Abstract
OBJECTIVE
To examine the relation of fatty acid–binding protein (FABP)4 and nonesterified fatty acids (NEFAs) to diabetes in older adults.
RESEARCH DESIGN AND METHODS
We ascertained incident diabetes among 3,740 Cardiovascular Health Study participants (1992–2007) based on the use of hypoglycemic medications, fasting glucose ≥126 mg/dL, or nonfasting glucose ≥200 mg/dL. FABP4 and NEFA were measured on specimens collected between 1992 and 1993.
RESULTS
Mean age of the 3,740 subjects studied was 74.8 years. For each SD increase in log FABP4, hazard ratios (HRs) for diabetes were 1.35 (95% CI 1.10–1.65) for women and 1.45 (1.13–1.85) for men controlling for age, race, education, physical activity, cystatin C, alcohol intake, smoking, self-reported health status, and estrogen use for women (P for sex-FABP4 interaction 0.10). BMI modified the FABP4-diabetes relation (P = 0.009 overall; 0.02 for women and 0.135 for men), in that statistically significant higher risk of diabetes was mainly seen in men with BMI <25 kg/m2 (HR per SD: 1.78 [95% CI 1.13–2.81]). There was a modest and nonsignificant association of NEFA with diabetes (Ptrend = 0.21). However, when restricted to the first 5 years of follow-up, multivariable-adjusted HRs for diabetes were 1.0 (ref.), 1.68 (95% CI 1.12–2.53), and 1.63 (1.07–2.50) across consecutive tertiles of NEFA (Ptrend = 0.03).
CONCLUSIONS
Plasma FABP4 was positively associated with incident diabetes in older adults, and such association was statistically significant in lean men only. A significant positive association between plasma NEFA and incident diabetes was observed during the first 5 years of follow-up.
Type 2 diabetes is associated with high costs and societal burden, with current estimated total cost in the U.S. of $174 billion (1,2). The growing epidemic of obesity threatens to expand this burden substantially, highlighting the crucial importance of better understanding the link between adiposity and type 2 diabetes. Indeed, we have previously demonstrated that various measures of adiposity including BMI and waist circumference are individually associated with an increased risk of diabetes in older U.S. adults (3). Adipose tissues produce multiple adipokines with diverse functions including modulation of inflammation, thrombogenicity, insulin resistance, and other metabolic effects (4,5). As one of these adipokines, fatty acid–binding protein (FABP)4 (or adipocyte FABP or adipocyte protein 2 [aP2]) serves as a carrier protein for fatty acids and other lipophilic substances between extra- and intracellular membranes (6,7). FABP4 is expressed by adipocytes, where it makes up ~8% of the total protein of mature adipocytes, and by macrophages (8,9). FABP4-deficient mice remain insulin sensitive even when challenged by high-fat diets that induce obesity (10). Conversely, in animal models, expression of FABP4 in adipocytes has been associated with overall insulin resistance (11–13).
While FABP4 has been associated with a higher risk of stroke (14), mortality (14), and metabolic syndrome (8,15), only a single human study among 544 middle-aged Chinese adults has reported a prospective association between plasma FABP4 and incident type 2 diabetes (16). Earlier studies have reported that FABP4 may inhibit stearoyl-CoA enzyme in the de novo lipogenesis as well as enhance the activity of hepatic sensitive lipase (17): two pathways that influence plasma concentrations of nonesterified fatty acids (NEFAs). It is unclear whether FABP4 influences the relationship of NEFA with diabetes.
While some studies have reported a positive association between NEFA and type 2 diabetes in middle-aged and younger adults (18,19), other investigators have shown an inverse association between NEFA and type 2 diabetes (20). Elevated NEFA may increase peripheral insulin resistance (21,22) and impair insulin secretion via their toxic effects on pancreatic β-cells (23,24) or increased endogenous glucose production (25). However, no previous study has evaluated whether plasma NEFA and FABP4 individually or jointly influence the risk of type 2 diabetes in older individuals. The current project sought to prospectively examine the relation of plasma FABP4 and NEFA with incident type 2 diabetes in a geographic and racially diverse cohort of older adults in the U.S.
RESEARCH DESIGN AND METHODS
Study participants were members of the Cardiovascular Health Study (CHS), a prospective cohort consisting of 5,888 men and women aged ≥65 years who were randomly selected from Medicare-eligibility lists in four U.S. communities (Forsyth County, NC; Washington County, MD; Sacramento County, CA; and Pittsburgh, PA). A detailed description of methods and procedures in the CHS has previously been published (26). Briefly, persons eligible to participate were not institutionalized or wheelchair dependent, did not require a proxy for consent, were not receiving treatment for cancer, and were expected to remain in their respective regions for 3 years. From 1989 to 1990, 5,201 participants were recruited in the original cohort. Between 1992 and 1993, 687 subjects (predominantly African American) were also recruited. Baseline evaluation of study participants included standardized questionnaires, physical examination, anthropometric measurements, resting electrocardiography, and laboratory examinations. From 1989 through 1999, participants were followed up every 6 months, alternating between telephone calls and clinic visits; biennial telephone calls have continued since then. The institutional review board at each center approved the study, and each participant gave informed consent. For this analysis, the 1992–1993 clinic visit was used as baseline. Of the 5,553 participants alive at the 1992–1993 clinic visit, we excluded 773 people with prevalent diabetes, 780 people with missing data on FABP4 and NEFA, 205 people with missing data on prevalent or incident type 2 diabetes, and 55 individuals with missing covariate information. Thus, 3,740 participants were available for analyses.
