Abstract
OBJECTIVE
In the general population, a low ankle-brachial index (ABI) (<0.9) is strongly associated with (cardiovascular) mortality. However, the association between the ABI and mortality may be weaker in individuals with diabetes, as ankle pressures may be elevated by medial arterial calcification and arterial stiffening, which occur more frequently in diabetes. Therefore, the aim of this study was to compare the association between ABI and mortality in individuals without and with diabetes.
RESEARCH DESIGN AND METHODS
We studied the associations between ABI and cardiovascular and all-cause mortality in 624 individuals from the Hoorn study, a population-based cohort of 50- to 75-year-old individuals (155 with diabetes and 469 without) followed for a median period of 17.2 years. Data were analyzed using Cox proportional hazards models.
RESULTS
During the follow-up period, 289 of 624 (46.3%) participants died (97 of 155 with and 192 of 469 without diabetes and 52 of 65 with and 237 of 559 without ABI <0.9): 85 (29.4%) of CVD (30 of 155 with and 55 of 469 without diabetes and 20 of 65 with and 65 of 559 without ABI <0.9). A low ABI was strongly associated with cardiovascular mortality (relative risk 2.57 [95% CI 1.50–4.40]) and all-cause mortality (2.02 [1.47–2.76]), after adjustment for Framingham risk factors. The associations of the ABI with mortality did not differ between individuals without and with diabetes for cardiovascular (Pinteraction = 0.45) or all-cause (Pinteraction = 0.63) mortality.
CONCLUSIONS
In the Hoorn Study, associations between ABI and cardiovascular and all-cause mortality were similar in individuals without and with diabetes. Future studies should investigate, in both individuals without and with diabetes, whether measurement of ABI can be used to guide treatment decisions.
Identifying individuals with a high risk of cardiovascular morbidity and mortality remains challenging, as many high-risk individuals are asymptomatic (1). The ankle-brachial blood pressure index (ABI) is a marker for systemic atherosclerosis, is strongly associated with mortality (2), and may thus improve identification of high-risk individuals. However, the validity of the ABI may be decreased in diabetes, as ankle pressures may be elevated by medial arterial calcification and arterial stiffening, which occur more frequently in diabetes (3). In diabetes, the ABI may thus fall within the normal range (≥0.9 to ≤1.4) when both atherosclerosis and arterial stiffening and calcifications of the lower limbs occur within the same individual. Therefore, the aim of this study was to investigate whether the associations between the ABI and cardiovascular and all-cause mortality are similar or, in fact, weaker in individuals with diabetes than in individuals without diabetes. We investigated the ABI at several cutoff points (ABI <0.9, <1.0, and <1.1) and as a continuous variable because development of atherosclerosis in the lower limbs is a continuous process (4). In addition, we investigated whether alternatives for the ABI, such as the toe-brachial index (TBI) and Doppler flow curves of the lower extremities, were more strongly associated with mortality than the ABI in individuals with diabetes, as these techniques may be less affected by arterial calcifications and stiffening than the ABI.
RESEARCH DESIGN AND METHODS
We used data from the Hoorn Study, a population-based cohort study on glucose metabolism and other cardiovascular risk factors in a general Caucasian population, which has previously been described in detail (5). The Hoorn Study was approved by the ethics review committee of VU University Medical Centre. Informed consent was obtained from all participants, and all study participants gave written informed consent to be included. In brief, men and women aged 50–75 years were randomly selected from the population register of the town of Hoorn, the Netherlands; 2,484 subjects participated (response rate 71%). Baseline examinations were conducted from October 1989 until February 1992. All subjects had a 75-g oral glucose tolerance test, except those in whom type 2 diabetes had previously been diagnosed. An extensive metabolic and cardiovascular investigation was performed in an age-, sex-, and glucose tolerance–stratified, random subsample of 631 participants (89% of those invited), which was used in the current study (5). Participants without data on the ABI or Framingham risk factors were excluded (n = 7). The current study, therefore, consisted of 624 individuals: 371 with normal glucose metabolism, 98 with impaired glucose metabolism (including those with impaired fasting glucose and/or impaired glucose tolerance), and 155 with type 2 diabetes (6).
