Abstract
NF-κB activation is central to the initiation and progression of inflammation, which contributes to the pathogenesis of CKD. Tissue plasminogen activator (tPA) modulates the NF-κB pathway, but the underlying mechanism remains unknown. We investigated the role of tPA signaling in macrophage NF-κB activation and found that tPA activated NF-κB in a time- and dose-dependent manner. tPA also induced the expression of the NF-κB–dependent chemokines IP-10 and MIP-1α. The protease-independent action of tPA required its membrane receptor, annexin A2. tPA induced the aggregation and interaction of annexin A2 with integrin CD11b, and ablation of CD11b or administration of anti-CD11b neutralizing antibody abolished the effect of tPA. Knockdown of the downstream effector of CD11b, integrin-linked kinase, or disruption of its engagement with CD11b also blocked tPA-induced NF-κB signaling. In vivo, tPA-knockout mice had reduced NF-κB signaling, fewer renal macrophages, and less collagen deposition than their counterparts. Taken together, these data suggest that tPA activates the NF-κB pathway in macrophages through a signaling pathway involving annexin A2/CD11b-mediated integrin-linked kinase.
Despite the initial etiology, inflammation induced at the site of injury is a hallmark of CKD and plays an essential role in the pathogenesis and progression of CKD. In disease conditions, inflammatory cells, such as monocytes/macrophages, are recruited in response to injury cues and express a panoply of proinflammatory genes through a combination of transcription factors, of which NF-κB is the most fundamental one.1 The NF-κB family of transcription factors regulates the induction, progression, and resolution of inflammation. They exist in the forms of homo- and heterodimers that form among the five members of NF-κB family: p50, p52, p65 (RelA), RelB, and c-Rel; they are retained in the cytoplasm by its specific inhibitor, inhibitory κ B (IκB).2,3 NF-κB can be activated through either canonical (p65/p50) or noncanonical (RelB/p52) pathways. Canonical NF-κB signal transduction can be activated through phosphorylation and degradation of IκB, resulting in the release and nuclear translocation of p65/p50 and subsequently, DNA binding and gene transcription.2,3 Mounting evidence shows that activation of NF-κB correlated with the progression of CKD and that inhibition of NF-κB ameliorates renal inflammation and CKD progression.3–7 However, the underlying mechanisms and the regulation of NF-κB pathways during CKD remain to be elucidated.
Tissue plasminogen activator (tPA), as a member of the serine protease family, participates in the activation of various zymogens and certain growth factors and plays a pivotal role in the homeostasis of blood coagulation/fibrinolysis and matrix regulation.8–11 However, recent studies show that tPA also acts as a profibrotic cytokine to promote the progression of CKD by triggering profound receptor-mediated intracellular signaling events.8,12–16 Emerging evidence suggests that tPA may also modulate the inflammatory response to tissue injury.17,18 First, in a carbon tetrachloride-induced hepatic damage model, tPA-deficient mice displayed a decreased CD4 and CD8 T cell infiltration in contrast to the wild-type mice.17 Second, in an ischemia/reperfusion model of acute renal injury, tPA-deficient mice displayed less leukocyte infiltration in the diseased kidneys compared with their wild-type counterparts.18 However, the underlying mechanisms, where tPA modulates renal inflammation during injury, remain largely unknown. Recent evidence suggests that tPA modulates NF-κB pathways in an ischemic brain injury model.19 Thus, we hypothesize that tPA may promote renal inflammation by activating NF-κB pathway.
Annexin A2 is a member of the Ca2+- and phospholipid-binding protein family that has unique structure that allows it to dock onto membrane in a peripheral and reversible manner.20 Annexin A2 has been identified as a major membrane receptor of tPA on endothelial,21 microglia cells,22 and other cancer cells,23 and it is implicated in mediating certain signal transductions.22,24,25 However, unlike the other well-known tPA receptor, LDL receptor-related protein 1 (LRP-1), annexin A2 is a membrane-associated protein, lacking transmembrane domain to which tPA can only dock.20,23 It remains unknown how annexin A2 transactivates the intracellular signaling cascade initiated by the binding of extracellular tPA.
In the present study, we show that tPA activates NF-κB pathway and induces the expression of the associated proinflammatory chemokines in macrophages through a novel signaling cascade involving annexin A2-mediated aggregation of integrin CD11b and the subsequent activation of integrin-linked kinase (ILK) pathway.
Results
tPA Induces Canonical NF-κB Activation in Macrophages
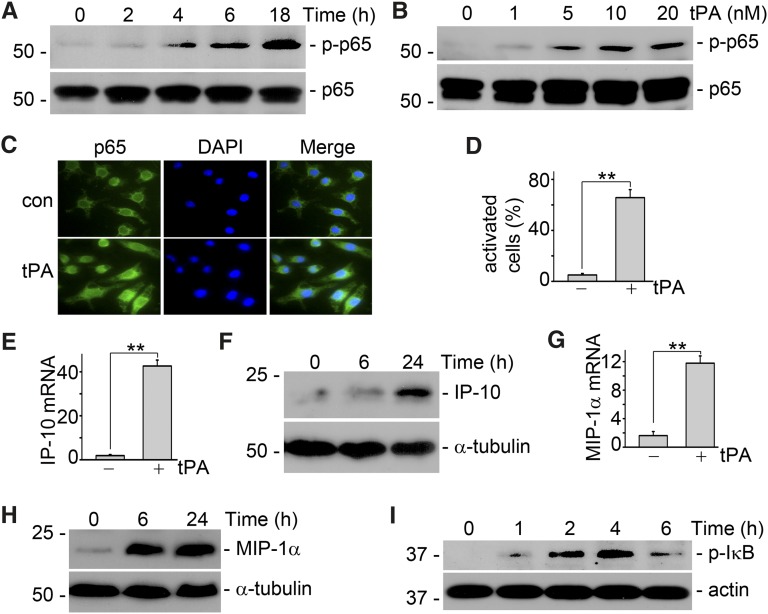
To investigate the role of tPA in NF-κB signaling, we evaluated the effect of tPA on p65 phosphorylation, a classic marker of NF-κB activation. We found that tPA activated NF-κB pathway in a time- (Figure 1A) and dose-dependent (Figure 1B) manner. We also confirmed that tPA induced the nuclear translocation of p65 in macrophages, another marker of NF-κB activation, which was illustrated by immunofluorescence staining (Figure 1, C and D). In addition, tPA stimulated the mRNA and protein expression of NF-κB–dependent IP-10 (Figure 1, E and F) and MIP-1α (Figure 1, G and H) chemokines in macrophages,2,26 which was shown by the quantitative real-time PCR and Western blot analyses. Of note, tPA also promoted the phosphorylation of IκB (Figure 1I), suggesting that tPA activates canonical NF-κB pathway in macrophages. Thus, tPA not only activates the classic NF-κB pathway but also induces the expression of NF-κB–dependent chemokines in macrophages.