Measurement of plasma FABP4 and NEFA
Plasma samples collected at the 1992–1993 examination were stored at −70°C until analyzed at the central laboratory at the University of Vermont. Plasma FABP4 concentration was measured using standard ELISA kits (Biovendor ELISA). The interassay coefficient of variation (CV) was 2.61–5.32% (detectable range 5–250 ng/mL). Plasma NEFA concentration was measured by the Wako enzymatic method. This technique relies on the acylation of CoA by the fatty acids in the presence of added acyl-CoA synthetase. Acyl-CoA produced is oxidized by added acyl-CoA oxidase with generation of hydrogen peroxide and in the presence of peroxidase permits the oxidative condensation of 3-methy-N-ethyl-N(β-hydroxyethyl)-aniline with 4-aminoantipyrine to form a purple-colored adduct. The latter is then measured colorimetrically at 550 nm. The interassay CV was 3.54–8.17% (detectable range 0.0156–1.50 mEq/L).
Assessment of incident type 2 diabetes
We assessed medication use at baseline and annually by a validated medication inventory (27) through 2007. In addition, fasting glucose was measured on blood specimens from the examinations in years 1992–1993, 1996–1997, 1998–1999, and 2005–2006; nonfasting glucose was measured on blood specimens from 1994 to 1995. A participant was classified as having type 2 diabetes if any of the following conditions were met: 1) use of insulin or oral hypoglycemic agents, 2) fasting glucose level of ≥7 mmol/L (126 mg/dL), or 3) a nonfasting glucose level of ≥11.1 mmol/L (200 mg/dL).
Other covariates
We used covariates from the 1992–1993 examination for adjustment. Age, sex, race, years of education, smoking status, and alcohol consumption were based on self-report. Leisure-time activity (kilocalories per week) was assessed using a modified Minnesota Leisure-Time Activities questionnaire (28). Weight, height, and waist circumference were measured using standardized protocols. BMI was calculated as weight in kilograms divided by the square of height in meters. Missing values for smoking and height to calculate BMI were carried forward from previous years if available. Fasting insulin and lipids were measured in fasting blood specimens (29). Serum albumin was assessed using the Kodak Ektachem 700 analyzer (Eastman Kodak, Rochester, NY). Insulin resistance was assessed using homeostasis model assessment (HOMA-IR). Plasma triglyceride was measured by enzymatic methods on an Olympus Demand system (Olympus, Lake Success, NY). HDL was measured by an enzymatic method after precipitation of apolipoprotein B–containing lipoproteins with dextran sulfate/magnesium sulfate. Interassay CVs using reference standards for triglycerides and HDL were 1.78 and 2.15%, respectively. LDL was calculated according to the Friedewald equation if triglyceride was ≤4.51 mmol/L. Cystatin C was measured by nephelometer using a particle-enhanced immunonephelometric assay (N Latex Cystatin C; Dade Behring, Newark, DE) (30) with a interassay CV of 2.9–3.3%. C-reactive protein was measured by immunoassay (interassay CV 6.2%).
Statistical analyses
Baseline characteristics of study participants were summarized according to categories of FABP4 and NEFA; continuous variables were presented as means ± SD and categorical variables as percentages. Incidence rates of type 2 diabetes were calculated per 10,000 person-years.
Cox proportional hazards regression was used to estimate the association of FABP4 and NEFA with incident type 2 diabetes to allow adjustment for covariates. Because the distribution of FABP4 differed appreciably between men and women, FABP4 was modeled as sex-specific tertiles with the lowest tertile as the referent category. FABP4 was also modeled continuously per SD of log FABP4. NEFA was similarly modeled as tertiles based on the overall distribution and also continuously per SD. Individuals were censored for death, loss to follow-up, or end of diabetes ascertainment (2006–2007). Potential confounders included in adjusted models were age, race, and sex (where appropriate) (model 1) with the addition of BMI (included in NEFA model only), education (less than high school versus high school or more), cystatin C (log transformed and included in FABP4 model only), serum albumin (included in NEFA model only), kilocalories of physical activity (log transformed), alcohol intake (none and <0.5, 0.5–1, and >1 drinks/day for women; none and <1, 1–2, and >2 drinks/day for men), smoking status (never, former, and current), self-reported health status (fair/poor versus better), and estrogen use for women (model 2). Given the production of FABP4 in adipocytes, we examined interaction between BMI and FABP4. To evaluate intermediate pathways by which FABP4 might lead to type 2 diabetes, we adjusted for C-reactive protein (log transformed), HOMA-IR, triglycerides (log transformed), HDL, and LDL. To evaluate the joint impact of FABP4 and NEFA on diabetes risk, we fit a model that included both analytes. We also tested for an interaction between FABP4 and NEFA on the risk of incident type 2 diabetes. In secondary analyses, we evaluated whether there were statistically significant interactions between log FABP4 and age or waist circumference or between NEFA and sex or BMI. Because measured weight may not always reflect adiposity in older adults, we excluded participants with unintentional weight loss (defined as self-reported loss of ≥10 pounds not due to diet or exercise during the past 12 months), prevalent cardiovascular disease (coronary heart disease or stroke), or cancer in a sensitivity analysis. Since plasma NEFA varies with dietary intake (31) and lipid metabolism and a single measure may not be a good estimate of average concentrations over the long term, we repeated the analysis while restricting follow-up to the first 5 years. Schoenfeld residuals and plots of the residuals over time were used to evaluate proportional hazards assumptions; there were no meaningful violations. Stata, version 11.1 (StataCorp, College Station, Texas), was used for all analyses, and P values <0.05 were considered statistically significant.
RESULTS
Among 3,740 participants, 2,259 were women (60%) and the mean age at baseline was 74.8 years (range 65–98). Median plasma FABP4 was 29.5 ng/mL (interquartile range 22.0–39.3), and women had substantially higher concentrations of FABP4 (median 34.4 ng/mL) than men (median 22.8 ng/mL). Median NEFA was 0.46 mEq/L (interquartile range 0.35–0.60).
