Abstract
The role of sialylation in kidney biology is not fully understood. The synthesis of sialoglycoconjugates, which form the outermost structures of animal cells, requires CMP-sialic acid, which is a product of the nuclear enzyme CMAS. We used a knock-in strategy to create a mouse with point mutations in the canonical nuclear localization signal of CMAS, which relocated the enzyme to the cytoplasm of transfected cells without affecting its activity. Although insufficient to prevent nuclear entry in mice, the mutation led to a drastically reduced concentration of nuclear-expressed enzyme. Mice homozygous for the mutation died from kidney failure within 72 hours after birth. The Cmasnls mouse exhibited podocyte foot process effacement, absence of slit diaphragms, and massive proteinuria, recapitulating features of nephrin-knockout mice and of patients with Finnish-type congenital nephrotic syndrome. Although the Cmasnls mouse displayed normal sialylation in all organs including kidney, a critical shortage of CMP-sialic acid prevented sialylation of nephrin and podocalyxin in the maturing podocyte where it is required during the formation of foot processes. Accordingly, the sialylation defects progressed with time and paralleled the morphologic changes. In summary, sialylation is critical during the development of the glomerular filtration barrier and required for the proper function of nephrin. Whether altered sialylation impairs nephrin function in human disease requires further study.
The acidic nine-carbon sugar sialic acid (Sia) terminates the bulk of oligosaccharide chains present on cell surface components and circulating glycoproteins. For a long time, they were regarded as mere carriers of negative charge. However, the last decades disclosed that Sia is involved in virtually all areas of vertebrate life1; that is, in the shaping of anatomic structures,2 the control of immune reactions,3 and in mediating interactions between cells4 as well as cells and pathogens.5 With respect to the vital functions, it was not unexpected that prevention of Sia biosynthesis in mice causes lethality in early gestation.6 Sia has been implicated in the formation of the glomerular filtration apparatus in the kidney.7 Podocytes forming the visceral layer of the filtration barrier express podocalyxin, a highly sialylated and sulfated mucine type protein at their apical pole. The dense array of negative charge linked to this scaffold is believed to keep the filtration slits open.8 In accordance with this hypothesis, neutralization of charge by injection of protamine sulfate or depletion of Sia by injection of sialidases caused abrupt onset of proteinuria.2,9 However, these methods generally affected surface sialylation, and did not provide access to the molecular mechanisms underlying filtration failure. Furthermore, they did not explain whether general physicochemical features of Sia or specific sialoglycoconjugates are needed to maintain foot process (FP) architecture and function.
A prerequisite for the biosynthesis of sialoglycoconjugates is the activation of Sia to CMP-Sia, catalyzed by the CMP-sialic acid synthetase (CMAS) (Supplemental Figure 1). Only the activated sugar can be transported into the Golgi apparatus and transferred onto glycoconjugates by linkage-specific sialyltransferases. A unique feature of the eukaryotic sialylation pathway (Supplemental Figure 1) is CMAS’ nuclear sequestration,10,11 which is dispensable for functionality at the cellular level.12
In this study, we describe a mouse model with mutant Cmas. Using a knock-in approach, the major nuclear localization signal was inactivated by point mutations (nls). The mutant enzyme retained nuclear localization, but CMASnls protein levels were drastically reduced in all tissues analyzed. Mice homozygous for the nls mutation died within 72 hours after birth due to podocytopathy. In a detailed biochemical and histologic study, we identified the major structural protein of the slit membrane, nephrin, as a target for sialylation. Moreover, we showed a selective and a progressive loss of sialylation on nephrin and podocalyxin to impair the establishment of a functional filtration barrier, in an otherwise regularly sialylated environment. With this in vivo model we provide significant new insight into the specific role of individual sialoglycoconjugates in podocyte maturation.
Results
Early Postnatal Lethality in nls/nls Mice
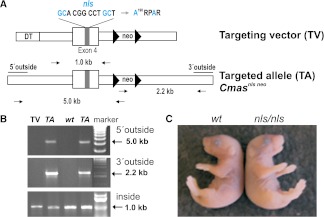
Originally we intended to investigate the role of CMAS nuclear sequestration. Therefore, we generated the Cmasnls mouse model by replacing the endogenous Cmas locus by the mutant Cmasnls. With the nls mutation, two point mutations were introduced into the canonical NLS K198RPRR known to be crucial for nuclear entry of CMAS. CMASnls (mutant sequence A198RPAR) clearly relocated to the cytoplasm of transfected cells but retained full enzymatic activity.12 To test the consequences of CMAS relocation in a mouse model, exon 4 of the endogenous Cmas was replaced by Cmasnls exon 4 (Figure 1A). The neomycin resistance gene was removed by crossing with the deleter strain Zp3-cre.13 Both homologous recombination in embryonic stem cells and deletion of the neomycin resistance gene were confirmed by PCR (Figure 1B and Supplemental Figure 2A, respectively). Homozygous mutants were born in the expected Mendelian ratio (Supplemental Figure 2B) and were indistinguishable from wild type (wt) at the day of birth (Figure 1C). However, nls/nls mice never survived beyond postnatal day (P) 3.5. After P1.5, some homozygotes showed ascites and a poorly filled bladder. Heterozygotes always appeared unaffected.
Figure 1.
