Abstract
OBJECTIVE
Metabolic activation of the innate immune system governed by interleukin (IL)-1β contributes to β-cell failure in type 2 diabetes. Gevokizumab is a novel, human-engineered monoclonal anti–IL-1β antibody. We evaluated the safety and biological activity of gevokizumab in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
In a placebo-controlled, dose-escalation study, a total of 98 patients were randomly assigned to placebo (17 subjects) or gevokizumab (81 subjects) at increasing doses and dosing schedules. The primary objective of the study was to evaluate the safety profile of gevokizumab in type 2 diabetes. The secondary objectives were to assess pharmacokinetics for different dose levels, routes of administration, and regimens and to assess biological activity.
RESULTS
The study drug was well tolerated with no serious adverse events. There was one hypoglycemic event whereupon concomitant insulin treatment had to be reduced. Clearance of gevokizumab was consistent with that for a human IgG2, with a half-life of 22 days. In the combined intermediate-dose group (single doses of 0.03 and 0.1 mg/kg), the mean placebo-corrected decrease in glycated hemoglobin was 0.11, 0.44, and 0.85% after 1, 2 (P = 0.017), and 3 (P = 0.049) months, respectively, along with enhanced C-peptide secretion, increased insulin sensitivity, and a reduction in C-reactive protein and spontaneous and inducible cytokines.
CONCLUSIONS
This novel IL-1β–neutralizing antibody improved glycemia, possibly via restored insulin production and action, and reduced inflammation in patients with type 2 diabetes. This therapeutic agent may be able to be used on a once-every-month or longer schedule.
Onset of type 2 diabetes occurs when pancreatic β-cells fail to adapt to the increased insulin demand caused by insulin resistance. Morphological and therapeutic intervention studies have uncovered an inflammatory process in islets of patients with type 2 diabetes characterized by the presence of cytokines, immune cells, β-cell apoptosis, amyloid deposits, and fibrosis. This is attributed to a pathological activation of the innate immune system by metabolic stress and is governed by interleukin (IL)-1 signaling (1–4).
Based on the above-described islet inflammation with a predominant role for IL-1β, we initiated clinical trials of IL-1 antagonism in type 2 diabetes. In a proof-of-concept study, the naturally occurring antagonist of IL-1β, IL-1 receptor agonist (IL-1Ra), was tested in a placebo-controlled study of 70 patients (5). At 13 weeks of treatment, glycated hemoglobin was significantly improved as a result of enhanced β-cell secretory function. Of interest, some improvement promoted by IL-1β blockade lasted for at least 39 weeks following treatment withdrawal (6). More recently, IL-1Ra also was shown to improve insulin secretion in prediabetic patients (7). This indicates the disease-modifying potential of this therapy (8).
Although the results obtained with IL-1Ra in patients with type 2 diabetes are encouraging, IL-1Ra has characteristics that make it unsuitable for the treatment of type 2 diabetes, such as its short half-life and injection-site reactions (9). It would have to be taken on a daily basis to maintain adequate suppression of IL-1β. Moreover, the amount of IL-1Ra required would make treatment an expensive option. Of importance, an anti–IL-1 strategy that spares IL-1α may offer safety benefits. Therefore, specifically reducing IL-1β activity with longer-lasting agents would provide a therapeutic improvement in terms of sustained inhibition of IL-1β–mediated islet-associated inflammation.
Gevokizumab is a recombinant human-engineered monoclonal antibody that binds and neutralizes human IL-1β with a Kd of 0.3 pmol/L (10). The predicted circulating half-life of the antibody was 22–25 days. The Fc of gevokizumab is the IgG2 isotype, which unlike IgG1 Fc does not activate the FcRIII on macrophages and other myeloid cells, thereby reducing the probability of antibody-dependent cell-mediated cytotoxicity. In this study, we evaluated the safety, pharmacokinetics, and diabetes- and inflammation-related parameters of gevokizumab in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
This first-in-humans, randomized, placebo-controlled, dose-escalation study was carried out concurrently in the U.S. and Switzerland. The U.S. arm of the study included 68 subjects (56 in the gevokizumab group and 12 in the placebo group) and took place between September 2007 and June 2009. The Swiss arm of the study included 30 subjects (25 in the gevokizumab group and 5 in the placebo group) and took place between November 2007 and July 2009. XOMA (U.S.), the manufacturer of gevokizumab, sponsored the study.