Baseline measurements
Main determinants.
The ABI was measured at baseline as previously described in detail (5). In short, Doppler-assisted systolic blood pressure (SBP) measurements were taken from the brachial and posterior tibial arteries on both sides using 12-cm cuffs (Medasonics Vasculab, Mountainview, CA). Recording started after a 15-min resting period in a supine position (room temperature 23°C). Whenever the ABI over the posterior tibial artery was <0.9 or the Doppler flow signal was not audible, the ankle pressure was also measured over the dorsalis pedis artery or the peroneal artery. The ABI was calculated for each leg using the highest ankle pressure divided by the highest systolic brachial pressure. An ABI of <0.9 in either leg was considered indicative of systemic atherosclerosis. In additional analyses, the associations between the ABI and mortality were reanalyzed with higher cutoff points (<1.1 and <1.0) and with the ABI as a continuous variable.
In diabetic individuals, the ABI may increase because of arterial stiffening and calcification of large vessels. Thus, in diabetic individuals methods alternative to the ABI, which may be less affected by these phenomena, may be more strongly associated with mortality than the ABI. Therefore, we measured toe pressures to calculate a TBI, which may give a more valid impression of flow impairment in diabetes, as smaller vessels are thought to be less affected by arterial calcifications than larger vessels. The TBI was calculated for each leg by dividing the toe pressure by the highest brachial pressure. For the TBI, a ratio of <0.7 is considered abnormal (7). In addition, we measured Doppler flow curves of the lower arteries, as even in calcified vessels atherosclerosis may yield abnormal flow patterns. With the use of a 5- or 8-mHz bidirectional continuous wave Doppler connected to a frequency analyzer, Doppler flow curves were determined at the left and right leg, at the level of the aorta-iliac, femoral-popliteal, dorsalis pedis, peroneal, and tibial arteries (5). Triphasic or biphasic curves were considered normal, whereas a monophasic curve or the absence of a curve in one or more of the arterial tracts was considered abnormal.
Covariates.
Urinary albumin concentration was measured in a first-voided sample by rate nephelometry (Array Protein System; Beckman Coulter, Galway, Ireland) with a detection threshold of 6.2 mg/L (intra- and interassay coefficients of variation 5 and 8%, respectively). Urinary creatinine was measured with a modified Jaffé method. Microalbuminuria was present if the albumin-to-creatinine ratio was in the range of 2.0–30 mg/mmol. Macroalbuminuria was present if the albumin-to-creatinine ratio was >30 mg/mmol. BMI; waist and hip circumference; SBP and diastolic (DBP) blood pressure; levels of fasting plasma glucose; HbA1c; insulin; total, HDL, and LDL cholesterol; triglycerides and plasma creatinine; and smoking status were measured as previously described (5). Estimated glomerular filtration rate (eGFR) was determined by the short Modification of Diet in Renal Disease equation (8). Hypertension was defined as an SBP ≥140 mmHg and/or a DBP ≥90 mmHg and/or the use of antihypertensive drugs. Prior cardiovascular disease (CVD) was defined when individuals had any of the following: a history of myocardial infarction, stroke or transient ischemic attack, abnormalities on a resting electrocardiogram (Minnesota codes 1.1–1.3, 4.1– 4.3, 5.1–5.3, or 7.1), intermittent claudication, nontraumatic amputation, previous coronary bypass surgery, or angioplasty.
Follow-up
Data on the participants’ vital status up to 1 January 2009 were collected from the mortality register of the municipality of Hoorn. Information on cause of death was extracted from the medical records of the general practitioners and the local hospital and coded according to the ICD-9. Cardiovascular mortality, including sudden death, was defined by ICD-9 codes 390–459 and 798. Information on cause of death could not be obtained for 32 of the deceased individuals. All subjects were followed until death or end of follow-up, at which time they were censored.