Targeted mutagenesis of CMAS. (A) The NLS (K198RPRR) in exon 4 of Cmas was targeted by mutations marked in blue (nls). A neomycin resistance (neo) cassette flanked by loxP sites (triangles) was inserted into intron 4 and a diphtheria-toxin (DT) cassette was inserted into intron 3. Correct homologous recombination was detected by 5′ outside and 3′ outside primers combined with neo primer pairs. A 1-kb fragment generated by inside primers was used as a control. (B) PCR products from targeting vector (TV), targeted allele (TA) from Cmasnls neo embryonic stem cells, and wild-type (wt) embryonic stem cells and DNA marker. (C) P0.5 homozygous Cmasnls mutants were indistinguishable from wt with respect to gross anatomy and coat color. nls refers to the mutant Cmasnls allele lacking the neo after CRE-mediated excision.
Renal Phenotype in nls/nls Mice
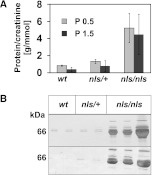
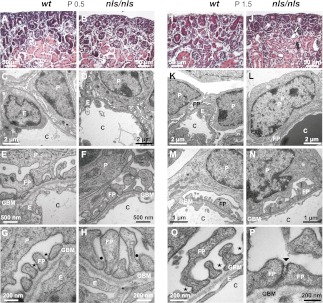
As a first clinical parameter, we analyzed urine from wt and nls/nls mice and identified a dramatic loss of protein in the homozygous mutants. Whereas the protein/creatinine ratio was 1.4 g protein/mmol creatinine in wt and heterozygous mice, the value increased to >5 in homozygous mutants (Figure 2A). The most prominent protein was albumin (Figure 2B), indicating glomerulopathy in nls/nls mice.14 Light microscopic analysis of kidney sections revealed no significant histologic differences between wt and mutants at P0.5 (Figure 3, A and B) and identical numbers of mature glomeruli were present in the inner cortex. In developing capillary loop stage glomeruli (typing according to Reeves et al.15), transmission electron microscopy displayed isolated podocyte cell bodies and incipient FPs. Moreover, occluding junctions as well as ladder-like structures appeared between adjacent FPs in both genotypes (Figure 3, C–H). Uniformly formed and regularly spaced FPs as visible already at P0.5 in the wt maturing glomeruli (Figure 3G) were completely absent in the mutant. In the latter, broader and malformed FPs with constricted interspaces occurred (Figure 3H) and only immature slit-spanning membranes between the lateral FP surfaces were seen that never reached the basal pole.
Figure 2.
Cmasnls mice develop massive proteinuria. (A) The protein/creatinine ratio was determined in individual samples at P0.5 (n=5) and P1.5 (n=5) of each genotype. (B) Silver staining (upper panel) and albumin immunoblot (lower panel) of urine from P1.5 wt (n=2), nls/+ (n=2), and nls/nls (n=3) individual mice.
Figure 3.
Destruction of the glomerular filtration barrier in nls/nls mice. (A, B, I and J) Hematoxylin and eosin–stained paraffin sections of renal cortex from (A and I) wt and (B and J) nls/nls mice. Whereas morphologic changes are not obvious at P0.5 in nls/nls mice (B), renal corpuscles with enlarged urinary space (white arrow) and dilated tubules with cast formation in the lumen (black arrow) are evident at P1.5 (J). (C, D, K, and L) Low-power TEM of renal corpuscles from (C) wt and (D) homozygous mutant at P0.5 and from (K) wt and (L) nls/nls mice at P1.5 showing the glomerular filtration barrier. No differences are obvious in immature podocytes at P0.5 in nls/nls mice (D), but dramatically effaced FPs are observed at P1.5 (L). (E–H and M–P) TEM of glomerular filtration barrier of wt (E and G) and homozygous mutants (F and H) at P0.5 showing regular slit membranes (asterisk) and FPs in wt (G), but either immature or malformed FPs with apically located slit-like junctions (black dots) in mutants (H). At P1.5, FPs with mature slit diaphragms (asterisk) are evident in wt (M and O) but immature FPs with occluding junctions or ladder-like structures (arrowhead) are observed in nls/nls mice (N and P). TEM, transmission electron microscopy; C, capillary; E, endothelial cell; P, podocyte.
At P1.5, kidney size and shape in nls/nls mice still appeared normal upon gross anatomic examinations. However, light microscopy of mature glomeruli of the inner cortex revealed a dramatic enlargement of the glomerular urinary space (Figure 3J). Lumina of proximal and distal tubules were expanded and often obstructed with protein casts. Ultrastructural analyses identified either immature or dramatically effaced FP morphology (Figure 3, L, N, and P). Regularly spaced mature FPs were never observed, not even in the most mature glomeruli. In the remaining constricted FP junctions, slit diaphragms were missing and occluding junctions or ladder-like structures occurred instead (Figure 3, F, H, N, and P). In contrast, the two other components forming the glomerular filtration barrier, the fenestrated endothelium, and the glomerular basement membrane (GBM) displayed wt morphology as visualized in transmission electron microscopy (Supplemental Figure 3A) and immunofluorescence with antibodies directed against podocalyxin and vWf (Supplemental Figure 3B). Finally, no sign of mesangial cell activation (Supplemental Figure 4) and no sign of abnormal development in any other organ of P1.5 nls/nls mice were found. Heterozygous mice were indistinguishable from wt.
Unaltered Nuclear Localization but Reduced Expression of CMASnls In Vivo
Kidneys explanted from P0.5 mice were used to interrogate the consequences of the nls mutation at histologic level (Figure 4A). In marked contrast to the cellular system analyzed previously,12 CMASnls was exclusively detected in the nuclear compartment. In a second approach, kidney homogenates of P0.5 mice were fractionated and analyzed by Western blotting. Beyond confirming the strict nuclear localization of CMASnls, this experiment displayed a dramatic reduction in the expression of the mutant CMASnls in kidney (Figure 4B) as well as other tested organs (Supplemental Figure 5). To determine whether the cellular concentrations of free Sia and CMP-Sia were also impacted, detailed quantitative analyses were carried out with the isolated cytosolic and nuclear fractions but did not reveal genotype specific differences in kidney (Table 1) or other organs (Supplemental Table 1). Similarly, an analysis assessing the capacity of wt and nls/nls mutants to synthesize sialoglycoconjugates did not display any obvious alteration (Figure 4C).
