Abstract
Bisphosphonates may prevent or treat the bone loss promoted by the immunosuppressive regimens used in renal transplantation. Risedronate is a commonly used third-generation amino-bisphosphonate, but little is known about its effects on the bone health of renal transplant recipients. We randomly assigned 42 new living-donor kidney recipients to either 35 mg of risedronate weekly or placebo for 12 months. We obtained bone biopsies at the time of renal transplant and after 12 months of protocol treatment. Treatment with risedronate did not affect bone mineral density (BMD) in the overall cohort. In subgroup analyses, it tended to preserve BMD in female participants but did not significantly affect the BMD of male participants. Risedronate did associate with increased osteoid volume and trabecular thickness in male participants, however. There was no evidence for the development of adynamic bone disease. In summary, further study is needed before the use of prophylactic bisphosphonates to attenuate bone loss can be recommended in renal transplant recipients.
Bisphosphonates are used in renal transplant recipients for prevention of bone loss. The Kidney Disease Outcomes Quality Initiative 2003 guidelines suggest that bisphosphonate treatment should be considered if bone mineral density (BMD) T scores are <−2.0 SD at the time of transplant or any other time.1 Similarly, the Kidney Disease Improving Global Outcomes 2009 guidelines suggest that renal transplant recipients with an estimated GFR (eGFR) >30 ml/min per 1.73 m2 and with low BMD be considered for therapy with vitamin D or bisphosphonates.2 However, there are significant concerns regarding their use to treat or prevent bone disease. Still unanswered are questions regarding the safety and efficacy of bisphosphonates on established osteopenia, timing of treatment postrenal transplant, and choice of parenteral versus oral formulations. We showed that parenteral pamidronate, a second-generation amino-bisphosphonate, preserved BMD but was associated with development of adynamic bone disease.3 Although there have been several prospective studies reporting the effects of parenteral4,5 and oral bisphosphonates6–9 on BMD, there are few studies in renal transplant patients using risedronate,a commonly used third-generation amino-bisphosphonate, and its effect on BMD and biochemical measures.10,11 There are no studies of serial histomorphometry in renal transplant recipients using risedronate. We therefore conducted a 12-month prospective, double-blind, placebo-controlled trial to evaluate the effects of weekly risedronate treatment in living-donor recipients on bone histomorphometry, BMD, and bone biochemical measures.
Results
Baseline Demographics
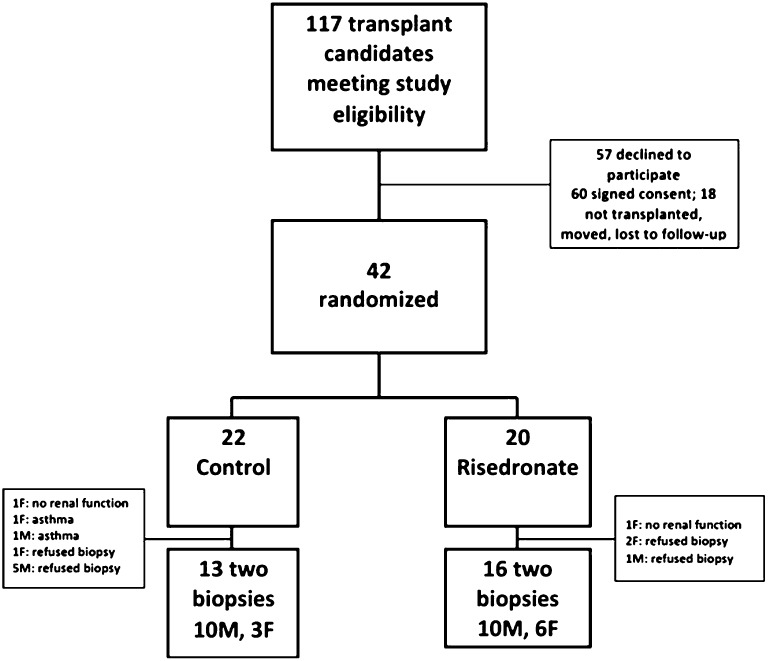
During the study period, 177 adult patients underwent living-donor transplantation; 117 patients met the eligibility criteria. There were 57 patients who refused to participate, 60 potential participants who signed consent, and 42 participants agreed to randomization, whereas the remaining 18 patients were either not transplanted at the time, moved to another center, or were lost to follow-up. There was no difference between the 42 study participants and the 18 nonparticipants in terms of age, sex, ESRD etiology, or dialysis months.
Study Group
We randomized 42 participants, who underwent baseline bone biopsy, bone densitometry, and biochemical studies. Twenty-nine participants had a second biopsy at 12 months. Participants who did not have a second bone biopsy included two participants with primary nonfunction of the allograft, seven participants who were lost to follow-up, two participants who had uncontrolled asthma with need for protracted high-dose corticosteroids, and two participants who refused to continue the protocol (Figure 1). There was no difference between those participants who had been randomized to the risedronate and control groups, in terms of age, sex distribution, ESRD etiology, or dialysis months. Controls had a higher body mass index (BMI) than the risedronate group, attributable to higher BMI in the female participants. Female controls also had a higher body surface area (BSA) (Table 1).
Figure 1.
Participant randomization. F, female; M, male.
Table 1.
Baseline demographics
| Risedronate Group (n=20) | Control Group (n=22) | P Value | |
|---|---|---|---|
| Male, n (%) | 11 (55) | 16 (72) | |
| Age (yr) | 42±11 | 48±14 | |
| men | 45.7±11 (34–69) | 47.7±15 (25–68) | 0.72 |
| women | 37.7±11 (22–54) | 50.3±9 (40–62) | 0.03 |
| BMI (kg/m2) | 24±4 | 27±5 | 0.03 |
| men | 25.1±4 | 26.7±5 | 0.38 |
| women | 22.8±3 | 30.3±3 | 0.003 |
| BSA (m2) | 1.76±0.2 | 1.94±0.2 | 0.02 |
| men | 1.90±0.2 | 1.97±0.2 | 0.39 |
| women | 1.58±0.2 | 1.84±0.2 | 0.03 |
| Race | |||
| African American | 3 | 3 | |
| Hispanic | 10 | 11 | |
| white | 5 | 6 | |
| other | 2 | 2 | |
| Dialysis (mo) | 11 (6–66) | 12 (6–35) | 0.72 |
| ESRD etiology | |||
| hypertensive nephrosclerosis | 6 | 7 | |
| diabetic nephropathy | 3 | 6 | |
| chronic GN | 10 (2 males) | 5 (4 males) | |
| adult polycystic kidney disease | 1 | 2 | |
| other | 0 | 2 | |
Continuous variable are presented as mean ± SD if normally distributed or median (interquartile range) if not normally distributed.
The study drug was started within 4–6 weeks after renal transplantation, as soon as serum creatinine (SCr) was ≤2 mg/dl. Because most of the rejection episodes were early after transplantation, and before the study drug had been started, only four participants required subsequent holding of the study drug for ≤2 weeks.
Immunosuppression
There was no difference in episodes of acute rejection or adverse events between the two groups. Although the total amount of glucocorticoids used was not different between the groups, women had a higher total glucocorticoid dose per kilogram of body weight (60.7±17 mg/kg in men versus 84.5±28 mg/kg in women; P=0.01). When analyzed in terms of BSA, the total glucocorticoid dose remained pronounced in women (2381±572 mg/m2 in men versus 3158±961 mg/m2 in women; P=0.01).
Renal Function
SCr and eGFR were similar between the study groups at 6 and 12 months of protocol. SCr levels were 1.64±0.4 versus 1.5±0.5 mg/dl at 6 months and 1.6±0.3 versus 1.3±0.4 mg/dl at 12 months in the risedronate group versus the control group, respectively; and eGFRs were 54.6±18 versus 57.7±19 ml/min per 1.73 m2 at 6 months and 54.3±15 versus 64.1±23 ml/min per 1.73 m2 at 12 months in the risedronate group versus the control group, respectively (all P>0.20). Risedronate did not affect SCr or eGFR at any time point in either male or female participants. There was no significant difference in proteinuria between the groups, as measured by urinary protein/creatinine ratio at 6 months (0.5±0.8 and 0.3±0.3, risedronate versus control, respectively) and 12 months (0.4±0.7 and 0.2±0.3, risedronate versus control, respectively; all P>0.70).