Statistical analyses
Baseline characteristics between survivors and nonsurvivors were compared with the use of Student t or χ2 tests. Logistic regression models were used to investigate the associations between cardiovascular risk factors and having an abnormal ABI, TBI, or Doppler flow curve. Cox proportional hazards regression models were used to calculate the crude and adjusted relative risks (RRs) and respective 95% CIs of cardiovascular and all-cause mortality associated with having, respectively, an abnormal ABI, TBI, or Doppler flow curve.
To investigate whether the associations between ABI, TBI, and Doppler flow curves and mortality were different between individuals without and with diabetes, interaction terms (presence of diabetes × presence of an abnormal ABI, TBI, or Doppler flow curve, respectively) were used. In addition, analyses stratified for the presence of diabetes were performed. When dummy variables for impaired glucose metabolism and diabetes were added to the model, the RRs for all-cause and cardiovascular mortality of individuals with impaired glucose tolerance did not differ significantly from individuals with normal glucose tolerance. Therefore, individuals with impaired glucose metabolism were combined with individuals with normal glucose metabolism and compared with individuals with diabetes. To prevent overadjustment for peripheral arterial disease (PAD), self-reported PAD (n = 1) and nontraumatic amputation (n = 2) were excluded from the definition of prior CVD when the associations between ABI, TBI, and Doppler flow curves and mortality were adjusted for prior CVD.
For all analyses, a two-sided P value of <0.05 was considered statistically significant, except for the interaction terms, for which a two-sided P value of <0.10 was considered statically significant. All analyses were performed with SPSS (version 17.0).
RESULTS
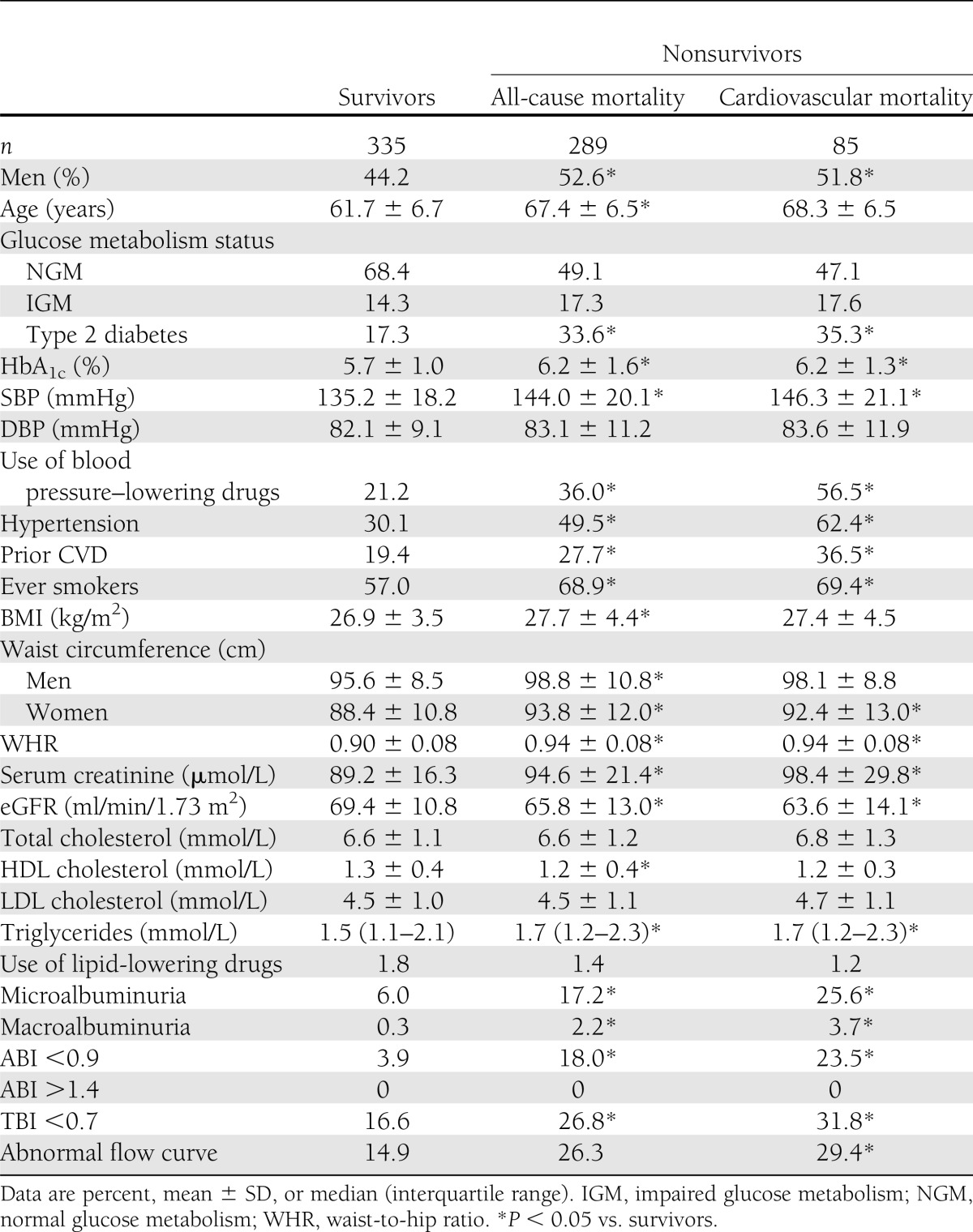
Median duration of follow-up was 17.2 years (range 0.5–19.2). During the follow-up period, 289 of 624 (46.3%) participants died (97 of 155 with and 192 of 469 without diabetes and 52 of 65 with and 237 of 559 without ABI <0.9): 85 (29.4%) of CVD (30 of 155 with and 55 of 469 without diabetes and 20 of 65 with and 65 of 559 without ABI <0.9). At baseline, individuals who died, compared with those who survived, more often had an ABI of <0.9, a TBI of <0.7, and abnormal Doppler flow curves. In addition, male sex, type 2 diabetes, hypertension, lower eGFR, (micro)albuminuria, lower HDL cholesterol, and prior CVD occurred more frequently, and age, waist circumference, and HbA1c were higher in the nonsurvivors compared with the survivors (Table 1). Smoking, higher age, higher SBP, lower HDL cholesterol, and prior CVD were independently associated with the ABI (data not shown). An ABI of <0.9 occurred more often in individuals with diabetes (14.8%) than in nondiabetic individuals (9.0%). In this cohort, an ABI of >1.4 did not occur.
