Abstract
OBJECTIVE
Metabolite predictors of deteriorating glucose tolerance may elucidate the pathogenesis of type 2 diabetes. We investigated associations of circulating metabolites from high-throughput profiling with fasting and postload glycemia cross-sectionally and prospectively on the population level.
RESEARCH DESIGN AND METHODS
Oral glucose tolerance was assessed in two Finnish, population-based studies consisting of 1,873 individuals (mean age 52 years, 58% women) and reexamined after 6.5 years for 618 individuals in one of the cohorts. Metabolites were quantified by nuclear magnetic resonance spectroscopy from fasting serum samples. Associations were studied by linear regression models adjusted for established risk factors.
RESULTS
Nineteen circulating metabolites, including amino acids, gluconeogenic substrates, and fatty acid measures, were cross-sectionally associated with fasting and/or postload glucose (P < 0.001). Among these metabolic intermediates, branched-chain amino acids, phenylalanine, and α1-acid glycoprotein were predictors of both fasting and 2-h glucose at 6.5-year follow-up (P < 0.05), whereas alanine, lactate, pyruvate, and tyrosine were uniquely associated with 6.5-year postload glucose (P = 0.003–0.04). None of the fatty acid measures were prospectively associated with glycemia. Changes in fatty acid concentrations were associated with changes in fasting and postload glycemia during follow-up; however, changes in branched-chain amino acids did not follow glucose dynamics, and gluconeogenic substrates only paralleled changes in fasting glucose.
CONCLUSIONS
Alterations in branched-chain and aromatic amino acid metabolism precede hyperglycemia in the general population. Further, alanine, lactate, and pyruvate were predictive of postchallenge glucose exclusively. These gluconeogenic precursors are potential markers of long-term impaired insulin sensitivity that may relate to attenuated glucose tolerance later in life.
Type 2 diabetes is characterized by a long progression period before overt disease onset (1,2). Metabolic perturbations characterizing and contributing to the disease development may be observed already in the prediabetic state (3,4). Knowledge on systemic metabolites associated with deteriorating glucose tolerance may elucidate the pathogenesis of diabetes and holds potential for prevention. Comprehensive metabolic profiling is therefore increasingly used to provide hypotheses implicating novel pathways in the disease etiology (5).
Using high-throughput metabolite quantification, we have demonstrated metabolic signatures of insulin resistance in young adults beyond the traditional characteristics of the metabolic syndrome (6). Further, metabolite profiling in the Framingham Heart Study recently linked five branched-chain (leucine, isoleucine, and valine) and aromatic (phenylalanine and tyrosine) amino acids with the risk for future diabetes (7). A lipid signature of triacylglycerols with lower carbon number and double-bond content was also associated with increased risk for diabetes (8). Nonetheless, it remains unknown whether these metabolites are associated with the development of hyperglycemia in nondiabetic individuals and whether the effects would be more specific to fasting or postload glycemia.
Elevated fasting and postchallenge glucose both increase the risk for diabetes; however the mechanisms regulating fasting and stimulated glycemia are partly distinct and the glucose measures have different consequences in terms of risk for cardiovascular mortality (9–11). Pathophysiological differences related to insulin sensitivity and secretion have been suggested to underpin the dissimilarities in glucose tolerance (1,3,11,12). Here, we studied metabolite profiles of glycemia in the general population. The aim was to investigate associations of circulating metabolites from high-throughput profiling separately for fasting and 2-h glucose cross-sectionally and prospectively in middle-aged Finnish men and women. We further assessed whether changes in metabolite levels paralleled changes in glycemia during the 6.5-year follow-up period.
RESEARCH DESIGN AND METHODS
The study was composed of two Finnish population-based cohorts, the Pieksämäki cohort and the Health 2000 Study. A flowchart of the study design is illustrated in Supplementary Fig. 1. The Pieksämäki cohort originally consisted of 1,294 subjects from the town of Pieksämäki in Eastern Finland (13,14). The eligible population consisted of all individuals born in 1942, 1947, 1952, 1957, and 1962, of which 71% (923) participated in the initial examination in 1997 and 690 of these attended a follow-up survey in 2003–2004. The Health 2000 Study is a Finnish cross-sectional health survey carried out in 2000–2001 (15). The overall Health 2000 Study (8,028 persons) was representative of the Finnish population 30 years of age and above. A supplemental study of 1,353 participants 46–76 years of age was carried out in order to study diabetes risk factors more thoroughly; this group was analyzed in the current study. In total, 2,090 participants from the two cohorts had glucose tolerance and comprehensive metabolite data measured. All individuals treated for diabetes (n = 46) or on lipid medication (n = 162) were excluded from analyses. The two studies served as mutual replication of cross-sectional findings. In the longitudinal substudy, 618 individuals (59% women) from the Pieksämäki cohort had data on oral glucose tolerance test (OGTT) at the 6.5-year follow-up (1997–2003) and were not treated pharmacologically for diabetes. All participants gave written informed consent, the study protocols were approved by the local ethics committees, and the studies were conducted in accordance with the Declaration of Helsinki.