FABP4 and type 2 diabetes risk
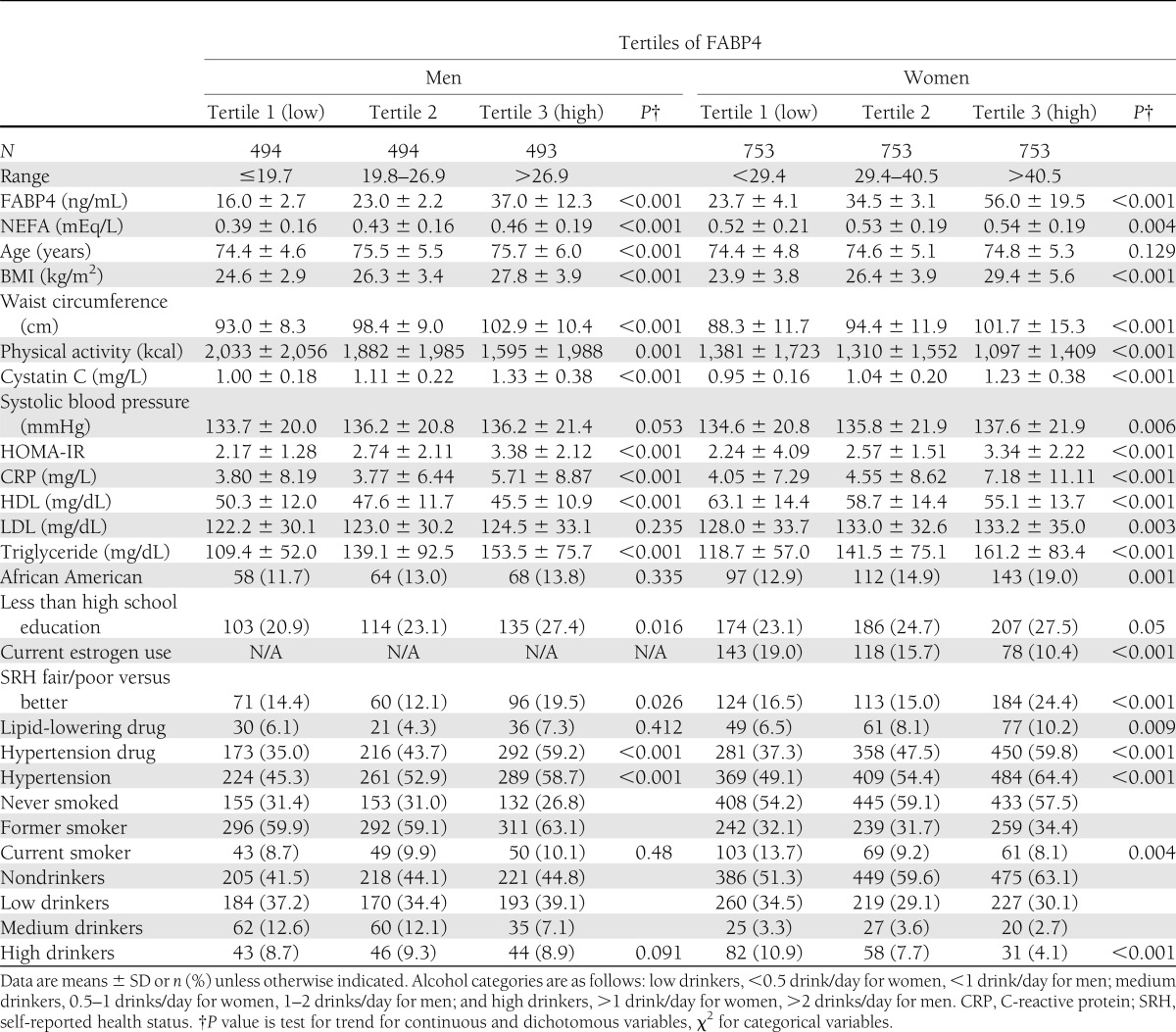
Table 1 presents baseline characteristics of subjects according to tertiles of FABP4 by sex. Compared with the lowest tertile of FABP4, those in the highest tertile had higher measures of adiposity and were more likely to have insulin resistance, higher prevalence of dyslipidemia, poorer health, higher levels of cystatin C, and lower physical activity. The Spearman correlation coefficient between BMI and log FABP4 was 0.38. Details on correlation coefficients between FABP4 and risk factors for type 2 diabetes are presented in Supplementary Table 1.
Table 1.
Characteristics of 3,740 participants by sex-specific tertiles of FABP4 in the CHS
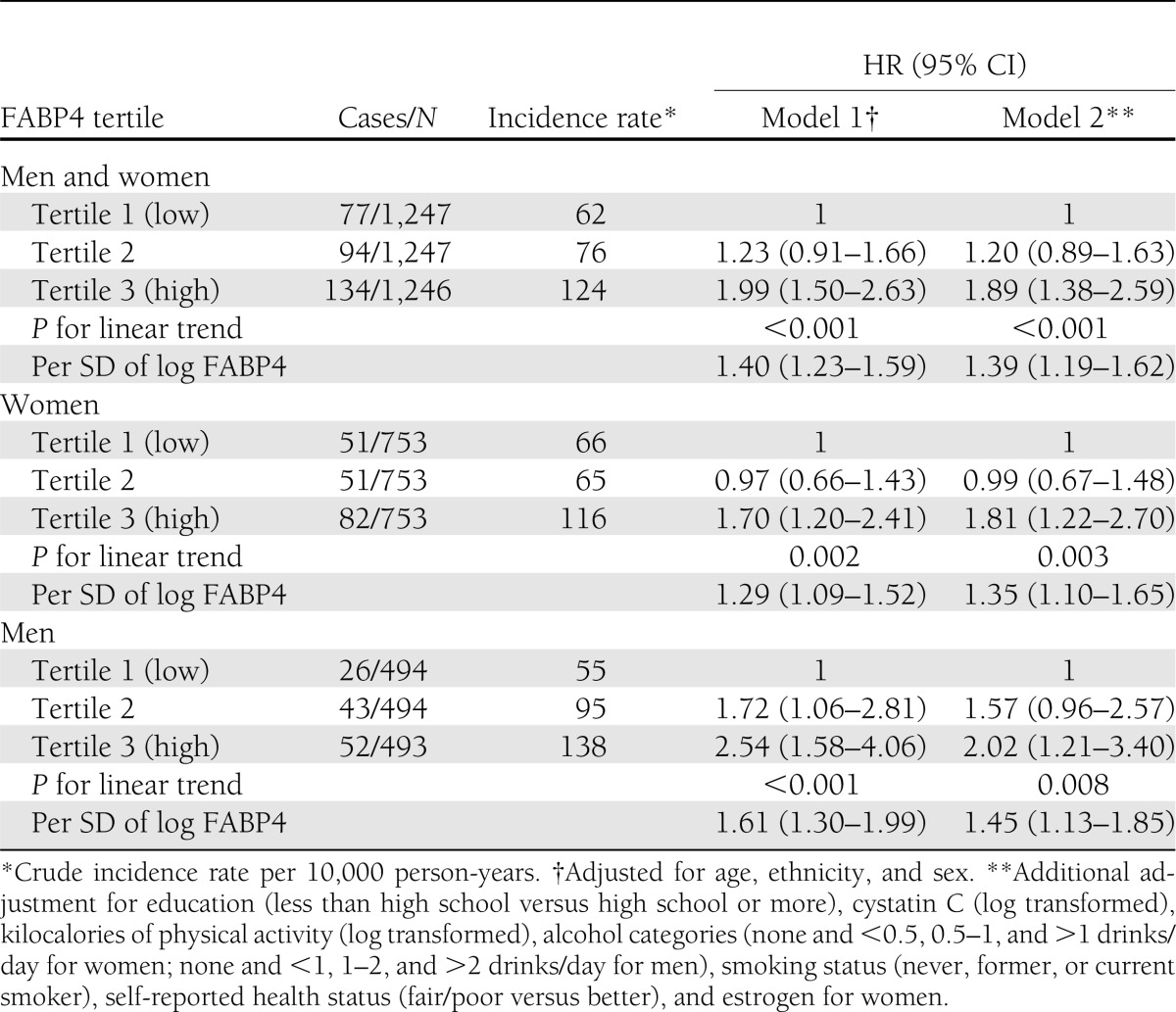
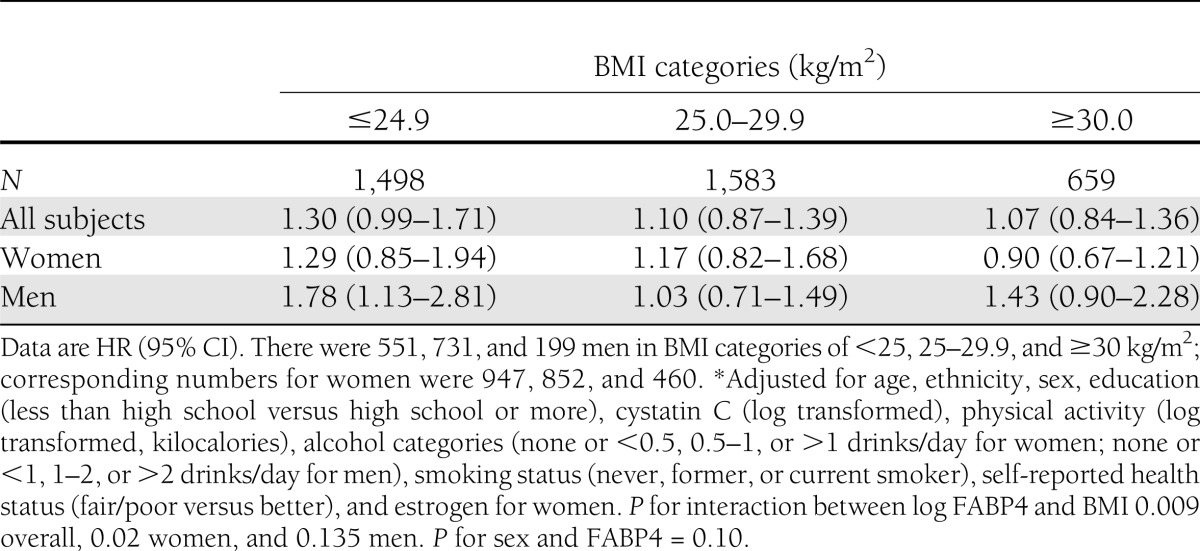
During a mean follow-up of 9.5 years, 305 cases of incident diabetes occurred. Hazard ratios (HRs) associated with each SD increase in log FABP4 were 1.39 (95% CI 1.19–1.62) overall, 1.35 (1.10–1.65) for women, and 1.45 (1.13–1.85) for men after adjustment for age, race, sex, education, physical activity, cystatin C, alcohol intake, smoking, self-reported health status, and estrogen use for women (Table 2), P for sex-by-FABP4 interaction 0.10). There was evidence for an interaction between BMI and FABP4 on the risk of diabetes (P value for interaction between