Abstract
OBJECTIVE
HbA1c levels are higher in most ethnic groups compared with white Europeans (WEs) independent of glycemic control. This comparison has not been performed between South Asians (SAs) and WEs. We analyzed the independent effect of ethnicity on HbA1c and fasting and 2-h plasma glucose (FPG and 2hrPG, respectively) between these groups.
RESEARCH DESIGN AND METHODS
Analysis of the ADDITION-Leicester study, in which 4,688 WEs and 1,352 SAs underwent oral glucose tolerance testing, HbA1c, and other risk factor measurements.
RESULTS
Significant associations with HbA1c included ethnicity, FPG, 2hrPG, and homeostasis model assessment of β-cell function (P < 0.001); age and sex (P < 0.01); and fasting insulin and potassium (P < 0.05). After adjusting for these and other risk factors, SAs demonstrated higher HbA1c (6.22 and 6.02%, mean difference 0.20%, 0.10–0.30, P < 0.001), FPG (5.15 and 5.30 mmol/L, mean difference 0.15 mmol/L, 0.09–0.21, P < 0.001), and 2hrPG (5.82 and 6.57 mmol/L, mean difference 0.75 mmol/L, 0.59–0.92, P < 0.001) compared with WEs, respectively.
CONCLUSIONS
HbA1c, FPG, and 2hrPG levels were higher in SAs independent of factors affecting glycemic control.
Glycated hemoglobin (HbA1c) is now recommended as a diagnostic tool for detecting type 2 diabetes, alongside fasting and 2-h plasma glucose (FPG and 2hrPG, respectively), and remains the standard test for monitoring disease progression (1). Previous studies demonstrate HbA1c values are higher in some black and minority ethnic groups compared with white Caucasians independent of glycemic control or factors that differ between ethnic groups (2–5). These studies suggest HbA1c levels are higher in African Americans by 0.2–0.4%, in Hispanics by 0.1–0.3%, and in Southeast Asians by 0.2–0.3% (2–5). Because this analysis has not been performed in South Asians (people of Indian, Pakistani, and Bangladeshi origin), our aim was to evaluate the independent effect of ethnicity on glycemia among South Asians and white Europeans and to quantify the magnitude of any differences.
RESEARCH DESIGN AND METHODS
The analysis was performed using cross-sectional data from the Anglo-Danish-Dutch Study of Intensive Treatment in People with Screen Detected Diabetes in Primary Care (ADDITION)-Leicester population-based diabetes screening study. An in-depth description of study methods has been published previously (6). In brief, primary care participants aged 40 to 75 years underwent an oral glucose tolerance test (OGTT), classified using World Health Organization 1999 criteria (7), and other measurements, including HbA1c, from 2005 to 2009. HbA1c samples were measured on a Bio-Rad VARIANT II high-performance liquid chromatography instrument (Hemel Hempstead, U.K.), which is standardized to current recommendations for diagnosis of diabetes and has a coefficient of variation <2% (1). This analyzer detected hemoglobinopathies (S and C) and such results were excluded.
Statistical analysis was performed using SPSS version 18.0 (Chicago, IL). Multiple regression analysis was used to determine all significant associations of HbA1c. Insulin resistance and β-cell function were calculated using homeostasis model assessment equations (8). Ethnicity was classified using U.K. national census categories (9). ANCOVA modeling was used to calculate the mean difference of HbA1c between South Asians and white Europeans using stepwise models. Model 1 compared unadjusted HbA1c values. Model 2 adjusted HbA1c levels for age, sex, BMI, waist circumference, systolic and diastolic blood pressure, LDL and HDL cholesterol, triglycerides, creatinine, albumin-to-creatinine ratio, FPG, and 2hrPG. Model 3 included fasting insulin as well. Model 4 was similar to model 2 but excluded FPG and 2hrPG. Adjustments for multiple comparisons were made using Bonferroni corrections. P < 0.05 was considered significant.