Glucose tolerance test and metabolite quantification
Blood samples were drawn in the morning after at least a 12-h fast. Serum was separated and frozen on site prior to storage at −70°C. All participants underwent a 2-h OGTT using a 75-g glucose load with the zero time point in a fasting condition. Plasma glucose concentrations were determined using an automated hexokinase method (intercoefficient of variation [inter-CV] 1.9%) (Peridochrom Glucose GOD-PAP; Boehringer Ingelheim, Mannheim, Germany) for the Pieksämäki cohort and a dehydrogenase assay for the Health 2000 Study (inter-CV 1.7%) (Merck, Darmstadt, Germany). Insulin concentrations at 0, 30, and 120 min during the OGTT were determined by radioimmunoassay (inter-CV 5.7%) (Phadeseph Insulin RIA; Pharmacia, Uppsala, Sweden) in the Health 2000 Study. Alanine aminotransferase was assayed by a kinetic method using a Cobas 6000 analyzer (Hitachi, Tokyo, Japan).
Circulating metabolites were quantified from fasting serum samples using high-throughput nuclear magnetic resonance (NMR) spectroscopy (16). This NMR platform has been applied in various epidemiological and genetic studies (6,17–21). Detection of small molecule solutes from native serum, such as various amino acids and glycolysis substrates, was enabled by spectroscopy settings that suppress the broad spectral signals from lipoprotein particles. Lipid constituents and the diversity of fatty acid saturation were measured from serum lipid extracts (17). Baseline and follow-up samples from the Pieksämäki cohort were measured in a single measurement series (13). Details of the NMR experimentation and metabolite quantification have been described previously (16–18).
Statistical analyses
Peripheral insulin sensitivity index was estimated based on Matsuda ISI (22) and insulin secretion index calculated as total insulin area under the curve per total glucose area under the curve during the OGTT (3). Metabolite measures, insulin indices, and glucose were log transformed prior to analyses. Metabolite tracking (the likelihood of maintaining the original fractile over time) was assessed with Spearman correlation coefficients between the baseline and follow-up concentrations. Associations of circulating metabolites with glucose levels as outcomes were assessed with linear regression models separately for each metabolite. All models were adjusted for sex, age, BMI, systolic blood pressure, HDL cholesterol (HDL-C), and triglycerides. Adjustment for HDL-C and triglycerides was omitted for serum lipid extract measurements to minimize collinearity. The 2-h glucose associations were tested with and without adjustment for fasting glucose. Cross-sectional analyses were conducted separately for the two cohorts and combined using inverse variance–weighted meta-analysis assuming fixed effect size. For these cross-sectional analyses, statistical significance was inferred at P < 0.001. To ensure replication, an additional criterion was that each metabolite was nominally significant (P < 0.05) in both cohorts with concordant direction of effect. We tested for sex differences but did not find significant metabolite × sex interactions. To ease comparison of effects, β-regression coefficients are reported throughout in units of 1-SD increase in glucose levels (outcome) per 1-SD increase in metabolite concentration (predictor).
Metabolites displaying cross-sectional associations with glycemia were analyzed for association with insulin sensitivity and insulin secretion indices for 975 individuals from the Health 2000 Study. The same metabolites were further analyzed for the subset of 618 individuals with longitudinal data on glucose tolerance at the 6.5-year follow-up. Changes in metabolite levels during the follow-up period were tested for association with changes in glucose levels. The dynamics analyses were additionally adjusted for baseline glucose and insulin. Finally, the metabolites were analyzed to assess whether baseline metabolite levels would be predictive of fasting and postload glucose at the 6.5-year follow-up. These longitudinal analyses were also adjusted for baseline glucose and insulin levels, and statistical significance was inferred for two-tailed P < 0.05.
RESULTS
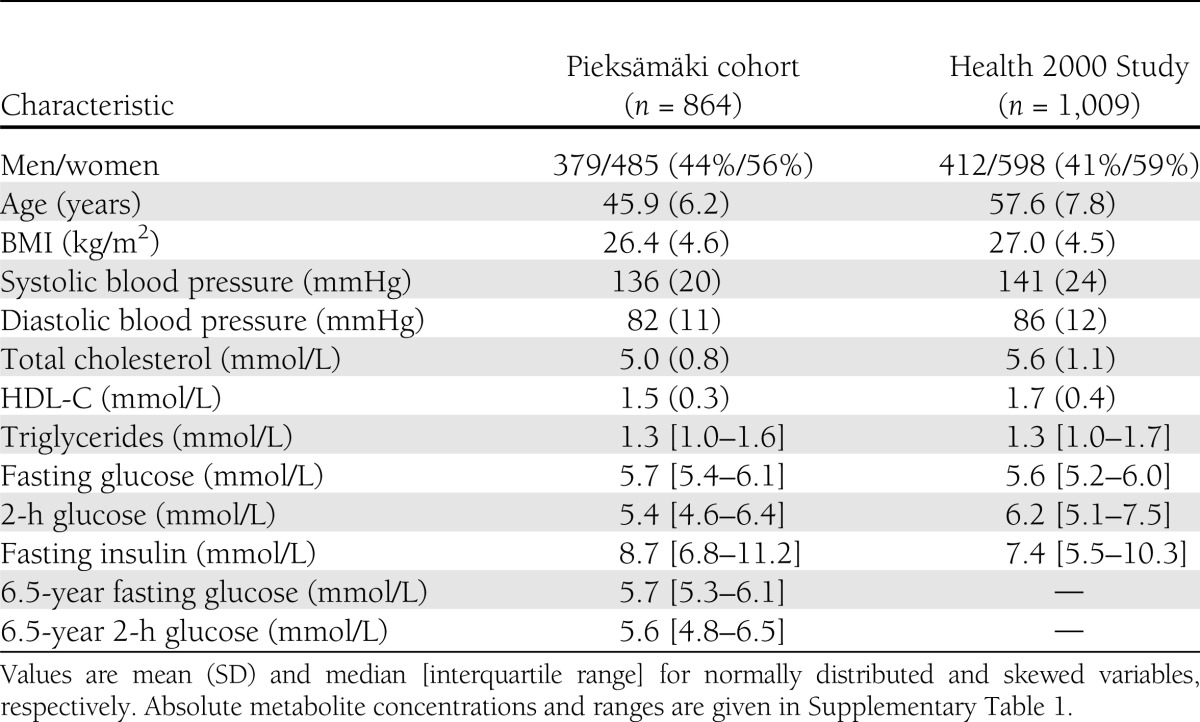
Baseline characteristics of the 1,873 individuals from the two population-based studies are shown in Table 1. The study population represents middle-aged, community-dwelling men and women free of lipid-lowering medication and diabetes treatment. There were similar risk factor profiles in the two cohorts. Absolute concentrations and interquartile ranges of the fasting metabolite levels in both cohorts and at the follow-up survey are given in Supplementary Table 1 along with tracking coefficients of the metabolites over the 6.5-year follow-up. Metabolite levels were similar between the two cohorts and between the two time points. Most metabolites displayed tracking comparable to that of glucose during the follow-up period, suggesting that the metabolite levels are not solely reflecting immediate dietary intake.
