their disease progression. If the graders identify the patient as having PDR (R3), treatment is given.
If a patient in the model is to be treated, then the treatment may or may not succeed. If the treatment does succeed, the patient’s probability of developing vision loss from state R3 is reduced. Either way, for simplification, the patient does not attend any further screening appointments.
Populating the retinopathy screening model
When populating the retinopathy model (ReSS), we considered two different scenarios: the 2S (or two-stream) scenario represents the current world order, in which patients are screened either annually or every 6 months, and the 3S (or three-stream) scenario represents the proposed strategy of screening R0 patients every 2 years, rather than annually. For simplification, we assume 1 year to be 365 days and 6 months to be 182 days. Each scenario was simulated for 15 years and replicated 10 times, with results averaged over the replications.
Royal Devon and Exeter supplied an anonymized patient dataset for the project, which was comprised of those records that could be cross-matched across both the internal screening administration system and the internal clinical database, to provide all required data. This provided us with 3,537 unique patient records and 33,810 unique screening appointments dating from 1991 to 2011, of which 2,201 patients were type 2 diabetic patients. Patient records included patient sex, date of diagnosis of diabetes, type of diabetes, screening dates, and latest screening grade. Appointment records specified the date and time of the appointment, the patient’s age at the appointment, whether or not the appointment was cancelled and the reason for cancellation, and the screening grade given as an outcome of the appointment. Of the 3,537 patients, 57.6% were male and 40.9% were female. The sex of 53 patients (1.5%) was not specified.
The Royal Devon and Exeter data show that patients are 86% likely to attend a screening appointment. If an appointment is missed, an alternative appointment is usually arranged within a few days, but can be much longer if the patient has to be fitted into a clinic at the hospital. As such, the average time given by the Royal Devon and Exeter data is 93 days. Although this is not representative of common shorter waits, we implement this figure in the model as a “worst-case scenario.” We used a diabetes incidence rate of 4.42/1,000 person-years in the model as an initial estimate (17) and increased this by 4.1% of the initial incidence rate at the end of each year (18). The initial simulated diabetic patient population size was 5,000.
The ages of patients in the model were drawn directly from the patient data supplied by Royal Devon and Exeter. Mortality rates for patients in the model were based on data from a 2005 study of mortality in diabetic patients (19), which also included a Cox proportional hazards model, from which we estimated rate adjustments for the various stages of retinopathy. Rates were averaged across both sexes for each age group and were converted to daily mortality rates.
The R-grade distributions at baseline in the model were taken from the first screening grades on record for each type 2 diabetic patient in the Royal Devon and Exeter data. Therefore, for patients within the model, 54.9% started at R0, 37.6% at R1, 4.9% at R2, and 2.6% at R3. This compares to 73% at R0, 18% at R1, 2% at moderate R2, and 0.5% at R3 in the Liverpool Diabetic Eye Study (7). The higher rates of background retinopathy and above at baseline in the Royal Devon and Exeter data may indicate an increased risk of retinopathy in this population.
Sensitivity and specificity data for the digital retinal photography screening test were supplied by Royal Devon and Exeter and are 98.86 and 99.52%, respectively. They also provided an NHS cost figure of £37 per screening episode, which is typical of screening units in the U.K. (cost code C5-local Specialty Patient Type 41 [Community/Domiciliary]). These values were used directly in the model. Positive results are typically assessed within 1 week after the screening appointment, but the dataset included data from a period of historical backlogs; therefore, the average across the dataset was 20 days. We used a value of 20 days in the model to represent a worst-case scenario. Grading assessments (after initial grading) are assumed to be 100% accurate, as they are checked multiple times by other graders. If a patient in the model is diagnosed with PDR (R3), they are sent for treatment 14 days later, which is the minimum requirement according to NHS quality assurance standards (20). A patient’s laser photocoagulation treatment occurs as a single episode in the model, for the purposes of simplification, and is 84% likely to succeed (21). If successful, the treatment reduces the risk of progression to vision loss by 90% (3).