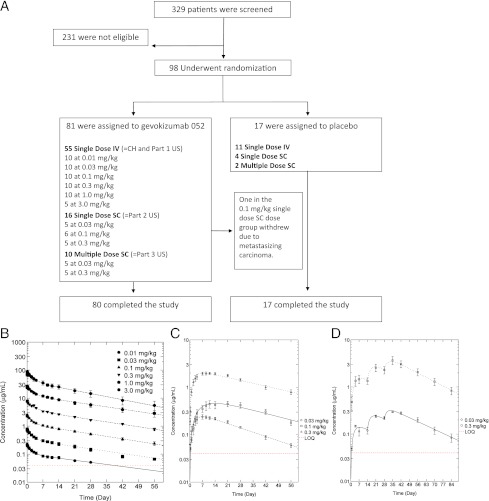
The eligibility criteria of both arms of the study were similar. Patients were requested to continue stable doses of baseline antidiabetes treatments (metformin, sulfonylureas, and/or insulin) and were asked not to change their diet and exercise habits during the study. Both arms of the study tested single intravenous doses of gevokizumab at the same five-dose levels (0.01, 0.03, 0.1, 0.3, and 1.0 mg/kg). In addition, there was a three-part study in the U.S. arm that tested a single intravenous dose of 3.0 mg/kg (part 1), a single subcutaneous dosing at three dose levels (part 2), and multiple subcutaneous dosing at two dose levels (part 3) (Fig. 1A). The follow-up of patients was 2 months in the U.S. arm and 3 months in the Swiss arm. Randomization was performed with the use of a common, central, computer-generated system with permutated blocks of six schedules.
Figure 1.
Enrollment, outcomes, and pharmacokinetics. A: A total of 329 patients with type 2 diabetes underwent physical and biochemical screening; 231 were found to be ineligible according to entry criteria. Ninety-eight patients were randomly assigned to receive either gevokizumab or placebo. In the gevokizumab group, one patient was withdrawn after <1 week. A total of 98 patients completed the 13-week treatment and evaluation of study end points. B, C, and D: Plasma concentrations following a single intravenous (IV) (n = 55) (B), subcutaneous (SC) (n = 16) (C), or multiple subcutaneous (n = 10) (D) doses of gevokizumab. (A high-quality color representation of this figure is available in the online issue.)
Throughout the course of the study, treatment assignment was blinded to study subjects and investigators but not to the sponsor (XOMA). The sponsor maintained blinding in all communications with investigators and other site personnel. The study was approved by investigational regulations committees in Switzerland and the U.S. Written informed consent was obtained from each patient prior to randomization.
Inclusion and exclusion criteria
The inclusion criteria were type 2 diabetes diagnosed according to the American Diabetes Association (11), with a duration of >3 months (>6 months for the U.S. arm); age ≥18 and ≤70 years; a glycated hemoglobin level at screening ≥7.5 and ≤12% (≤12.5% for the U.S. arm); BMI ≥23 and ≤40 kg/m2; stable disease, defined as no recent change in type 2 diabetes medications; and the patient’s agreement not to change his or her diet and exercise during the study.
The exclusion criteria were use of anti-inflammatory therapy other than aspirin (≤100 mg/day); use of immunosuppressive therapy; use of β2 and nonselective adrenergic blockers; use of thiazolidinediones, glucagon-like peptide agonists, or dipeptidyl peptidase-4 inhibitors; fasting C-peptide <400 pmol/L (<1.20 µg/L); hemoglobin <8.0 g/dL; white blood cells (WBCs) <3.0 × 103/mm3; platelet count <125 × 103/mm3; creatinine >1.5 mg/dL; aspartate aminotransferase/alanine aminotransferase >2 × upper limit of normal; alkaline phosphatase >2 × upper limit of normal; positive test for GAD65 or insulinoma-associated protein 2 autoantibodies; known HIV antibody positivity, hepatitis B surface antigens, and/or hepatitis C antibodies; history or evidence of thyroid abnormalities, including active hyperthyroidism needing medication; abnormal T3, T4, thyroglobulin, or thyroid-stimulating hormone levels; infectious disease (C-reactive protein [CRP] >30 mg/L), fever, or infection requiring treatment with antibiotics within 3 weeks; history of recurrent infection or predisposition to infection; (active leg or foot ulcer); malignancy within 5 years prior to study entry other than carcinoma in situ of the cervix or adequately treated, nonmetastatic squamous or basal cell carcinoma of the skin; history of severe allergic or anaphylactic reactions to humanized or murine monoclonal antibodies; history of tuberculosis, positive purified protein derivative test, or active atopic disease requiring medication; asthma; immunodeficiency; history or symptoms of a demyelinating disease; clinically significant diabetic macular edema and/or proliferative diabetic retinopathy by history or fundoscopy; use of a therapeutic monoclonal antibody within 90 days; participation in an investigational drug or device trial within 30 days; major surgery within 28 days prior to day 0; receipt of a live (attenuated) vaccine within 3 months; and female subjects who were pregnant, planning to become pregnant during the course of the study, or who were breastfeeding.