Table 1.
Baseline characteristics of the study population
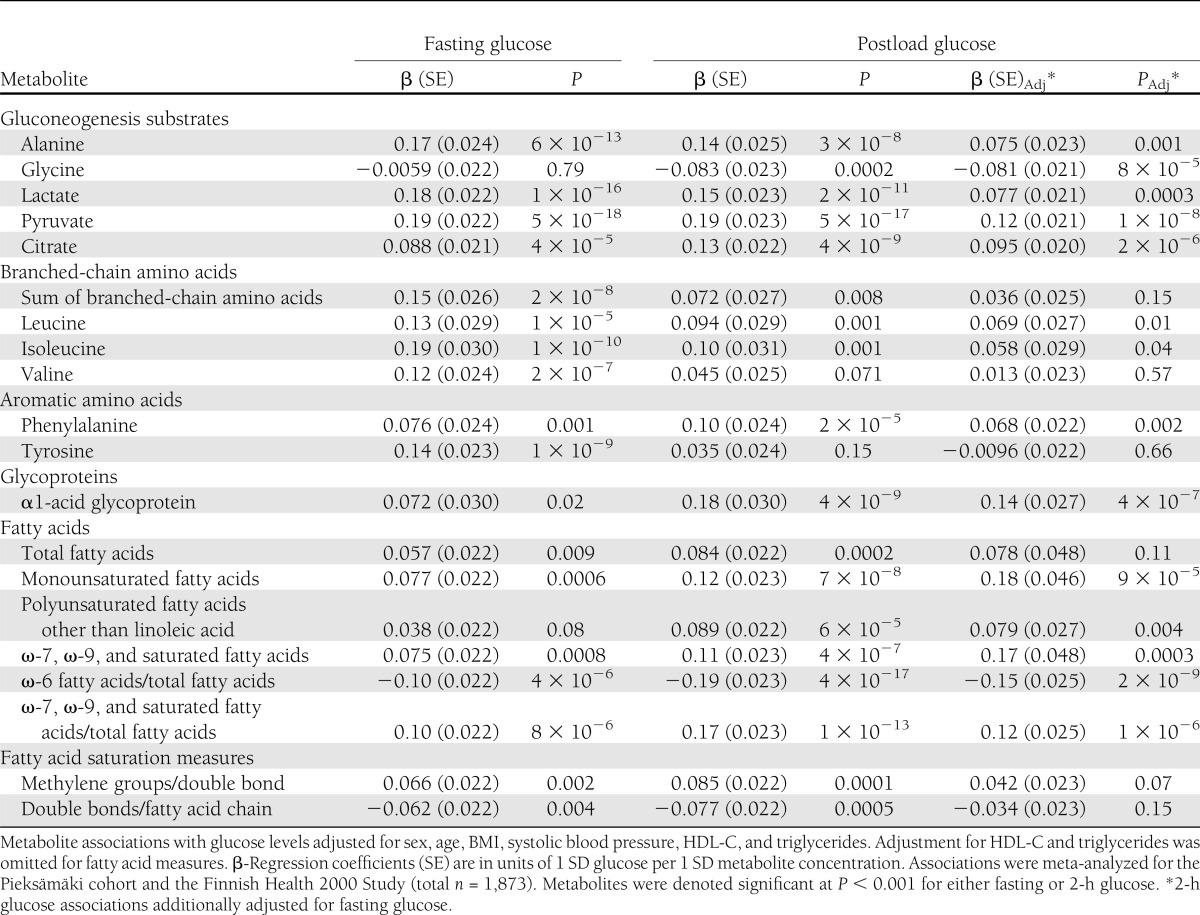
Metabolites associated with fasting and/or 2-h glucose at baseline are listed in Table 2. In total, 19 metabolite measures were cross-sectionally associated with glycemia (meta-analyzed P < 0.001) and nominally significant with the same direction of effect in both cohorts. The significant metabolites comprise a spectrum of gluconeogenesis substrates, branched-chain and aromatic amino acids, α1-acid glycoprotein, as well as fatty acid species and saturation measures. Metabolites were positively associated with increased glycemia with the exceptions of glycine, ω-6 fatty acids/total fatty acids, and double bonds/fatty acid chain. Association magnitudes reached up to β = 0.19 for fasting as well as postload glucose, where the unit of β-regression coefficient indicates increase in SDs of glucose (0.9 and 2.1 mmol/L for fasting and 2-h glucose, respectively) per 1-SD increase in metabolite concentration. For comparison, the association magnitude of BMI with glycemia was β = 0.21 for both fasting and postload glucose, yet the metabolite associations were adjusted for this established risk factor. The majority of metabolites displayed similar magnitude of association for fasting and postload glucose; however, fatty acid metabolites tended to be more strongly associated with postload glycemia. Further, the associations were generally stronger for amino acids and gluconeogenesis precursors than for the fatty acid measures. To assess whether the fasting state metabolite levels reflect postload glycemia independently of fasting glucose, we tested 2-h glucose associations with additional adjustment for fasting glucose, as shown in Table 2. Albeit attenuated, most metabolite associations remained significant, in particular for gluconeogenic substrates and fatty acid measures. Associations of all analyzed metabolites with fasting and 2-h glucose are illustrated separately for the two cohorts along with cross-sectional associations at the follow-up survey in Supplementary Fig. 2. The metabolite associations were coherent for both cohorts, and the associations remained essentially unaltered at the follow-up survey, thus indicating consistency of the associations across study setting and time.