continuous BMI and log FABP4: P = 0.009 overall, 0.02 for women, and 0.135 for men) (Table 3). FABP4 was associated with diabetes in people with lower BMI in both sexes, but the only significantly elevated risk was among lean men (Table 3).
Table 2.
Incidence rate and HRs of type 2 diabetes according to FABP4 tertiles or SD of log FABP4 (0.46) in the CHS
Table 3.
HR of diabetes per SD increase in log FABP4 stratified by BMI in the CHS*
As expected, additional adjustment for potential mediating factors (C-reactive protein, HOMA-IR, LDL and HDL cholesterol, and triglycerides) attenuated the observed association further (relative risk [RR] per SD increase of log FABP4 of 1.25 [95% CI 1.01–1.55]) for women and 1.17 [0.90–1.53] for men). Relations between each potential mediator and type 2 diabetes when added individually to the full model are shown in Supplementary Table 2. There was no statistically significant interaction between FABP4 and NEFA (P = 0.16), and results were similar with the addition of NEFA to these models (data not shown). In a sensitivity analysis, exclusion of individuals with reported unintentional weight loss, prevalent cardiovascular disease, and cancer tended to strengthen the association (HR per SD increase in log FABP4 from 1.39 [95% CI 1.19–1.62] before exclusion to 2.42 [1.56–3.73] after exclusion for men and women combined).