Study end points
The primary objective of the study was to evaluate the safety of gevokizumab in type 2 diabetic patients. The safety assessments included evaluation of adverse events, clinically significant changes from baseline in vital signs, electrocardiograms, and laboratory values (hematology, chemistry, and urinalysis); infusion reactions; and immune responses to gevokizumab. The Swiss arm additionally included diabetic foot exams, thyroid physical exams, and ophthalmic exams, and the U.S. arm included assessments of forced expiratory volumes.
Secondary objectives comprised pharmacokinetics of gevokizumab, standard diabetic parameters (glycated hemoglobin, fasting glucose, and C-peptide), and inflammatory markers (CRP, WBC counts, and ex vivo cytokine production from whole-blood cultures in response to inflammatory stimulants). In the Swiss arm, oral and intravenous stimulation tests of C-peptide release were included. A glucose stimulation test was performed at baseline and at 4 and 13 weeks to assess β-cell function and insulin production/reserve. Subjects ingested 75 g glucose and were sampled for plasma glucose and C-peptide measurements at –10, 0, 30, 60, 90, and 120 min. Immediately at the end of the oral glucose tolerance test, an intravenous injection of 0.3 g glucose/kg body wt, 0.5 mg glucagon, and 4.5 g arginine was administered. Blood then was sampled at 0, 3, 6, 9, and 12 min. The patients were asked to forgo their antidiabetes medication and fast for 8–9 h prior to the test. Whole-body insulin sensitivity index was calculated as 10,000/square root of [fasting glucose (mmol/L) × fasting insulin (pmol/L) × (mean glucose {mmol/L} × mean insulin {pmol/L} during the oral glucose tolerance test)] (12).
Whole-blood cultures for ex vivo cytokine production
Heparinized blood was obtained before and on days 1, 7, and 28 after the administration of gevokizumab. At the same time, blood was sent for routine hematology. A total of 250 µL of blood were added to 1.5-mL sterile cryotubes (Fisher) containing 750 µL of RPMI (Grand Island) or RPMI with premeasured stimulants as follows: lipopolysaccharides (LPS) (Sigma), such that the final concentration was 100 ng/mL; heat-killed Staphylococcus epidermidis, such that the final concentration was 10 bacteria per WBC (assuming the average WBC is 7,500 mm3); the combination of IL-12 plus IL-18, such that the final concentration of IL-12 (R&D Systems) was 2 ng/mL and the final concentration of IL-18 was 20 ng/mL; or LPS plus muramyl dipeptide (Sigma), such that the final concentration of LPS was 10 ng/mL and the final concentration of muramyl dipeptide was 10 μg/mL.
The tubes were capped tightly and incubated at 37°C for 24 h. Thereafter, the tubes were inverted several times, 100 µL of 5% Triton X (Sigma) were added, and, after thorough mixing, the tubes were frozen at –20°C. After last-patient last-visit, the tubes were thawed and assayed for total cytokines. Cytokines assayed were IL-1α, tumor necrosis factor (TNF) α, interferon (IFN) γ, IL-6, and IL-1Ra using electrochemiluminesence (BioVeris, Gaithersburg, MD). The electrochemiluminesence assay for cytokines has been described previously (13). The coefficient of variance for each cytokine assay is <20%.
Cytokine levels in picograms per milliliter of the lysed blood culture were converted to percentage change in cytokine levels per million WBCs based on the WBCs at the time of the blood draw for each time point. The data then were analyzed by setting the cytokine concentration per million WBCs before the infusion of gevokizumab at 100% for each cytokine. For each time point (days 1, 7, and 28) following the infusion, the percentage change from the preinfusion level was calculated for each subject. The mean percent change (±SEM) for each cytokine and each dose group or placebo was then calculated for each time point.
Statistical analyses
Safety and biological activity analyses included all subjects who received any amount of study drug (intent-to-treat analysis). No interim analyses were carried out in the study. Values are expressed as means ± SEM. Because of the small sample size, as a result of the dose-escalation design of the study, patients with the single intravenous or subcutaneous injection of gevokizumab were combined to placebo/low-, intermediate-, and high-dose groups, as follows, in order to allow statistical testing. Patients with placebo and the extremely low dose of 0.01 mg/kg were combined as the placebo/low-dose group; patients with 0.03 and 0.1 mg/kg as the intermediate-dose group; and patients with 0.3, 1.0, and 3.0 mg/kg as the high-dose group. Statistical differences were tested with the use of a Wilcoxon rank sum test. A P value of <0.05 was considered to indicate statistical significance. All reported P values are two sided.