Figure 1.
tPA activates NF-κB pathway in macrophages. (A) J744 macrophages were treated with 10 nM tPA for 0, 2, 4, 6, and 18 hours. Western blot showed that tPA induced phosphorylation of p65, a classic marker of NF-κB activation, as early as 4 hours. (B) J774 cells were incubated with 0, 1, 5, 10, and 20 nM tPA for 6 hours followed by Western blot for phospho-p65 and total p65. (C) Macrophages were incubated with vehicle or 10 nM tPA for 6 hours followed by immunofluorescence staining with anti-p65 antibody and DAPI. Original magnification, ×400. (D) Quantitative illustration of tPA-induced p65 nuclear translocation in J774 macrophages. Unit was expressed as the number of macrophages with p65 nuclear translocation per 100 cells. **P<0.01 (n=3 experiments). (E). Macrophages were treated with vehicle or 10 nM tPA for 6 hours followed by quantitative PCR assay for chemokine interferon-inducible protein-10. **P<0.01 (n=3). (F) Interferon-inducible protein-10 protein level was determined by Western blot. Macrophages were incubated with 10 nM tPA for indicated periods. (G) Macrophage-inflammatory protein-1α mRNA level was evaluated by quantitative PCR assay after treatment with 10 nM tPA for 6 hours. **P<0.01 (n=3). (H) Macrophage inflammatory protein-1α protein level was assessed by Western blot. J774 cells were treated with 10 nM tPA for 0, 6, and 24 hours. In the cases to determine the cellular protein level of chemokines, J774 macrophages were incubated with Brefeldin A solution (eBioscience, San Diego, CA) 5 hours before harvest to blockade the releasing of chemokines from the cells. (I) tPA induced phosphorylation of IκB, a marker of canonic activation of NF- κB, as indicated by Western blot analysis.
tPA-Induced NF-κB Activation Is through Its Cytokine Functions and Mediated by Annexin A2
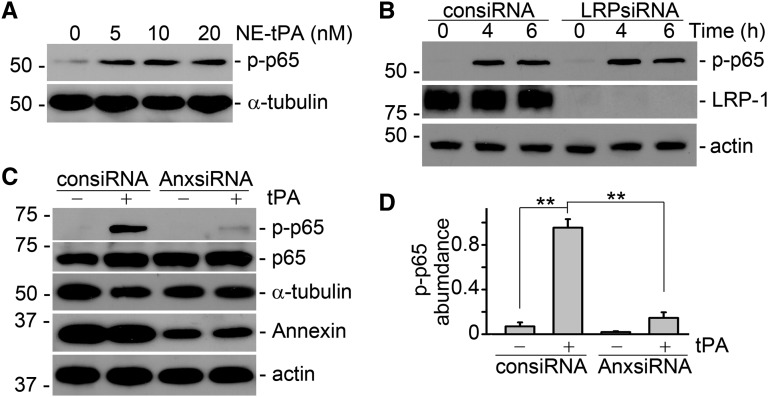
tPA is a hybrid molecule of protease and cytokine.8,12–15 We wanted to know if tPA-induced activation of the NF-κB pathway depends on its protease activity or if it is mediated through its cytokine function-initiated intracellular signaling cascade. To this end, J774 macrophages were treated with vehicle and a mutant, catalytically inactive tPA (in which the serine within the active site of the enzyme was replaced with alanine27 at various concentrations of 5, 10, and 20 nM, comparable with the wild-type tPA). As shown in Figure 2A, the nonenzymatic tPA also induced NF-κB activation in a dose-dependent manner, indicating that activation of the NF-κB pathway is independent of the protease activity of tPA. We next investigated the role of tPA receptor in NF-κB activation. We surprisingly found that small interfering RNA (siRNA) knockdown of LRP-1, the major cell membrane receptor of tPA mediating multiple cytokine actions of tPA in fibroblasts,8,12–15 had little effect on p65 phosphorylation in macrophages (Figure 2B), suggesting that LRP-1 does not mediate the tPA-activated NF-κB pathway in macrophages. Instead, knockdown of the expression of annexin A2, another cell-surface receptor for tPA21,22 that has the signaling potential,22,24,25 abolished tPA-induced NF-κB activation in macrophages (Figure 2, C and D). Thus, it is clear that annexin A2 but not LRP-1 mediates tPA-activated NF-κB signaling cascade in macrophages. However, it remains unknown that how annexin A2, a cell-surface receptor without transmembrane domain, mediates tPA-initiated signaling transduction.
Figure 2.
tPA-activated NF-κB signaling is receptor annexin A2-mediated and independent of its protease activity. (A) Nonenzymatic tPA (NE-tPA) induced phosphorylation of p65 in a dose-dependent manner. J774 cells were incubated with NE-tPA of indicated concentrations for 6 hours. (B) Knockdown of LRP-1 by RNA silence did not affect tPA-induced NF-κB activation in J774 macrophages. (C) Western blot analysis showed that knockdown of annexin A2 in J774 macrophages abolished tPA-induced NF-κB activation. (D) Quantitative presentation of the relative abundance of phospho-p65 in macrophages treated with control (consiRNA) and annexin A2 (AnxsiRNA) siRNAs. **P<0.01 (n=3). J774 macrophages were incubated with vehicle or 10 nM tPA for 6 hours unless indicated elsewhere.
tPA Promotes the Aggregation of Annexin A2 and CD11b Integrin
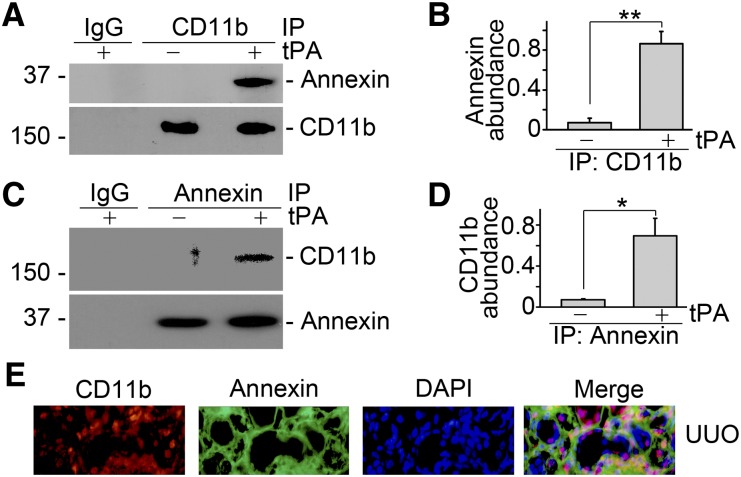
Because annexin A2 docks onto cell membrane in a peripheral manner and lacks the transmembrane domain,20 it is presumable that annexin A2 may function as a coreceptor of the known outside-in signal transduction pathway, such as the integrin pathway. Based on the recent finding that tPA directly binds to integrin Mac-1 (CD11b/CD18) in macrophages,28 we hypothesized that tPA could promote the clustering and activation of integrin outside-in signaling by promoting formation of the complex of annexin A2 and CD11b. To test this hypothesis, we investigated the possible interaction between annexin A2 and CD11b by coimmunoprecipitation. As shown in Figure 3, A–D, tPA promoted the aggregation of annexin A2 and CD11b in J774 macrophages. Of note, annexin A2 also colocalized with CD11b in diseased kidney with unilateral ureteral obstruction (UUO) as indicated by double immune staining (Figure 3E).