Figure 4.
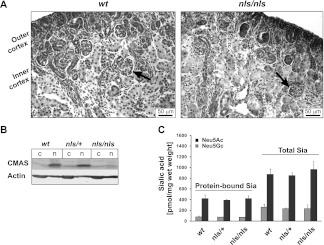
Nuclear but reduced expression of CMASnls does not affect the general sialylation in Cmasnls kidney homogenates. (A) CMAS staining with S59 in P0.5 kidney sections. Mature glomeruli (black arrows) of the inner cortex are marked. Both CMAS and Cmasnls are localized in the nuclear compartment at single cell level. (B) Immunostaining of CMAS with S56 in cytosolic (c) and nuclear (n) fractions of kidney homogenates at P1.5. Actin expression as internal standard. (C) Total and protein-bound Sia were quantified in kidney homogenates of P1.5 mice by the DMB-HPLC method. Values represent means ± SD of six animals for each genotype. N-acetylneuraminic acid (Neu5Ac, black bars) and N-glycolylneuraminic acid (Neu5Gc, gray bars) were separately assessed.
Table 1.
Quantification of sialic acid and CMP-sialic acid in kidney of wt and mutant mice
| Nuclear (Cytosolic) Concentration in Kidney | ||||||
|---|---|---|---|---|---|---|
| Sia (nmol/mg protein) | Neu5Ac (%) | Neu5Gc (%) | CMP-Sia (nmol/mg protein) | CMP-Neu5Ac (%) | CMP-Neu5Gc (%) | |
| +/+ | 1.99±0.27 (2.51±0.44) | 76 (74) | 24 (26) | 6.66±0.65 (6.44±0.37) | 70 (58) | 30 (42) |
| nls/+ | 2.76±0.42 (2.11±0.31) | 80 (73) | 20 (27) | 7.44±0.46 (7.07±0.83) | 65 (59) | 35 (41) |
| nls/nls | 3.20±0.94 (2.71±0.83) | 68 (70) | 32 (30) | 6.92±0.80 (7.97±1.32) | 65 (61) | 35 (39) |
Nuclear and cytosolic tissue fractions were prepared from P1.5 kidney. Samples equivalent to 25 µg protein were dried, hydrolyzed, DMB labeled, and separated by HPLC. For the quantification of CMP-Sia, the keto group of free nonactivated Sia was reduced before hydrolysis to prevent DMB labeling. Concentrations of Sia and CMP-Sia were quantified and the percentages of Neu5Ac and Neu5Gc as well as of the activated sugars CMP-Neu5Ac and CMP-Neu5Gc were specified. Cytosolic values are shown in parentheses. Values represent means ± SD of three animals for each genotype.
Sialylation Is Reduced in Glomeruli of nls/nls Mice
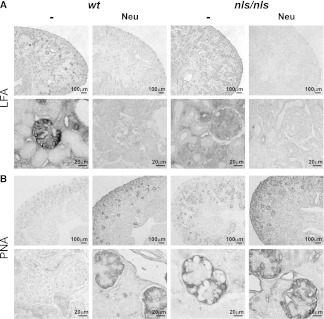
Subsequently, the spatial sialylation patterns in wt and nls/nls kidney were analyzed by staining kidney sections with the lectin Limax flavus agglutinin (LFA), which recognizes sialylated structures independently of the carrier (lipid or protein) and the glycosidic linkage.16 The strong LFA signal visible in P1.5 wt glomeruli—presumably in the visceral epithelial layer—was completely absent in nls/nls kidney (Figure 5A), whereas the basal LFA staining seen in the entire section was maintained. The specificity of LFA for Sia was confirmed by treatment with neuraminidase, which removes Sia (Figure 5A). Complementary to the LFA data, a counterstain with peanut agglutinin (PNA) demonstrated the predominance of galactosyl-β1,3-N-acetylgalactosamine in nls/nls kidney. These structures were masked by Sia in the wt and accessible only after neuraminidase treatment. To note, the identity of the PNA staining in wt and mutant kidney after neuraminidase treatment confirmed that Sia, and not the underlying glycans, was reduced in the mutant organ (Figure 5B).
Figure 5.
Spatial reduction of sialylation in nls/nls glomeruli. (A) Staining of terminal Sia with LFA in P1.5 kidney sections before (−) and after neuraminidase (Neu) treatment. To visualize even weak staining, the tissues were not counterstained. Neu-treated sections are devoid of Sia and serve as negative controls for the LFA staining. Lower lanes represent a larger magnification of the inner rim of the cortex. (B) Staining of terminal galactosyl-β-1,3-N-acetylgalactosamine with PNA in P1.5 kidney sections. Sialylation inhibits PNA binding, whereas Neu treatment uncovers the recognition structure.