The data supplied by Royal Devon and Exeter did not include a way of tracking disease progression rates. Therefore, the progression of diabetic retinopathy was modeled using data from the literature. We conducted an extensive search of papers published after the Wisconsin Epidemiologic Study of Diabetic Retinopathy (12), upon which the POST study was based (1,11), to see if there had been any newer, but similar, large-scale cohort studies that drew from a wide diabetic population and looked at the progression of diabetic retinopathy. We found the closest match to be a cohort study of diabetic patients in Melbourne (13), in which baseline checks were performed between 1992 and 1994, with follow-up checks performed 5 years later. Over 5 years, the probability of progression from no retinopathy to background retinopathy was 0.096, from background to preproliferative retinopathy was 0.187, from preproliferative to proliferative retinopathy was 0.25, and from proliferative retinopathy to vision loss was 0.22. Daily probabilities of progression were estimated from the 5-year progression data of the study. The Melbourne cohort included a 22.6% prevalence of insulin-treated diabetic patients and a higher mean hemoglobin A1c (HbA1c), a potential indication of poor diabetes control, in people who progressed to retinopathy (13). The progression rates used in this study therefore implicitly include those patients who are at higher risk of developing diabetic retinopathy (13).
The maculopathy model
Diabetic maculopathy is a form of retinopathy that is common in the elderly and typically occurs after BDR has developed (11). Patients are screened for maculopathy in their retinopathy screening sessions, as patients can develop combinations of retinopathy and diabetic macular edema (DMO) or clinically significant macular edema (CSMO) (11) in conjunction with their diabetes. Of all patients in the Royal Devon and Exeter data, 8.5% were recorded as having some form of diabetic maculopathy (grade M1 of the U.K. National Screening Committee grading system) (5).
To minimize complexity in ReSS, and because our focus was on the diabetic retinopathy complication, which more commonly causes blindness and affected more patients than maculopathy in the Royal Devon and Exeter dataset, we did not include a simulation of the progression of maculopathy in ReSS. However, to ensure that the results obtained from ReSS would remain applicable even when considering patients who develop maculopathy, we developed a small spreadsheet model using Microsoft Excel that includes maculopathy and calculates the expected number of patients in each possible stage of disease progression for each year in a 15-year period.
Unlike ReSS, the maculopathy comparison model only simulates the progression of patients’ diabetes complications and does not consider screening, as this requires a more complex modeling framework, such as that used for ReSS. However, this is not problematic, because we were simply seeking to assess how maculopathy progression rates affect the proportion of patients that lose their vision.
Maculopathy can occur at any stage of progression of diabetic retinopathy (11) and therefore cannot be simulated using a single linear disease progression path. Instead, we took into account all of the possible permutations of retinopathy and maculopathy (both DMO and CSMO severities) and modeled each potential route of progression as a separate “path.” Specifically, a diabetic patient can develop retinopathy and/or maculopathy in any one of seven different ways (Table 1), each of which can be considered a path of progression of the disease (11).
Table 1.
Progression paths in the maculopathy comparison model
For each path, a population of 5,000 type 2 diabetic patients was modeled over a period of 15 years. We used progression rates from the POST study (1,11) to determine the predicted number of patients at each stage in the path in each year. All patients start without retinopathy or maculopathy (R0M0) in the maculopathy comparison model, no patients die for simple comparison between paths, and, for simplification and to mimic a cohort-style study, no new patients are added over time.
RESULTS
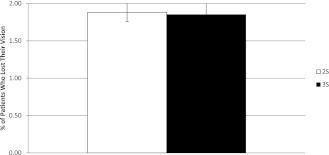
The proportion of type 2 diabetic patients in the ReSS model who are predicted to lose their vision within 15 years is 1.9% (±0.1 SD) and is identical under both scenarios (Fig. 2).
Figure 2.
Proportion of type 2 diabetic patients who lost their vision. The figure shows the total proportion of type 2 diabetic patients in the model who lost their vision within the simulated period of 15 years. Results are shown for both the current screening policy (2S) and the proposed three-tier screening policy (3S). Results are averaged over 10 replications of the simulation.
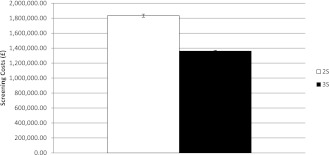
Over 15 years, the proposed new screening policy (the 3S scenario) is predicted to incur £1,360,516 (±£10,036 SD) in costs for screening type 2 diabetic patients, compared with £1,834,060 (±£21,286 SD) with the current screening policy (the 2S scenario) (Fig. 3). This represents a 25.8% reduction in screening costs if type 2 diabetic patients without retinopathy are screened every 2 years instead of annually. Furthermore, ReSS predicts that only 12,561 screening appointments (±227 SD) would be carried out for type 2 diabetic patients without retinopathy over 15 years under the new screening policy, compared with 23,611 appointments (±454 SD) under the existing policy, a reduction of 11,050 appointments over 15 years or 737 appointments per year.