NEFA and type 2 diabetes risk
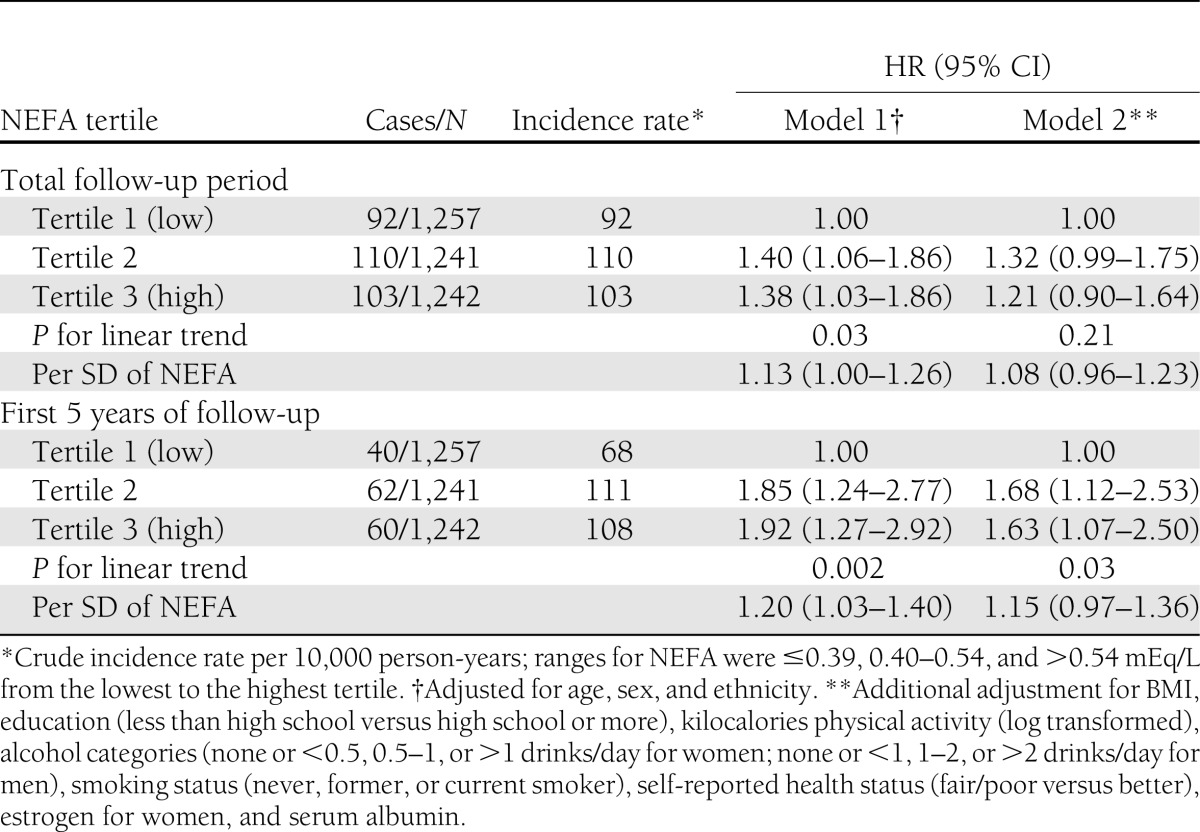
Participants with higher concentrations of NEFA were older, were more likely to be female, had higher measures of adiposity, and had higher levels of total cholesterol, triglycerides, HDL, and C-reactive protein levels (Supplementary Table 3). In a multivariable model adjusted for age, sex, ethnicity, education, BMI, leisure-time physical activity (kilocalories), smoking, alcohol use, health status, estrogen use (in women), and serum albumin, the HR associated with each SD of NEFA (0.20 mEq/L) was 1.08 (95% CI 0.96–1.23) (Table 4). Results were similar with the addition of FABP4 to these models (data not shown). When stratified by BMI (<25, 25–29.9, and ≥30 kg/m2), the results did not change materially and the P value for BMI-by-NEFA interaction was 0.13. In a secondary analysis restricted to the first 5 years of follow up, each SD of higher NEFA was associated with an HR of 1.15 (0.97–1.36) in a fully adjusted model, and there was evidence for increased risk of diabetes at higher levels of NEFA when analyzed by tertiles of NEFA (1.0 [ref.], 1.68 [95% CI 1.12–2.53], and 1.63 [1.07–2.50]) (Table 4). There was no evidence for sex-by-NEFA interaction (P = 0.97).
Table 4.
HR (95% CI) of diabetes by category of and per SD (0.20 mEq/L) increase in NEFAs in the CHS
CONCLUSIONS
In this prospective study of 3,740 people, we demonstrate a positive association between plasma FABP4 and incident type 2 diabetes in older adults and such association was modified by BMI in a way that a statistically significant higher risk of diabetes with FABP4 was observed in lean men only. Additional adjustment for NEFA did not alter the results. We also observed a significant positive association between plasma NEFA and incident type 2 diabetes during the first 5 years that was attenuated and not statistically significant when the total follow-up period was considered. This association was not materially altered with adjustments for FABP4, and there was no interaction between FABP4 and NEFA with incident type 2 diabetes.
To the best of our knowledge, this is the first large prospective study to evaluate associations between plasma FABP4 and incident type 2 diabetes among older men and women in a community setting. While several animal studies have examined the effects of FABP4 expression on adiposity, insulin resistance, and type 2 diabetes risk (7,10,11,32), there are only limited data available in humans. In a cross-sectional study of 98 patients with coronary artery disease, serum levels of FABP4 were positively correlated with the prevalence of metabolic syndrome (MetS) (P = 0.037) and the number of MetS components (P = 0.035) (33). In another cross-sectional study of 806 middle-aged men and women, each SD of higher log FABP4 was associated with a 1.85-fold increased odds of MetS (95% CI 1.53–2.23) controlling for age, sex, and race (5). Two other small and cross-sectional studies by Xu et al. (8) (n = 229) and Stejskal and Karpisek (6) (n = 138) have reported positive associations between serum concentrations of FABP4 and MetS. Baseline FABP4 was also shown to be associated with incident MetS after 5 years of follow-up in 356 Chinese individuals (9).