Table 1.
Baseline characteristics according to survival status after a median follow-up of 17.2 years (n = 624)
ABI and cardiovascular and all-cause mortality
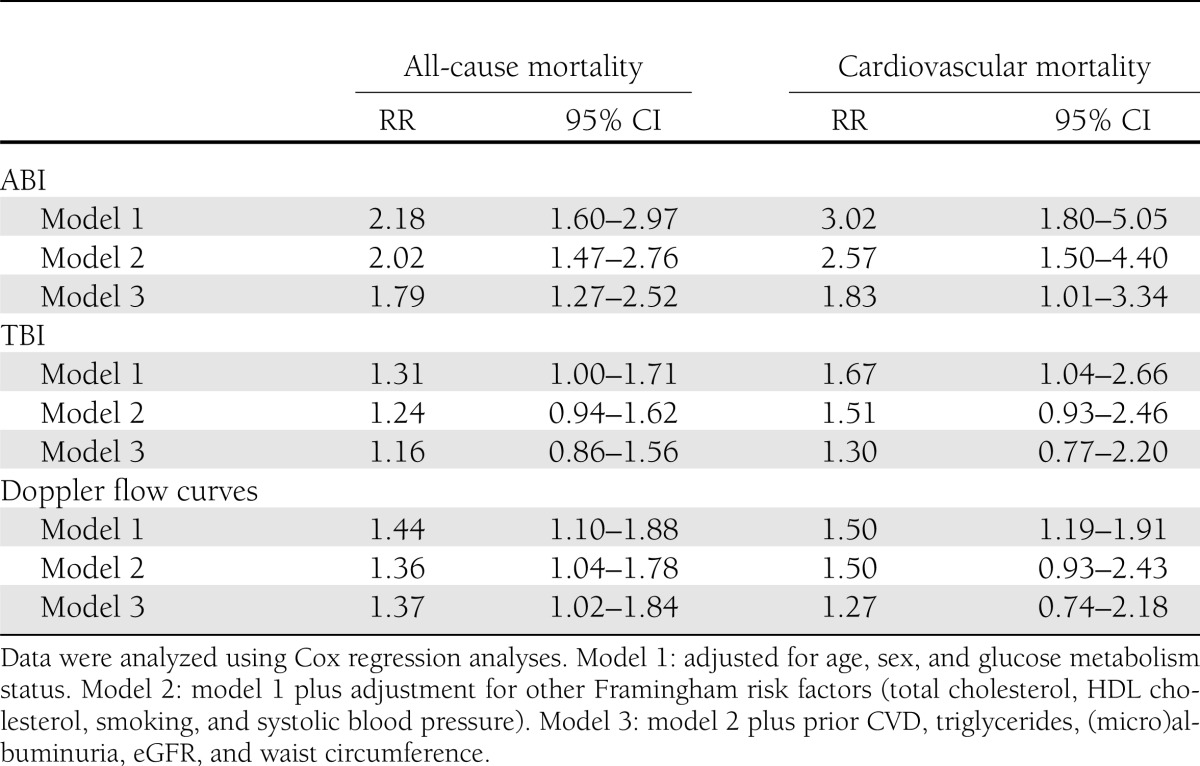
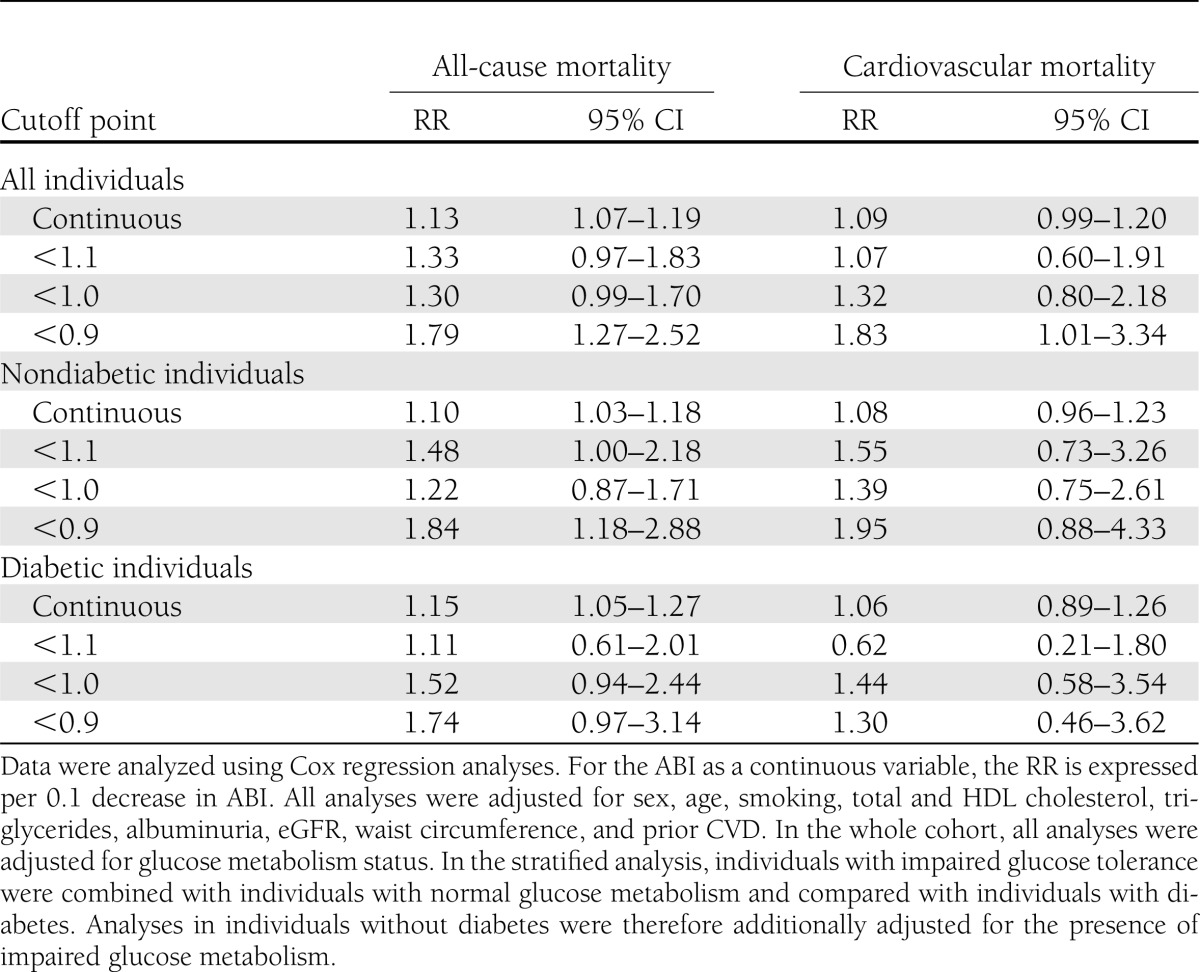
A low ABI (<0.9) was strongly associated with cardiovascular mortality (RR 2.57 [95% CI 1.50–4.40]) and all-cause mortality (2.02 [1.47–2.76]), independently of the Framingham risk factors (Table 2, model 2). Additional adjustment for (micro)albuminuria, eGFR, triglycerides, and prior CVD attenuated the association with cardiovascular mortality (1.83 [1.01–3.34]) and somewhat with all-cause mortality (1.79 [1.27–2.52]) (Table 2, model 3). The associations of the ABI (<0.9) with mortality did not differ between individuals without and with diabetes for cardiovascular (Pinteraction = 0.45) or all-cause (Pinteraction = 0.63) mortality. When <1.1 was selected as a cutoff point for the ABI, the associations of the ABI with cardiovascular mortality (Pinteraction = 0.04) and all-cause mortality (Pinteraction = 0.14) were weaker in individuals with diabetes than in individuals without diabetes (Table 3). This was not the case when <1.0 was selected as a cutoff point for the ABI (both Pinteraction ≥ 0.43) or when the ABI was analyzed as a continuous variable (both Pinteraction ≥ 0.54) (Table 3).
Table 2.
Associations of ABI, TBI, or abnormal Doppler flow curves with cardiovascular and all-cause mortality
Table 3.
Associations of the ABI at different cutoff points and as a continuous variable with all-cause and cardiovascular mortality
Alternative methods to the ABI and their associations with cardiovascular and all-cause mortality
A low TBI was positively associated with cardiovascular mortality (RR 1.30 [95% CI 0.77–2.20]) and with all-cause mortality (1.16 [0.86–1.56]). Abnormal Doppler flow curves were also positively associated with cardiovascular mortality (1.27 [0.74–2.18]) and with all-cause mortality (1.37 [1.02–1.84]). However, compared with the ABI, the TBI and Doppler flow curves were not more strongly associated with all-cause and cardiovascular mortality than the ABI (Table 2). This was still the case if we stratified the above associations for the presence of diabetes (data not shown).
CONCLUSIONS
This study had two main findings. Firstly, a low ABI was independently associated with cardiovascular and all-cause mortality in individuals without and with diabetes. Secondly, in individuals with diabetes, the TBI and Doppler flow curves were not superior to the ABI, i.e., were not more strongly related to mortality than the ABI.