Biochemical and Hormonal Markers
Whereas the individual groups showed differences from baseline to 12 months, there was no difference in changes in calcium, phosphorus, bicarbonate, and magnesium between the arms of the study or between the sexes. Similarly, risedronate did not affect biochemical or hormonal parameters of bone turnover (Tables 2, 3 and 4). There was no difference in phosphate repletion between the two groups.
Table 2.
Bone biochemical parameters in the total cohort: risedronate versus control
| Parameter | Baseline | 6 mo | 12 mo | ∆12 mo | Baseline versus 12 mo | ∆12 mo Risedronate versus Control |
|---|---|---|---|---|---|---|
| P Value | ||||||
| Risedronate | ||||||
| calcium (8.5–10.5 mg/dl) | 9.5±0.8 | 9.7±0.5 | 9.7±0.6 | 0.19±0.6 | 0.18 | 0.21 |
| phosphorus (2.5–4.5 mg/dl) | 5.8±1.4 | 2.8±0.8 | 3.0±0.5 | −2.8±1.3 | 0.001 | 0.63 |
| bicarbonate (24–30 mEq/L) | 21.2±4 | 23.5±3.8 | 23.2±3.2 | 2.0±4.4 | 0.07 | 0.61 |
| magnesium (1.7–2.8 mg/dl) | 1.8±0.3 | 1.6±0.2 | 1.5±0.2 | −0.28±0.3 | 0.05 | 0.30 |
| PTH (10–65 pg/ml) | 445 (233–842) | 93 (56–258) | 99 (59–244) | −287 (−636 to −132) | 0.001 | 0.39 |
| vitamin D25OH (10–68 ng/ml) | 13±9 | 17±8 | 19±6 | 5±8 | 0.03 | 0.33 |
| vitamin D1,25OH (6–62 pg/ml) | 19±9 | 49±9 | 36±29 | 17±27 | 0.06 | 0.28 |
| BSAP (U/L) (ref 13–85 IU) | 68±105 | 48±45 | 59±46 | −13±111 | 0.69 | 0.70 |
| OC (ref 10.7–35 ng/ml) | 26±17 | 17±11 | 21±19 | −8±16 | 0.13 | 0.87 |
| UNTx (ref <36 nm/mM Cr) | 143±166 | 124±231 | 79±92 | −10±118 | 0.82 | 0.49 |
| Control | ||||||
| calcium (8.5–10.5 mg/dl) | 9.3±0.9 | 9.8±0.6 | 9.8±0.6 | 0.49±0.8 | 0.01 | |
| phosphorus (2.5–4.5 mg/dl) | 5.5±1.7 | 2.8±0.7 | 2.9±0.7 | −2.5±1.9 | 0.001 | |
| bicarbonate (24–30 mEq/L) | 22.0±5 | 24.1±3 | 24.8±3 | 2.8±5 | 0.02 | |
| magnesium (1.7–2.8 mg/dl) | 1.6±0.3 | 1.5±0.3 | 1.5±0.3 | −0.07±0.4 | 0.66 | |
| PTH (10–65 pg/ml) | 363 (161–577) | 105 (59–152) | 88 (38–154) | −191 (−460 to −8) | 0.002 | |
| vitamin D 25OH (10–68 ng/ml) | 13±8 | 18±9 | 19±6 | 8.3±10 | 0.01 | |
| vitamin D 1,25OH (6–62 pg/ml) | 18±11 | 46±17 | 47±28 | 29.6±31 | 0.003 | |
| BSAP (U/L) (ref 13–85 IU) | 47±46 | 48±25 | 50±32 | −0.82±38 | 0.94 | |
| OC (ref 10.7–35 ng/ml) | 37±55 | 18±13 | 20±16 | −7±22 | 0.30 | |
| UNTx (ref <36 nm/mM Cr) | 100±59 | 88±53 | 198±307 | 67±301 | 0.58 |
Values shown in the first column are normal values. Continuous variable are presented as mean ± SD if normally distributed or median (interquartile range) if not normally distributed. BSAP, bone-specific alkaline phosphatase; ref, reference value; OC, serum osteocalcin; UNTx, urinary N-telopeptide.
Table 3.
Bone biochemical parameters in male participants: risedronate versus control
| Parameter | Baseline | 6 mo | 12 mo | ∆12 mo | Baseline versus 12 mo | ∆12 mo Risedronate versus Control | ∆12 mo Men verus Women |
|---|---|---|---|---|---|---|---|
| P Value | |||||||
| Risedronate | |||||||
| calcium (8.5–10.5 mg/dl) | 9.7±0.9 | 9.9±0.4 | 9.8±0.7 | 0.09±0.6 | 0.60 | 0.43 | 0.36 |
| phosphorus (2.5–4.5 mg/dl) | 5.5±1.3 | 2.7±0.7 | 2.9±0.5 | −2.6±1.4 | 0.001 | 0.63 | 0.41 |
| bicarbonate (24–30 mEq/L) | 21.0±5 | 23.7±5 | 23.7±4 | 2.7±5 | 0.20 | 0.30 | 0.40 |
| magnesium (1.7–2.8 mg/dl) | 1.8±0.3 | 1.6±0.2 | 1.5±0.2 | −0.23±0.3 | 0.10 | 0.61 | 0.31 |
| PTH (10–65 pg/ml) | 326 (157–841) | 80 (53–238) | 93 (55–274) | −314 (−641 to −71) | 0.01 | 0.47 | |
| vitamin D 25OH (10–68 ng/ml) | 15±11 | 17±9 | 18±9 | 2.7±8 | 0.32 | 0.18 | |
| vitamin D1,25OH (6–62 pg/ml) | 18±8 | 53±33 | 41±36 | 22.3±34 | 0.15 | 0.94 | |
| BSAP (U/L) (ref 13–85 IU) | 80±124 | 52±55 | 63±50 | −18.7±135 | 0.69 | 0.65 | |
| OC (ref 10.7–35 ng/ml) | 23±16 | 15±11 | 22±24 | −7.9±16 | 0.34 | 0.93 | |
| UNTx (ref <36 nm/mM Cr) | 91±82 | 143±293 | 57±50 | −43±83 | 0.82 | 0.40 | |
| Control | |||||||
| calcium (8.5–10.5 mg/dl) | 9.4±0.9 | 9.7±0.4 | 9.8±0.4 | 0.44±0.9 | 0.08 | 0.63 | |
| phosphorus (2.5–4.5 mg/dl) | 5.3±1.8 | 2.7±0.7 | 2.8±0.7 | −2.6±2.2 | 0.003 | 0.76 | |
| bicarbonate (24–30 mEq/L) | 22.8±4 | 24.1±3 | 24.6±4 | 2.7±5 | 0.16 | 0.06 | |
| magnesium (1.7–2.8 mg/dl) | 1.7±0.3 | 1.5±0.3 | 1.4±0.3 | −0.22±0.4 | 0.28 | 0.14 | |
| PTH (10–65 pg/ml) | 363 (150–573) | 105 (59–152) | 88 (38–148) | −186 (−460 to −8) | 0.01 | ||
| vitamin D 25OH (10–68 ng/ml) | 14±8 | 20±8 | 22±7 | 8.2±10 | 0.01 | ||
| vitamin D 1,25OH (6–62 pg/ml) | 22±12 | 46±20 | 42±25 | 24±24 | 0.01 | ||
| BSAP (U/L) (ref 13–85 IU) | 44±34 | 43±23 | 45±22 | −0.07±41 | 1.00 | ||
| OC (ref 10.7–35 ng/ml) | 43±61 | 23±17 | 21±16 | −8.8±21 | 0.25 | ||
| UNTx (ref <36 nm/mM Cr) | 98±59 | 73±39 | 208±330 | 89.2±324 | 0.53 |
Values shown in the first column are normal values. Continuous variable are presented as mean ± SD if normally distributed or median (interquartile range) if not normally distributed. BSAP, bone-specific alkaline phosphatase; ref, reference value; OC, serum osteocalcin; UNTx, urinary N-telopeptide.
Table 4.