Podocyte-Specific Sialylation Defects in nls/nls Kidney
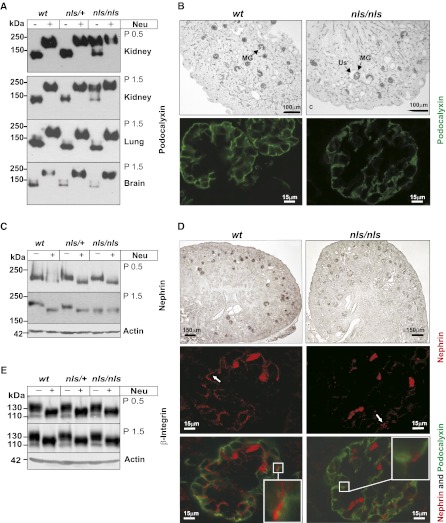
The obvious podocytopathy in the absence of other pathologies prompted us to monitor the sialylation status of individual podocyte proteins. The analysis was started with the major sialoglycoprotein podocalyxin. In accordance with the literature,17 the podocalyxin band, migrating with an apparent molecular mass of approximately 140 kD, shifted to approximately 200 kD after neuraminidase treatment in samples from wt and heterozygous kidney (Figure 6A). In contrast, in nls/nls samples the major podocalyxin band migrated at the level of the desialylated protein. Already evident at P0.5, the hyposialylation was aggravated in P1.5 kidney, but completely absent from lung and brain. Irrespective of the sialylation defect, podocalyxin was correctly expressed in mature glomeruli of the inner rim of the cortex (Figure 6B, upper panel) and located at the podocytes’ surface (Figure 6B, lower panel). Because regular slit diaphragms were almost not detectable in nls/nls mice, we scrutinized nephrin-180, proposed to form the scaffold of this structure by homophilic trans-interactions.18 The significant molecular mass shift induced by neuraminidase treatment clearly identified the protein as a sialoglycoconjugate (Figure 6C). Importantly, a wt-like nephrin sialylation was observed in P0.5 nls/nls samples; however, the band co-migrated with asialo-nephrin (nephrin after neuraminidase treatment) 1 day later, demonstrating abolishment of nephrin sialylation at P1.5. The observed progressive loss of Sia on nephrin paralleled the progression of morphologic disturbances in the podocyte ultrastructure (Figure 3). Nevertheless, similar to asialo-podocalyxin, asialo-nephrin was also expressed in mature glomeruli of the inner rim of the cortex (Figure 6D, upper panel) and transported to the podocyte surface (Figure 6D, middle panel). However, in contrast to wt where nephrin signals light up like pearls on a string, isolated nephrin spots indicated the lack of slit diaphragms in the glomeruli of nls/nls mice. In keeping with the regular destination of podocalyxin and nephrin in nls/nls podocytes, colocalization of the two proteins was never observed (Figure 6D, lower panel), indicating that the separate subcellular sorting routes were maintained.
Figure 6.
Kidney-specific sialylation analysis of distinct proteins in nls/nls mice. (A) Podocalyxin immunoblot of total lysates from P0.5 and P1.5 organs of all genotypes before (−) and after (+) removal of sialic acids by neuraminidase (Neu) treatment. (B) Immunostaining of podocalyxin in paraffin-embedded kidney sections of wt and nls/nls mice analyzed by light microscopy (upper panel) and fluorescence staining of podocalyxin (lower panel, green) in P1.5 wt and nls/nls kidney paraffin sections analyzed by confocal microscopy. MGs were stained positive for podocalyxin, whereas ureteric buds, renal vesicles, or comma-shaped bodies remain unstained. (C) Nephrin immunoblot of total kidney lysates at P0.5 and P1.5 before (−) and after (+) Neu treatment. (D) Immunostaining of nephrin in paraffin-embedded kidney sections of wt and nls/nls mice analyzed by light microscopy (upper panel), fluorescence staining of nephrin in red (middle panel), and fluorescence double staining (bottom panel) of podocalyxin (green)/nephrin (red) in P1.5 wt and nls/nls kidney paraffin sections analyzed by confocal microscopy. Selected areas are shown with larger magnification in white boxes. Nephrin dots are marked with white arrows. Erythrocytes are stained unspecifically. (E) β1-integrin immunoblot of P0.5 and P1.5 untreated (−) or Neu-treated (+) kidney lysates. Actin is the loading control. Us, enlarged urinary space.
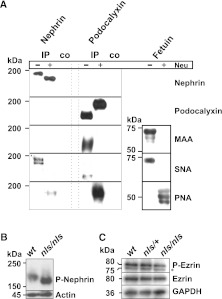
β1-integrin was investigated as another major sialoglycoconjugate of podocytes, but showed no genotype- or age-related alterations (Figure 6E). Because β1-integrin is known to carry Sia in α2,3-linkage19 and glomerular injury was described after enzymatic removal of α2,6-linked Sia,2 we hypothesized that podocytopathy in nls/nls mice resulted from the selective disturbance of α2,6-sialylated products and consequently analyzed the linkage-specificity of nephrin and podocalyxin sialylation (Figure 7A). Both proteins were precipitated from wt kidney homogenates, subjected to Western blotting and probed with the lectins Maackia amurensis agglutinin and Sambucus nigra agglutinin. M. amurensis agglutinin, which recognizes Sia in α2,3-linkage was only reactive to podocalyxin, whereas the α2,6-specific S. nigra agglutinin stained both nephrin and podocalyxin. Pretreatment with neuraminidase abolished the signals produced by the Sia-specific lectins and exposed the penultimate sugar galactosyl-β1,3-N-acetylgalactosamine, recognized by PNA. Fetuin was used as a positive control. The data obtained prove that nephrin sialylation in mice exclusively occurs in α2,6-linkage, whereas podocalyxin carries Sia in α2,3- and α2,6-linkage. A subsequent screen of other podocyte-associated sialoglycoconjugates (i.e., TRPC6, dystroglycan, densin, and synaptopodin) did not reveal any genotype-related differences in sialylation patterns (Supplemental Figure 6).
