Abstract
Regulatory T cells (Tregs) help protect against autoimmune renal injury. The use of agonist antibodies and antibody/cytokine combinations to expand Tregs in vivo may have therapeutic potential for renal disease. Here, we investigated the effects of administering IL-2/IL-2Ab complexes in mice with adriamycin nephropathy, a model of proteinuric kidney disease that resembles human focal segmental glomerulosclerosis. Injecting IL-2/IL-2Ab complexes before or, to a lesser extent, after induction of disease promoted expansion of Tregs. Furthermore, administration of this complex was renoprotective, evidenced by improved renal function, maintenance of body weight, less histologic injury, and reduced inflammation. IL-2/IL-2Ab reduced serum IL-6 and renal expression of IL-6 and IL-17 but enhanced expression of IL-10 and Foxp3 in the spleen. In vitro, the addition of IL-2/IL-2Ab complexes induced rapid STAT-5 phosphorylation in CD4 T cells. In summary, these data suggest that inducing the expansion of Tregs by administering IL-2/IL-2Ab complexes is a possible strategy to treat renal disease.
The key role of regulatory T cells (Tregs) has been identified in immune homeostasis and in suppressing unwanted inflammatory responses toward self-antigens.1,2 In a variety of autoimmune diseases, there are reduced numbers of Tregs predisposing to disease.3 In renal disease we and others have shown that the Foxp3-expressing subset of Tregs protect against renal disease, that removal of Tregs exacerbates disease, and that T cells transduced with Foxp3 to become Tregs are protective.4 The protection occurs in both immunodeficient (severe combined immunodeficiency) and immune-intact mice (BALB/c), suggesting that Tregs, in addition to suppressing cognate immune cells, can act on the innate immune response and potentially directly on injured renal tissue.4,5 The regulatory and production difficulties of ex vivo expansion of Tregs for therapeutic use in humans make in vivo expansion a potentially attractive option. However, enthusiasm for this has been tempered by the experience with TGN1412, the CD28 agonist that led to Treg expansion in rat models and protection against crescentic GN but caused pathologic T cell activation in human trials.6–9
IL-2 acts through the high-affinity IL-2 receptor composed of α, β, and γ subunits. It activates CD4+ and CD8+ T cells and Tregs.10 It can also activate cells with low-affinity βγ IL-2Rs, such as memory CD8+ and natural killer (NK) cells.11,12 Tregs are exquisitely dependent on IL-2 for survival, and Tregs can be eliminated by neutralizing anti–IL-2 mAb. The potency of IL-2 appears to be enhanced when it is complexed with certain antibodies. Of particular interest is the substantial selective Treg expansion in mice after injection of IL-2 complexed to a specific IL-2 mAb clone (JES6-1).13,14 This induces a temporary three- to four-fold increase in Treg numbers in vivo, potentially through enhanced binding to CD25, and is protective in experimental models of allergy, experimental allergic encephalitis (EAE), and islet transplantation.13–15 This is in comparison to S4B6 anti–IL-2 mAb, which blocks CD25 binding but enhances IL-2Rβ binding and thereby leads to CD8 rather than Treg activation.16
In this study, we used IL-2 complexed to JES6-1 IL-2 mAb to treat adriamycin nephropathy (AN), a model of chronic proteinuric renal disease, before and after AN induction with adriamycin.4,5 We found that mice treated with IL-2/IL-2Ab complex before and after adriamycin administration showed significant reduction in renal injury. This may allow Treg therapy that uses in vivo expansion rather than ex vivo cellular therapy to protect against kidney disease.