Figure 3.
tPA promotes annexin A2 and CD11b integrin interaction in macrophages and the obstructive kidneys. (A and B) Coimmunoprecipitation showed that tPA (10 nM) promoted the physical interaction of annexin A2 and CD11b integrin in J774 macrophages. Cell lysates were precipitated with anti-CD11b antibody followed by Western blot with antibodies against annexin A2 or CD11b, respectively. (A) Representative Western blot and (B) quantitative analysis of relative level of the annexin A2/CD11b complex were presented. **P<0.01 (n=3). Reverse immunoprecipitation was performed using antiannexin A2 antibody as precipitating antibody and anti-CD11b as probing antibody. (C) Representative Western blot. (D) Quantitative illustration of relative abundance of CD11b/annexin A2 complex. *P<0.05 (n=3). (E) Double immunofluorescence staining showed the colocalization of CD11b and annexin A2 in the diseased kidneys after 7 days of UUO. Nuclei were indicated by DAPI. Original magnification, ×400.
Blockade of CD11b Signaling Abolishes tPA-Induced NF-κB Activation
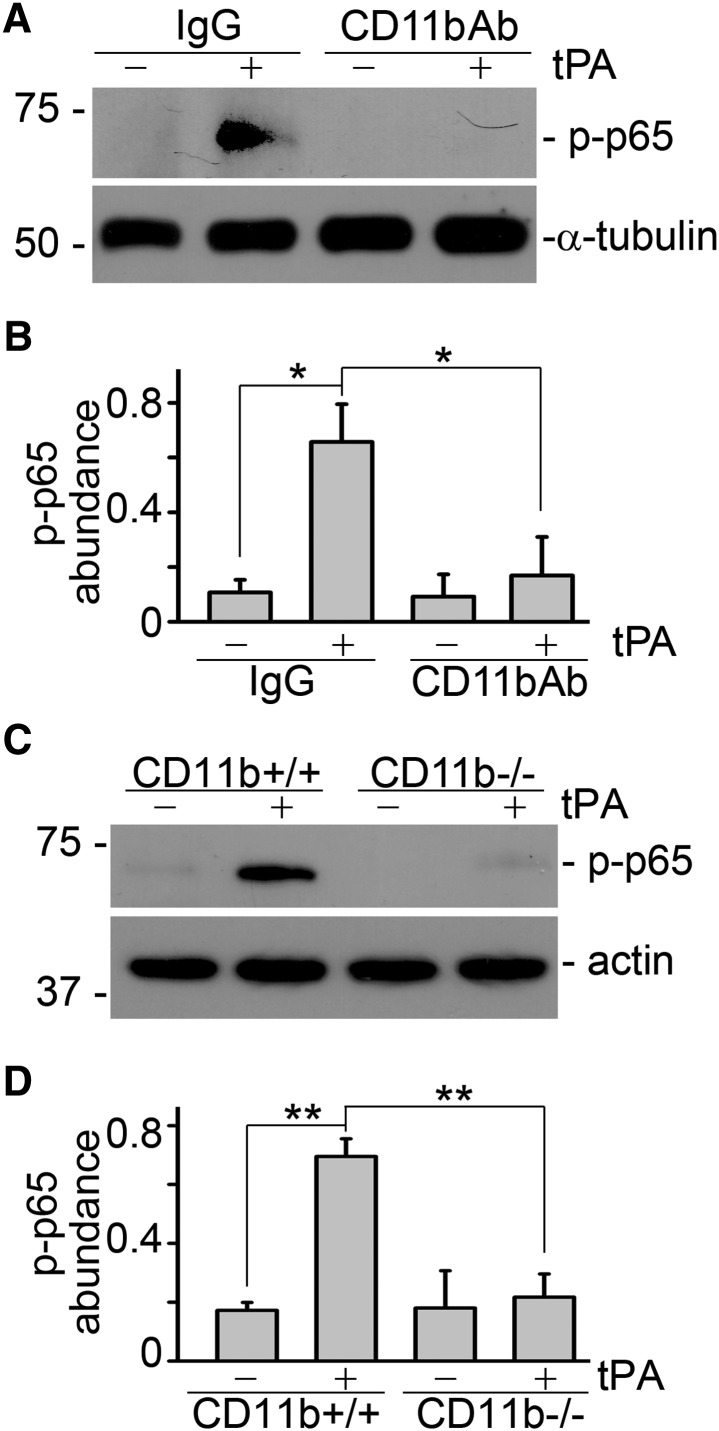
tPA-mediated aggregation and interaction between annexin A2 and CD11b may lead to the clustering of CD11b followed by its conformational changes and the subsequent activation of integrin outside-in signaling. To test our hypothesis, we investigated the effect of inhibiting CD11b signaling using neutralizing antibody. We found that CD11b neutralizing antibody significantly suppressed tPA-mediated NF-κB activation, whereas, control IgG had no effect (Figure 4, A and B). To further confirm the role of CD11b in tPA-induced activation of NF-κB pathway, we also compared the capacity of tPA to activate NF-κB signaling in the primary peritoneal macrophages isolated from wild-type (CD11b+/+) and CD11b knockout (CD11b−/−) mice. As shown in Figure 4, C and D, tPA induced the activation of NF-κB pathway in wild-type (CD11b+/+) macrophages but failed to do so in CD11b-deficient macrophages. Hence, CD11b signaling is indispensable to tPA-mediated NF-κB activation in macrophages.
Figure 4.
CD11b signaling is indispensable to tPA-induced activation of NF-κB pathway in macrophages. J774 macrophages were treated with control IgG or anti-CD11b neutralizing antibody (2.5 µg/ml) followed by incubation with vehicle or tPA (10 nM) for 6 h. Representative (A) Western blot and (B) quantitative analysis of relative abundance of phospho-p65 were presented. *P<0.05 (n=3). (C and D) Peritoneal macrophages isolated from CD11b knockout (CD11b−/−) mice were incubated with vehicle or 10 nM tPA for 6 hours followed by Western blot for phospho-p65 and actin. (C) Representative blot. (D) Quantitative illustration of relative abundance of phospho-p65. **P<0.01 (n=3).