Table 2.
Cross-sectional associations of metabolites with fasting and postload glucose
Deficiencies of insulin action and insulin secretion may underpin the metabolite associations with fasting and postload glucose (6,23). Associations of the metabolites with peripheral insulin sensitivity and total insulin secretion indices in the Health 2000 Study are shown in Supplementary Fig. 3. Most of the metabolites were associated with both insulin sensitivity and total insulin secretion during OGTT; however, all associations with insulin secretion vanished when conditioning on insulin sensitivity, whereas the insulin sensitivity associations remained significant upon conditioning on insulin secretion.
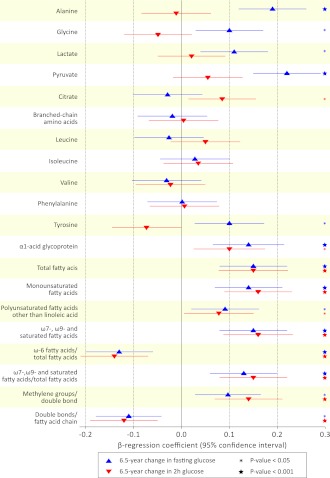
The regulation of fasting metabolite levels may follow changes in glucose tolerance over time (24). Associations between the changes in metabolites and changes in glucose during the follow-up period are shown in Fig. 1. The changes in the majority of the metabolites were associated with changes in fasting glucose during the 6.5-year follow-up period, and changes in fatty acid metabolites also paralleled changes in postload glucose. Notably, changes in branched-chain amino acids and phenylalanine levels were not associated with changes in glycemia during follow-up.
Figure 1.
Associations of 6.5-year changes in metabolite levels with changes in fasting and 2-h glucose. Changes in metabolite concentrations vs. change in fasting glucose (blue triangle) and 2-h glucose (red triangles) during 6.5-year follow-up adjusted for sex, age, baseline BMI, systolic blood pressure, HDL-C, triglycerides, insulin, and glucose. Fatty acid measures were not adjusted for HDL-C and triglycerides. Association magnitudes are in units of 1-SD change in glucose level per 1-SD change in metabolite concentration.
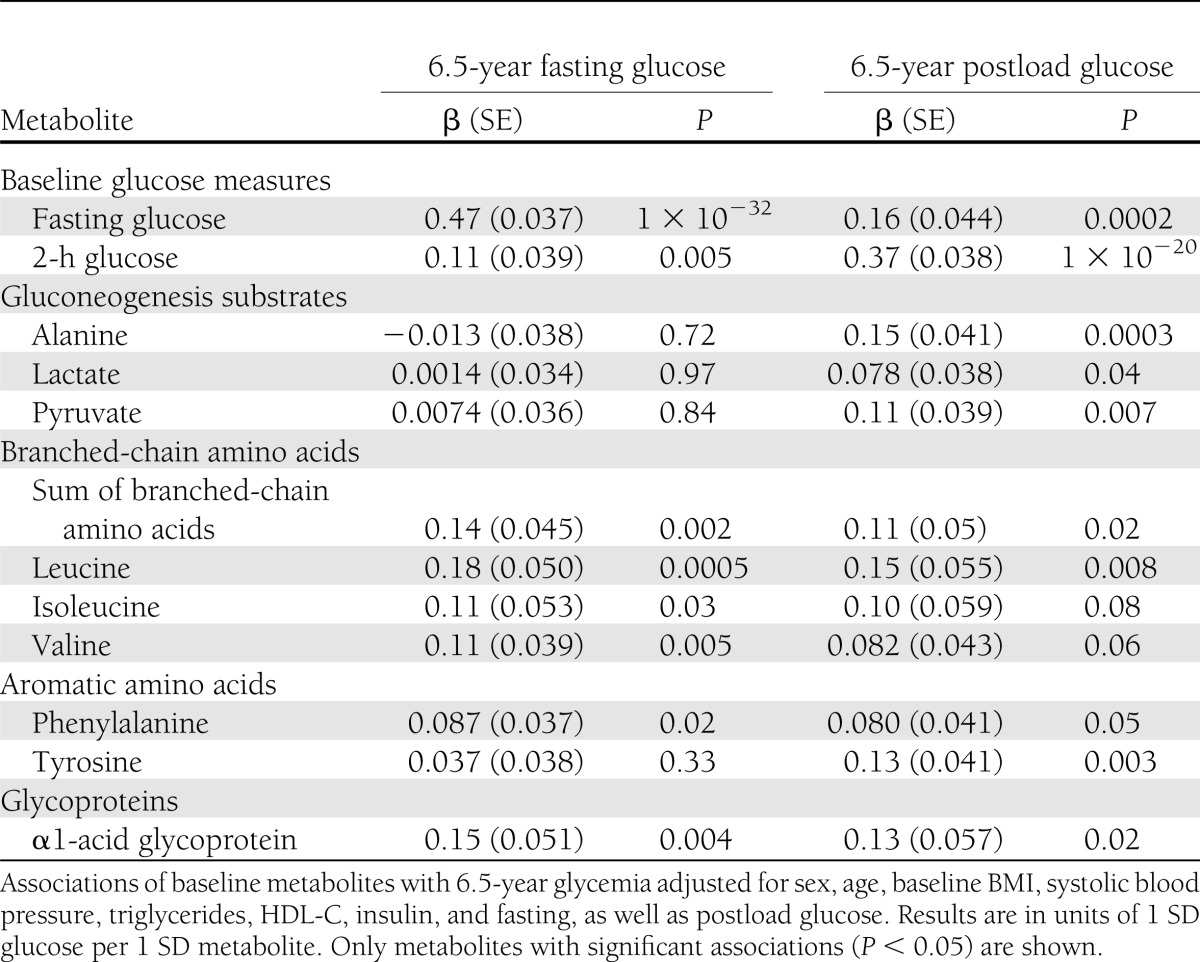
The metabolites linked with glycemia in cross-sectional analyses (Table 2) could potentially be markers of perturbations in glucose homeostasis associated with glucose tolerance later in life. To address this question, we tested whether the metabolites would be predictive of 6.5-year fasting and 2-h glucose in 618 individuals from the Pieksämäki cohort who attended follow-up. Results of these longitudinal analyses are shown for significant metabolites (P < 0.05) in Table 3. Branched-chain amino acids predicted both 6.5-year fasting and postload glycemia (β = 0.14/0.11 for fasting/2-h glucose for the sum of leucine, isoleucine, and valine). The most prominent association was observed for leucine (β = 0.18/0.15, corresponding to 0.11/0.24 mmol higher glucose per 17 µmol/L leucine higher than the median). In addition, phenylalanine and α1-acid glycoprotein were prospectively associated both with fasting and 2-h glucose (P < 0.05). In contrast, alanine, lactate, and pyruvate as well as tyrosine were exclusive predictors of 6.5-year postload glucose (P < 0.05 for all). Of notice, none of the fatty acid measures exhibited significant associations in prospective settings. Interestingly, the