RESULTS
There were 6,040 people (4,688 white Europeans and 1,352 South Asians) included in the analysis. The significant associations of HbA1c were ethnicity, FPG, 2hrPG, and homeostasis model assessment of β-cell function (P < 0.001); age and sex (P < 0.01); and insulin and potassium (P < 0.05), producing an adjusted R2 of 0.639.
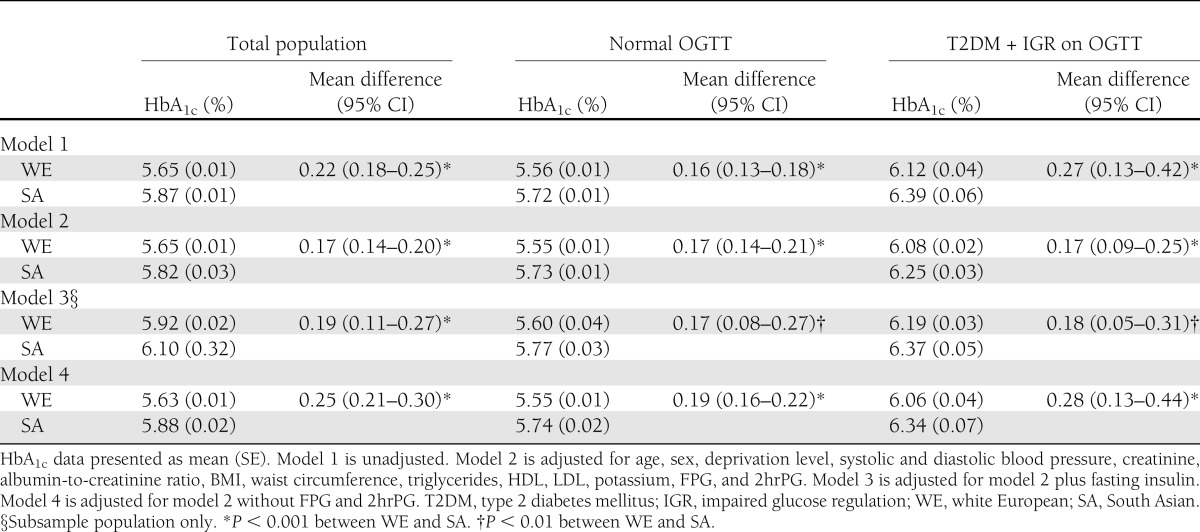
The mean (SE) crude HbA1c in white Europeans and South Asians was 5.65 (0.01) and 5.81% (0.01), respectively, producing a mean difference of 0.22% (95% CI 0.18–0.25; P < 0.001) (Table 1). After adjustment for risk factors, HbA1c remained higher in South Asians, with a mean difference of 0.19% (0.11–0.27; P < 0.001). Stratification by OGTT result demonstrated similar findings. When FPG was the dependent variable, mean crude values were 5.18 (0.01) and 5.27 mmol/L (0.03) in white Europeans and South Asians, respectively, a mean difference of 0.09 mmol/L (0.03–0.14; P < 0.01). After adjustment, these values were 5.15 (0.01) and 5.30 mmol/L (0.03), a mean difference of 0.15 mmol/L (0.09–0.21; P < 0.001) higher in South Asians. Using 2hrPG as the dependent variable, the mean crude values were 5.89 (0.08) and 6.46 mmol/L (0.07) in white Europeans and South Asians, respectively, producing a mean difference of 0.58 mmol/L (0.43–0.73; P < 0.001). After adjustment, these values were 5.82 (0.04) and 6.57 mmol/L (0.07), a mean difference higher in South Asians of 0.75 mmol/L (0.59–0.92; P < 0.001).
Table 1.