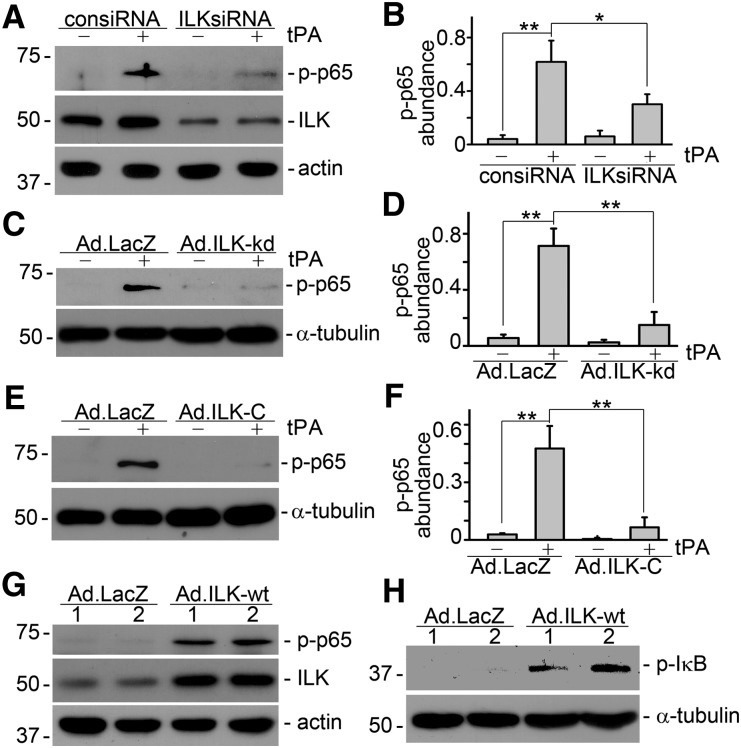
ILK, a Downstream Effector of Integrin Signaling, Mediates tPa-Induced Activation of NF-κB Pathway
Our previous study discovered that tPA activates β1 integrin/ILK signaling in fibroblasts.13 We also explored the role of ILK, the immediate downstream effector of integrin signal transduction, in tPA-mediated CD11b signaling and subsequent activation of NF-κB pathway. First, we examined the functional consequence of inhibition of endogenous expression of ILK in macrophages by siRNA knockdown. As shown in Figure 5, A and B, ILKsiRNA strongly suppressed tPA-activated NF-κB signaling, suggesting that endogenous ILK is indispensable to tPA-mediated NF-κB activation. Efficiency of ILK knockdown was confirmed by Western blot analysis (Figure 5A). We next investigated the role of ILK-associated kinase activity in tPA-mediated NF-κB activation by ectopic expression of dominant-negative ILK-kd. The ILK-kd adenoviral vector (Ad.ILK-kd) contains a single-point mutation by substitution of G for A at nucleotide 1,075 of the ILK, which disrupts ILK-associated kinase activity. J774 macrophages were infected with either control adenovirus (Ad.LacZ) or Ad.ILK-kd, followed by tPA treatment. It was found that infection with Ad.ILK-kd abrogated tPA-mediated NF-κB activation in macrophages (Figure 5, C and D). Then, we also examined the role of ILK in tPA-induced NF-κB activation by disrupting the interaction and engagement of CD11b and ILK using recombinant adenovirus harboring the dominant-negative C-terminal ILK (Ad.ILK-C), of which the N terminus was truncated, rendering its signaling incompetent as described in our previous study.13 As shown in Figure 5, E and F, infection J774 macrophages with Ad.ILK-C eliminated the phosphorylation of p65 and NF-κB signaling induced by tPA. In a word, both abundance of endogenous ILK and its associated kinase activity, as well as CD11b/ILK engagement, are imperative for tPA to activate NF-κB pathway in macrophages.
Figure 5.
ILK mediates tPA-induced activation of NF-κB pathway in macrophages. J774 macrophages were transfected with control (consiRNA) or ILK siRNA (ILKsiRNA), followed by the treatment with vehicle or 10 nM tPA for 6 hours. Western blot showed that knockdown of ILK abolished tPA-induced activation of NF-κB (A and B). Representative (A) Western blot and (B) quantitative analysis of relative abundance of phospho-p65 were presented. *P<0.05, **P<0.01 (n=3). Infection of macrophages with adenovirus vectors, containing either (C and D) dominant-negative mutant ILK-kd (Ad.ILK-kd) or (E and F) C-terminal ILK (Ad.ILK-C), abrogated tPA-induced NF-κB activation. (C and E) Representative Western blots. (D and F) Quantitative analyses of relative abundance of phospho-p65. **P<0.01 (n=3). Infection with adenovirus vectors containing wild-type ILK (Ad.ILK-wt) mimicked tPA and induced the phosphorylation of (G) p65 and (H) IκB. Number indicates concentration in particles per milliliter (×107) of the vectors.
Ecotopic Expression of ILK Mimics tPA and Activates Canonical NF-κB Pathway
We also examined the role of ILK activation in tPA-activated NF-κB signaling by ectopic overexpression of wild-type ILK. J774 macrophages were infected with adenovirus vectors containing wild-type ILK (Ad.ILK-wt) or Ad.LacZ. NF-κB activation was assessed by Western blot for phospho-p65. As shown in Figure 5G, infection with Ad.ILK-wt mimicked the effect of tPA on NF-κB activation. Of note, Ad.ILK-wt also induced phosphorylation of IκB (Figure 5H), suggesting that ectopic expression of ILK activates canonical NF-κB signaling.
tPA Mediates NF-κB Signaling In Vivo
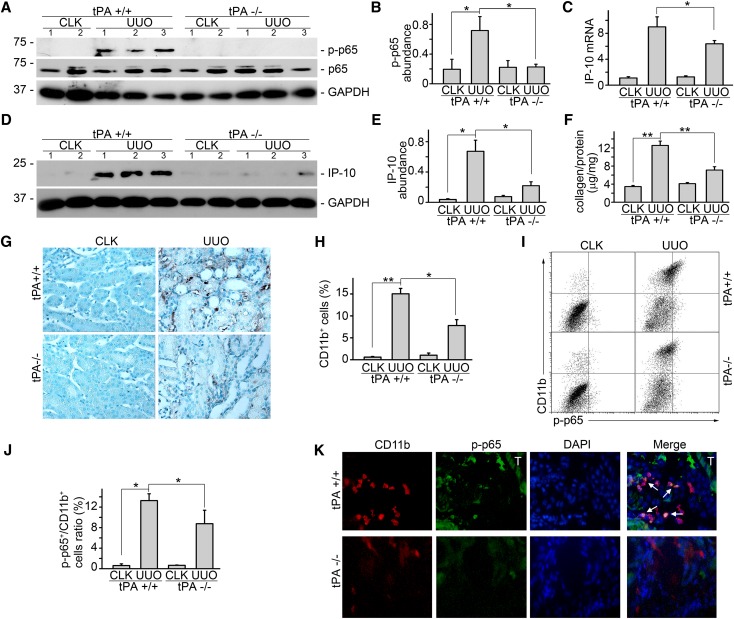
To investigate the role of tPA in NF-κB signaling in vivo, we investigated the NF-κB signaling in the obstruction-induced fibrotic kidneys harvested from tPA wild-type and knockout mice. It was found that NF-κB signaling was strongly activated, which was indicated by p65 phosphorylation (Figure 6, A and B) and the concurrent induction of chemokine IP-10 (Figure 6, C–E) in the fibrotic kidneys from the wild-type mice at 7 d after UUO. Phosphorylation of p65 (Figure 6, A and B) and IP-10 level (Figure 6, C–E) were significantly reduced in tPA knockout mice compared with these factors of tPA wild-type control, suggesting that NF-κB activation was attenuated in tPA-deficient mice. Of note, collagen deposition (Figure 6F) and infiltration of CD11b-positive macrophages (Figure 6, G and H) were also alleviated in tPA knockout mice. In addition, flow cytometry analysis showed that obstruction-induced accumulation of phospho-p65–positive CD11b macrophages in tPA wild-type mice was clearly attenuated in tPA knockout mice (Figure 6, I and J). Moreover, double immunofluorescence staining of phospho-p65 (green) and CD11b (red) confirmed that interstitial cells with activated NF-κB signaling in the diseased kidneys were largely CD11b-positive macrophages (Figure 6K).