Bone biochemical parameters in female participants: risedronate versus control
| Parameter | Baseline | 6 mo | 12 mo | ∆12 mo | Baseline versus 12 mo | ∆12 mo Risedronate versus Control |
|---|---|---|---|---|---|---|
| P Value | ||||||
| Risedronate | ||||||
| calcium (8.5–10.5 mg/dl) | 9.3±0.5 | 9.6±0.7 | 9.7±0.5 | 0.35±0.6 | 0.19 | 0.34 |
| phosphorus (2.5–4.5 mg/dl) | 6.2±1.5 | 3.0±1.1 | 3.1±0.5 | −3.2±0.7 | 0.003 | 0.11 |
| bicarbonate (24–30 mEq/L) | 21.4±1.7 | 23.3±3 | 22.3±2 | 0.86±2 | 0.38 | 0.30 |
| magnesium (1.7–2.8 mg/dl) | 1.8±0.3 | 1.6±0.2 | 1.5±0.2 | −0.23±0.3 | 0.10 | 0.61 |
| PTH (10–65 pg/ml) | 477 (286–966) | 145 (79–325) | 101 (58–242) | −286 (−753 to −231) | 0.03 | 0.85 |
| vitamin D 25OH (10–68 ng/ml) | 12±7 | 14.4±7 | 21±9 | 9.6±6 | 0.02 | 0.89 |
| vitamin D1,25OH (6–62 pg/ml) | 27±25 | 37±21 | 29±11 | 9.2±7 | 0.05 | 0.43 |
| BSAP (U/L) (ref 13–85 IU) | 43±18 | 45±22 | 49±39 | 0.65±28 | 0.97 | 0.85 |
| OC (ref 10.7–35 ng/ml) | 26±16 | 22±9 | 19±9 | −8.1±17 | 0.31 | 0.65 |
| UNTx (ref <36 nm/mM Cr) | 210±200 | 103±88 | 117±142 | 33±155 | 0.70 | 0.62 |
| Control | ||||||
| calcium (8.5–10.5 mg/dl) | 9.2±0.8 | 10.2±0.8 | 9.8±1.0 | 0.66±0.2 | 0.003 | |
| phosphorus (2.5–4.5 mg/dl) | 6.0±1.4 | 3.0±0.7 | 3.2±0.6 | −2.2±0.8 | 0.01 | |
| bicarbonate (24–30 mEq/L) | 19.4±5 | 24.6±3 | 25.5±2 | 7.0±4 | 0.05 | |
| magnesium (1.7–2.8 mg/dl) | 1.7±0.3 | 1.5±0.3 | 1.4±0.3 | −0.23±0.4 | n/a | |
| PTH (10–65 pg/ml) | 347 (145–2253) | 109 (60–174) | 94 (27–146) | −283 (−942 to 30) | 0.14 | |
| vitamin D 25OH (10–68 ng/ml) | 10±5 | 20±12 | 16±11 | 9±13 | 0.36 | |
| vitamin D 1,25OH (6–62 pg/ml) | 114±147 | 46±8 | 63±39 | 68±53 | 0.90 | |
| BSAP (U/L) (ref 13–85 IU) | 43±34 | 60±26 | 67±59 | −4±34 | 0.86 | |
| OC (ref 10.7–35 ng/ml) | 23±18 | 15±17 | 18±19 | −1.8±26 | 0.90 | |
| UNTx (ref <36 nm/mM Cr) | 213±139 | 128±70 | 101±90 | −64±14 | 0.70 | |
Values shown in the first column are normal values. Continuous variable are presented as mean ± SD if normally distributed or median (interquartile range) if not normally distributed. BSAP, bone-specific alkaline phosphatase; NA, not available; ref, reference value; OC, serum osteocalcin; UNTx, urinary N-telopeptide.
Sex Hormones
Of the female participants, 53% had not menstruated in the past 5 years, whereas the rest had menstruated in the previous 30–90 days. Comparing menstruating versus postmenopausal participants at baseline, there was no significant difference in estrogen, testosterone, and luteinizing hormone. Follicle-stimulating hormone at baseline approached significance (P=0.05). Serum estrogen was higher at 12 months in the menstruating participants.
Men, hypogonadal at baseline as indicated by low total and free testosterone levels, corrected sex hormone status to normal ranges by 12 months (Table 5). Serum estrogen levels did not differ at any time point between men and women.
Table 5.
Sex hormone parameters at baseline
| Men | Women | P Value, Men versus Women | |
|---|---|---|---|
| Estrogen (females, 61–350 pg/ml) | 141±67 | 172±90 | 0.14 |
| Testosterone (males, 220–980 ng/dl) | 137±131 | 25±25 | 0.03 |
| Free testosterone (males, 35–155 pg/ml) | 5±5 | 0.42±0.47 | 0.02 |
| Luteinizing hormone (0.8–7.6 mIU/ml) | 6±5 | 19±24 | 0.02 |
| Follicle-stimulating hormone (mIU/ml) | 4±2 | 38±43 | 0.001 |
BMD
Most of the participants had healthy or osteopenic T scores at baseline (Tables 6, 7 and 8). Twelve-month T scores did not change significantly with treatment assignment or sex. Parathyroid hormone (PTH), sex hormones, or vitamin D levels did not correlate with BMD at any time point. Z scores, which compare age-matched peers, were not different at baseline or at any time point regardless of treatment assignment or sex.
Table 6.
Sex hormone parameters at 12 months
| Parameter | Baseline | 12 mo | ∆12 mo | Baseline versus 12 mo | ∆12 mo Risedronate versus Control | ∆12 mo Men versus Women |
|---|---|---|---|---|---|---|
| P Value | ||||||
| Men | ||||||
| risedronate | ||||||
| estrogen | 175.4±81 | 143.8±36 | −43±90 | 0.26 | 0.13 | |
| testosterone | 130.5±161 | 428.8±194 | 291±191 | 0.001 | 0.44 | |
| free testosterone | 6.2±7 | 39.1±37 | 33±29 | 0.02 | 0.44 | |
| control | ||||||
| estrogen | 116.8±40 | 131.8±43 | 13±58 | 0.49 | ||
| testosterone | 124.4±93 | 506.7±263 | 370±265 | 0.001 | ||
| free testosterone | 4.2±3 | 31.3±29 | 21±28 | 0.06 | ||
| Women | ||||||
| risedronate | ||||||
| estrogen | 174.8±92 | 220.0±94 | 21±177 | 0.26 | 0.86 | 0.42 |
| testosterone | 33.0±24 | 20.5±13 | −15±37 | 0.001 | 0.23 | 0.004 |
| free testosterone | 0.44±0.5 | 1.6±2 | 0.20±0.5 | 0.02 | 0.24 | 0.09 |
| control | ||||||
| estrogen | 184.3±132 | 188.1±188 | 4±59 | 0.90 | 0.80 | |
| testosterone | 23.8±15 | 40.0±56 | 21±47 | 0.43 | 0.02 | |
| free testosterone | 0.6±0.4 | 4.3±6 | 4±6 | 0.36 | 0.11 |
Table 7.
BMD T scores at baseline
| Healthy (0 to −1 SD) | Osteopenic (>−1 to −2.4 SD) | Osteoporotic (>−2.5 SD) | |
|---|---|---|---|
| Men | |||
| spine | 13 | 9 | 2 |
| hip | 14 | 8 | 2 |
| forearm | 8 | 5 | 3 |
| Women | |||
| spine | 4 | 6 | 2 |
| hip | 8 | 5 | 0 |
| forearm | 5 | 1 | 3 |
Baseline T scores are given in SDs below average for a young healthy 30-year-old at peak bone density.
Table 8.