prospective associations displayed a pattern opposite to that observed for the changes of metabolites with glycemia; the changes in those metabolites, which were predictors of either fasting or postload glycemia, were not associated with the changes in the corresponding glucose level during the follow-up period (Fig. 1). The ability of the metabolites to predict 6.5-year glycemia may be compared with the predictive ability of baseline glucose for estimating glycemia at follow-up. The prospective metabolite associations were up to 40% of the magnitude of that for baseline glucose versus 6.5-year glycemia (Table 3). The moderate association magnitudes are partly accounted for by the adjustment for baseline glucose as well as insulin and other established risk factors. All results were essentially similar when excluding individuals with newly diagnosed diabetes at baseline. Thus, the associations were not solely driven by the small number of individuals with overt diabetes. The results were virtually unchanged when excluding individuals on blood pressure–lowering medication and hormone treatment (data not shown).
Table 3.
Baseline metabolites as predictors of 6.5-year fasting and postload glucose
CONCLUSIONS
This metabolic profiling study identified 19 circulating metabolite measures associated with fasting or postload glucose in a general population setting of middle-aged men and women. Amino acids, gluconeogenesis precursors, and fatty acids displayed metabolic footprints of both elevated fasting glucose and attenuated glucose tolerance. Differences in metabolite associations emerged between fasting and postload glucose in longitudinal analyses. Alanine, lactate, pyruvate, and tyrosine were selective predictors of 6.5-year postload glucose, suggesting that these metabolites could be markers of deteriorating glucose tolerance later in life. Branched-chain amino acids predicted both fasting and postchallenge glucose levels, confirming their suspected role as markers of future glycemia (7).
Multiple metabolic abnormalities are associated with the progression toward type 2 diabetes (25). Hence, it is to be expected that circulating levels of numerous metabolites are reflective of the degree of glycemia (5). Our results exemplify the diversity of metabolites associated with glucose levels in a nondiabetic population independent of conventional metabolic risk factors (Table 2). Despite differences in cardiovascular morbidity and mortality between individuals with impaired fasting glucose and impaired glucose tolerance, their cardiometabolic risk factor profiles are similar (4,26). Correspondingly, we found the cross-sectional metabolite associations with baseline fasting and 2-h glucose to be of comparable magnitude. Importantly, the fasting metabolite concentrations were reflecting the degree of postchallenge glucose, thus indicating that glucose tolerance may partly be observed in the fasting metabolite profile. However, the small effect sizes also suggest that OGTT response cannot be accurately inferred from the fasting metabolite profile.