RESULTS
Baseline characteristics and compliance
Ninety-eight of 329 patients initially screened were randomly assigned to placebo or treatment with gevokizumab (Fig. 1A). The most frequent reason for ineligibility was an HbA1c level <7.5%. Table 1 shows the baseline characteristics of patients after randomization. Most demographic and baseline characteristics were well balanced between the groups. The exceptions were median glycated hemoglobin in the single-dose intravenous subjects, which at 8.6% was slightly lower than the other groups; duration of diabetes ranking from 5.8 to 9.7 years; and differences in treatment. None of these differences were statistically significant.
Table 1.
Baseline characteristics of the patients
All subjects but one completed the study. That patient was diagnosed with metastasizing carcinoma of the appendix <1 week after receiving 0.1 mg/kg gevokizumab. The cancer was discovered after the patient disclosed a chronic recurrent pain of the upper abdomen for many months; this information was not stated by the patient at screening. The patient remained in the study for safety monitoring but did not perform the glucose tolerance tests.
The patients did not change their antidiabetes medications throughout the study, as reflected by recorded types and doses of antidiabetes medication, expect for one patient who developed a hypoglycemic event 7 days after receiving 0.1 mg/kg gevokizumab. In this case, insulin dose was reduced by 4 units.
Primary end point
Safety.
Gevokizumab administered over two logarithmic dose intervals was safe and well tolerated. There were two serious adverse events, including a carcinoma of the appendix (see above) and a carotid artery occlusion, which occurred 44 days after the last injection of the study drug at a dose of 0.03 mg/kg. Both adverse events were not judged as related to the study drug. One patient experienced hypoglycemia. He also was treated with insulin and remained asymptomatic after reduction of the insulin dose.
Secondary end points
Pharmacokinetics.
The pharmacokinetics of intravenous gevokizumab demonstrated a dose-proportional biexponential decline, with a mean β half-life of 22.4 ± 0.9 days and a mean clearance of 2.5 ± 0.1 mL/day/kg (Fig. 1B). The concentration-time profile for the single subcutaneous dose was characterized by an absorption phase followed by a one-compartment elimination phase, with a mean elimination half-life of 23.9 ± 0.9 days and a mean clearance of 3.5 ± 0.3 mL/day/kg (Fig. 1C). A supplementary population pharmacokinetic analysis determined the absorption rate constant to be 0.13 ± 0.02 mL/day/kg and the bioavailability to be 69 ± 4%. Initial and terminal half-lives, clearance, and volume of distribution were typical for a human antibody subject to a nonspecific clearance mechanism. Gevokizumab showed predictable dose linearity with maximum concentrations proportional through the observed dose range. Samples from three subjects were confirmed positive with antibody against gevokizumab. The titer levels for all three subjects were low (<250 pg/L) and did not impact on the half-life of gevokizumab.
Glycemia.
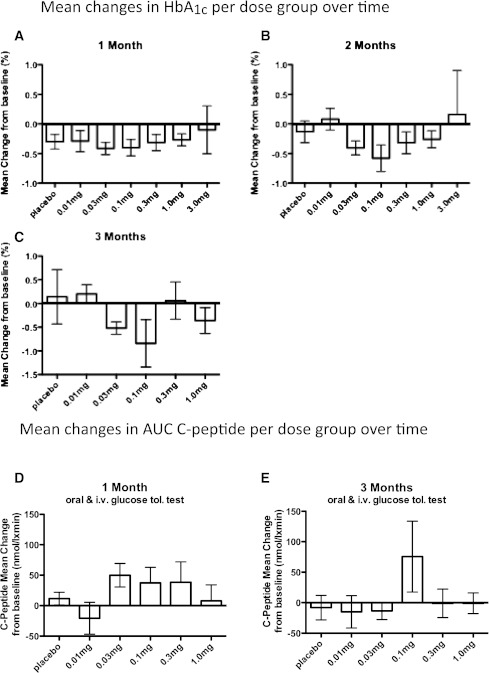
Glycated hemoglobin decreased in a dose-dependent, U-shaped manner, with the greatest decline from baseline glycated hemoglobin seen in the 0.1 mg/kg dose group at all time points. After 1 month, the mean HbA1c reduction from baseline in the intermediate-dose group (0.03 and 0.1 mg/kg) was −0.41 (±0.09) and −0.30% (±0.10) in the placebo/low-dose group (placebo and 0.01 mg/kg), respectively (P = 0.46) (Fig. 2A). After 2 months, the intermediate-dose group had a mean reduction from baseline in HbA1c of −0.49% (±0.12) compared with −0.05% (±0.13) in the placebo/low-dose group, which was a statistically significant difference (P = 0.017) (Fig. 2B). This significant reduction in HbA1c levels still was present after 3 months (decrease of −0.68% [±0.25] from baseline in the intermediate-dose group compared with an increase of +0.17% [±0.29] in the placebo/low-dose group; P = 0.049) (Fig. 2C). Thus, the placebo-corrected mean glycated hemoglobin decrease was −0.11, −0.44, and −0.85% after 1, 2, and 3 months, respectively, between the intermediate-dose and placebo/low-dose groups.