BMD calcium (g/cm2)
| Baseline | 6 mo | 12 mo | ∆12 mo | Baseline versus 12 mo | ∆12 mo Risedronate versus Control | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P Value | ||||||||||||||||||||||||||
| Totala | Womenb | Menc | Total | Women | Men | Total | Women | Men | Total | Women | Men | Total | Women | Men | Total | Women | Men | |||||||||
| Per protocol | ||||||||||||||||||||||||||
| RIS, n | 19 | 6 | 10 | 17 | 6 | 10 | 16 | 6 | 6 | |||||||||||||||||
| V | 1.02±0.18 | 0.93±0.20 | 1.07±0.18 | 0.98±0.18 | 0.93±0.20 | 1.10±0.17 | 1.00±0.19 | 0.95±0.22 | 0.95±0.22 | −0.02±0.07 | −0.03±0.04 | −0.04±0.08 | 0.36 | 0.21 | 0.13 | 0.97 | 0.04 | 0.24 | ||||||||
| H | 0.90±0.15 | 0.80±0.11 | 0.98±0.15 | 0.81±0.11 | 0.88±0.14 | 0.93±0.14 | 0.91±0.14 | 0.82±0.12 | 0.82±0.12 | −0.01±0.05 | −0.01±0.05 | −0.02±0.05 | 0.62 | 0.49 | 0.36 | 0.71 | 0.08 | 0.60 | ||||||||
| F | 0.54±0.11 | 0.54±0.11 | 0.60±0.11 | 0.57±0.12 | 0.57±0.12 | 0.63±0.11 | 0.55±0.13 | 0.55±0.13 | 0.55±0.13 | −0.01±0.07 | −0.01±0.07 | −0.01±0.03 | 0.17 | 0.12 | 0.42 | 0.92 | 0.02 | 0.83 | ||||||||
| C, n | 22 | 5 | 13 | 17 | 5 | 12 | 18 | 5 | 5 | |||||||||||||||||
| V | 1.01±0.17 | 0.89±0.22 | 1.03±0.17 | 0.98±0.16 | 0.85±0.20 | 0.99±0.14 | 0.83±0.16 | 0.83±0.15 | 0.83±0.15 | −0.02±0.06 | −0.06±0.07 | −0.02±0.04 | 0.26 | 0.22 | 0.66 | |||||||||||
| H | 0.92±0.18 | 0.86±0.08 | 0.93±0.19 | 0.87±0.15 | 0.77±0.09 | 0.90±0.15 | 0.87±0.16 | 0.80±0.07 | 0.80±0.07 | −0.02±0.07 | −0.06±0.07 | −0.002±0.07 | 0.41 | 0.25 | 0.90 | |||||||||||
| F | 0.60±0.11 | 0.61±0.03 | 0.63±0.10 | 0.58±0.12 | 0.50±0.16 | 0.60±0.11 | 0.58±0.10 | 0.48±0.15 | 0.48±0.15 | −0.09±0.08 | −0.004±0.01 | 0.001±0.10 | 0.71 | 0.33 | 0.92 | |||||||||||
| Per ITT with imputed values | ||||||||||||||||||||||||||
| RIS, n | 20 | 9 | 11 | 20 | 9 | 11 | 20 | 9 | 9 | |||||||||||||||||
| V | 1.01±0.17 | 0.94±0.16 | 1.07±0.17 | 0.98±0.16 | 0.93±0.16 | 1.02±0.16 | 1.00±0.18 | 0.95±0.18 | 0.95±0.18 | −0.02±0.07 | 0.01±0.05 | −0.04±0.08 | 0.27 | 0.42 | 0.09 | 0.53 | 0.04 | 0.57 | ||||||||
| H | 0.90±0.15 | 0.80±0.003 | 0.98±0.15 | 0.88±0.13 | 0.82±0.03 | 0.93±0.13 | 0.91±0.13 | 0.83±0.12 | 0.83±0.12 | 0.01±0.06 | 0.003±0.01 | −0.01±0.06 | 0.60 | 0.19 | 0.49 | 0.05 | 0.03 | 0.28 | ||||||||
| F | 0.54±0.09 | 0.48±0.07 | 0.60±0.80 | 0.57±0.11 | 0.49±0.07 | 0.63±0.10 | 0.55±0.12 | 0.48±0.07 | 0.48±0.07 | 0.003±0.08 | 0.01±0.07 | −0.01±0.1 | 0.86 | 0.37 | 0.98 | 0.23 | 0.34 | 0.45 | ||||||||
| C, n | 22 | 6 | 16 | 22 | 6 | 16 | 22 | 6 | 6 | |||||||||||||||||
| V | 1.01±0.17 | 0.96±0.18 | 1.03±0.16 | 0.97±0.14 | 0.93±0.16 | 0.99±0.17 | 1.00±0.18 | 0.95±0.18 | 0.95±0.18 | −0.02±0.08 | −0.07±0.09 | −0.04±0.07 | 0.13 | 0.13 | 0.44 | |||||||||||
| H | 0.92±0.17 | 0.84±0.08 | 0.95±0.20 | 0.86±0.13 | 0.78±0.06 | 0.89±0.14 | 0.87±0.15 | 0.83±0.10 | 0.83±0.10 | −0.05±0.11 | −0.06±0.07 | −0.02±0.05 | 0.05 | 0.12 | 0.16 | |||||||||||
| F | 0.61±0.09 | 0.55±0.09 | 0.63±0.08 | 0.58±0.11 | 0.53±0.11 | 0.60±0.11 | 0.58±0.09 | 0.52±0.11 | 0.52±0.11 | −0.03±0.10 | −0.03±0.12 | −0.03±0.10 | 0.16 | 0.54 | 0.21 | |||||||||||
RIS, risedronate group; V, vertebral; H, hip; F, forearm; C, control group.
Among the total cohort, there was no difference in BMD at any time point between the two groups.
The female control group lost BMD compared with its baseline, whereas risedronate preserved or increased BMD compared with its baseline. The effect was maintained whether the cohort was analyzed per protocol or with ITT with imputed values.
Among men, there was no difference in changes in BMD between the two groups whether analyzed per protocol or per ITT with imputed values.
When the entire group was reviewed either per protocol (with three participants in the risedronate arm and four controls missing 12-month values) or per intention to treat (ITT) analysis, with missing values imputed, there was no significant difference in changes in BMD between the risedronate and control groups at any site or time point (Tables 6–8). One vertebral fracture was present in controls at baseline and two new vertebral fractures at 12 months (both in men in the risedronate arm with no change in bone histomorphometry from baseline, one mixed uremic osteodystrophy and one high turnover osteodystrophy). There were no hip fractures during the study.
Female participants did not gain weight (risedronate arm: 57.6±12 kg versus 57.2±14 kg; control arm: 73.5±10 kg versus 76.8±11 kg at 12 months versus baseline; risedronate versus control; P=0.38). Risedronate tended to preserve BMD at all of the study targets, whereas controls tended to lose BMD whether analyzed per protocol or per ITT (Table 7). Risedronate increased vertebral BMD at 12 months, whereas controls had decreased BMD. Risedronate preserved forearm BMD at 12 months and preserved hip and vertebral BMD when analyzed by ITT (Table 7).
Men had higher BMD at each of the study targets at baseline in both groups than did women (Table 8). BMD decreased at the vertebral spine and hip in both groups.
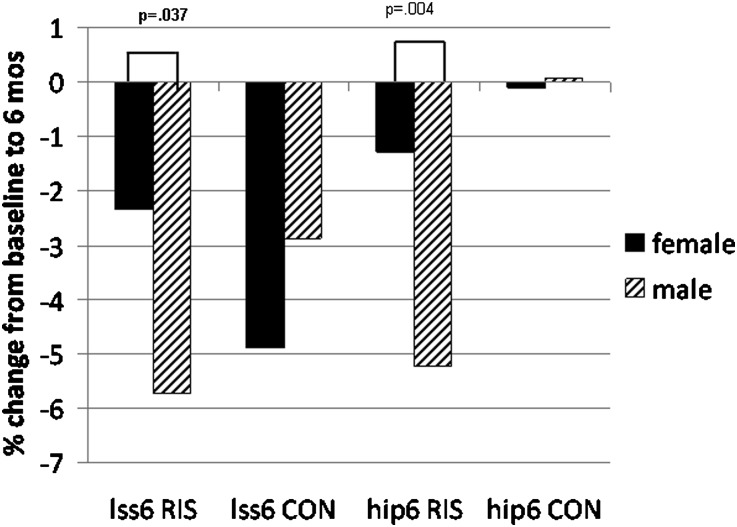
Women in the risedronate arm preserved BMD in the hip and vertebral spine more than men at 6 months (Figure 2). There was a potential risedronate-sex interaction affecting the vertebral spine and hip from baseline to 6 months and baseline to 12 months (Table 9).
Figure 2.
In the risedronate arm, female participants preserved more BMD than male participants at the lumbar spine (LSS6, P=0.04) and hip (hip6, P=0.004) at 6 months. RIS, risedronate; CON, control.
Table 9.
Potential risedronate-sex effect on changes in BMD from baseline to 6 months and baseline to 12 months in vertebral spine and hip BMD
| Change from Baseline | B | P Value | 95% CI |
|---|---|---|---|
| Vertebral 6 mo | 0.07 | 0.07 | 0.15, 0.01 |
| Vertebral 12 mo | 0.09 | 0.05 | 0.19, 0.002 |
| Hip 6 mo | 0.10 | 0.09 | 0.21, 0.01 |
| Hip 12 mo | 0.01 | 0.13 | 0.02, 0.11 |
P values <0.200 may indicate potential interaction. “B” coefficient and 95% CI refer to the coefficient of product interaction of sex and risedronate in models simultaneously adjusted for sex and risedronate. 95% CI, 95% confidence interval.