Only one study has evaluated the association of FABP4 levels with incident type 2 diabetes. In a 10-year prospective study of 544 Chinese participants with a mean age of 50 years, FABP4 levels above the population median (15.3 ng/mL for men and 20.4 ng/ml for women) were associated with a twofold increased risk of type 2 diabetes after controlling for BMI and other confounding factors (RR 2.25 [95% CI 1.40–3.65]) (16). We found that the association of FABP4 with type 2 diabetes risk was most pronounced in persons with BMI <25 kg/m2. In light of this finding, it is notable that the above Chinese study has a lower BMI (mean ± SD 24.3 ± 3.7 kg/m2) than the CHS study (26.4 ± 4.6 kg/m2) (16). The interaction between BMI and FABP4 is consistent with the positive association seen in the leaner Chinese cohort. Our working hypothesis is that expression of FABP4 may be directly associated with the size of adipocytes; furthermore, we are hypothesizing that overweight/obese subjects have larger adipocyte size than lean people. Further studies are needed to elucidate this conjecture.
Our findings of no overall significant association between NEFA and type 2 diabetes in older adults are contrary to other reports. Pankow et al. (19) reported a 63% higher risk of type 2 diabetes in the fourth relative to the first quartile of NEFA (RR 1.63 [1.04 –2.57]) in middle-aged adults. Similar results were noted in the Pima study (18) with a 2.3-fold greater risk of type 2 diabetes comparing the highest with the lowest decile of plasma NEFA. The mean duration of follow-up in our study (9.5 years) was at least twice as long as that reported in the Pima study (18) and nearly five times longer than the 2 years reported in the Paris Prospective Study (34). Furthermore, subjects in our study were much older (mean age 74.8 years) compared with a mean age of 26 years in the Pima study (18), 48.9 years for the Paris study (34), and 52.8 years for Atherosclerosis Risk in Communities (ARIC) study (19). The longer follow-up duration and older age of participants in our cohort might explain the lack of an association in our data. In particular, it is possible that a single NEFA concentration may be less strongly associated with type 2 diabetes risk over long-term follow-up compared with in the short term. NEFA may better predict diabetes risk within a shorter period of follow-up as observed in the Paris and Pima studies (18,34). This hypothesis is consistent with the larger effect size (HR 1.63 [95% CI 1.07–2.50] comparing the 3rd to the 1st tertile of NEFA) observed in our analysis when follow-up time was restricted to the first 5 years of follow-up. If this hypothesis is correct, it suggests that it might be necessary to update NEFA measurements over time to fully characterize NEFA exposure. Nonetheless, our findings also differ from the 53% lower risk of type 2 diabetes (95% CI 19–73) per unit of log NEFA observed in the Insulin Resistance Atherosclerosis Study (IRAS) (20). IRAS showed that 2-h glucose was a major confounder; unfortunately, we did not conduct an oral glucose tolerance test in our cohort at the time of FABP4 and NEFA measurement (20). Further, IRAS was multiethnic with younger average age (∼55 vs. 75 years in the CHS). These differences in model adjustment and subject characteristics may partially explain diverging results.
What potential biologic mechanisms could causally relate FABP4 and NEFA to type 2 diabetes? As a lipid chaperone, FABP4 is expressed in adipocytes and macrophages and plays an important role in lipid metabolism and perhaps glucose utilization (35). As such, it is possible that FABP4 is a mediator of the obesity–type 2 diabetes association. Such a hypothesis would be consistent with the fact that compared with wild-type mice, FABP4 knockout mice do not develop insulin resistance despite extreme adiposity when fed a high-fat diet (10). On the other hand, FABP4 might influence the size of fat cells through de novo lipogenesis and lead to obesity. At this point, it appears that FABP4 is a consequence of obesity. Additional studies with repeated measures of BMI and FABP4 are warranted for clarification.
FABP4 also inhibits stearoyl-CoA desaturase-1, an enzyme that plays a key role in de novo lipogenesis (36) and could influence plasma NEFA. During de novo lipogenesis, plasma glucose is metabolized to saturated fatty acids (myristic acid [c14] and palmitic acid [c16]) (37). Stearoyl-CoA desaturase-1 catalyzes the conversion of palmitic to palmitoleic acid and stearic (c18) to oleic acid (c18), a glucose-requiring process (36). In FABP4-deficient mice, there is an increased ratio of short-chain fatty acids (C14) to longer chain (C18) in muscle and adipose tissues; a more favorable ratio leads to enhanced insulin receptor signaling, AMP-activated kinase activity, and insulin-stimulated glucose uptake (38). In addition, FABP4 attenuates the inhibition of hepatic sensitive lipase through its binding of fatty acids (17). Hepatic sensitive lipase activity can lead to lipolysis with elevated triglycerides and release of free fatty acids, thus leading to insulin resistance.
Higher NEFA concentrations can increase insulin resistance (21,22), exert toxic effects on pancreatic β-cells (23,24), and increase glucose production (25). In addition, treatment with pioglitazone (which reduces insulin resistance) has been associated with reduced NEFA and lipotoxicity (39). Additional adjustment for FABP4 did not alter the point estimate for NEFA in this study, suggesting that the contribution of FABP4 to NEFA concentration may be negligible.