Figure 6.
tPA mediates NF-κB activation in vivo. tPA knockout mice displayed significantly reduced induction of (A and B) phospho-p65 and (C) interferon-inducible protein-10 mRNA and (D and E) protein production. (A and D) Representative Western blots. Number indicates individual mouse. (B, C, and E) Quantitative analysis of relative abundance. *P<0.05 (n=5 mice per group). (F) Total renal collagen content was evaluated by Sirius Red/Fast Green collagen assay kit and expressed as micrograms collage per milligram protein. **P<0.01 (n=5 mice per group). (G) Representative immunohistochemical staining of CD11b for macrophages. (H) Quantitative demonstration of interstitial infiltration of CD11b-positive macrophages. *P<0.05, **P<0.01 (n=5 mice per group). (I) Representative flow cytometry analysis. Upper right indicates CD11b-positive macrophages with activated NF-κB signaling. (J) Quantitative analysis of relative amount of CD11b macrophages with activated p65 phosphorylation. Unit was expressed as percentage of both phospho-p65– and CD11b-positive cells in gated cells. *P<0.05 (n=4 mice per group). (K) Double immunostaining of phospho-p65 (green) and CD11b (red) in the diseased kidney after 7 days obstruction. Nuclei were indicated by DAPI. Original magnification, ×400. Cells with both positive staining of phospho-p65 and CD11b were indicated by arrows. Tubular staining was marked with T. CLK, contralateral kidney.
Discussion
Inflammatory infiltration is one of the histologic hallmarks of CKD. In response to chronic injury, infiltrating inflammatory cells, including monocytes/macrophages and other leukocytes, migrate to the sites of injury and produce and release a profound profile of chemokines, growth factors, and reactive oxygen species through activation of various transcription factors and their target genes. These detrimental factors not only create a fibrogenic microenvironment, leading to the generation of the matrix-producing effector cells through fibroblast activation and transdifferentiation, but also form a vicious loop of self-amplification. NF-κB family is the most prominent transcription factor that is involved in the transcriptional regulation of inflammation, immunity, apoptosis/proliferation, and cell differentiation. Increased activation of NF-κB has been found in various experimental and human renal diseases,3–5 which coincides with the concomitant induction of tPA signaling in the diseased kidney,13,14 suggesting that tPA may be a key endogenous factor that modulates NF-κB pathway during CKD.
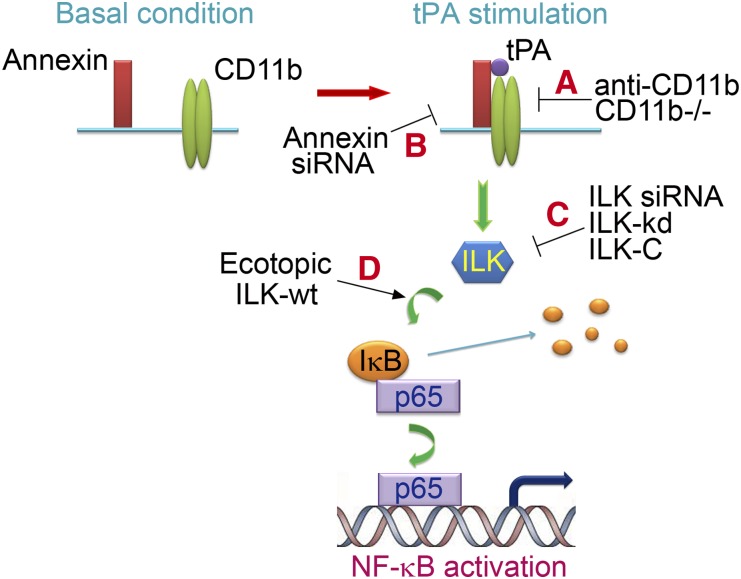
In this study, we discovered new function of tPA in the regulation of renal inflammation and defined a novel signaling pathway involved in the modulation of NF-κB pathway. As illustrated in Figure 7, tPA binds to its receptor annexin A2 and induces the interaction and aggregation of annexin A2 and CD11b, leading to the clustering and activation of outside-in signaling of CD11b integrin, which in turn, activates its downstream ILK and phosphorylates IκB. Phosphorylation of IκB leads to its degradation and the release of NF-κB dimers into nuclei, resulting in the subsequent DNA binding and transcription of target proinflammatory genes. Blockade of any step within this signaling cascade by various strategies (Figure 7, a–c) eliminates tPA-induced NF-κB activation. Of note, tPA also modulates NF-κB signaling in vivo as obstruction-induced phosphorylation of p65, and chemokine expressions are dramatically reduced in the kidneys from tPA knockout mice and accompanied with decreased collagen deposition and macrophage infiltration (Figure 6). Therefore, tPA is one of the key endogenous factors modulating the NF-κB signaling in CKD.
Figure 7.
Schematic illustration of the signaling transduction pathway in tPA-induced NF-κB activation. tPA promotes the interaction and aggregation of annexin A2 and CD11b, inducing the clustering and outside-in signaling of CD11b integrin, which leads to the activation of the downstream ILK, phosphorylation of IκB, and subsequent activation of NF-κB pathway. (A–C) Blockade of any step within this signaling cascade by various strategies eliminates tPA-induced NF-κB activation. (D) Ectopic expression of wild-type ILK mimics tPA effect.
Recent findings have defined tPA as a hybrid molecule with protease and cytokine functions.12–15,19,28–35 As a member of the serine protease family, tPA also activates numerous growth factors, including TGF-β1 and platelet-derived growth factor CC,9–11 in addition to its ability to activate numerous zymogens into active enzymes that are involved in fibrinolysis and extracellular matrix degradation. In the present study, we determined that tPA-induced activation of the NF-κB pathway is independent of its protease activity, because the mutant nonenzymatic tPA, in which the serine within the active site of the enzyme was replaced with alanine,27 also induced NF-κB activation in a dose-dependent manner (Figure 2A). This result confirmed that tPA activates the NF-κB pathway through its receptor-mediated cytokine function.
Unlike its close cousin urokinase plasminogen activator, tPA does not have a dedicated, specific receptor thus far. However, extensive studies have pointed to several candidates that act functionally and biologically as tPA receptors by initiating intracellular signaling and eliciting downstream cellular responses. LRP-1 is the most well known tPA receptor, and it mediates most of the cytokine functions of tPA in various cell types.12–16,19,28–30,33–35 Intriguingly, LRP-1 does not mediate tPA-induced activation of NF-κB pathway in macrophages, because knockdown of LRP-1 has no effect (Figure 2B). Instead, annexin A2, the other known receptor of tPA,21–23 mediates tPA effect, because knockdown the expression of annexin A2 abolished tPA-induced NF-κB activation in macrophages (Figure 2, C and D). In contrast to our present result, tPA has been shown to induce endothelial matrix metalloproteinase-3 expression through binding to LRP-1 and activating NF-κB.36 LRP-1 has also been implicated in tPA-mediated NF-κB activation in the ischemic brain tissue.19 However, LRP-1 has been reported to suppress NF-κB signaling and the associate chemokine expression in macrophages through indirectly regulating TNF receptor-1.37 Thus, the role of LRP-1 in tPA-induced NF-κB signaling may be context-dependent. Given our previous observations that LRP-1 mediates multiple profibrotic effects of tPA in renal fibroblasts,12–15 together with the present finding, it is assumable that tPA executes multiple cell type-specific biologic functions by binding to different membrane receptors (LRP-1 or annexin A2) and triggering specific receptor-mediated intracellular signaling events.