Figure 3.
Estimated cost of screening over 15 years. The figure compares the estimated total type 2 diabetic patient screening costs over 15 years of the current screening policy (2S) with the proposed three-tier screening policy (3S). Results are averaged over 10 replications of the simulation.
In general, we found that the results were insensitive to variations in all of the key parameter values, with the exception of progression rates of retinopathy. Unsurprisingly, here the outputs were highly sensitive to variation and gave vision loss percentages ranging from 0.71 to 5.71% of the population when halving and doubling the implemented progression rates, respectively. The Melbourne study does report a significantly lower incidence of new retinopathy diagnosis than other studies (13) but points out that the retinopathy progression rates were similar to other studies (within a maximum of 11% variation), including the Wisconsin Epidemiologic Study of Diabetic Retinopathy (12). We are therefore confident that the progression rates reported by the Melbourne study and used to parameterize our model are sufficiently representative.
Of all the permutations tested in the maculopathy comparison model, the highest percentage of patients who are predicted to lose their vision within 15 years is 1.2%, which refers to patients who develop both DMO and CSMO in the background stages of retinopathy (path F). This compares to 2.1% of patients for the group that never develops maculopathy, only retinopathy (path A).
When building any model, it is vital to extensively verify and validate it to maximize the reliability of the predictions that it generates. Verification is the process of checking that the model performs as expected by the design and that it is free of errors, whereas validation checks that the model produces the kind of behavior and outputs that would be expected. Both models correctly produced the expected results for each of 29 tests.
CONCLUSIONS
Our model predicts that it is safe to screen type 2 diabetic patients who have not been diagnosed with retinopathy every 2 years, rather than annually, because the proportion of patients who develop retinopathy-mediated vision loss is unaffected. Furthermore, such a policy could reduce the cost of screening by ∼25%, thereby mitigating the problem of increased demand on screening services arising from significant increases in diabetic prevalence. The proportion of patients developing vision loss remains the same under the new policy because retinopathy progresses slowly, and a screening interval of 2 years is sufficiently frequent to identify patients at or before the proliferative stage, where treatment can be given.
By increasing the screening interval for patients without retinopathy, it is possible that early stages of retinopathy will be diagnosed later. However, even if a patient progresses through one or two stages of retinopathy in the 2-year interval, they will be screened more frequently once this is identified. It is unlikely that a lower-risk patient would progress from no retinopathy to the proliferative stage within a 2-year period, given the slow rate of progress of the disease for many patients.
The proportion of patients who lose their vision when considering maculopathy complications never exceeds that of the population that never develops maculopathy. Therefore, the 2-year screening interval predicted to be safe by ReSS would not be problematic for these patients.
Although the predictions generated by ReSS are compelling, it is prudent to highlight potential caveats to a proposed policy of 2-year screening intervals. First, diabetic care is most effective when individualized (22–24), and the importance of retinal clinicians using discretion on a case-by-case basis cannot be overstated. Diabetic retinopathy has been shown to be closely linked to individual risk factors (25–28), and in some cases such risks (longer duration of diabetes, poor metabolic control, or a dramatic change in glycemic levels) may necessitate more frequent screening because of the potentially higher rates of progression in these patients. Even if these patients do not show signs of retinopathy, more frequent screening may be prudent due to the increased risk for this subpopulation.
Second, there is concern that longer screening intervals could lead to patients misunderstanding the severity of retinopathy or forgetting to attend screening appointments (16). However, this is unlikely to be problematic if a computerized call-recall system is used to administer the screening program, but could be explored by comparing the impact of nonattendance with the cost and resource benefits of a 2-year screening interval. Nevertheless, it may be wise to ensure that any potential screening interval changes are coupled with a strategy of educating patients about retinopathy and emphasizing the importance of good control in managing the diabetic condition.
Third, some of the key risk factors for diabetic retinopathy, such as the level of glycemic control and blood pressure, are also associated with other nonocular complications, such as nephropathy, neuropathy, and strokes (2). More frequent screening may be beneficial in identifying such complications earlier, although patients with diabetes would continue to receive annual check-ups for their condition even with a reduction in retinopathy screening, and therefore such risk factors could be identified at these sessions.