Figure 7.
Quality of nephrin and podocalyxin sialylation influences downstream targets in nls/nls mice. (A) Lectin analysis of nephrin and podocalyxin sialylation. Nephrin and podocalyxin were immunoprecipitated from wt kidney homogenates and analyzed by Western blotting before and after neuraminidase (Neu) treatment (upper panels). α2,6-linked Sia were detected on both proteins with SNA, whereas α2,3-linked Sia was only visible on podocalyxin (MAA staining). PNA detects terminal galactosyl (β-1,3) N-acetylgalactosamine (lower panels). Fetuin served as a positive control for lectin staining. (B) Nephrin phosphorylation and (C) expression of ezrin and phosphorylated ezrin were analyzed in P1.5 kidney homogenates of the indicated genotypes by SDS-PAGE and Western blotting. Cross-reaction with phosphorylated moesin (asterisk) was observed. Actin and GAPDH were used as loading standards. IP, nephrin immunoprecipitate; co, beads without antibody-load served as negative control; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; SNA, S. nigra agglutinin; MAA, M. amurensis agglutinin.
Because the results described thus far assign lethal sialylation defects to the capillary loop stage, the integrity of the sialylation reaction at earlier time points of kidney development was investigated. In comma- and S-shaped bodies, the neural cell adhesion molecule (NCAM) is highly expressed and known to carry long chains of α2,8-linked polysialic acid.20 The prevalence of polysialylated NCAM was qualitatively as well as quantitatively identical in wt and mutants and thus again highlighted the nls/nls mouse as a unique model exhibiting so far unparalleled sialylation defects in a tissue-, time-, and even molecule-specific mode (Supplemental Figure 7).
Discussion
The nls/nls mouse model, originally designed to relocate a functional CMAS to the cytoplasm, demonstrated that in contrast to the cellular system, genetic depletion of only one of two potential NLS motifs present in CMAS12 was insufficient to prevent nuclear entry in mice. Instead, the introduced point mutations caused a significant reduction in the expression level of the mutant protein CMASnls. At a functional level, the reduction manifested exclusively in podocytes, leading to a stop in maturation that could be explained by specific sialylation deficits in the major podocyte proteins nephrin and podocalyxin.
This selective phenotypic manifestation clearly differentiates the new mouse model from the earlier described GneM712T/M712T mouse,21 in which podocytopathy developed on the background of a constitutively and systemically depressed sialylation (see the defective NCAM polysialylation in GneM712T/M712T mouse brain). Moreover, GneM712T/M712T mice are smaller than wt littermates at the day of birth, showing petechial hemorrhages and split GBMs. In marked contrast, prenatal development is normal in Cmasnls/nls mice and alterations manifest progressively after birth along with podocyte maturation. A morphology-guided screen allowed us to identify the defects in the major podocyte proteins nephrin and podocalyxin. Other important podocyte proteins (i.e., TRPC6, dystroglycan, densin, and synaptopodin) retained patterns indistinguishable from wt.
Significant similarities between the current mouse model and nephrin-knockout mice,22 as well as patients with congenital nephrotic syndrome of the Finnish type harboring mutations in the human nephrin gene,23 indicate the requirement for nephrin sialylation. In fact, anatomic structures representing the initial steps in podocyte maturation15,24 were identified at P0.5 when nephrin was still sialylated. At P1.5, the sialylation of nephrin was abolished, slit diaphragms were absent, and the maturation block was evident. Furthermore, at this time point, a significantly increased Tyr-phosphorylation of asialo-nephrin indicated an abnormal intracellular signaling to downstream targets in nls/nls mice (Figure 7B).25 In contrast, the lack of Sia did not affect nephrin expression and subcellular transport, as is reported for the inhibition of nephrin N-glycosylation.26
In contrast to the intriguing parallels that exist between nls/nls mice and nephrin-knockouts,22 important features distinguish the model from podocalyxin knockouts.27 The latter die within 24 hours after birth due to anuric renal failure. At a morphologic level, these mice fail to form FPs and develop impermeable tight junctions instead of slit diaphragms.8,27 Nevertheless, effacement and reduced intracellular space between cell bodies and pedicels as well as between adjacent pedicels in nls/nls mice underline the crucial role that electrostatic forces (provided by the highly sialylated podocalyxin) play for the morphogenesis of FPs. Intracellular coupling of podocalyxin to the cytoskeleton is mediated by ezrin phosphorylation.28 Enzymatic desialylation has been shown to impair signaling29 and reduced levels of P-ezrin also indicated a functional loss in nls/nls mice (Figure 7C). Hyposialylated podocalyxin still allowed the initiation of FPs, albeit formation of irregular connections, whereas maturation of the complex slit diaphragm depended on nephrin sialylation. Thus, progression of maturation deficiencies reflects the impaired sialylation of both nephrin and podocalyxin.
The unexpected finding that sialylation defects in the nls/nls mice are limited to podocytes is based on the fact that the onset of extensive FP formation (a process that proceeds in the postnatal phase in mice) requests high local CMP-Sia concentrations as confirmed by the strong LFA signal in wt capillary loop stage and maturing glomeruli. By use of lectins that recognize Sia in specific glycosidic linkages, we demonstrate here for the first time that nephrin sialylation occurs exclusively in α2,6-linkage in vivo in mice. Similarly, α2,6-linked Sia was found to be prominent also on podocalyxin (Figure 7A). Both proteins progressively lost Sia between P0.5 and P1.5, whereas other major podocyte proteins showed perfectly normal sialylation. This finding suggests that the α2,6-sialyltransferase(s) responsible for the modification of nephrin and podocalyxin are disadvantaged in the concurrence for the substrate CMP-Sia. Because the expression of CMASnls is drastically reduced, CMP-Sia cannot be supplied in a sufficient quantity to meet the requirements of all linkage-specific sialyltransferases in the critical phase of podocyte maturation.