Figure 2.
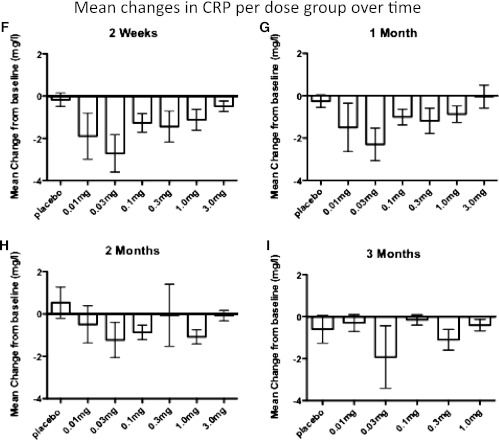
Changes in glycated hemoglobin, β-cell secretory function, and CRP during the study period. Average absolute differences in glycated hemoglobin levels between baseline and 1 (A), 2 (B), and 3 (C) months in each study group (n = 15 for placebo, n = 10 for 0.01 mg/kg, n = 15 for 0.03 mg/kg, n = 16 for 0.1 mg/kg, n = 15 for 0.3 mg/kg, n = 10 for 1.0 mg/kg, and n = 5 for 3.0 mg/kg at 1 and 2 months and n = 5 for all groups at the 3-month time point). β-Cell secretory function was assessed by a 2-h oral glucose tolerance test, followed by intravenous stimulation with 0.3 g glucose/kg body wt, 0.5 mg glucagon, and 4.5 g arginine. Average absolute differences between baseline and 1 (D) and 3 (E) months are shown (n = 5 for placebo and 0.01, 0.03, 0.1, 0.3, and 1.0 mg/kg). Average absolute differences in CRP between baseline and 2 weeks (F) and 1 (G), 2 (H), and 3 (I) months in each study group (n = 15 for placebo, n = 10 for 0.01 mg/kg, n = 15 for 0.03 mg/kg, n = 16 for 0.1 mg/kg, n = 15 for 0.3 mg/kg, n = 10 for 1.0 mg/kg, and n = 5 for 3.0 mg/kg at 2 weeks and 1 and 2 months and n = 5 for groups at the 3-month time point). The data are depicted as means ± SEM.
The decrease from baseline in mean glycated hemoglobin in the high-dose group (0.3, 1.0, and 3.0 mg/kg) did not reach statistical significance after 1, 2, or 3 months, respectively, consistent with the U-shaped dose-response curve. In addition, the small subgroup of patients (n = 5 for each 0.03 and 0.3 mg/kg) receiving three consecutive (baseline and days 14 and 28) subcutaneous injections of gevokizumab did not reach statistical significant changes in HbA1c.
β-Cell secretory function.
Along with the changes in glycated hemoglobin, mean C-peptide release in the combined oral glucose and intravenous stimulation tests was significantly increased from baseline in the intermediate-dose group after 1 month (+43.4 ± 14.8 nmol/L ⋅ min) but not in the placebo/low-dose group (−3.6 ± 13.4 nmol/L ⋅ min; P = 0.045) (Fig. 2D). After 3 months, an increase in mean C-peptide release from baseline was observed in the intermediate-dose group (+25.5 ± 28.9 nmol/L ⋅ min) compared with the placebo/low-dose group (−10.3 ± 14.9 nmol/L ⋅ min), although not reaching statistical significance (P = 0.39) (Fig. 2E).
Insulin sensitivity measures.
Whole-body insulin sensitivity index was slightly, but not significantly, increased in the intermediate-dose group after 1 month (mean increase +0.10 ± 0.12 compared with baseline; P = 0.2). At the same time, there was a decrease in the high-dose and placebo/low-dose groups (−0.33 ± 0.29 and −0.16 ± 0.14 decrease compared with baseline, respectively).
Systemic and leukocyte inflammation.