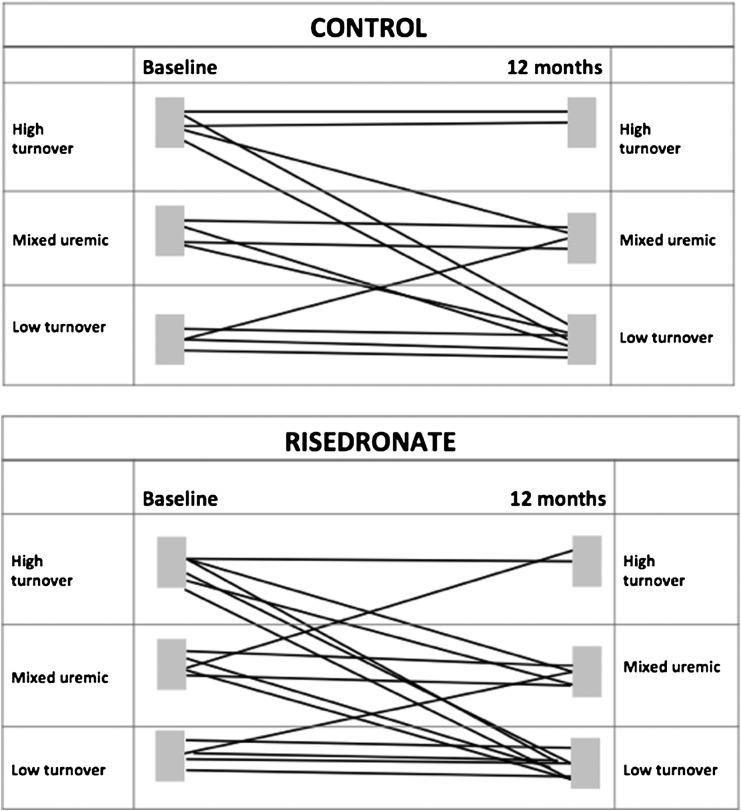
Bone Histomorphometry
Twenty-nine participants had baseline and follow-up biopsies. At baseline, histology was distributed equally among low, mixed, and high turnover bone disease (Figure 3). There was no difference in baseline histomorphometric values between the two study groups (Table 10). T scores did not correlate with nor predict bone histology at baseline or at 12 months in any group. At baseline, there was positive correlation in men only between Z scores in spine and hip with bone volume/tissue volume (BV/TV) (P=0.04), osteoid volume (OV) (P=0.03), osteoid volume/tissue volume (OV/TV) (P=0.004), mineralized volume/tissue volume (MdV/TV) (P=0.05), and trabecular number (P=0.02). At 12 months, the correlation with histomorphometry was not evident in either treatment arm or when corrected for sex.
Figure 3.
Distribution of bone histomorphometry at baseline and 12 months in the risedronate and control study groups.
Table 10.
Bone histomorphometry parameters in the risedronate and control groups
| Baseline | 12 mo | ∆12 mo | Baseline versus 12 mo | ∆12 mo Risedronate versus Control | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P Value | |||||||||||||||
| Total | Women | Men | Total | Women | Men | Total | Women | Men | Total | Women | Men | Total | Women | Men | |
| Risedronate, n | 16 | 6 | 10 | ||||||||||||
| BV/TV (%) | 10.7±6 | 8.6±4 | 12.4±7 | 11.3±5 | 11.5±4 | 11.2±5 | 0.54±7.1 | 2.9±4.9 | −0.89±8 | 0.76 | 0.21 | 0.73 | 0.17 | 0.07 | 0.54 |
| MdV (%) | 1.2±0.4 | 1.1±0.5 | 1.3±0.4 | 1.2±0.5 | 1.3±0.9 | 1.1±0.3 | −0.05±0.6 | 0.15±8 | −18±0.5 | 0.78 | 0.68 | 0.02 | 0.40 | 0.51 | 0.71 |
| MdV/BV (%) | 95±3 | 95±4 | 95.6±3 | 92±7 | 95±5 | 89.3±7 | −4.1±7 | -0.9±5 | −6.1±7 | 0.07 | 0.67 | 0.02 | 0.11 | 0.25 | 0.06 |
| TbTh (plate, µm) | 72.6±17 | 68.2±17 | 79.0±19 | 72.1±28 | 74.3±32 | 70.8±27 | −0.63±25 | 6.1±30 | −4.6±23 | 0.92 | 0.63 | 0.53 | 0.08 | 0.49 | 0.16 |
| OV | 0.06±0.04 | 041±0.03 | 0.06±0.04 | 0.10±0.09 | 0.05±0.05 | 0.13±0.1 | 0.04±8.2 | 0.01±0.04 | 0.10±0.03 | 0.06 | 0.57 | 0.07 | 0.03 | 0.22 | 0.05 |
| OV/BV (%) | 4.1±2.6 | 3.7±2.3 | 4.4±3 | 8.7±7.2 | 4.8±5 | 10.7±7.5 | 4.6±6.5 | 1.7±4.8 | 6.1±7 | 0.02 | 0.66 | 0.02 | 0.11 | 0.21 | 0.17 |
| OV/TV (%) | 0.49±0.4 | 0.32±0.3 | 0.56±0.4 | 0.90±8 | 0.49±0.4 | 1.2±8 | 0.42±0.54 | 0.17±0.4 | 0.56±0.60 | 0.01 | 0.34 | 0.01 | 0.13 | 0.06 | 0.26 |
| OS/BS (%) | 23±18 | 20.4±17 | 23.7±19 | 24±27 | 6.9±5 | 35±29 | 1.8±29 | −14±19 | 11±34 | 0.81 | 0.15 | 0.30 | 0.79 | 0.20 | 0.05 |
| OB/BS | 2.3±3 | 2.3±3 | 2.1±3 | 1.23±1.6 | 1.4±2 | 1.2±1.5 | −1.1±2.7 | −0.98±2.9 | −1.5±3 | 0.13 | 0.44 | 0.22 | 0.55 | 0.54 | 0.73 |
| OC/BS | 1.5±1.9 | 1.6±1.9 | 1.5±1.9 | 0.28±0.43 | 0.3±0.19 | 0.28±0.6 | −4.0±6.1 | −1.3±1.9 | −1.1±1.9 | 0.02 | 0.26 | 0.10 | 0.21 | 0.53 | 0.41 |
| ES/BS | 9±11 | 11.9±16 | 2.1±2.2 | 2.7±3 | 3.1±3.8 | 0.53±0.7 | −6.3±12 | −8.9±17 | −1.7±2.4 | 0.05 | 0.27 | 0.08 | 0.49 | 0.68 | 0.35 |
| BFR | 0.02±0.05 | 0.00±0.05 | 0.02±0.05 | 0.10±0.16 | 0.06±0.08 | 0.10±0.16 | n/a | n/a | n/a | ||||||
| Control, n | 13 | 3 | 10 | ||||||||||||
| BV/TV (%) | 13.3±9 | 11.7±2 | 15.4±14 | 9.7±4 | 7.9±0.7 | 10.2±4 | −3.4±9 | −3.7±2.8 | −3.3±9 | 0.13 | 0.15 | 0.19 | |||
| MdV (%) | 1.3±1 | 1.0±0.7 | 1.5±1.2 | 1.0±0.5 | 0.68±0.45 | 1.1±0.5 | −0.31±0.9 | 96.2±1.7 | −0.32±1 | 0.27 | 0.34 | 0.35 | |||
| MdV/BV (%) | 95±4 | 94±3 | 95.1±5 | 96±6 | 96±2 | 95.4±76 | 0.12±7 | 3±4 | −0.79±8 | 0.95 | 0.67 | 0.35 | |||
| TbTh (plate, µm) | 90.9±39 | 82.9±50 | 92.1±42 | 62.2±20 | 68.9±12 | 60.2±22 | −29±55 | −14.1±57 | −33±57 | 0.08 | 0.71 | 0.10 | |||
| OV | 0.06±0.06 | 0.07±0.08 | 0.10±0.12 | 0.03±0.02 | 0.02±0.01 | 0.04±0.03 | −0.03±0.07 | −0.05±0.09 | −0.02±0.07 | 0.23 | 0.46 | 0.41 | |||
| OV/BV (%) | 4.6±3.4 | 6.9±4.2 | 4.9±5 | 4.9±5 | 3.8±1.5 | 5.3±5.9 | 0.35±7.2 | −3.2±4.3 | 1.4±8 | 0.87 | 0.34 | 0.58 | |||
| OV/TV (%) | 0.58±0.58 | 0.87±0.6 | 0.75±1.1; | 0.51±0.8 | 0.319±0.12 | 0.56±95; | −0.08±1.1 | −0.57±0.6 | 1.2±0.4; | 0.53 | 0.25 | 0.74 | |||
| OS/BS (%) | 15±8 | 13±10 | 15.6±8.6 | 14±10 | 17±1.6 | 13.6±11 | −0.64±17 | 4.1±12 | −2±18 | 0.89 | 0.60 | 0.26 | |||
| OB/BS | 1.3±1.3 | 0.37±0.6 | 1.4±1.3 | 0.75±0.76 | 0.52±0.55 | 0.82±0.83 | −0.96±2.6 | 0.16±1.1 | −0.8±1.5 | 0.16 | 0.83 | 0.73 | |||
| OC/BS | 0.97±1.2 | 1.07±1.6 | 0.86±1.1 | 0.68±1.23 | 0.8±1.23 | 0.65±1.3 | −0.29±1.9 | 0.27±2.6 | −0.29±1.7 | 0.36 | 0.87 | 0.61 | |||
| ES/BS | 1.36±1.7 | 6.7±6.6 | 1.2±1.5 | 0.82±1.4 | 2.7±4.1 | 0.82±1.5 | −3.2±12 | −4±10 | −0.57±2.6 | 0.46 | 0.58 | 0.61 | |||
| BFR | 0.04±0.1 | 0.1±0.15 | 0.02±0.01 | 0.05±0.06 | 0.05±0.07 | 0.04±0.06 | n/a | n/a | n/a | 0.51 |
BV/TV, bone volume/tissue volume; MdV, mineralized bone volume; TbTh, trabecular thickness; OV, osteoid volume; OV/BV, osteoid volume/bone volume; OV/TV, osteoid volume/tissue volume; MdV/BV, mineralized volume/bone volume; OB/S, osteoblasts/bone surface; OC/S, osteoclasts/bone surface; ES/BS, erosion surface/bone surface; BFR, bone formation rat.