Even with these caveats, the predictions generated by the model certainly present an appealing argument for extending the screening interval for diabetic retinopathy in a subset of type 2 diabetic patients. Although our study did not look at linking patients’ clinical risk factors with their recommended screening intervals, our results imply that significant benefits can be brought about simply by reducing the screening interval for patients who have not yet developed retinopathy. Our predictions also support the findings of other studies that have found longer screening intervals for diabetic retinopathy to be cost-effective (7,15,16). We would therefore recommend a review of the current NICE guidelines, which suggest an annual retinopathy screening, at minimum, for diabetic patients, as a way of addressing the projected increased burden that will be placed on NHS screening services without increasing the risk of poor outcomes. In addition, extensions to this research that explicitly consider the impact of risk factors, such as through individualization of screening intervals (29), may be beneficial in assessing whether diabetic patients who have developed retinopathy can also be screened less frequently, depending on their clinical assessment and control of their condition.
Acknowledgments
This study was funded by the National Institute for Health Research (NIHR) Collaborations for Leadership in Applied Health Research and Care (CLAHRC).
The views and opinions expressed in this article are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
No potential conflicts of interest relevant to this article were reported.
D.C. initiated the study; managed the project; designed, implemented, tested, and presented the model, experiments, and results; and analyzed the patient data. M.P. and K.S. initiated the study, oversaw the progress of the project, and offered guidance. B.V. offered advice from a clinical perspective for the design of the project and provided feedback. All authors contributed to the final manuscript and had access to the data in the study. D.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Gillian Bullen and Richard Blackwell (Royal Devon and Exeter NHS Foundation Trust) for offering invaluable input and access to anonymized patient data for this study, and Mark Daly (Peninsula College of Medicine and Dentistry) for his assistance in shaping the design of the project and for offering feedback on the manuscript. The authors also thank Julie Tomlinson (PenCLAHRC) for her input in the question generation process.
References
- 1.Davies R, Roderick P, Canning C, Brailsford S. The evaluation of screening policies for diabetic retinopathy using simulation. Diabet Med 2002;19:762–770 [DOI] [PubMed] [Google Scholar]
- 2.Fong DS, Aiello L, Gardner TW, et al. American Diabetes Association Retinopathy in diabetes. Diabetes Care 2004;27(Suppl. 1):S84–S87 [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Bastida J, Cabrera-Lopez F, Serrano-Aguilar P. Sensitivity and specificity of digital retinal imaging for screening diabetic retinopathy. Diabet Med 2007;24:403–407 [DOI] [PubMed] [Google Scholar]
- 4.NICE. Management of type 2 diabetes (retinopathy - screening and early management) [article online], 2002. Available from http://www.nice.org.uk/nicemedia/pdf/diabetesretinopathyguideline.pdf Accessed 12 January 2012
- 5.National Screening Programme for Diabetic Retinopathy Enriched Grading Form [article online], 2010. Available from: http://www.retinalscreening.nhs.uk/userFiles/File/Enriched%20Grading%20form%20Version%2021%2003%2010.pdf Accessed 12 January 2012
- 6.Wong TY, Mwamburi M, Klein R, et al. Rates of progression in diabetic retinopathy during different time periods: a systematic review and meta-analysis. Diabetes Care 2009;32:2307–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Younis N, Broadbent DM, Vora JP, Harding SP, Liverpool Diabetic Eye Study Incidence of sight-threatening retinopathy in patients with type 2 diabetes in the Liverpool Diabetic Eye Study: a cohort study. Lancet 2003;361:195–200 [DOI] [PubMed] [Google Scholar]
- 8.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4–14 [DOI] [PubMed] [Google Scholar]