In parallel to HbA1c values, levels of CRP were lowered in a dose-dependent, U-shaped fashion (Fig. 2F–I), with the most pronounced decreases at the earlier time points. When dose groups were combined as described above and CRP levels >20 mg/dL were excluded from the analysis because they suggested ongoing infection, there was a statistically different change in CRP at day 14 in the intermediate-dose group compared with baseline (P = 0.0063), with strong trends after 1 and 2 months (P = 0.08 and 0.05, respectively). This effect was less pronounced in the high-dose group, reflecting the same dose effect as for HbA1c and area under the curve of C-peptide. As expected, neutrophil and platelet counts were slightly decreased by gevokizumab but remained within the normal range (data not shown).
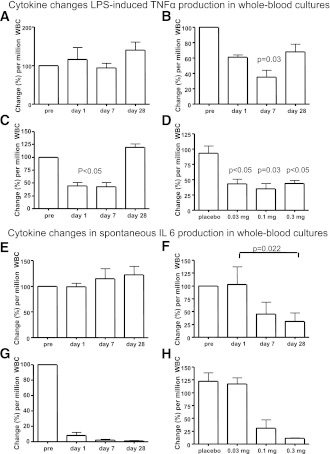
The anti-inflammatory effect of gevokizumab on ex vivo cytokine production also was tested in whole-blood cultures in patients from the Swiss arm of the study. Gevokizumab decreased total TNFα production induced by LPS (Fig. 3A–D). Moreover, spontaneous production of IL-6 from peripheral blood cells of patients treated with gevokizumab was time dependently decreased, most potently at 0.3 mg/kg on day 28 (Fig. 3E–H). In contrast, the anti-inflammatory cytokines IL-1Ra as well as IFNγ remained unaffected by the treatment (not shown).
Figure 3.
Cytokine changes in LPS-induced TNFα and spontaneous IL-6 production in whole-blood cultures. A: LPS-induced TNFα in patients receiving placebo (n = 5). B: LPS-induced TNFα in patients receiving 0.1 mg/kg gevokizumab (n = 5). C: LPS-induced TNFα in patients receiving 0.03 mg/kg gevokizumab (n = 5). D: Dose-response of gevokizumab on LPS-induced TNFα on day 7 in patients treated with gevokizumab. E: Spontaneous production of IL-6 in patients receiving placebo (n = 5). F: Spontaneous production of IL-6 in patients receiving 0.1 mg/kg gevokizumab (n = 5). G: Spontaneous production of IL-6 in patients receiving 0.3 mg/kg gevokizumab (n = 5). H: Dose response of gevokizumab on spontaneous production of IL-6 on day 28. The data are expressed as mean (±SEM) percentage change on days 1, 7, and 28 from baseline levels per million WBCs before the infusion of either placebo or gevokizumab. Details on the calculations are described in research design and methods.
CONCLUSIONS
This single- and multiple-dose study with the anti–IL-1β antibody, gevokizumab, provides preliminary data for a disease-modifying approach to type 2 diabetes by targeting the master inflammatory cytokine impairing the insulin-producing β-cells. Of note, because the IL-1 receptor antagonist used in an earlier trial in type 2 diabetes (5,6) blocks receptor binding and signaling in response to both IL-1α and -β, this is the first study to show that specific neutralization of IL-1β yields similar effects. If confirmed, this observation provides evidence that IL-1β antagonism alone may be sufficient to reduce β-cell failure, allowing for a therapy that spares the action of IL-1α, providing a safety advantage over IL-1 receptor blockade.
No evidence of safety or pharmacokinetic issues for gevokizumab was identified. The effects observed on glycemia, C-peptide secretion, and CRP support previous studies using IL-1Ra (5–7) and are in line with more recent studies using other neutralizing antibodies to IL-1β (14,15).
We compared ex vivo cytokine production from cultured whole blood from patients treated with either gevokizumab or placebo and observed a consistent reduction in the total TNFα as well as IL-6 synthesized at all doses of gevokizumab. Although such a reduction was anticipated since IL-1β can induce TNFα and IL-6, the greatest reduction in IL-6, but not of TNFα, was observed 28 days after the infusion of gevokizumab, and IL-6 was not reduced at the dose of gevokizumab most potently reducing CRP, suggesting a reprogramming effect of 28 days of IL-1β inhibition in the myeloid compartment of the bone marrow independent of effects on systemic inflammation. However, the production of the anti-inflammatory IL-1Ra was unaffected by the same doses of gevokizumab that reduced CRP and glycated hemoglobin. Of importance, the production of IFNγ induced by the combination of IL-12 plus IL-18 was unchanged in gevokizumab-treated patients (not shown), in accordance with other studies of gevokizumab measuring the production of IFNγ (16) as well as in mice deficient in IL-1β. IFNγ is a key cytokine in protecting the host from opportunistic infections (17,18). In over 100,000 patients with rheumatoid arthritis treated with anakinra, there are no reports of increased opportunistic infection, which is in sharp contrast to patients treated with TNFα-blocking therapies (18). These data suggest that the IFNγ-sparing effects of blocking IL-1β is associated with an advantageous risk profile of gevokizumab and other IL-1 antagonists with regard to opportunistic infections.