Only 3 of 29 participants showed increased bone activity in the second biopsy, 1 in the control group and 2 in the risedronate group. There was no change from baseline bone activity in 7 of 16 risedronate recipients and 7 of 13 controls (Figure 3), whereas 7 of 16 risedronate recipients and 5 of 13 controls had decreased bone activity. However, none of the participants developed adynamic bone disease.
In the risedronate arm, histomorphometry showed maintenance of bone volume, mineralized volume per bone volume (MdV/BV), and trabecular thickness at 12 months. Osteoblast function was preserved with no change in osteoblast number per bone surface (OB/BS) at 12 months, even as the OV per bone volume (OV/BV) and tissue volume (OV/TV) increased significantly. Osteoclast function was suppressed with decreased osteoclast number per bone surface (OC/BS and decreased erosion surface per bone surface (ES/BS) at 12 months. The bone formation rate (BFR) could not be compared because of small numbers. Compared with risedronate, OV was significantly decreased at 12 months in controls (Table 10).
Women in the risedronate arm showed insignificant changes in activity from baseline to 12 months. They preserved bone as evidenced by maintenance of total BV and OV, as well as mineralized bone. Compared with risedronate, controls tended to lose more bone at 12 months (decreased BV/TV and MdV) (Table 10).
There was a significant decrease in bone activity in the second biopsy in men in the risedronate arm, whereas controls maintained bone activity (Table 10). Histomorphometry showed that risedronate-treated participants developed significant increases in uncalcified bone, with increased osteoid (OV/BV and OV/TV) and decreased MdV/BV. OV was significantly increased only in risedronate, whereas the decrease in MdV/BV in risedronate-treated participants approached significance compared with controls at 12 months. Osteoclast function, as evidenced by osteoclast number and erosion surface, was similarly decreased in both male controls and risedronate recipients (Table 10). Among the groups treated with risedronate, men had greater osteoid surface/bone surface (OS/BS) in the second biopsy than women (34.8±30 in men versus 6.9±4.9 in women; P=0.04). Risedronate-treated men had greater OS/BS compared with control men (P=0.05). On regression analysis, we found potential risedronate-sex interactions associated with osteoid parameters (Table 11).
Table 11.
Potential risedronate-sex interaction on histomorphometry
| B | P | 95% CI | |
|---|---|---|---|
| BV2 | −0.71 | 0.17 | −1.8, 0.33 |
| OV2 | 0.06 | 0.28 | −0.05, 0.18 |
| dOV2 | 0.08 | 0.20 | −0.04, 0.21 |
| MDV2 | −0.66 | 0.03 | −1.2, −0.09 |
| BS2 | −27 | 0.01 | −48, −6.4 |
| OV/BV2 | 4.66 | 0.16 | −1.9, 11.2 |
| OS/BS2 | 31.30 | 0.004 | 10.5, 52 |
| OB/OS2 | −21 | 0.01 | −38, −5 |
P values < 0.200 may indicate potential interaction. “B” coefficient and 95% CI refer to the coefficient of product interaction of sex and risedronate in models simultaneously adjusted for sex and risedronate. 95% CI, 95% confidence interval.
Sex Hormone Correlation with Histomorphometry
On multivariate analyses, sex hormone levels did not significantly predict histomorphometric parameters of bone activity, regardless of sex or risedronate use.
PTH Association with Histomorphometry
PTH was elevated at baseline (median 414 pg/ml). This was associated with evidence for increased bone activity at baseline, as follows: increased OV (P=0.05) and thickness (P<0.04) and decreased MdV (P=0.02), with tendency toward increased numbers of osteoblasts and osteoclasts (P=0.05). By 12 months, median PTH was 95 pg/ml in risedronate-treated patients verus 81 pg/ml in controls (P=0.39). There was evidence for increased osteoblast function with increased numbers of osteoblasts and osteoid formation in risedronate-treated men with higher PTH compared with those with lower PTH.
Discussion
This is the first randomized, double-blinded, placebo-controlled study of 12 months of risedronate treatment on bone histomorphometry and BMD in new renal transplant recipients. To note, most of our cohort was not osteoporotic at baseline. We found that a usual weekly dose of risedronate preserved BMD in women but not in men. On histomorphometry, we found that risedronate suppressed bone activity more in men than women.
Our previous study showed that pamidronate preserved BMD but led to the development of adynamic bone disease in most participants receiving 6 months of pamidronate treatment.3 The results of this study suggest that risedronate preserved BMD without causing adynamic bone disease. At 12 months, risedronate suppressed bone activity in men, as measured by increased OV, decreased mineralization, and decreased numbers of osteoclasts. Women treated with risedronate did not show the same degree of suppression. In a previous study, zoledronic acid, another third-generation amino-bisphosphonate, given at the time of renal transplant and 3 months later, improved BMD at 6 months but showed increased osteoid surface at follow-up bone biopsies in seven zoledronic participants (six males) compared with six controls (three males).5
Our study showed that risedronate-treated male participants had bone loss comparable with that of male controls. However, histomorphometry showed different reasons. For example, risedronate-treated male participants had increased osteoid formation, whereas male controls had increased resorption characteristics. Male controls lost BMD in a similar fashion to female controls. Interaction analysis between risedronate and sex supported our observations of possible risedronate sex effects. Most studies do not report sex results. For example, a recent open-labeled trial of risedronate in 101 kidney recipients reported positive effects on BMD in the risedronate group but did not comment further on sex.11
There was no clear evidence that risedronate affected sex hormones. In our study, both men and women were hypogonadal at baseline. Risedronate use was not associated with long-term changes in sex hormones in men. The control and risedronate-treated men showed similar and improved testosterone levels at 12 months. A cross-sectional study of patients who were transplanted for 10±5 years, with varying degrees of renal graft function, showed that serum testosterone did not predict BMD or bone activity in men, whereas serum estradiol levels correlated significantly with increased bone formation activity in women.12 We did not find similar correlations.
To note, bisphosphonate use in male senile osteoporosis increases BMD, similar to the effect in females.13,14 However, in senile osteoporosis, older men are usually hypogonadal, with no natural reconstitution of their sex hormones. The same is not true in male renal transplant recipients. Our study showed that endogenous testosterone, very low at transplant, increased to normal levels by 12 months after renal transplant. In addition to estrogen receptors, androgen receptors are also expressed on the osteoblast surface in human bone.15,16 Androgens may stimulate osteoblast proliferation and function, including increased bone matrix production. We speculate that bisphosphonate use in nonelderly male renal transplant recipients could exaggerate the apparent uncoupling between bone formation caused by improved hormonal status and bone resorption halted by the medication, leading to excess unmineralized osteoid production.