In the MERITO II study (9), the association between a low ABI and cardiovascular mortality was significantly weaker in individuals with diabetes than in individuals without diabetes. However, that study had a follow-up duration of only 1 year, which may explain the difference from the current study. On the other hand, in the Prevalence of Peripheral Arterial Disease in Patients with Acute Coronary Syndrome (PAMISCA) study, in individuals with acute myocardial infarction, the association between PAD and all-cause mortality at 1-year follow-up was not weaker in individuals without compared with those with diabetes (10). Although in this study, individuals with a low ABI and symptomatic PAD (such as revascularization procedures or ischemic amputations) were analyzed as a single group, it is unlikely that this changed the associations between ABI and mortality in this study, as of the 421 individuals with PAD, only 30 had such symptomatic disease. Also in line with our findings, in the Strong Heart Study and the “Men born in 1914” study, the association between a low ABI and mortality was similar in individuals without and with diabetes at longer follow-up times (11,12). Taken together, these studies support our finding that the association between the ABI and mortality does not differ between individuals without and with diabetes, especially at long-term follow-up.
Although the TBI and Doppler flow curves have been advocated as alternatives to the ABI in individuals with an elevated (>1.4) ABI (7) and although the TBI has been shown to predict mortality in individuals with an elevated ABI (13), we show here that in a population without elevated ABI values the TBI and Doppler flow curves are not more strongly associated with mortality than the ABI, both in individuals without and with diabetes. In addition, and in line with the “Men born in 1914” study (11), we show that individuals with elevated ABI values are uncommon in the general Caucasian population. Thus, the current study suggests that both the TBI and Doppler flow curves are not more strongly associated with mortality than the ABI in individuals with diabetes and an ABI in the nonelevated range, but additional studies are needed to confirm this.
This study was performed in a middle-aged to elderly Caucasian population, and thus its results may not apply to other populations. In addition, due to the trend of increasing obesity in the Western world during the follow-up of our study, it may be argued that the participants in our study are perhaps at baseline less representative in terms of BMI of the patients with diabetes currently seen in clinical practice. However, there is no biological reason why this should affect the association between ABI and cardiovascular outcomes. In addition, our study lacked power to perform analyses to investigate whether the ABI improves risk prediction of mortality. The advantage of our study over the MERITO II and PAMISCA studies is its longer follow-up time (9,10). Compared with the Strong Heart Study, our study adds data on the performance of the ABI in an older Caucasian population and, to the “Men born in 1914” study, the inclusion of women and a larger variance in age categories.
Taken together, our data show that the ABI is a suitable tool to investigate the association between systemic atherosclerosis and mortality in individuals with diabetes, as it is in individuals without diabetes (2). This study underlines that, at its conventional cutoff point of <0.9, the association between ABI and all-cause and cardiovascular mortality does not differ between individuals without and with diabetes. We did find an interaction between the ABI and diabetes at a cutoff point of 1.1. Although this finding may be due to chance, as we did not find an interaction between the ABI and diabetes when we analyzed the ABI at lower cutoff points (<1.0 and <0.9) or as a continuous variable, this finding may indicate that arterial stiffening and calcification in diabetes may elevate the ABI only in the normal to high range (>1.0) but not in the lower ranges (0 to 1.0) and is not of clinical importance at the regular cutoff point of the ABI (<0.9). In addition, alternatives to the ABI are not more strongly associated with mortality than is the ABI in individuals with diabetes.
The ABI may be an important tool to improve risk prediction. In line with this, the American College of Cardiology Foundation/American Heart Association 2010 guideline states that measurement of the ABI in individuals at intermediate risk is reasonable to improve cardiovascular risk prediction (14). In addition, the American Diabetes Association recommends measurement of the ABI in individuals with diabetes to help prevent complications of PAD and identify patients at high cardiovascular risk (15). However, there are currently no studies that investigated whether measurement of the ABI is actually effective in motivating patients to comply with measures to reduce cardiovascular risk or whether serial measurement of the ABI can be used to monitor or guide treatment approaches (14), and future studies should investigate this.