Annexin A2 belongs to the Ca2+- and phospholipid-binding protein family. The core domain in the C terminus of annexin A2 consists of four highly α-helical annexin repeats that mediate its membrane binding.20 tPA has been shown to bind to the hexapeptide LCKLSL (residues 7–12) in the N terminus of annexin A2.38 Although several studies indicate that annexin A2 has the potential of signal transduction,22,24,25 it remains unknown how annexin A2 transduces outside signal into the intracellular nuclei because of the fact that annexin A2 is a membrane-associate protein that can only dock onto the cell membrane in the peripheral manner.20,23 Another novel finding in this study is that tPA promotes the aggregation and interaction of annexin A2 and CD11b integrin, leading to the clustering and activation of CD11b signaling in macrophages, which was demonstrated by a coimmunoprecipitation assay (Figure 3, A–D). Such interaction between annexin A2 and CD11b apparently has clinical significance, because their aggregation happens during the course of obstructive renal injury, which was indicated by double immunofluorescence staining (Figure 3E). CD11b, a member of the integrin superfamily, is also named as integrin αM or Mac-1 α chain. CD11b is mainly expressed on leukocytes, including macrophages and neutrophils. As a matrix receptor, CD11b binds to various extracellular matrix components and numerous cytokines, including tPA.28 It is presumable that tPA may promote the aggregation by directly interacting with annexin A2 and CD11b. Aggregation of CD11b causes its conformational change to active conformer and subsequently activation of outside-in signaling, a process initiated by outside ligand binding to integrin followed by intracellular signal activation.39 This hypothesis was confirmed by the coimmunoprecipitation of annexin A2 and CD11b (Figure 3, A–D) and the fact that inhibition of CD11b signaling by either neutralizing antibody or gene knockout approach abrogates tPA-induced activation of NF-κB in macrophages (Figure 4). Therefore, annexin A2 may act as the coreceptor of CD11b to transduce the outside tPA signal into the nucleus. Of note, urokinase plasminogen activator receptor also acts as a coreceptor of CD11b in mediating its signaling.40 The present finding established a new signal transduction mechanism used by tPA to function as cytokine.
As a major downstream effector of integrin signaling, ILK interacts with the cytoplasmic domain of integrins through its C terminus and mediates their signaling by activating downstream mediators. It is found in the present study that knockdown of ILK by siRNA or disruption of its engagement with CD11b integrin by infection with dominant-negative C-terminal ILK adenovirus blockades tPA-induced NF-κB signaling (Figure 5, A, B, E, and F). In addition, infecting macrophages with Ad.ILK-kd, which contains a single-point mutation causing the disruption of the ILK-associated kinase activity, eliminates the effect of tPA (Figure 5, C and D). Thus, both physical interaction of ILK with CD11b integrin and ILK-associated kinase activity are indispensable to tPA-induced NF-κB activation. Conversely, ectopic expression of wild-type ILK by adenovirus infection induces the phosphorylation of IκB and p65 and the subsequent activation of NF-κB signaling (Figure 5, G and H). It is clear that ILK is both necessary and sufficient to mediate tPA-induced activation of NF-κB pathway in macrophages.
In summary, the present study has established the previously unrecognized function of tPA in the activation of NF-κB signaling in macrophages. The above finding also defined a novel signaling transduction pathway mediated by tPA receptor annexin A2, which involves the aggregation and clustering of CD11b and subsequent activation of ILK, leading to the phosphorylation and degradation of IκB and resultant canonical activation of NF-κB pathway in macrophages (Figure 7).
Concise Methods
Antibodies and Reagents
The antibodies against phospho-specific and total p65, ILK, and phospho-specific IκBα were purchased from Cell Signaling Technology (Beverly, MA). Mouse anti–α-tubulin and normal rat IgG were obtained from Sigma (St. Louis, MO). Monoclonal annexin A2 antibody and neutralizing CD11b antibody were provided by BD Biosciences (San Diego, CA). Monoclonal anti-mouse CD 11b PE-Cy5 antibody was purchased from eBioscience (San Diego, CA). Polyclonal anti-CD11b antibody was bought from Abcam (Cambridge, MA). Mouse monoclonal anti–LRP-1 (11H4) antibody was prepared as described previously.12–15 The secondary horseradish peroxidase-conjugated antibodies, fetal bovine serum, and supplements were obtained from Fisher Scientific (Pittsburgh, PA). Recombinant human single-chain tPA was purchased from American Diagnostica Inc. (Stamford, CT). The nonenzymatic tPA was supplied by Molecular Innovations Inc. (Southfield, MI). The protein A/G plus agarose bead was purchased from Santa Cruz Biotechology (Santa Cruz, CA). The Dulbecco’s modified Eagle’s medium was obtained from American Type Culture Collection (Manassas, VA). All other chemicals of analytic grade were obtained from Sigma or Fisher Scientific unless otherwise indicated.
Cell Culture
Mouse macrophages J774.A1 were purchased from American Type Culture Collection. Primary peritoneal macrophages were isolated from CD11b knockout (CD11b−/−) and wild-type (CD11b+/+) mice using the method modified from a previous report.41 Briefly, mouse peritoneal macrophages were isolated by peritoneal lavage and purified by removing nonadherent cells after 1 hour of culture in complete medium with 10% fetal bovine serum. Macrophages were maintained in Dulbecco’s modified Eagle’s medium containing 4 mM l-glutamine, 4500 mg/L glucose, 1 mM sodium pyruvate, 1500 mg/L sodium bicarbonate, and 10% fetal bovine serum. After 24-hour serum-free starvation, the macrophages were treated with vehicle and tPA for various periods of time as indicated, and then, they were collected for different assays.
siRNA Inhibition and Adenovirus Infection
The adenoviral vectors containing the wild-type ILK (Ad.ILK-wt) or the dominant-negative ILK-kd (Ad. ILK-kd) containing a single-point mutation by substitution of G for A at nucleotide 1,075 of the ILK and the C-terminal truncated ILK fragment (Ad.ILK-C) were described elsewhere.42,43 Mouse LRP-1 siRNA was purchased from Thermo Scientific with target sequence as GACCAGUGUUCUCUGAAUA, whereas siRNAs targeting mouse annexin A2 and ILK were obtained from Invitrogen. Transient transfection of siRNAs and adenovirus infection were performed as previously described.12–15
Western Blot Analysis
Whole-cell lysates were prepared and separated on 10% SDS polyacrylamide gels as previously described.12–15 The poly(vinylidene difluoride) membrane with transferred proteins was incubated overnight at 4°C with various primary antibodies in Tris-buffered saline with Tween buffer (20 mM Tris⋅HCl, 150 mM NaCl, and 0.1% Tween 20) containing 5% nonfat milk followed by incubation with horseradish peroxidase-conjugated secondary antibodies for 1 hour. After extensive washes in Tris-buffered saline with Tween buffer, the signals on the membrane were visualized by the SuperSignal West Pico Chemiluminescent Substrate kit (Thermo Fisher Scientific).
Coimmunoprecipitation
Cells were treated with 10 nM tPA for 1.5 hours and then subjected to immunoprecipitation as described elsewhere.13–15 Briefly, cell lysates were incubated with primary antibodies or IgG of the same host species at 4°C overnight followed by incubation with protein A/G PLUS agarose beads for additional 3 hours. After washes with lysis buffer, the proteins on the beads were extracted in reducing sample buffer, separated on 10% SDS polyacrylamide gel, and analyzed by Western blotting.