Glucocorticoids decrease bone formation and increase bone resorption, resulting in net bone loss.17 Prophylactic bisphosphonates are commonly used to preserve BMD and decrease fracture rates in patients treated with glucocorticoids for various chronic illnesses.18,19 In our study, although the total dose of glucocorticoids was the same in men and women, women had a higher BSA as well as a higher dose of glucocorticoids per kilogram of body weight. In these women, risedronate may have attenuated steroid-related bone loss.
It is not clear that bisphosphonates are ultimately useful in the prophylactic treatment of BMD in the transplant recipient. In our study, risedronate, given at the usual doses used for osteoporosis in the general population, resulted in maintenance of BMD and bone activity mainly in women, albeit the small cohort number. The men, however, with larger numbers in the cohort, showed no preservation of BMD regardless of treatment group: histomorphometry showed that the decrease in BMD in risedronate was due to increased unmineralized osteoid, whereas controls had decreased mineralized bone volume. We think that this observation is important to note: it may be that men may not benefit from antiresorptive bone therapy with risedronate, at least not in the early transplant period.
Multiple studies report that bisphosphonates increase BMD, but there is no proven effect in terms of preventing fractures in the long term.20 In a recent retrospective study, bisphosphonates given 1–2 years after renal transplant were not associated with improved fracture rate, even though BMD increased. Rather, patients on bisphosphonates developed more fractures. The authors raised the possibility that these patients may have lower bone activity at baseline and bisphosphonates could further suppress bone activity.21 In our study, two new vertebral fractures were seen in the risedronate group (both were men who had not developed lower bone activity). There have been reports of atypical fractures with long-term use of bisphosphonates for osteoporosis in the general population, suggesting profound disturbances in bone activity and structure.22
Although it was randomized, double blinded, and placebo controlled, our study has limitations that may have influenced the observed results. This was a single-center study with a relatively small cohort. There were imbalances in the baseline demographics because of chance randomization, with fewer women participating in the study. In addition, the risedronate-treated women were younger than controls. Our study evaluated the use of a common and widely used oral third-generation amino-bisphosphonate, risedronate, while being powered for effects seen in our previous study using an intravenous second-generation amino-bisphosphonate, pamidronate. It is possible that bisphosphonates have different potencies in their mechanism of action: amino-bisphosphonates inhibit the enzyme farnesyl pyrophosphate synthase, resulting in abnormalities in osteoclast cytoskeleton and disruption in their resorptive activities, leading to osteoclast death. There may be a difference between second- and third-generation bisphosphonate enzyme suppressive activities.23–25 Several studies of parenteral pamidronate reported preservation of BMD26–28 but there were no histomorphometry or sex comparisons. Finally, although we observed a possible risedronate-sex interaction, our study was not designed to assess this. We acknowledge that conclusions in such a study must be considered tentative; however, we think that our observations and statistically suggestive risedronate-sex interaction merit further study.
In summary, we have shown that risedronate used in prophylactic treatment of renal transplant–associated bone loss decreases bone activity while preserving BMD, without development of adynamic bone disease. Specifically, this drug may be more useful in women than men. Further study is needed before prophylactic bisphosphonate use to attenuate bone loss can be recommended, especially in male renal transplant recipients.
Concise Methods
Study Design
A prospective, double-blinded, placebo-controlled trial was designed to evaluate the effect of weekly risedronate treatment on BMD, bone biochemical parameters, and bone histomorphometry in adult patients who received a living-donor kidney transplant at Montefiore Medical Center. The study was approved by the Institutional Review Board of Montefiore Medical Center and was registered at Clinicaltrials.gov (identifier NCT00266708).
Participants
Participants were recruited from eligible transplant recipients during 2002–2006. Eligibility criteria included adult age, ability to give informed consent, and undergoing living-donor renal transplant. Exclusion criteria included inability to return for regular follow-up or participation in another clinical trial. Participants were not different from those patients who declined to participate in terms of age, sex, ESRD etiology, or dialysis months.
Protocol
The participants were randomized to receive 35 mg of risedronate weekly or identical placebo. Baseline BMD was not an inclusion/exclusion criterion. Randomization was done by the study pharmacist using computer-generated randomization. Blinding of the assigned treatment was achieved by overencapsulation of the risedronate tablets to appear similar to the placebo capsules, which were dispensed monthly by the study pharmacist. Study drug treatment started as soon as the renal function improved to a SCr of <2.0 mg/dl after transplant and was held if there was an interim deterioration of renal function.
Participants received standard immunosuppression with glucocorticoids, tacrolimus, rapamycin, and mycophenolate mofetil. Cyclosporine was used in four participants instead of tacrolimus. Participants received induction immunosuppression with basiliximab, 500 mg of intraoperative intravenous methylprednisolone followed by maintenance prednisone (20 mg/d tapered to 5 mg/d by 90 days postoperatively), tacrolimus (adjusted to maintenance levels of 8–10 ng/ml for 3 months and then 5–8 ng/ml), and rapamycin (starting 1 week postoperatively, adjusted to maintain levels of 10–15 ng/ml) or mycophenolate mofetil (1 g orally twice daily). Episodes of acute rejection, total dosage of glucocorticoids, and tacrolimus levels were recorded. All participants received repletion of phosphorus, magnesium, and calcium as required to maintain adequate electrolyte levels. All participants received 0.25 μg of calcitriol daily.
Bone Mineral Densitometry and Radiographic Studies
BMD of the vertebral spine (L1–L4), total hip, and distal third of the nondialysis access forearm were measured at baseline, 6 months, and 12 months, using the same Hologic 4500 QDC scanner. We noted T scores, which are defined by the World Health Organization as the SDs below average for a young healthy 30-year-old individual at peak bone density, with 0 to –1 SD regarded as healthy, <−1 to −2.4 SD as osteopenia, and <−2.5 SD as osteoporosis. We also recorded Z scores, which compare the fracture risk with age-matched peers. Vertebral and hip radiographs were obtained at baseline and at 12 months to evaluate for radiographic evidence of bone fractures.
Biochemical and Hormonal Determinations
Bone biochemical markers—including intact PTH (Immulite 2000; Siemens), vitamin D levels (Liaison; DiaSorin), osteocalcin (microtiter plate, Quidel Corp), bone-specific alkaline phosphatase (enzyme immunoassay), and urinary N-telopeptide (microtiter plate; Wampole Laboratories)—were obtained at baseline and months 6 and 12 of the protocol. Sex hormones—including total estrogens, free and total testosterone, luteinizing hormone, and follicle-stimulating hormone—were obtained at baseline, 6 months, and 12 months.
Mineralized Bone Histology and Bone Histomorphometry
Bone biopsies extracted from the anterior iliac crest were performed at the time of renal transplantation and after 1 year of study drug treatment. When possible, participants underwent tetracycline labeling of bone before baseline biopsy (we were unable to complete tetracycline labeling in some cases because of unpredictability of transplant timing). Participants underwent tetracycline labeling before follow-up bone biopsy per the following protocol: 2-day oral administration of 500 mg of tetracycline twice daily, followed by a free interval of 12 days and subsequent administration of 500 mg of tetracycline twice daily for 4 days. Bone biopsy was performed 4 days thereafter. The first bone biopsy was done just before renal transplantation surgery while the patient was under general anesthesia. The second biopsy was done 12 months later using local anesthesia and mild conscious sedation as needed.
Bone Processing
The bone core was fixed and embedded in polymethacrylate for histologic sectioning and histomorphometry. Histomorphometry was performed using BioQuant Osteo II MR software (version 8.20.40; BioQuant, Nashville, TN). All slides were analyzed in a blinded manner by a single reviewer (J.P.), without knowledge of treatment assignment. Bone activity was defined as low turnover (decreased cellular activity, bone formation and mineralization), high turnover (increase in cellular activity with increased bone formation and erratic mineralization), and mixed uremic (hyperparathyroid with mineralization defect).29
Statistical Analyses
Sample size calculations were based on our previously reported differences in adynamic bone disease between the treatment group and the control group, which showed that 80% of the participants treated with pamidronate developed adynamic bone compared with the control group.3 Assuming a similar bisphosphonate effect, 20 participants (total of 40) in each group would show a 25% difference in adynamic bone histology between the treatment and placebo groups. The data were analyzed on an intention-to-treat basis, following Consolidated Standards of Reporting Trials guidelines.30
Because 13 participants (4 risedronate-treated patients and 9 controls) did not have a second biopsy, we imputed the sex-specific mean parameters within each treatment arm. For example, mean risedronate values for men were assigned to the missing risedronate male values, whereas mean control values for men were assigned to the missing male control values. The females were likewise imputed.