Our study has some limitations. We measured plasma FABP4 and NEFA only once in this cohort. Hence, we were unable to account for change in these biomarkers resulting from change in weight or other factors over time. Weight loss is associated with a reduction in serum FABP4 in humans (40), and in our study, subjects in the highest tertile of FABP4 were more likely to report unintentional weight loss. The fact that we observed a stronger association between FABP4 and diabetes risk after exclusion of subjects who reported unintentional weight loss, cancer, or CVD suggests that repeated measures of FABP4 might lead to even stronger relationships. As an observational study, we cannot exclude residual or unmeasured confounding as an alternative explanation of observed associations. Our sample consisted of Caucasian and, to a lesser degree, African American adults, all of whom were aged ≥65 years; results may not generalize to younger individuals or other race/ethnicities. We did not have measures of fasting glucose in all years, and, hence, we likely missed type 2 diabetes cases in intervening years. We did not have 2-h glucose at the time of FABP4 and NEFA measurement for further adjustment. We cannot exclude the possibility that obese people were less likely to attend clinic and have their blood glucose measured; missing data on glucose that was differential based on obesity may have led to an underestimate of type 2 diabetes events. However, the fact that a similar proportion of obese subjects attended the baseline (17.6%) and 1996–1997 (18.1%) examination is reassuring. Despite these limitations, our study has numerous strengths including a large sample size, a representative sample of older adults, inclusion of both men and women, the use of a valid and reproducible method to assess FABP4 and NEFA, availability of data on numerous potential confounders, and long-term and nearly complete follow-up.
In summary, we observed a positive association between plasma FABP4 and incident type 2 diabetes that was statistically significant only among lean men. The interaction observed between BMI and FABP4 merits further evaluation. Lastly, our data support a statistically significant association between a single measure of NEFA and incident type 2 diabetes in older adults during a shorter but not a longer follow-up period.
Supplementary Material
Acknowledgments
The research reported in this article was supported by the National Heart, Lung, and Blood Institute (R01HL094555 to L.D., J.H.I., K.J.M., S.J.Z., and J.R.K.). Additional support was provided by the National Institute on Aging (AG-023629). The CHS was supported by contracts N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01-HC-15103, N01-HC-55222, N01-HC-75150, and N01-HC-45133 and grant U01-HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by grants R01-AG15928, R01-AG20098, R01-HL085710-01, and R01-AG027058 from the National Institute on Aging; R01-HL075366 from the National Heart, Lung, and Blood Institute; and the University of Pittsburgh Claude D. Pepper Older Americans Independence Center (P30-AG-024827).
The funding agencies did not have any role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
No potential conflicts of interest relevant to this article were reported.
L.D. developed the study concept and design, participated in the acquisition of data, participated in the interpretation of data, drafted the manuscript, critically revised the manuscript for important intellectual content, and obtained funding. O.K. participated in the interpretation of data and critically revised the manuscript for important intellectual content. T.M.B. and M.L.B. performed statistical analysis, participated in the interpretation of data, and critically revised the manuscript for important intellectual content. J.H.I., S.J.Z., J.R.K., and R.P.T. participated in the acquisition of data, participated in the interpretation of data, critically revised the manuscript for important intellectual content, and obtained funding. D.S.S. participated in the acquisition of data, participated in the interpretation of data, criti-cally revised the manuscript for important intellectual content, obtained funding, and supervised the study. K.J.M. participated in the acquisition of data, participated in the interpretation of data, critically revised the manuscript for important intellectual content, and obtained funding. T.M.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors are indebted to the participants and staff of the CHS. A full list of the participating CHS investigators and institutions can be found at http://www.chs-nhlbi.org.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1690/-/DC1.
References
- 1.Fu AZ, Qiu Y, Radican L, Wells BJ. Health care and productivity costs associated with diabetic patients with macrovascular comorbid conditions. Diabetes Care 2009;32:2187–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011 [Google Scholar]
- 3.Biggs ML, Mukamal KJ, Luchsinger JA, et al. Association between adiposity in midlife and older age and risk of diabetes in older adults. JAMA 2010;303:2504–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004;89:2548–2556 [DOI] [PubMed] [Google Scholar]
- 5.Bagheri R, Qasim AN, Mehta NN, et al. Relation of plasma fatty acid binding proteins 4 and 5 with the metabolic syndrome, inflammation and coronary calcium in patients with type-2 diabetes mellitus. Am J Cardiol 2010;106:1118–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stejskal D, Karpisek M. Adipocyte fatty acid binding protein in a Caucasian population: a new marker of metabolic syndrome? Eur J Clin Invest 2006;36:621–625 [DOI] [PubMed] [Google Scholar]
- 7.Yang R, Castriota G, Chen Y, et al. RNAi-mediated germline knockdown of FABP4 increases body weight but does not improve the deranged nutrient metabolism of diet-induced obese mice. Int J Obes (Lond) 2011;35:217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu A, Wang Y, Xu JY, et al. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem 2006;52:405–413 [DOI] [PubMed] [Google Scholar]
- 9.Xu A, Tso AW, Cheung BM, et al. Circulating adipocyte-fatty acid binding protein levels predict the development of the metabolic syndrome: a 5-year prospective study. Circulation 2007;115:1537–1543 [DOI] [PubMed] [Google Scholar]