The most beneficial and consistent effects on glycemia, C-peptide secretion, and inflammatory parameters appeared at intermediate doses of gevokizumab. Unexpectedly, with increasing doses, a U-shaped dose-response relationship was observed. Although no toxic effects were observed at the higher concentrations of the anti–IL-1β antibody, a decrease in efficacy was apparent. Assuming that this preliminary observation is confirmed, one explanation may be the physiologic role of inflammation. Indeed, very low concentrations of IL-1β promote insulin secretion and β-cell proliferation (19). At higher doses, the potency of gevokizumab in neutralizing endogenous IL-1β may deprive the β-cell of these physiologic levels of IL-1β. Likewise, excessive IL-1β plays a pathological role in many inflammatory diseases (9), but low levels serve host defense, promote healing, and enhance growth factor production. Furthermore, IL-1β governs a large panel of cytokines and chemokines, some having beneficial effects under specific conditions: IL-1β causes recruitment of macrophages to islets, which, depending on the level of activation, may be beneficial or deleterious to the β-cell secretory function (20,21). Furthermore, IL-6, which also is regulated by IL-1β, promotes β-cell function via glucagon-like peptide-1 production (22). We conclude, therefore, that an appropriate dose and duration of gevokizumab treatment will be crucial in order to reshape the immune system in patients with type 2 diabetes. In particular, the very high affinity of gevokizumab with responses observable at concentrations as low as femtomolar concentrations offers obvious pharmacological advantages, such as dosage volume, but its use should be limited at high doses.
A growing number of animal studies have demonstrated a role for IL-1β in insulin resistance (23–26). In humans, studies hint at a role for IL-1β in insulin resistance (23,27), and a study in patients with prediabetes treated with anakinra pointed to improvement in insulin sensitivity; however, only in patients without injection site reactions (7). In the current study, a tendency toward such effects also was observed. Because of differences in level and type of inflammation, and blood flow between islets and insulin-sensitive tissues, other doses and duration of treatment may be necessary to uncover and make use of these effects. In addition, patients were not subjected to hyperinsulinemic-euglycemic clamps, which may have uncovered such effects.
The improvement of HbA1c could be attributed to enhanced insulin secretion or action. On the one hand, the improvement of HbA1c did not show a clear correlation with the increase of C-peptide secretion. On the other hand, the improvement in insulin sensitivity failed to reach statistical significance. However, the small sample size of our study does not allow for a definitive conclusion on the underlying mechanism of improvement of glycemic parameters.
Levels of CRP were consistently decreased by gevokizumab in the current study and consistent with other IL-1 blocking agents (9). A reduction in CRP often is interpreted as beneficial and associated with less cardiovascular risk in type 2 diabetes. Accordingly, a large phase 3 study using an anti–IL-1β antibody in 17,000 patients recently has been launched (28). This should allow uncovering the full potential of IL-1β antagonism in the treatment of cardiovascular disease and diabetes.
Supplementary Material
Acknowledgments
This work was supported by the Swiss National Science Foundation, National Institutes of Health Grant AI-15614 (to C.A.D.), the Novo Nordisk Foundation (to T.M.-P.), and XOMA.
M.A.S. and H.Z. are employees and stockholders of XOMA. M.Y.D. has a patent for the use of IL-1 antagonism in type 2 diabetes. No other potential conflicts of interest relevant to this article were reported.
C.C.-W., T.M.-P., C.A.D., and M.Y.D. designed the study. C.C.-W., A.B.-B., C.K., M.A.S., and C.A.D. performed the experiments and researched data. C.C.-W., A.M.S., T.M.-P., C.A.D., and M.Y.D. analyzed data, contributed to the discussion, and wrote, reviewed, and edited the manuscript. M.Y.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Patrizia Zala (University Hospital Basel) for her help in performing the study and to Leonid Reznikov and Tania Azam (University of Colorado Denver) for assistance in the whole-blood assay.
Footnotes
A slide set summarizing this article is available online.
Clinical trial reg. no. NCT00541983, clinicaltrials.gov.