Differences between the risedronate and control groups were analyzed using t tests. The paired t test detected differences in the same patient at two different time points, and the independent t test compared the same time point in two different populations. Because distributions of results were less skewed and followed a normal curve, Δ changes in each category were analyzed and compared with each other in the two arms of the trial. In normally distributed results, regression analysis was used to determine which independent variables significantly influenced the dependent variable. In non-normally distributed results, the median is presented with interquartile ranges. These results were compared for significant differences using the Mann–Whitney U test between groups and Wilcoxon rank test for intragroup comparisons. Independent variables included in the multivariate analyses were those significant on univariate analysis. Any interaction between sex and risedronate on various parameters was evaluated with the creation of a product interaction term and tested via regression analysis with risedronate and sex included in the model. We used SPSS software for statistical analyses. We report means ± SDs. Significance is defined as P<0.05. P values <0.20 were considered evidence of potential interaction. The “B” coefficient and 95% confidence intervals in Tables 9 and 11 refer to the coefficient of product interaction of sex and risedronate in models simultaneously adjusting for sex and risedronate.
Disclosures
None.
Acknowledgments
We thank Ernestine Middleton for her expertise in the processing of the bone samples.
This study was supported by grants from the Kidney and Urology Foundation of America and the Institute for Clinical and Translational Research of Albert Einstein College of Medicine.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group: KDIGO clinical practice guideline for the diagnosis, evaluation, prevention and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int 76: S121–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Coco M, Glicklich D, Faugere MC, Burris L, Bognar I, Durkin P, Tellis V, Greenstein S, Schechner R, Figueroa K, McDonough P, Wang G, Malluche H: Prevention of bone loss in renal transplant recipients: A prospective, randomized trial of intravenous pamidronate. J Am Soc Nephrol 14: 2669–2676, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Grotz W, Nagel C, Poeschel D, Cybulla M, Petersen KG, Uhl M, Strey C, Kirste G, Olschewski M, Reichelt A, Rump LC: Effect of ibandronate on bone loss and renal function after kidney transplantation. J Am Soc Nephrol 12: 1530–1537, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Haas M, Leko-Mohr Z, Roschger P, Kletzmayr J, Schwarz C, Mitterbauer C, Steininger R, Grampp S, Klaushofer K, Delling G, Oberbauer R: Zoledronic acid to prevent bone loss in the first 6 months after renal transplantation. Kidney Int 63: 1130–1136, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Jeffery JR, Leslie WD, Karpinski ME, Nickerson PW, Rush DN: Prevalence and treatment of decreased bone density in renal transplant recipients: A randomized prospective trial of calcitriol versus alendronate. Transplantation 76: 1498–1502, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Nayak B, Guleria S, Varma M, Tandon N, Aggarwal S, Bhowmick D, Agarwal SK, Mahajan S, Gupta S, Tiwari SC: Effect of bisphosphonates on bone mineral density after renal transplantation as assessed by bone mineral densitometry. Transplant Proc 39: 750–752, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Lan G, Peng L, Xie X, Peng F, Wang Y, Yu S: Alendronate is effective to treat bone loss in renal transplantation recipients. Transplant Proc 40: 3496–3498, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Trabulus S, Altiparmak MR, Apaydin S, Serdengecti K, Sariyar M: Treatment of renal transplant recipients with low bone mineral density: A randomized prospective trial of alendronate, alfacalcidol, and alendronate combined with alfacalcidol. Transplant Proc 40: 160–166, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Torregrosa JV, Fuster D, Pedroso S, Diekmann F, Campistol JM, Rubí S, Oppenheimer F: Weekly risedronate in kidney transplant patients with osteopenia. Transpl Int 20: 708–711, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Torregrosa JV, Fuster D, Gentil MA, Marcen R, Guirado L, Zarraga S, Bravo J, Burgos D, Monegal A, Muxí A, García S: Open-label trial: effect of weekly risedronate immediately after transplantation in kidney recipients. Transplantation 89: 1476–1481, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Cueto-Manzano AM, Freemont AJ, Adams JE, Mawer B, Gokal R, Hutchison AJ: Association of sex hormone status with the bone loss of renal transplant patients. Nephrol Dial Transplant 16: 1245–1250, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, Adami S, Weber K, Lorenc R, Pietschmann P, Vandormael K, Lombardi A: Alendronate for the treatment of osteoporosis in men. N Engl J Med 343: 604–610, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Ringe JD, Orwoll E, Daifotis A, Lombardi A: Treatment of male osteoporosis: Recent advances with alendronate. Osteoporos Int 13: 195–199, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Abu EO, Horner A, Kusec V, Triffitt JT, Compston JE: The localization of androgen receptors in human bone. J Clin Endocrinol Metab 82: 3493–3497, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Leder BZ, LeBlanc KM, Schoenfeld DA, Eastell R, Finkelstein JS: Differential effects of androgens and estrogens on bone turnover in normal men. J Clin Endocrinol Metab 88: 204–210, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Lukert BP, Raisz LG: Glucocorticoid-induced osteoporosis: Pathogenesis and management. Ann Intern Med 112: 352–364, 1990 [DOI] [PubMed] [Google Scholar]
- 18.de Nijs RN, Jacobs JW, Lems WF, Laan RF, Algra A, Huisman AM, Buskens E, de Laet CE, Oostveen AC, Geusens PP, Bruyn GA, Dijkmans BA, Bijlsma JW, STOP Investigators : Alendronate or alfacalcidol in glucocorticoid-induced osteoporosis. N Engl J Med 355: 675–684, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, Thamsborg G, Liberman UA, Delmas PD, Malice MP, Czachur M, Daifotis AG, Glucocorticoid-Induced Osteoporosis Intervention Study Group : Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. N Engl J Med 339: 292–299, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Schwarz C, Mitterbauer C, Heinze G, Woloszczuk W, Haas M, Oberbauer R: Nonsustained effect of short-term bisphosphonate therapy on bone turnover three years after renal transplantation. Kidney Int 65: 304–309, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Conley E, Muth B, Samaniego M, Lotfi M, Voss B, Armbrust M, Pirsch J, Djamali A: Bisphosphonates and bone fractures in long-term kidney transplant recipients. Transplantation 86: 231–237, 2008 [DOI] [PMC free article] [PubMed]
- 22.Black DM, Kelly MP, Genant HK, Palermo L, Eastell R, Bucci-Rechtweg C, Cauley J, Leung PC, Boonen S, Santora A, de Papp A, Bauer DC, Fracture Intervention Trial Steering Committee. HORIZON Pivotal Fracture Trial Steering Committee : Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med 362: 1761–1771, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, Ebetino FH, Rogers MJ: Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther 296: 235–242, 2001 [PubMed] [Google Scholar]
- 24.Russell RG, Xia Z, Dunford JE, Oppermann U, Kwaasi A, Hulley PA, Kavanagh KL, Triffitt JT, Lundy MW, Phipps RJ, Barnett BL, Coxon FP, Rogers MJ, Watts NB, Ebetino FH: Bisphosphonates: An update on mechanisms of action and how these relate to clinical efficacy. Ann N Y Acad Sci 1117: 209–257, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Allen MR, Burr DB: Bisphosphonate effects on bone turnover, microdamage, and mechanical properties: What we think we know and what we know that we don’t know. Bone 49: 56–65, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Fan SL, Almond MK, Ball E, Evans K, Cunningham J: Pamidronate therapy as prevention of bone loss following renal transplantation. Kidney Int 57: 684–690, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Fan SL, Kumar S, Cunningham J: Long-term effects on bone mineral density of pamidronate given at the time of renal transplantation. Kidney Int 63: 2275–2279, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Walsh SB, Altmann P, Pattison J, Wilkie M, Yaqoob MM, Dudley C, Cockwell P, Sweny P, Banks LM, Hall-Craggs M, Noonan K, Andrews C, Cunningham J: Effect of pamidronate on bone loss after kidney transplantation: A randomized trial. Am J Kidney Dis 53: 856–865, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Malluche H, Faugere MC: Renal bone disease 1990: An unmet challenge for the nephrologist. Kidney Int 38: 193–211, 1990 [DOI] [PubMed] [Google Scholar]
- 30.Schulz K, Altman D, Moher D, CONSORT Group: CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann Intern Med 152: 726–732, 2010 [DOI] [PubMed] [Google Scholar]