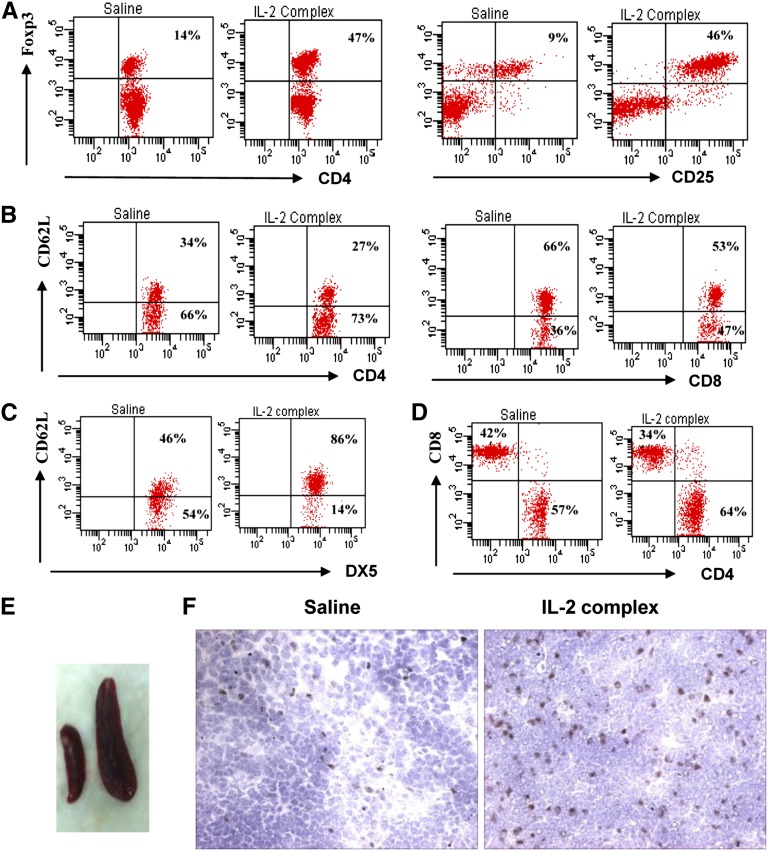
AN is a murine model of chronic proteinuric renal disease that resembles human focal segmental glomerulosclerosis. We compared pretreatment and post-treatment findings using the IL-2/IL-2Ab complex in AN mice. We used a clinically designed protocol with a short three-dose regimen before AN and a longer post-AN six-dose regimen to test efficacy and potential therapeutic utility. Mice were injected with the IL-2/IL-2Ab complex. Splenocytes of treated animals were analyzed by flow cytometry for CD4+CD25+Foxp3+ Tregs. Five to 6 days after IL-2/IL-2Ab complex injection, spleen size increased (Figure 1E) and CD4+ Foxp3+ cells increased from a mean ± SD of 14±3% to 47±4% of the splenic CD4+ T cells (n=7), and CD4+CD25+Foxp3+ T cells increased from 9±2% to 46±3% of the splenic CD4+ T cells (n=7) (Figure 1A). Immunohistochemical sections from mice injected with IL-2/IL-2Ab complex showed increased numbers of Foxp3+ Treg cells in the spleen at days 5–6 after IL-2/IL-2Ab complex treatment (Figure 1F). This expansion is similar to that reported previously using C57Bl/6 mice.13
Figure 1.
IL-2/IL-2Ab complex induces Foxp3+ Treg expansion in vivo. Mice received IL-2/IL-2Ab complex or saline intraperitoneally daily for 3 days. CD4+CD25+Foxp3+ Tregs from splenocytes were stained and analyzed by flow cytometry at day 6. CD4+ Foxp3+ cells increased from 14% to 47%. CD4+CD25+Foxp3+ T cells increased from 9% to 46% (A). CD4+ T cell expansion was greater (57% increased to 64%) than CD8+ T cells (42% decreased to 34%) and NK cells (54% decreased to 14%) (B and D). NK cells were less activated with higher CD62L expression, whereas there was a slight increase in activated CD8+ T cells (CD62L lo) (C). There was an increase in spleen size (E) and increased numbers of Foxp3+ T cells in spleen. (F) Immunohistochemical staining 5 days after IL-2/IL-2Ab complex treatment. Data are representative of three independent experiments (n=5–7). Original magnification, ×400.
At day 6 after IL-2 complex stimulation in vivo, other splenic subsets were assessed, including CD4, CD8, and NK cell numbers. CD4+ T cells (increased from 57% to 64%) showed greater expansion than did CD8+ T cells (decreased from 42% to 34%) and NK cells (decreased from 54% to 14%). Activation as assessed by loss of CD62L expression was reduced in NK cells in IL-2 complex–treated mice, whereas CD8 T cells showed an increase in the activated CD62L lo proportion (Figure 1, B–D).