Gluconeogenesis plays a pivotal role in glucose homeostasis (27). In this study, three precursors of gluconeogenesis, alanine, lactate, and pyruvate, were predictors of postchallenge glucose after 6.5-year follow-up (Table 3). The molecular mechanisms of how these gluconeogenic substrates are related to future glucose tolerance remain elusive. Lactate has been associated with the prevalence of diabetes in older adults, and decreased oxidative capacity has been proposed to underpin this association (28). Fasting levels of alanine and pyruvate are highly correlated (r = 0.59), which could suggest that the metabolites are reporters of the same pathophysiological effect. On the other hand, pyruvate is involved in multiple glycolytic pathways, including pyruvate cycling pathways in the mitochondria that might underlie the prospective association (25,29). The interconversion between pyruvate and alanine is catalyzed by alanine amino aminotransferase, a liver enzyme for which the plasma levels have been linked with obesity, liver fat extent, and the risk for diabetes (21,30); however, the correlations between alanine aminotransferase and alanine and pyruvate were modest (r = 0.12 for both metabolites).
Alanine, lactate, and pyruvate were predictive of postchallenge glucose, but not fasting glycemia, at the follow-up survey. Elevation of postload glucose is characterized by peripheral insulin resistance, whereas individuals with isolated high fasting glucose have been linked with attenuated insulin secretion and hepatic insulin resistance (1,3,8–12). The prominent associations of the gluconeogenesis substrates with the peripheral insulin sensitivity index support this connection (Supplementary Fig. 3). On the other hand, essentially all the metabolites linked with glycemia were associated with impaired insulin sensitivity rather than insulin secretion. These results therefore do not fully elucidate the differences between fasting and postload glycemia observed in the longitudinal analyses. Despite the cross-sectional associations with insulin sensitivity, the gluconeogenesis substrates remained predictors of postload glucose even when adjusting for baseline glucose and insulin. Because glucose regulation is essential in metabolic fuel homeostasis, the circulating levels of gluconeogenesis precursors could potentially reflect impaired insulin sensitivity prior to elevation of fasting glucose and insulin levels. Analyses of the changes in metabolites during follow-up indicated that alanine, lactate, and pyruvate levels were following changes in fasting but not postload glucose (Fig. 1). These findings support that the gluconeogenic substrates contain a component of information that is not only related to immediate glucose levels but also reflects future glucose tolerance. In a nested case-control setting, alanine was predictive of incident diabetes more than a decade later (P = 0.04) (7). Together with our population-based results, this suggests that metabolic perturbations causing elevated circulating alanine, lactate, and pyruvate could be early markers of attenuated glucose tolerance that may eventually progress to diabetes.
Circulating levels of five branched-chain and aromatic amino acids have been associated with insulin resistance (6,23,31,32) and reported to predict the development of future diabetes (7). Leucine, isoleucine, valine, phenylalanine, and tyrosine were all associated with glycemia and insulin sensitivity at baseline and predictors of fasting and/or postchallenge glucose in this study. These findings indicate that the amino acids are not only markers of the risk for overt diabetes but also predictors of future glycemia in the general population. Importantly, the changes in branched-chain amino acids and phenylalanine concentrations did not parallel changes in fasting glucose during the follow-up. Thus, whereas fasting glucose changes are accompanied by altered levels of fatty acids and gluconeogenic substrates, the regulation of branched-chain amino acids appears to be less tightly connected to changes in glycemia (Fig. 1). These results suggest that abnormalities in branched-chain amino acid metabolism precede the development of hyperglycemia, and this may partly explain how the amino acids mediate the risk for future diabetes. Experimental animal studies have suggested a mechanistic role of branched-chain amino acids in the development of insulin resistance (32); however, it remains unsettled whether the amino acids are causally implicated in humans. Although our results are compatible with a functional role of branched-chain and aromatic amino acids in the pathogenesis of hyperglycemia, the elevated amino acid concentrations could also potentially be secondary markers of metabolic abnormalities that may not be fully observed in baseline glucose and insulin levels from a single time point. In this relation, we have recently shown that branched-chain amino acids as well as alanine, pyruvate, and α1-acid glycoprotein all are regulated by a variant in the gene encoding glucokinase regulatory protein (GCKR), which is suspected to affect the glucose sensory mechanism and indirectly affect lipid and amino acid metabolism (6,20,21,33). In a similar manner, the prospective associations of amino acids and gluconeogenesis substrates with glycemia could reflect perturbations in glucose sensing, which eventually may lead to hyperglycemia.
Low-grade, systemic inflammation has been associated with the risk for diabetes (34,35). The acute-phase protein α1-acid glycoprotein (orosomucoid) is an abundant immunomodulator protein in circulation, which is induced by stressful conditions such as infections and inflammation, and elevated levels have been found in diabetic patients (36,37). Expression of α1-acid glycoprotein has been linked with metabolic signaling, including hyperglycemia, and has been suggested to modulate immune responses to protect adipose tissue from inflammation and metabolic dysfunction (37). In this study, α1-acid glycoprotein was prospectively associated with both fasting and postchallenge glucose (Table 3). α1-acid glycoprotein has previously been linked with the risk for incidence of diabetes in the Atherosclerosis Risk in Communities (ARIC) study; however, the association was not independent of baseline BMI, insulin, and glucose (35). Our results suggest that α1-acid glycoprotein is not only related to metabolic dysfunction cross-sectionally but also a predictor of future glycemia, and thus highlight the role of prolonged inflammation as a risk marker for attenuating glucose tolerance.