The kidney is well known as an organ of minimum tolerance against alterations in the sialylation machinery.21 Not surprising thus that animal models exhibiting mutations that lead to a general decrease in cellular Sia levels develop nephropathies.21,30,31 The nls/nls mouse model, however, is the first that unequivocally identified the candidates and processes that crucially depend on sialylation during the construction of the size and charge controlled filtration barrier. Although CMAS mutations in humans presumably result in embryonic lethality and may not be of postnatal clinical relevance, the pathology of acquired sialylation deficiencies caused by neuraminidase producing germs may include targets as described for nls/nls mice.
In conclusion, our data prove that proper sialylation of nephrin and podocalyxin is as essential for the morphogenesis, maturation, and maintenance of the visceral part of the distinctly layered kidney filtration barrier as are the carrier proteins themselves. This new animal model should be of major relevance to study the progression of nephropathies on a molecular level and to develop treatment strategies.
Concise Methods
Antibodies
Details on antibodies and lectins are described in Supplemental Table 2.
Mice
A targeting vector (Figure 1 A) was introduced into E14–1 embryonic stem cells. The entire vector was sequence verified. Embryonic stem cells were injected into C57Bl/6J blastocysts and resulting offspring were backcrossed for six generations onto C57Bl/6J. Animals were hosted in the animal facility of the Hannover Medical School under specific pathogen-free conditions. All animal experiments were carried out in compliance with German law for protection of animals and were approved by the local authorities.
Urine Analyses
Creatinine and total protein of urine samples were measured in an Olympus AU 400 analyzer (Olympus Deutschland GmbH, Hamburg, Germany). The creatinine concentration was determined by a kinetic color reaction (Jaffé method), and the protein concentration was measured photometrically with pyrogallol red and molybdate reagents.
Preparation of Kidney Lysates
Total lysates were prepared in Triton buffer (2% Triton X-100, 20 mM Tris pH 8.0, 150 mM NaCl, 5 mM EDTA, and protease inhibitors). Cytosolic and nuclear fractions were prepared according to Dignam et al.32 Vibrio cholerae neuraminidase was purchased from Roche.
SDS-PAGE and Western Blot Analyses
SDS-PAGE was performed according to Laemmli, and Western blots were developed as described.12,33 Appropriate horseradish peroxidase–coupled secondary antibodies and an enhanced chemiluminescence assay were used for detection. For immunoprecipitation, kidney homogenates (2 mg total protein) were incubated with 1 µg antinephrin antibody or 2 µg antipodocalyxin antibody overnight at 4°C. After incubation with ProteinA or ProteinG coupled to Sepharose, the nephrin immunoprecipitates were washed, Laemmli sample buffer was added, and the samples were applied to SDS-PAGE and subsequent Western blotting. Lectin staining was carried out according to the manufacturer’s instructions (Roche).
Analyses of Sia, CMP-Sia, and Polysialic Acid
Samples were prepared and the determination of total and protein-bound Sia by the 1,2-diamino-4,5-methylenedioxybenzene (DMB)–HPLC application was performed as described previously.34,35 To quantify Sia and CMP-Sia in the cytosol or nucleus, extracts were prepared according to Dignam et al.32 and samples equivalent to 25 µg protein were dried. Hydrolysis and DMB labeling as well as HPLC separation were performed as mentioned for total and protein-bound Sia. For the quantification of CMP-Sia, the keto group of free nonactivated Sia was reduced before hydrolysis to prevent DMB labeling.36 The degree of polymerization was carried out according to previous protocols by the use of 5 mg original tissue.34,37,38 Endosialidase from bacteriophage K1F was purified as described previously39 and used for polySia degradation.12
Immunohistochemistry
Mouse tissues were fixed in 4% paraformaldehyde/0.1 M PBS and were embedded in paraffin; 3-µm sections were stained with hematoxylin and eosin following standard procedures. For immunohistochemistry, tissue sections were heated for 1 hour at 80°C, deparaffinized, incubated overnight in 0.01 M citrate buffer pH 6.0 at 75°C, and endogenous peroxidase activity was blocked with 0.75% H2O2 in methanol. Binding of anti-CMAS and secondary antibodies was detected with Elite Vectastain ABC (Vector Labs) and visualized by the DAB Peroxidase Substrate Kit (Vector Lab). For α-smooth muscle actin immunostaining, the sections were deparaffinized, blocked by blocking solution from the Dako ARK kit, washed with PBS/Tween, and incubated with first and secondary antibodies followed by DAB substrate. Hemalaun was used for nuclear staining. Lectin staining was carried out according to Martin et al.40 In brief, deparaffinized tissue sections were preincubated for 2.5 hours at 37°C with or without Arthrobacter ureafaciens neuraminidase (EY Labs). Peroxidase-coupled LFA was incubated overnight at 4°C and developed with DAB/nickel. Digoxigenin-labeled PNA was incubated overnight at 4°C, and a peroxidase-coupled antidigoxigenin antibody was incubated for 1 hour and developed with DAB/nickel. Endogenous peroxidase was blocked by blocking solution from the Dako ARK kit. The slices were mounted with vitroclud (Langenbrinck). For immunofluorescence, sections were blocked with 5% BSA or 5% skim milk. Antibodies against nephrin, podocalyxin, and vWF were incubated overnight at 4°C and washed in PBS. Subsequently, sections were incubated with the Cy 3–labeled donkey anti-guinea pig IgG for nephrin detection, with FITC- or Alexa568-labeled donkey anti-goat IgG for podocalyxin detection and with Alexa488-labeled donkey anti-rabbit IgG for detection of vWF. Slices were mounted with Vectashield (Vector Labs). Microscopy was performed with a Zeiss Axiovert 200 M microscope equipped with an AxioCam MRm digital camera and AxioVison software (Zeiss). Confocal laser scanning microscopy of fluorescent-labeled sections was performed with a Zeiss Axiovert 200 M microscope (Zeiss) equipped with a Zeiss LSM 510 Meta scan head, an Argon laser, and a He/Ne laser. Images were acquired using LSM 5 image software (Zeiss).