A comparison of crude and adjusted differences for HbA1c in white Europeans and South Asians
CONCLUSIONS
In this multiethnic cohort of adults undergoing an OGTT, HbA1c values were 0.2% higher in South Asians than white Europeans, even in analysis stratified by glucose intolerance status. The current study is the first to demonstrate this effect persisted after adjusting for factors that may affect glycemia or that differed between these ethnic groups. The strengths of this study include the large numbers of white Europeans and South Asians who underwent robust measurement of risk factors, allowing detection of any clinically significant differences. The diabetes risk factors included in the multiple regression analysis explained 63.9% of the variation in HbA1c, which is relatively higher than other studies (3). However, there may be other unmeasured factors that influence HbA1c. FPG and 2hrPG levels may not give a robust representation of 24-h glucose profile, a problem recognized in similar studies (3,4). Other examples include dietary intake, genetic influences, and iron deficiency anemia (10,11). Therefore, our finding that sex independently associates with HbA1c should be interpreted with caution. Studies that account for either hematocrit or hemoglobin provide contradictory reports of an independent effect of sex on HbA1c (3,4). Our results showing a higher HbA1c level of 0.2% in South Asians was consistent when separated by males and females (data not shown).
Ethnic variation in HbA1c levels could be attributed predominantly to biological variation in hemoglobin glycation and differential erythrocyte survival. However, African Americans, who also possess higher HbA1c levels than white Caucasians, have more adverse profiles of glycemic markers unaffected by hematological factors, suggesting this does not explain HbA1c differences (2).
Implications for policy makers and clinicians
First, international organizations have recommended using ethnic-specific cut points for South Asians in relation to BMI, waist circumference, and metabolic syndrome, which came as a response to high rates of diabetes within this group (12). However, there is no suggestion of ethnic-specific cut points for diagnosis of diabetes using HbA1c (1). The prevalence of diabetes using HbA1c ≥6.5% is higher in South Asians than white Europeans compared with using an OGTT, with a similar finding for detecting high-risk individuals (13,14). Second, it is reported that a greater proportion of South Asians with established diabetes do not achieve glycemic guideline targets in comparison with white Europeans (15). Because our study demonstrates independently higher HbA1c, FPG, and 2hrPG levels in South Asians, this result may be partially explained by factors related to glycemia. Future research should address the relationship between HbA1c and the onset of diabetes complications, including prevalent retinopathy, between South Asians and white Europeans in well-designed outcome studies to determine if ethnic-specific cut points are required for diabetes diagnosis in South Asians.
Acknowledgments
The ADDITION-Leicester study was funded for support and treatment costs by U.K. National Health Service (NHS) Department of Health (Clinical trial reg. no. NCT00318032). S.A.M. and D.R.W. have received fellowships from Novo Nordisk U.K. Research Foundation. M.J.D. and K.K. (Chair) are members of the National Institute for Health and Clinical Excellence Public Health Guidance on Prevention of Type 2 Diabetes Among People With Prediabetes and are advisors to the U.K. Department of Health for the NHS Health Checks Programme.
The views expressed in this publication are those of the authors and not necessarily those of the funder, who played no role in the conduct of the study, collection of data, management of the study, analysis of data, interpretation of data, or preparation of the manuscript.
M.J.D. has received funds for research and honoraria for speaking at meetings and has served on advisory boards for Eli Lilly, sanofi-aventis, Merck Sharp & Dohme, Novo Nordisk, Bristol-Myers Squibb, Boehringer Ingelheim, and Roche. K.K. has received funds for research and honoraria for speaking at meetings and/or has served on advisory boards for Astra Zeneca, Eli Lilly, Novartis, Pfizer, Servier, sanofi-aventis, Merck Sharp & Dohme, and Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
S.A.M. conceived and designed the study, had access to the databases, conducted the statistical analysis under supervision, and wrote the manuscript. M.J.D. and K.K. conceived and designed the study; obtained funding for ADDITION-Leicester and provided administrative, technical, and material support; and contributed to results interpretation and drafting of the manuscript. D.R.W. and B.T.S. contributed to results interpretation and drafting of the manuscript. L.J.G. (statistician) had access to the databases, supervised statistical analysis, and contributed to results interpretation and drafting of the manuscript. S.A.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in an oral presentation abstract at the International Diabetes Federation World Diabetes Congress, Dubai, United Arab Emirates, 4–8 December 2011.