- 10.Hotamisligil GS, Johnson RS, Distel RJ, Ellis R, Papaioannou VE, Spiegelman BM. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science 1996;274:1377–1379 [DOI] [PubMed] [Google Scholar]
- 11.Boord JB, Maeda K, Makowski L, et al. Combined adipocyte-macrophage fatty acid-binding protein deficiency improves metabolism, atherosclerosis, and survival in apolipoprotein E-deficient mice. Circulation 2004;110:1492–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuncman G, Erbay E, Hom X, et al. A genetic variant at the fatty acid-binding protein aP2 locus reduces the risk for hypertriglyceridemia, type 2 diabetes, and cardiovascular disease. Proc Natl Acad Sci USA 2006;103:6970–6975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simón I, Escoté X, Vilarrasa N, et al. Adipocyte fatty acid-binding protein as a determinant of insulin sensitivity in morbid-obese women. Obesity (Silver Spring) 2009;17:1124–1128 [DOI] [PubMed] [Google Scholar]
- 14.Tso AW, Lam TK, Xu A, et al. Serum adipocyte fatty acid-binding protein associated with ischemic stroke and early death. Neurology 2011;76:1968–1975 [DOI] [PubMed] [Google Scholar]
- 15.Akbal E, Özbek M, Güneş F, Akyürek O, Üreten K, Delibaşi T. Serum heart type fatty acid binding protein levels in metabolic syndrome. Endocrine 2009;36:433–437 [DOI] [PubMed] [Google Scholar]
- 16.Tso AW, Xu A, Sham PC, et al. Serum adipocyte fatty acid binding protein as a new biomarker predicting the development of type 2 diabetes: a 10-year prospective study in a Chinese cohort. Diabetes Care 2007;30:2667–2672 [DOI] [PubMed] [Google Scholar]
- 17.Shen WJ, Liang Y, Hong R, et al. Characterization of the functional interaction of adipocyte lipid-binding protein with hormone-sensitive lipase. J Biol Chem 2001;276:49443–49448 [DOI] [PubMed] [Google Scholar]
- 18.Paolisso G, Tataranni PA, Foley JE, Bogardus C, Howard BV, Ravussin E. A high concentration of fasting plasma non-esterified fatty acids is a risk factor for the development of NIDDM. Diabetologia 1995;38:1213–1217 [DOI] [PubMed] [Google Scholar]
- 19.Pankow JS, Duncan BB, Schmidt MI, et al. Atherosclerosis Risk in Communities Study Fasting plasma free fatty acids and risk of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes Care 2004;27:77–82 [DOI] [PubMed] [Google Scholar]
- 20.Il’yasova D, Wang F, D’Agostino RB, Jr, Hanley A, Wagenknecht LE. Prospective association between fasting NEFA and type 2 diabetes: impact of post-load glucose. Diabetologia 2010;53:866–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groop LC, Barzilai N, Ratheiser K, et al. Dose-dependent effects of glyburide on insulin secretion and glucose uptake in humans. Diabetes Care 1991;14:724–727 [DOI] [PubMed] [Google Scholar]
- 22.Randle PJ. Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev 1998;14:263–283 [DOI] [PubMed] [Google Scholar]
- 23.Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes 1995;44:863–870 [DOI] [PubMed] [Google Scholar]
- 24.Carpentier A, Mittelman SD, Bergman RN, Giacca A, Lewis GF. Prolonged elevation of plasma free fatty acids impairs pancreatic beta-cell function in obese nondiabetic humans but not in individuals with type 2 diabetes. Diabetes 2000;49:399–408 [DOI] [PubMed] [Google Scholar]
- 25.Rebrin K, Steil GM, Getty L, Bergman RN. Free fatty acid as a link in the regulation of hepatic glucose output by peripheral insulin. Diabetes 1995;44:1038–1045 [DOI] [PubMed] [Google Scholar]
- 26.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–276 [DOI] [PubMed] [Google Scholar]
- 27.Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M, The Cardiovascular Health Study Collaborative Research Group Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. J Clin Epidemiol 1992;45:683–692 [DOI] [PubMed] [Google Scholar]
- 28.Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 1978;31:741–755 [DOI] [PubMed] [Google Scholar]
- 29.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem 1995;41:264–270 [PubMed] [Google Scholar]
- 30.Wasén E, Isoaho R, Mattila K, Vahlberg T, Kivelä SL, Irjala K. Estimation of glomerular filtration rate in the elderly: a comparison of creatinine-based formulae with serum cystatin C. J Intern Med 2004;256:70–78 [DOI] [PubMed] [Google Scholar]
- 31.Svensson J, Rosenquist A, Ohlsson L. Postprandial lipid responses to an alpha-linolenic acid-rich oil, olive oil and butter in women: a randomized crossover trial. Lipids Health Dis 2011;10:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leff T, Granneman JG. Adipose Tissue in Health and Disease. Wiley-VCH Verlag GMBh & Co, KGaA, Weinheim, Germany, 2010
- 33.Hsu BG, Chen YC, Lee RP, Lee CC, Lee CJ, Wang JH. Fasting serum level of fatty-acid-binding protein 4 positively correlates with metabolic syndrome in patients with coronary artery disease. Circ J 2010;74:327–331 [DOI] [PubMed] [Google Scholar]
- 34.Charles MA, Eschwège E, Thibult N, et al. The role of non-esterified fatty acids in the deterioration of glucose tolerance in Caucasian subjects: results of the Paris Prospective Study. Diabetologia 1997;40:1101–1106 [DOI] [PubMed] [Google Scholar]
- 35.Krusinová E, Pelikánová T. Fatty acid binding proteins in adipose tissue: a promising link between metabolic syndrome and atherosclerosis? Diabetes Res Clin Pract 2008;82(Suppl. 2):S127–S134 [DOI] [PubMed] [Google Scholar]
- 36.Roberts R, Hodson L, Dennis AL, et al. Markers of de novo lipogenesis in adipose tissue: associations with small adipocytes and insulin sensitivity in humans. Diabetologia 2009;52:882–890 [DOI] [PubMed] [Google Scholar]
- 37.Hellerstein MK, Schwarz JM, Neese RA. Regulation of hepatic de novo lipogenesis in humans. Annu Rev Nutr 1996;16:523–557 [DOI] [PubMed] [Google Scholar]
- 38.Maeda K, Cao H, Kono K, et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab 2005;1:107–119 [DOI] [PubMed] [Google Scholar]
- 39.Roden M, Mariz S, Brazzale AR, Pacini G. Free fatty acid kinetics during long-term treatment with pioglitazone added to sulfonylurea or metformin in Type 2 diabetes. J Intern Med 2009;265:476–487 [DOI] [PubMed] [Google Scholar]
- 40.Corripio R, Gónzalez-Clemente JM, Pérez-Sánchez J, et al. Weight loss in prepubertal obese children is associated with a decrease in adipocyte fatty-acid-binding protein without changes in lipocalin-2: a 2-year longitudinal study. Eur J Endocrinol 2010;163:887–893 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.