Electron Microscopy
Tissues were fixed in 3% glutaraldehyde buffered with 0.1M sodium cacodylate/HCl pH 7.4 overnight and washed in cacodylate buffer. The specimens were postfixed for 2 hours at 4°C in 2% OsO4, washed in cacodylate buffer, and consecutively treated with solutions of 1% tannic acid and 1% Na2SO4, both dissolved in 0.1M sodium cacodylate/HCl pH 7.4. After dehydration with graded series of ethanol followed by propylenoxide, specimens were embedded in epoxy resin. Ultrathin sections (70 nm) were stained with uranyl acetate and lead citrate,41 and observed with a Morgagni 268 electron microscope (FEI, Europe) at 80 kV.
Disclosures
None.
Acknowledgments
We are grateful to H. Bakker, H. Hildebrandt, H. Haller, C. Klein, M. Mühlenhoff, and K.M.H. Philippens, Hannover Medical School, for fruitful discussion, as well as to M. Levandowski, University of California, for Zp3-cre mice. We thank I. Albers, W. Mink, and D. Wittenberg for expert technical assistance. We appreciate the help of Nissi Varki in establishing the lectin staining on tissue sections.
This work received financial support from the German Research Foundation (DFG) to A.K.M.-K. (MU1849/1), R.G.-S. (DFG Research Unit 548), and M. Schiffer (SCHI 587/3-1). W.S. is supported by funding from the DFG for the Cluster of Excellence REBIRTH.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011090947/-/DCSupplemental.
References
- 1.Varki A: Sialic acids in human health and disease. Trends Mol Med 14: 351–360, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gelberg H, Healy L, Whiteley H, Miller LA, Vimr E: In vivo enzymatic removal of alpha 2—>6-linked sialic acid from the glomerular filtration barrier results in podocyte charge alteration and glomerular injury. Lab Invest 74: 907–920, 1996 [PubMed] [Google Scholar]
- 3.Crocker PR, Varki A: Siglecs, sialic acids and innate immunity. Trends Immunol 22: 337–342, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Schauer R: Sialic acids as regulators of molecular and cellular interactions. Curr Opin Struct Biol 19: 507–514, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varki NM, Varki A: Diversity in cell surface sialic acid presentations: Implications for biology and disease. Lab Invest 87: 851–857, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwarzkopf M, Knobeloch KP, Rohde E, Hinderlich S, Wiechens N, Lucka L, Horak I, Reutter W, Horstkorte R: Sialylation is essential for early development in mice. Proc Natl Acad Sci U S A 99: 5267–5270, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews PM: Glomerular epithelial alterations resulting from sialic acid surface coat removal. Kidney Int 15: 376–385, 1979 [DOI] [PubMed] [Google Scholar]
- 8.Takeda T, Go WY, Orlando RA, Farquhar MG: Expression of podocalyxin inhibits cell-cell adhesion and modifies junctional properties in Madin-Darby canine kidney cells. Mol Biol Cell 11: 3219–3232, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurihara H, Anderson JM, Kerjaschki D, Farquhar MG: The altered glomerular filtration slits seen in puromycin aminonucleoside nephrosis and protamine sulfate-treated rats contain the tight junction protein ZO-1. Am J Pathol 141: 805–816, 1992 [PMC free article] [PubMed] [Google Scholar]
- 10.Kean EL, Münster-Kühnel AK, Gerardy-Schahn R: CMP-sialic acid synthetase of the nucleus. Biochim Biophys Acta 1673: 56–65, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Münster-Kühnel AK, Tiralongo J, Krapp S, Weinhold B, Ritz-Sedlacek V, Jacob U, Gerardy-Schahn R: Structure and function of vertebrate CMP-sialic acid synthetases. Glycobiology 14: 43R–51R, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Münster AK, Weinhold B, Gotza B, Mühlenhoff M, Frosch M, Gerardy-Schahn R: Nuclear localization signal of murine CMP-Neu5Ac synthetase includes residues required for both nuclear targeting and enzymatic activity. J Biol Chem 277: 19688–19696, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Lewandoski M, Wassarman KM, Martin GR: Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr Biol 7: 148–151, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Ratge D, Wisser H: Urinary protein profiling by high-performance gel permeation chromatography. J Chromatogr A 230: 47–56, 1982 [DOI] [PubMed] [Google Scholar]
- 15.Reeves W, Caulfield JP, Farquhar MG: Differentiation of epithelial foot processes and filtration slits: Sequential appearance of occluding junctions, epithelial polyanion, and slit membranes in developing glomeruli. Lab Invest 39: 90–100, 1978 [PubMed] [Google Scholar]
- 16.Knibbs RN, Goldstein IJ, Ratcliffe RM, Shibuya N: Characterization of the carbohydrate binding specificity of the leukoagglutinating lectin from Maackia amurensis. Comparison with other sialic acid-specific lectins. J Biol Chem 266: 83–88, 1991 [PubMed] [Google Scholar]