The authors acknowledge all members of the ADDITION-Leicester team, including Steve Hiles, Joe Henson, Emer Brady, and Tom Yates (all members of the University Hospitals of Leicester Diabetes Research Team, U.K.), and the participants for their contributions. They would also like to acknowledge support from the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care—Leicestershire, Northamptonshire, and Rutland, and from the National Institute for Health Research Leicester—Loughborough Diet, Lifestyle and Physical Activity Biomedical Research Unit.
Footnotes
Clinical trial reg. no. NCT00318032, clinicaltrials.gov.
References
- 1.Executive summary: standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl. 1):S4–S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selvin E, Steffes MW, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL. Racial differences in glycemic markers: a cross-sectional analysis of community-based data. Ann Intern Med 2011;154:303–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herman WH, Ma Y, Uwaifo G, et al. Diabetes Prevention Program Research Group Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care 2007;30:2453–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziemer DC, Kolm P, Weintraub WS, et al. Glucose-independent, black-white differences in hemoglobin A1c levels: a cross-sectional analysis of 2 studies. Ann Intern Med 2010;152:770–777 [DOI] [PubMed] [Google Scholar]
- 5.Herman WH, Dungan KM, Wolffenbuttel BH, et al. Racial and ethnic differences in mean plasma glucose, hemoglobin A1c, and 1,5-anhydroglucitol in over 2000 patients with type 2 diabetes. J Clin Endocrinol Metab 2009;94:1689–1694 [DOI] [PubMed] [Google Scholar]
- 6.Webb DR, Khunti K, Srinivasan B, et al. Rationale and design of the ADDITION-Leicester study, a systematic screening programme and randomised controlled trial of multi-factorial cardiovascular risk intervention in people with type 2 diabetes mellitus detected by screening. Trials 2010;11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Geneva, World Health Organization, 1999 [Google Scholar]
- 8.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 9.U.K. Office for National Statistics. Population estimates by ethnic group: methodology paper [article online], May 2011. Available from http://www.ons.gov.uk/ons/taxonomy/index.html?nscl=Population+Estimates+by+Ethnic+Group. Accessed 18 May 2012
- 10.Kim C, Bullard KM, Herman WH, Beckles GL. Association between iron deficiency and A1C levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999-2006. Diabetes Care 2010;33:780–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paré G, Chasman DI, Parker AN, et al. Novel association of HK1 with glycated hemoglobin in a non-diabetic population: a genome-wide evaluation of 14,618 participants in the Women’s Genome Health Study. PLoS Genet 2008;4:e1000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–163 [DOI] [PubMed] [Google Scholar]
- 13.Mostafa SA, Khunti K, Srinivasan BT, Webb D, Gray LJ, Davies MJ. The potential impact and optimal cut-points of using glycated haemoglobin, HbA1c, to detect people with impaired glucose regulation in a UK multi-ethnic cohort. Diabetes Res Clin Pract 2010;90:100–108 [DOI] [PubMed] [Google Scholar]
- 14.Mostafa SA, Davies MJ, Webb D, et al. The potential impact of using glycated haemoglobin as the preferred diagnostic tool for detecting type 2 diabetes mellitus. Diabet Med 2010;27:762–769 [DOI] [PubMed] [Google Scholar]
- 15.Millett C, Netuveli G, Saxena S, Majeed A. Impact of pay for performance on ethnic disparities in intermediate outcomes for diabetes: a longitudinal study. Diabetes Care 2009;32:404–409 [DOI] [PMC free article] [PubMed] [Google Scholar]