The role of metabolic dysfunction in the pathogenesis of diabetes has primarily focused on lipoproteins and lipid-induced mechanisms (5,38,39). In contrast to this notion, the metabolites predictive of glycemia in this study were amino acids and gluconeogenesis substrates rather than lipid composition measures. Comprehensive lipid profiling was recently used to identify a pattern of triacylglycerides associated with insulin resistance and the risk for future diabetes (8). Our results are somewhat surprising in this respect; whereas several fatty acid species and saturation measures were cross-sectionally associated with glycemia and impaired insulin sensitivity (Table 2 and Supplementary Fig. 3), none of the lipid measures predicted future glycemia in this study. However, the triacylglycerol signature linked with the risk for diabetes was composed of lipid species not quantifiable by the high-throughput metabolic profiling platform. These results may suggest that information on the molecular character of lipid components, including specific chain length and saturation content, is beneficial for elucidating the etiology of lipid-mediated hyperglycemia.
As a limitation, we note that our study was inadequately powered to assess the risk for incidence of diabetes. Although the cross-sectional associations were mutually replicated in two cohorts, the prospective associations merit further validation in independent populations. Insulin action was approximated by OGTT-based surrogates, which have limited ability to separate contributions from peripheral and hepatic insulin resistance (1). Our study was conducted in a homogenous Finnish population and care must be taken before generalization to other age groups and ethnicities.
In conclusion, high-throughput profiling revealed a diverse metabolic footprint of glycemia in middle-aged men and women. Alanine, lactate, and pyruvate were predictors of postchallenge glucose after a 6.5-year follow-up, indicating that early signs of attenuating glucose tolerance may be observed in gluconeogenesis precursors quantified from fasting serum samples. α1-acid glycoprotein as well as branched-chain and aromatic amino acids predicted both fasting and postload glycemia, but the changes in amino acid levels did not follow glucose changes over time. These findings support the notion that alterations in amino acid metabolism precede perturbations in glucose homeostasis, and the circulating amino acid levels may serve as predictors of future glycemia in a general population setting.
Supplementary Material
Acknowledgments
This study was supported by the Academy of Finland (grants 250422, 137870, and 129429) and the Research Programme of the Academy of Finland Responding to Public Health Challenges, the Instrumentarium Science Foundation, the Antti and Jenny Wihuri Foundation, the Tampere University Hospital Medical Fund, the Emil Aaltonen Foundation, the Orion-Farmos Research Foundation, the Paulo Foundation, and the Finnish Foundation for Cardiovascular Research.
No potential conflicts of interest relevant to this article were reported.
P.W. researched and interpreted the data and wrote the manuscript. M.T., A.J.K., and P.S. designed the metabolic profiling platform and analyzed the NMR experiments. V.-P.M. contributed to interpretation of the data and discussion. J.S., S.K.-K., P.M., T.L., M.L., A.J., M.K., and M.V. provided clinical or laboratory data and interpretations. M.A.-K. contributed to interpretation of the data and discussion, designed the metabolic profiling platform, and analyzed the NMR experiments. All authors reviewed, commented on, and accepted the manuscript. P.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
A slide set summarizing this article is available online.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1838/-/DC1.
References
- 1.Faerch K, Borch-Johnsen K, Holst JJ, Vaag A. Pathophysiology and aetiology of impaired fasting glycaemia and impaired glucose tolerance: does it matter for prevention and treatment of type 2 diabetes? Diabetologia 2009;52:1714–1723 [DOI] [PubMed] [Google Scholar]
- 2.Tabák AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimäki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet 2009;373:2215–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stančáková A, Javorský M, Kuulasmaa T, Haffner SM, Kuusisto J, Laakso M. Changes in insulin sensitivity and insulin release in relation to glycemia and glucose tolerance in 6,414 Finnish men. Diabetes 2009;58:1212–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nóvoa FJ, Boronat M, Saavedra P, et al. Differences in cardiovascular risk factors, insulin resistance, and insulin secretion in individuals with normal glucose tolerance and in subjects with impaired glucose regulation: the Telde Study. Diabetes Care 2005;28:2388–2393 [DOI] [PubMed] [Google Scholar]
- 5.Bain JR, Stevens RD, Wenner BR, Ilkayeva O, Muoio DM, Newgard CB. Metabolomics applied to diabetes research: moving from information to knowledge. Diabetes 2009;58:2429–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Würtz P, Mäkinen VP, Soininen P, et al. Metabolic signatures of insulin resistance in 7,098 young adults. Diabetes 2012;61:1372–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhee EP, Cheng S, Larson MG, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest 2011;121:1402–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saxena R, Hivert MF, Langenberg C, et al. GIANT consortium. MAGIC investigators Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat Genet 2010;42:142–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genuth S, Alberti KG, Bennett P, et al. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–3167 [DOI] [PubMed] [Google Scholar]