- 17.Dekan G, Gabel C, Farquhar MG: Sulfate contributes to the negative charge of podocalyxin, the major sialoglycoprotein of the glomerular filtration slits. Proc Natl Acad Sci U S A 88: 5398–5402, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patrakka J, Tryggvason K: Nephrin—a unique structural and signaling protein of the kidney filter. Trends Mol Med 13: 396–403, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Gu JG, Taniguchi N: Regulation of integrin functions by N-glycans. Glycoconj J 21: 9–15, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Lackie PM, Zuber C, Roth J: Polysialic acid and N-CAM localisation in embryonic rat kidney: Mesenchymal and epithelial elements show different patterns of expression. Development 110: 933–947, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Galeano B, Klootwijk R, Manoli I, Sun M, Ciccone C, Darvish D, Starost MF, Zerfas PM, Hoffmann VJ, Hoogstraten-Miller S, Krasnewich DM, Gahl WA, Huizing M: Mutation in the key enzyme of sialic acid biosynthesis causes severe glomerular proteinuria and is rescued by N-acetylmannosamine. J Clin Invest 117: 1585–1594, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Putaala H, Soininen R, Kilpeläinen P, Wartiovaara J, Tryggvason K: The murine nephrin gene is specifically expressed in kidney, brain and pancreas: Inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet 10: 1–8, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Tryggvason K, Patrakka J, Wartiovaara J: Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354: 1387–1401, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Doné SC, Takemoto M, He L, Sun Y, Hultenby K, Betsholtz C, Tryggvason K: Nephrin is involved in podocyte maturation but not survival during glomerular development. Kidney Int 73: 697–704, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB: Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest 116: 1346–1359, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan K, Khoshnoodi J, Ruotsalainen V, Tryggvason K: N-linked glycosylation is critical for the plasma membrane localization of nephrin. J Am Soc Nephrol 13: 1385–1389, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Doyonnas R, Kershaw DB, Duhme C, Merkens H, Chelliah S, Graf T, McNagny KM: Anuria, omphalocele, and perinatal lethality in mice lacking the CD34-related protein podocalyxin. J Exp Med 194: 13–27, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orlando RA, Takeda T, Zak B, Schmieder S, Benoit VM, McQuistan T, Furthmayr H, Farquhar MG: The glomerular epithelial cell anti-adhesin podocalyxin associates with the actin cytoskeleton through interactions with ezrin. J Am Soc Nephrol 12: 1589–1598, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Takeda T, McQuistan T, Orlando RA, Farquhar MG: Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest 108: 289–301, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kakani S, Yardeni T, Poling J, Ciccone C, Niethamer T, Klootwijk ED, Manoli I, Darvish D, Hoogstraten-Miller S, Zerfas P, Tian E, Ten Hagen KG, Kopp JB, Gahl WA, Huizing M: The Gne M712T mouse as a model for human glomerulopathy. Am J Pathol 180: 1431–1440, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malicdan MCV, Noguchi S, Nonaka I, Hayashi YK, Nishino I: A Gne knockout mouse expressing human GNE D176V mutation develops features similar to distal myopathy with rimmed vacuoles or hereditary inclusion body myopathy. Hum Mol Genet 16: 2669–2682, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Dignam JD, Lebovitz RM, Roeder RG: Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 11: 1475–1489, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Münster AK, Eckhardt M, Potvin B, Mühlenhoff M, Stanley P, Gerardy-Schahn R: Mammalian cytidine 5′-monophosphate N-acetylneuraminic acid synthetase: A nuclear protein with evolutionarily conserved structural motifs. Proc Natl Acad Sci U S A 95: 9140–9145, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galuska SP, Oltmann-Norden I, Geyer H, Weinhold B, Kuchelmeister K, Hildebrandt H, Gerardy-Schahn R, Geyer R, Mühlenhoff M: Polysialic acid profiles of mice expressing variant allelic combinations of the polysialyltransferases ST8SiaII and ST8SiaIV. J Biol Chem 281: 31605–31615, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Galuska SP, Geyer R, Gerardy-Schahn R, Mühlenhoff M, Geyer H: Enzyme-dependent variations in the polysialylation of the neural cell adhesion molecule (NCAM) in vivo. J Biol Chem 283: 17–28, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Galuska SP, Geyer H, Weinhold B, Kontou M, Röhrich RC, Bernard U, Gerardy-Schahn R, Reutter W, Münster-Kühnel A, Geyer R: Quantification of nucleotide-activated sialic acids by a combination of reduction and fluorescent labeling. Anal Chem 82: 4591–4598, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Inoue S, Lin SL, Lee YC, Inoue Y: An ultrasensitive chemical method for polysialic acid analysis. Glycobiology 11: 759–767, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Nakata D, Troy FA, 2nd: Degree of polymerization (DP) of polysialic acid (polySia) on neural cell adhesion molecules (N-CAMS): Development and application of a new strategy to accurately determine the DP of polySia chains on N-CAMS. J Biol Chem 280: 38305–38316, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Stummeyer K, Dickmanns A, Mühlenhoff M, Gerardy-Schahn R, Ficner R: Crystal structure of the polysialic acid-degrading endosialidase of bacteriophage K1F. Nat Struct Mol Biol 12: 90–96, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Martin LT, Marth JD, Varki A, Varki NM: Genetically altered mice with different sialyltransferase deficiencies show tissue-specific alterations in sialylation and sialic acid 9-O-acetylation. J Biol Chem 277: 32930–32938, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Reynolds ES: The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17: 208–212, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]