Acknowledgments
This research was performed within the framework of the Center for Translational Molecular Medicine, project Biomarkers for the Prediction and Early Diagnosis of Diabetes and Diabetes-related Cardiovascular Complications (PREDICCt) (grant 01C-104), and supported by the Dutch Heart Foundation, Dutch Diabetes Research Foundation, and Dutch Kidney Foundation.
The funders did not have any role in the design, conduct, analysis, or write-up of the research reported.
No potential conflicts of interest relevant to this article were reported.
N.M.J.H. analyzed data and wrote the manuscript. M.S.H. designed the research and edited the manuscript. C.G.S. edited the manuscript. G.N. and J.M.D. designed the research and edited the manuscript. C.D.A.S. analyzed data, designed the research, and wrote the manuscript. C.D.A.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the American Heart Association Scientific Sessions, Orlando, Florida, 4–7 November 2007.
References
- 1.Myerburg RJ, Kessler KM, Castellanos A. Sudden cardiac death: epidemiology, transient risk, and intervention assessment. Ann Intern Med 1993;119:1187–1197 [DOI] [PubMed] [Google Scholar]
- 2.Fowkes FG, Murray GD, Butcher I, et al. Ankle Brachial Index Collaboration Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA 2008;300:197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young MJ, Adams JE, Anderson GF, Boulton AJ, Cavanagh PR. Medial arterial calcification in the feet of diabetic patients and matched non-diabetic control subjects. Diabetologia 1993;36:615–621 [DOI] [PubMed] [Google Scholar]
- 4.Aboyans V, Criqui MH, Denenberg JO, Knoke JD, Ridker PM, Fronek A. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation 2006;113:2623–2629 [DOI] [PubMed] [Google Scholar]
- 5.Beks PJ, Mackaay AJ, de Neeling JN, de Vries H, Bouter LM, Heine RJ. Peripheral arterial disease in relation to glycaemic level in an elderly Caucasian population: the Hoorn study. Diabetologia 1995;38:86–96 [DOI] [PubMed] [Google Scholar]
- 6.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 7.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007;45(Suppl. S):S5–S67 [DOI] [PubMed]
- 8.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 9.Mostaza JM, Manzano L, Suarez C, et al. Different prognostic value of silent peripheral artery disease in type 2 diabetic and non-diabetic subjects with stable cardiovascular disease. Atherosclerosis 2011;214:191–195 [DOI] [PubMed] [Google Scholar]
- 10.Quiles J, Morillas P, Bertomeu V, et al. Prevalence of Peripheral Arterial Disease in Patients with Acute Coronary Syndrome (PAMISCA) Investigators Combination of ankle brachial index and diabetes mellitus to predict cardiovascular events and mortality after an acute coronary syndrome. Int J Cardiol 2011;151:84–88 [DOI] [PubMed] [Google Scholar]
- 11.Ogren M, Hedblad B, Engström G, Janzon L. Prevalence and prognostic significance of asymptomatic peripheral arterial disease in 68-year-old men with diabetes. Results from the population study ‘Men born in 1914’ from Malmö, Sweden. Eur J Vasc Endovasc Surg 2005;29:182–189 [DOI] [PubMed] [Google Scholar]
- 12.Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation 2004;109:733–739 [DOI] [PubMed] [Google Scholar]
- 13.Suominen V, Uurto I, Saarinen J, Venermo M, Salenius J. PAD as a risk factor for mortality among patients with elevated ABI—a clinical study. Eur J Vasc Endovasc Surg 2010;39:316–322 [DOI] [PubMed] [Google Scholar]
- 14.Greenland P, Alpert JS, Beller GA, et al. American College of Cardiology Foundation. American Heart Association 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010;56:e50–e103 [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association Peripheral arterial disease in people with diabetes. Diabetes Care 2003;26:3333–3341 [DOI] [PubMed] [Google Scholar]