References
- 1.Donath MY, Böni-Schnetzler M, Ellingsgaard H, Ehses JA. Islet inflammation impairs the pancreatic beta-cell in type 2 diabetes. Physiology (Bethesda) 2009;24:325–331 [DOI] [PubMed] [Google Scholar]
- 2.Donath MY, Schumann DM, Faulenbach M, Ellingsgaard H, Perren A, Ehses JA. Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care 2008;31(Suppl. 2):S161–S164 [DOI] [PubMed] [Google Scholar]
- 3.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98–107 [DOI] [PubMed] [Google Scholar]
- 4.Maedler K, Sergeev P, Ris F, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest 2002;110:851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 2007;356:1517–1526 [DOI] [PubMed] [Google Scholar]
- 6.Larsen CM, Faulenbach M, Vaag A, Ehses JA, Donath MY, Mandrup-Poulsen T. Sustained effects of interleukin-1 receptor antagonist treatment in type 2 diabetes. Diabetes Care 2009;32:1663–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Asseldonk EJ, Stienstra R, Koenen TB, Joosten LA, Netea MG, Tack CJ. Treatment with anakinra improves disposition index but not insulin sensitivity in nondiabetic subjects with the metabolic syndrome: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 2011;96:2119–2126 [DOI] [PubMed] [Google Scholar]
- 8.Donath MY, Mandrup-Poulsen T. The use of interleukin-1-receptor antagonists in the treatment of diabetes mellitus. Nat Clin Pract Endocrinol Metab 2008;4:240–241 [DOI] [PubMed] [Google Scholar]
- 9.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011;117:3720–3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roell MK, Issafras H, Bauer RJ, et al. Kinetic approach to pathway attenuation using XOMA 052, a regulatory therapeutic antibody that modulates interleukin-1beta activity. J Biol Chem 2010;285:20607–20614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2011;34(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 13.Puren AJ, Fantuzzi G, Gu Y, Su MS, Dinarello CA. Interleukin-18 (IFNgamma-inducing factor) induces IL-8 and IL-1beta via TNFalpha production from non-CD14+ human blood mononuclear cells. J Clin Invest 1998;101:711–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rissanen A, Botha J, Thuren T. IL-1β antibody (canakinumab) improves insulin secretion rates in subjects with impaired glucose tolerance (IGT) and type 2 diabetes (T2DM). Late-breaking abstract presented at the 71st Scientific Sessions of the American Diabetes Association, 24–28 June 2011, San Diego Convention Center, San Diego, California [Google Scholar]
- 15.Sloan-Lancaster J, Polzer J, Miller JW, Scherer JC, Berg JK, Landschulz WH. Safety, tolerability and efficacy of subcutaneous LY2189102, a neutralizing IL-1β antibody, in patients with type 2 diabetes. Late-breaking abstract presented at the 71st Scientific Sessions of the American Diabetes Association, 24–28 June 2011, San Diego Convention Center, San Diego, California [Google Scholar]
- 16.Gül A, Tugal-Tutkun I, Dinarello CA, et al. Interleukin-1β-regulating antibody XOMA 052 (gevokizumab) in the treatment of acute exacerbations of resistant uveitis of Behcet's disease: an open-label pilot study. Ann Rheum Dis 2012;71:563–566 [DOI] [PubMed] [Google Scholar]
- 17.Ottenhoff TH, Verreck FA, Lichtenauer-Kaligis EG, Hoeve MA, Sanal O, van Dissel JT. Genetics, cytokines and human infectious disease: lessons from weakly pathogenic mycobacteria and salmonellae. Nat Genet 2002;32:97–105 [DOI] [PubMed] [Google Scholar]
- 18.Wallis RS. Reactivation of latent tuberculosis by TNF blockade: the role of interferon gamma. J Investig Dermatol Symp Proc 2007;12:16–21 [DOI] [PubMed] [Google Scholar]
- 19.Maedler K, Schumann DM, Sauter N, et al. Low concentration of interleukin-1β induces FLICE-inhibitory protein-mediated β-cell proliferation in human pancreatic islets. Diabetes 2006;55:2713–2722 [DOI] [PubMed] [Google Scholar]
- 20.Ehses JA, Lacraz G, Giroix MH, et al. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc Natl Acad Sci USA 2009;106:13998–14003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehses JA, Perren A, Eppler E, et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 2007;56:2356–2370 [DOI] [PubMed] [Google Scholar]
- 22.Ellingsgaard H, Hauselmann I, Schuler B, et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med 2011;17:1481–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koenen TB, Stienstra R, van Tits LJ, et al. Hyperglycemia activates caspase-1 and TXNIP-mediated IL-1β transcription in human adipose tissue. Diabetes 2011;60:517–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stienstra R, Joosten LA, Koenen T, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab 2010;12:593–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 2011;17:179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wen H, Gris D, Lei Y, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 2011;12:408–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghanim H, Abuaysheh S, Sia CL, et al. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care 2009;32:2281–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J 2011;162:597–605 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.