- 9.Zavrelova H, Hoekstra T, Alssema M, et al. Progression and regression: distinct developmental patterns of diabetic retinopathy in patients with type 2 diabetes treated in the diabetes care system West-Friesland, the Netherlands. Diabetes Care 2011;34:867–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kytö JP, Harjutsalo V, Forsblom C, Hietala K, Summanen PA, Groop PH, FinnDiane Study Group Decline in the cumulative incidence of severe diabetic retinopathy in patients with type 1 diabetes. Diabetes Care 2011;34:2005–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies R, Brailsford S, Roderick P, Canning C, Crabbe D. Using simulation modelling for evaluating screening services for diabetic retinopathy. J Oper Res Soc 2000;51:476–484 [Google Scholar]
- 12.Klein R, Klein BEMoss SE, Davis MD, De Mets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. X. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is 30 years or more. Arch Ophthalmology 1989;107:244–249 [DOI] [PubMed] [Google Scholar]
- 13.McCarty DJ, Fu CL, Harper CA, Taylor HR, McCarty CA. Five-year incidence of diabetic retinopathy in the Melbourne Visual Impairment Project. Clin Experiment Ophthalmol 2003;31:397–402 [DOI] [PubMed] [Google Scholar]
- 14.Younis N, Broadbent DM, Harding SP, Vora JP. Incidence of sight-threatening retinopathy in type 1 diabetes in a systematic screening programme. Diabet Med 2003;20:758–765 [DOI] [PubMed] [Google Scholar]
- 15.Olafsdóttir E, Stefánsson E. Biennial eye screening in patients with diabetes without retinopathy: 10-year experience. Br J Ophthalmol 2007;91:1599–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones S, Edwards RT. Diabetic retinopathy screening: a systematic review of the economic evidence. Diabet Med 2010;27:249–256 [DOI] [PubMed] [Google Scholar]
- 17.González ELM, Johansson S, Wallander M-A, Rodríguez LAG. Trends in the prevalence and incidence of diabetes in the UK: 1996-2005. J Epidemiol Community Health 2009;63:332–336 [DOI] [PubMed] [Google Scholar]
- 18.Diabetes in the UK 2010: key statistics on diabetes [article online], 2010. Available from http://www.diabetes.org.uk/Documents/Reports/Diabetes_in_the_UK_2010.pdf Accessed 12 January 2012
- 19.Cusick M, Meleth AD, Agrón E, et al. Early Treatment Diabetc Retinopathy Study Research Group Associations of mortality and diabetes complications in patients with type 1 and type 2 diabetes: early treatment diabetic retinopathy study report no. 27. Diabetes Care 2005;28:617–625 [DOI] [PubMed] [Google Scholar]
- 20.Diabetic Retinopathy Screening Programme Quality Assurance Standards Version NHS. 1.2 Release 7 [article online], 2011. Available from http://www.retinalscreening.nhs.uk/userFiles/File/Phase%201%20Revised%20Quality%20Assurance%20Standards%2029th%20June%202011%20Release%207%20v1%202%20(4)%20sc.pdf Accessed 12 January 2012
- 21.Chew EY, Ferris FL, 3rd, Csaky KG, et al. The long-term effects of laser photocoagulation treatment in patients with diabetic retinopathy: the early treatment diabetic retinopathy follow-up study. Ophthalmology 2003;110:1683–1689 [DOI] [PubMed] [Google Scholar]
- 22.Winocour PH. Effective diabetes care: a need for realistic targets. BMJ 2002;324:1577–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleming BB, Greenfield S, Engelgau MM, Pogach LM, Clauser SB, Parrott MA. The Diabetes Quality Improvement Project: moving science into health policy to gain an edge on the diabetes epidemic. Diabetes Care 2001;24:1815–1820 [DOI] [PubMed] [Google Scholar]
- 24.Kavanagh DJGS, Gooley S, Wilson PH. Prediction of adherence and control in diabetes. J Behav Med 1993;16:509–522 [DOI] [PubMed] [Google Scholar]
- 25.Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in type II diabetes over 6 years from diagnosis. Diabetologia 2001;44:156–163 [DOI] [PubMed] [Google Scholar]
- 26.Davis MD, Fisher MR, Gangnon RE, et al. Risk factors for high risk proliferative diabetic retinopathy and severe visual loss: Early Treatment Diabetic Study Report 18. Invest Ophthalmol Vis Sci 1998;39:233–252 [PubMed] [Google Scholar]
- 27.Kohner EM, Aldington SJ, Stratton IM, et al. United Kingdom Prospective Diabetes Study, 30: diabetic retinopathy at diagnosis of non-insulin-dependent diabetes mellitus and associated risk factors. Arch Opthalmol 1998;116:297–303 [DOI] [PubMed] [Google Scholar]
- 28.Porta M, Bandello F. Diabetic retinopathy: a clinical update. Diabetologia 2002;45:1617–1634 [DOI] [PubMed] [Google Scholar]
- 29.Aspelund T, Thornórisdóttir O, Ólafsdottir E, et al. Individual risk assessment and information technology to optimise screening frequency for diabetic retinopathy. Diabetologia 2011;54:2525–2532 [DOI] [PubMed] [Google Scholar]