- 11.Meyer C, Pimenta W, Woerle HJ, et al. Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care 2006;29:1909–1914 [DOI] [PubMed] [Google Scholar]
- 12.Carnevale Schianca GP, Rossi A, Sainaghi PP, Maduli E, Bartoli E. The significance of impaired fasting glucose versus impaired glucose tolerance: importance of insulin secretion and resistance. Diabetes Care 2003;26:1333–1337 [DOI] [PubMed] [Google Scholar]
- 13.Vanhala MJ, Kumpula LS, Soininen P, et al. High serum adiponectin is associated with favorable lipoprotein subclass profile in 6.4-year follow-up. Eur J Endocrinol 2011;164:549–552 [DOI] [PubMed] [Google Scholar]
- 14.Ahonen TM, Kautiainen HJ, Keinänen-Kiukaanniemi SM, Kumpusalo EA, Vanhala MJ. Gender difference among smoking, adiponectin, and high-sensitivity C-reactive protein. Am J Prev Med 2008;35:598–601 [DOI] [PubMed] [Google Scholar]
- 15.Sipilä K, Moilanen L, Nieminen T, et al. Metabolic syndrome and carotid intima media thickness in the Health 2000 Survey. Atherosclerosis 2009;204:276–281 [DOI] [PubMed] [Google Scholar]
- 16.Soininen P, Kangas AJ, Würtz P, et al. High-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolism. Analyst (Lond) 2009;134:1781–1785 [DOI] [PubMed] [Google Scholar]
- 17.Inouye M, Kettunen J, Soininen P, et al. Metabonomic, transcriptomic, and genomic variation of a population cohort. Mol Syst Biol 2010;6:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Würtz P, Soininen P, Kangas AJ, et al. Characterization of systemic metabolic phenotypes associated with subclinical atherosclerosis. Mol Biosyst 2011;7:385–393 [DOI] [PubMed] [Google Scholar]
- 19.Würtz P, Raiko JR, Magnussen CG, et al. High-throughput quantification of circulating metabolites improves prediction of subclinical atherosclerosis. Eur Heart J. 26 March 2012 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Kettunen J, Tukiainen T, Sarin AP, et al. Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat Genet 2012;44:269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chambers JC, Zhang W, Sehmi J, et al. Alcohol Genome-wide Association (AlcGen) Consortium. Diabetes Genetics Replication and Meta-analyses (DIAGRAM+) Study. Genetic Investigation of Anthropometric Traits (GIANT) Consortium. Global Lipids Genetics Consortium. Genetics of Liver Disease (GOLD) Consortium. International Consortium for Blood Pressure (ICBP-GWAS) Meta-analyses of Glucose and Insulin-Related Traits Consortium (MAGIC) Genome-wide association study identifies loci influencing concentrations of liver enzymes in plasma. Nat Genet 2011;43:1131–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 23.Huffman KM, Shah SH, Stevens RD, et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care 2009;32:1678–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laferrère B, Reilly D, Arias S, et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med 2011;3:80re2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 2008;9:193–205 [DOI] [PubMed] [Google Scholar]
- 26.Saukkonen T, Cederberg H, Jokelainen J, et al. Limited overlap between intermediate hyperglycemia as defined by A1C 5.7-6.4%, impaired fasting glucose, and impaired glucose tolerance. Diabetes Care 2011;34:2314–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hers HG, Hue L. Gluconeogenesis and related aspects of glycolysis. Annu Rev Biochem 1983;52:617–653 [DOI] [PubMed] [Google Scholar]
- 28.Crawford SO, Hoogeveen RC, Brancati FL, et al. Association of blood lactate with type 2 diabetes: the Atherosclerosis Risk in Communities Carotid MRI Study. Int J Epidemiol 2010;39:1647–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 2008;7:45–56 [DOI] [PubMed] [Google Scholar]
- 30.Sattar N, Scherbakova O, Ford I, et al. West of Scotland Coronary Prevention Study Elevated alanine aminotransferase predicts new-onset type 2 diabetes independently of classical risk factors, metabolic syndrome, and C-reactive protein in the West of Scotland Coronary Prevention Study. Diabetes 2004;53:2855–2860 [DOI] [PubMed] [Google Scholar]
- 31.Felig P, Marliss E, Cahill GF., Jr Plasma amino acid levels and insulin secretion in obesity. N Engl J Med 1969;281:811–816 [DOI] [PubMed] [Google Scholar]
- 32.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orho-Melander M, Melander O, Guiducci C, et al. Common missense variant in the glucokinase regulatory protein gene is associated with increased plasma triglyceride and C-reactive protein but lower fasting glucose concentrations. Diabetes 2008;57:3112–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–867 [DOI] [PubMed] [Google Scholar]
- 35.Duncan BB, Schmidt MI, Pankow JS, et al. Low-grade systemic inflammation and the development of type 2 diabetes: the Atherosclerosis Risk in Communities Study. Diabetes 2003;52:1799–1805 [DOI] [PubMed] [Google Scholar]
- 36.Fournier T, Medjoubi-N N, Porquet D. Alpha-1-acid glycoprotein. Biochim Biophys Acta 2000;1482:157–171 [DOI] [PubMed] [Google Scholar]
- 37.Lee YS, Choi JW, Hwang I, et al. Adipocytokine orosomucoid integrates inflammatory and metabolic signals to preserve energy homeostasis by resolving immoderate inflammation. J Biol Chem 2010;285:22174–22185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 2007;87:507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krauss RM. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care 2004;27:1496–1504 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.