Animal Model
Homozygous tPA knockout (tPA−/−) and wild-type (tPA+/+) mice on C57BL/6 background were purchased from the Jackson Laboratory (Bar Harbor, Maine) and maintained as previously described.12,13,15 Animal studies were performed by using an approved protocol by the Institutional Animal Care and Use Committee at the Penn State University College of Medicine. UUO was performed in 20- to 22-g sex-matched mice (four to five animals per group) using established procedures previously described.12,13,15 The right unobstructed kidneys served as controls. At day 7 after UUO, mice were euthanized, and kidney samples were harvested for analysis.
Indirect Immunofluorescence Staining
Snap-frozen kidney was cryosectioned at 5 μM thickness and subjected to immunofluorescence staining as previously described.13,15 Stained tissues were mounted with Vectashield antifade mounting media with 4′,6-diamidino-2-phenylindole HCl (DAPI; Vector Laboratories Inc., Burlingame, CA) and viewed under an Olympus IX81 fluorescence microscope (Olympus America Inc, Melville, NY). Sections stained without primary antibodies were served as negative controls.
Quantitative RT-PCR
Total RNA was extracted and reverse-transcribed into cDNA as previously described.13 cDNA was then amplified using SYBR Green PCR kit (Qiagen, Valencia, CA) and specific primers for mouse macrophage-inflammatory protein-1α,44 interferon-inducible protein-10, and β-actin.45 The sequence of the primers was reported elsewhere.44,45 The specificity of PCR reaction was confirmed by melting-curve analysis. Relative level of mRNAs was quantified and normalized to β-actin.
Flow Cytometry
Single-cell suspensions, generated from whole kidneys, were prepared as preciously described.46 In brief, kidneys from male tPA wild-type and knockout mice (four mice per group) were subjected to 20-ml saline perfusion to remove intravascular leukocytes and were then minced into small pieces around 1 mm3 followed by digestion with 2 mg/ml collagenase D and 100 U/ml DNase I. After passing through 100- and 40-μm mesh, the cells were incubated with red blood cells lysis buffer and then stained with anti-CD11b PE-Cy5 and antiphospho-p65 antibodies followed by flow cytometry analysis using FACSCaliber machine (BD Biosciences, San Diego, CA) and FlowJo software (Tree Star Inc., Ashland, OR).
Immunohistochemistry
Paraffin-embedded kidney tissue was sectioned at 4 µm and then subjected to staining with CD11b antibody.12 Briefly, tissue sections were deparaffinized, hydrated, and antigen-retrieved followed by incubation with primary and secondary antibodies. Sections were then incubated with avidin biotin complex reagents, subjected to NovaRED substrate, and counterstained with methyl green (Vector Laboratories). A total of 500 interstitial cells were counted per slide, and the percentage of interstitial CD11b-positive cells in total interstitial cell population was calculated for five mice per group.
Determination of Renal Total Collagen Content
Total collagen content was evaluated using the Sirius Red/Fast Green Collagen detection kit (Chondrex Inc., Redmond, WA) according to the manufacturer’s instruction. Briefly, tissue sections were stained with Sirius Red and Fast Green for collagen and noncollagen protein. The dyes were extracted using 0.05 M NaOH in methanol and measured at OD540 (Sirius Red) and OD605 (Fast Green), respectively. This method provided a simple and sensitive way to semiquantify the ratio of collagen/total protein.47,48 The relative collagen content was calculated and expressed as micrograms per milligram, total protein.
Statistical Analyses
All the experimental data were presented as means ± SEM. Statistical analyses of the data were performed using SigmaStat software (Jandel Scientific Software). Comparison between multiple groups was performed by using one-way ANOVA followed by the Newman–Keuls or t test between two groups. P<0.05 was considered statistically significant.
Disclosures
None.
Acknowledgments
This work is supported by American Heart Association Grant 10SDG3900029, a Kidney Foundation of Central Pennsylvania grant (to K.H.), and National Institutes of Health Grant DK54639 (to C.W.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Mosser DM, Edwards JP: Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8: 958–969, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonizzi G, Karin M: The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol 25: 280–288, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Sanz AB, Sanchez-Niño MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A: NF-kappaB in renal inflammation. J Am Soc Nephrol 21: 1254–1262, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Mezzano SA, Barría M, Droguett MA, Burgos ME, Ardiles LG, Flores C, Egido J: Tubular NF-kappaB and AP-1 activation in human proteinuric renal disease. Kidney Int 60: 1366–1377, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Sakai N, Wada T, Furuichi K, Iwata Y, Yoshimoto K, Kitagawa K, Kokubo S, Kobayashi M, Takeda S, Kida H, Kobayashi K, Mukaida N, Matsushima K, Yokoyama H: p38 MAPK phosphorylation and NF-kappa B activation in human crescentic glomerulonephritis. Nephrol Dial Transplant 17: 998–1004, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Tomita N, Morishita R, Lan HY, Yamamoto K, Hashizume M, Notake M, Toyosawa K, Fujitani B, Mu W, Nikolic-Paterson DJ, Atkins RC, Kaneda Y, Higaki J, Ogihara T: In vivo administration of a nuclear transcription factor-kappaB decoy suppresses experimental crescentic glomerulonephritis. J Am Soc Nephrol 11: 1244–1252, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Kim KH, Lee ES, Cha SH, Park JH, Park JS, Chang YC, Park KK: Transcriptional regulation of NF-kappaB by ring type decoy oligodeoxynucleotide in an animal model of nephropathy. Exp Mol Pathol 86: 114–120, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Hu K, Mars WM, Liu Y: Novel actions of tissue-type plasminogen activator in chronic kidney disease. Front Biosci 13: 5174–5186, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mars WM, Zarnegar R, Michalopoulos GK: Activation of hepatocyte growth factor by the plasminogen activators uPA and tPA. Am J Pathol 143: 949–958, 1993 [PMC free article] [PubMed] [Google Scholar]
- 10.Yee JA, Yan L, Dominguez JC, Allan EH, Martin TJ: Plasminogen-dependent activation of latent transforming growth factor beta (TGF beta) by growing cultures of osteoblast-like cells. J Cell Physiol 157: 528–534, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Fredriksson L, Li H, Fieber C, Li X, Eriksson U: Tissue plasminogen activator is a potent activator of PDGF-CC. EMBO J 23: 3793–3802, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu K, Lin L, Tan X, Yang J, Bu G, Mars WM, Liu Y: tPA protects renal interstitial fibroblasts and myofibroblasts from apoptosis. J Am Soc Nephrol 19: 503–514, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu K, Wu C, Mars WM, Liu Y: Tissue-type plasminogen activator promotes murine myofibroblast activation through LDL receptor-related protein 1-mediated integrin signaling. J Clin Invest 117: 3821–3832, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu K, Yang J, Tanaka S, Gonias SL, Mars WM, Liu Y: Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J Biol Chem 281: 2120–2127, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Lin L, Bu G, Mars WM, Reeves WB, Tanaka S, Hu K: tPA activates LDL receptor-related protein 1-mediated mitogenic signaling involving the p90RSK and GSK3beta pathway. Am J Pathol 177: 1687–1696, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y, Mantuano E, Inoue G, Campana WM, Gonias SL: Ligand binding to LRP1 transactivates Trk receptors by a Src family kinase-dependent pathway. Sci Signal 2: ra18, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higazi AA, El-Haj M, Melhem A, Horani A, Pappo O, Alvarez CE, Muhanna N, Friedman SL, Safadi R: Immunomodulatory effects of plasminogen activators on hepatic fibrogenesis. Clin Exp Immunol 152: 163–173, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roelofs JJ, Rouschop KM, Leemans JC, Claessen N, de Boer AM, Frederiks WM, Lijnen HR, Weening JJ, Florquin S: Tissue-type plasminogen activator modulates inflammatory responses and renal function in ischemia reperfusion injury. J Am Soc Nephrol 17: 131–140, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Polavarapu R, She H, Mao Z, Yepes M: Tissue-type plasminogen activator and the low-density lipoprotein receptor-related protein mediate cerebral ischemia-induced nuclear factor-kappaB pathway activation. Am J Pathol 171: 1281–1290, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rescher U, Gerke V: Annexins—unique membrane binding proteins with diverse functions. J Cell Sci 117: 2631–2639, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Cesarman GM, Guevara CA, Hajjar KA: An endothelial cell receptor for plasminogen/tissue plasminogen activator (t-PA). II. Annexin II-mediated enhancement of t-PA-dependent plasminogen activation. J Biol Chem 269: 21198–21203, 1994 [PubMed] [Google Scholar]
- 22.Siao CJ, Tsirka SE: Tissue plasminogen activator mediates microglial activation via its finger domain through annexin II. J Neurosci 22: 3352–3358, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J, Hajjar KA: Annexin II: A plasminogen-plasminogen activator co-receptor. Front Biosci 7: d341–d348, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Ortiz-Zapater E, Peiró S, Roda O, Corominas JM, Aguilar S, Ampurdanés C, Real FX, Navarro P: Tissue plasminogen activator induces pancreatic cancer cell proliferation by a non-catalytic mechanism that requires extracellular signal-regulated kinase 1/2 activation through epidermal growth factor receptor and annexin A2. Am J Pathol 170: 1573–1584, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Babbin BA, Parkos CA, Mandell KJ, Winfree LM, Laur O, Ivanov AI, Nusrat A: Annexin 2 regulates intestinal epithelial cell spreading and wound closure through Rho-related signaling. Am J Pathol 170: 951–966, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohmori Y, Hamilton TA: Cooperative interaction between interferon (IFN) stimulus response element and kappa B sequence motifs controls IFN gamma- and lipopolysaccharide-stimulated transcription from the murine IP-10 promoter. J Biol Chem 268: 6677–6688, 1993 [PubMed] [Google Scholar]
- 27.Olson ST, Swanson R, Day D, Verhamme I, Kvassman J, Shore JD: Resolution of Michaelis complex, acylation, and conformational change steps in the reactions of the serpin, plasminogen activator inhibitor-1, with tissue plasminogen activator and trypsin. Biochemistry 40: 11742–11756, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Cao C, Lawrence DA, Li Y, Von Arnim CA, Herz J, Su EJ, Makarova A, Hyman BT, Strickland DK, Zhang L: Endocytic receptor LRP together with tPA and PAI-1 coordinates Mac-1-dependent macrophage migration. EMBO J 25: 1860–1870, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akkawi S, Nassar T, Tarshis M, Cines DB, Higazi AA: LRP and alphavbeta3 mediate tPA activation of smooth muscle cells. Am J Physiol Heart Circ Physiol 291: H1351–H1359, 2006 [DOI] [PubMed] [Google Scholar]
- 30.An J, Zhang C, Polavarapu R, Zhang X, Zhang X, Yepes M: Tissue-type plasminogen activator and the low-density lipoprotein receptor-related protein induce Akt phosphorylation in the ischemic brain. Blood 112: 2787–2794, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee HY, Hwang IY, Im H, Koh JY, Kim YH: Non-proteolytic neurotrophic effects of tissue plasminogen activator on cultured mouse cerebrocortical neurons. J Neurochem 101: 1236–1247, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Liot G, Roussel BD, Lebeurrier N, Benchenane K, López-Atalaya JP, Vivien D, Ali C: Tissue-type plasminogen activator rescues neurones from serum deprivation-induced apoptosis through a mechanism independent of its proteolytic activity. J Neurochem 98: 1458–1464, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Lee SR, Arai K, Lee SR, Tsuji K, Rebeck GW, Lo EH: Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med 9: 1313–1317, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA: Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest 112: 1533–1540, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhuo M, Holtzman DM, Li Y, Osaka H, DeMaro J, Jacquin M, Bu G: Role of tissue plasminogen activator receptor LRP in hippocampal long-term potentiation. J Neurosci 20: 542–549, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki Y, Nagai N, Yamakawa K, Kawakami J, Lijnen HR, Umemura K: Tissue-type plasminogen activator (t-PA) induces stromelysin-1 (MMP-3) in endothelial cells through activation of lipoprotein receptor-related protein. Blood 114: 3352–3358, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Gaultier A, Arandjelovic S, Niessen S, Overton CD, Linton MF, Fazio S, Campana WM, Cravatt BF, 3rd, Gonias SL: Regulation of tumor necrosis factor receptor-1 and the IKK-NF-kappaB pathway by LDL receptor-related protein explains the antiinflammatory activity of this receptor. Blood 111: 5316–5325, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hajjar KA, Mauri L, Jacovina AT, Zhong F, Mirza UA, Padovan JC, Chait BT: Tissue plasminogen activator binding to the annexin II tail domain. Direct modulation by homocysteine. J Biol Chem 273: 9987–9993, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Lefort CT, Hyun YM, Schultz JB, Law FY, Waugh RE, Knauf PA, Kim M: Outside-in signal transmission by conformational changes in integrin Mac-1. J Immunol 183: 6460–6468, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith HW, Marshall CJ: Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol 11: 23–36, 2010 [DOI] [PubMed] [Google Scholar]
- 41.dos Santos TAT, Portes JdA, Damasceno-Sa JC, Caldas LA, de Souza W, DaMatta RA, Seabra SH: Phosphatidylserine exposure by Toxoplasma gondii is fundamental to balance the immune response granting survival of the parasite and of the host. PLoS One 6: e27867, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo L, Wu C: Regulation of fibronectin matrix deposition and cell proliferation by the PINCH-ILK-CH-ILKBP complex. FASEB J 16: 1298–1300, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Guo L, Chen K, Wu C: A critical role of the PINCH-integrin-linked kinase interaction in the regulation of cell shape change and migration. J Biol Chem 277: 318–326, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Lange-Sperandio B, Trautmann A, Eickelberg O, Jayachandran A, Oberle S, Schmidutz F, Rodenbeck B, Hömme M, Horuk R, Schaefer F: Leukocytes induce epithelial to mesenchymal transition after unilateral ureteral obstruction in neonatal mice. Am J Pathol 171: 861–871, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang B, Ramesh G, Uematsu S, Akira S, Reeves WB: TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J Am Soc Nephrol 19: 923–932, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tadagavadi RK, Reeves WB: Renal dendritic cells ameliorate nephrotoxic acute kidney injury. J Am Soc Nephrol 21: 53–63, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.López-De León A, Rojkind M: A simple micromethod for collagen and total protein determination in formalin-fixed paraffin-embedded sections. J Histochem Cytochem 33: 737–743, 1985 [DOI] [PubMed] [Google Scholar]
- 48.Tan X, Li Y, Liu Y: Paricalcitol attenuates renal interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol 17: 3382–3393, 2006 [DOI] [PubMed] [Google Scholar]