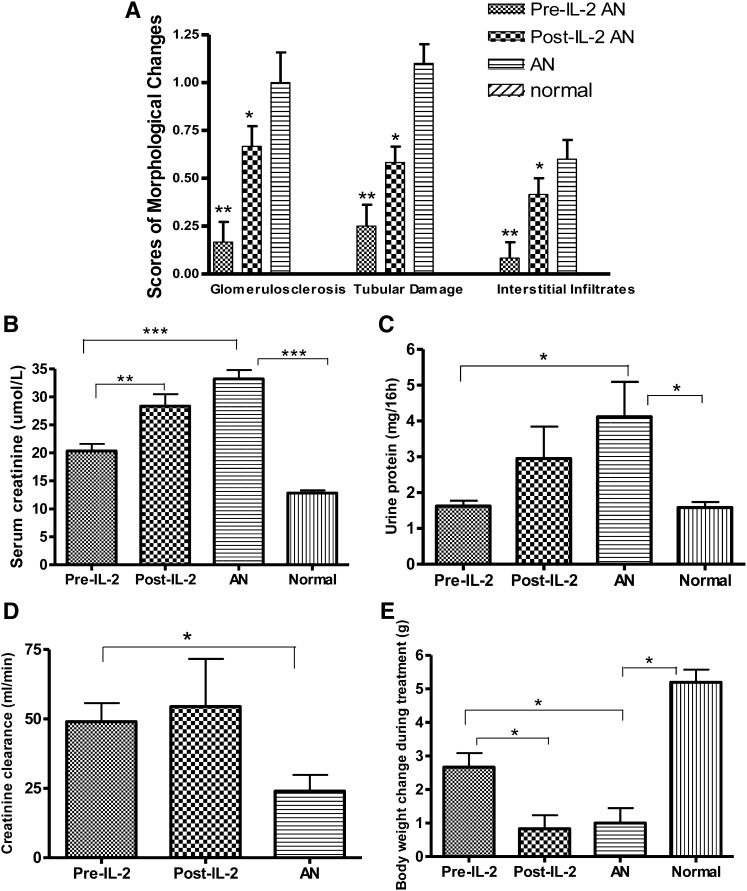
In the AN group, mice had severe renal injury, characterized by heavy proteinuria, elevated serum creatinine, and body weight loss. Histologic examination revealed tubular cell atrophy, significant glomerular injury, and a moderate interstitial infiltrate of inflammatory cells. Mice in the pre–IL-2 group had only mild damage of the glomeruli and tubules (P<0.01; Figure 2A, Figure 3), lower serum creatinine, and less proteinuria (P<0.01, P<0.05; Figure 2, B–D) and better maintenance of body weight (P<0.05; Figure 2E). Mice in the post–IL-2 group also showed reduced renal injury, with less glomerular and tubular damage and interstitial infiltration, but the complex was less protective than when given before adriamycin (P<0.05; Figure 2A).
Figure 2.
IL-2/IL-2Ab complex treatment protects against renal damage induced by adriamycin. Semiquantitative scores of morphologic changes of histopathology showed significantly less damage in glomeruli and tubules in pre–IL-2 group than in the AN group and the post–IL-2 group (A); mice from AN and post–IL-2 groups had significantly higher levels of serum creatinine (B) and urine protein excretion over 16 hours (C) and lower levels of creatinine clearance (D) compared with the normal (scores of morphologic changes for normal as designed as 0) and the pre–IL-2 groups. The pre–IL-2 group did not show the weight loss found in the other adriamycin-treated groups (E). *P<0.05, **P<0.01, ***P<0.001.
Figure 3.
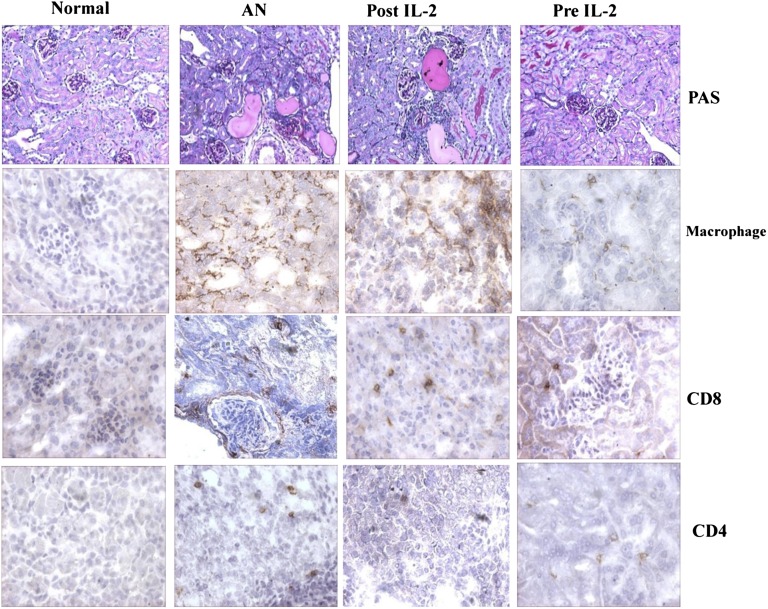
IL-2/IL-2Ab complex treatment reduces renal damage in AN. Representative histologic sections stained with periodic acid-Schiff (PAS) showed reduced renal injury and interstitial cellular infiltration in mice receiving IL-2/IL-2Ab complex injection (magnification ×200). There was reduced interstitial monocyte infiltration with macrophage, CD8+, and CD4+ T cells in both pre–IL-2 and post–IL-2 groups compared with the AN group (original magnification ×400).
In the AN group, dense mononuclear cell infiltrates were found in the renal interstitium, consisting of macrophages, CD8+ cells, and CD4+ T cells. In the pre–IL-2 group, there was a reduced interstitial monocytic infiltrate of the kidney with minimal macrophage, CD8+ cells, and CD4+ T cells. In the post–IL-2 group, although there was less infiltrate than with AN alone, there was greater infiltrate than in mice that received pretreatment with the complex (Figure 3).
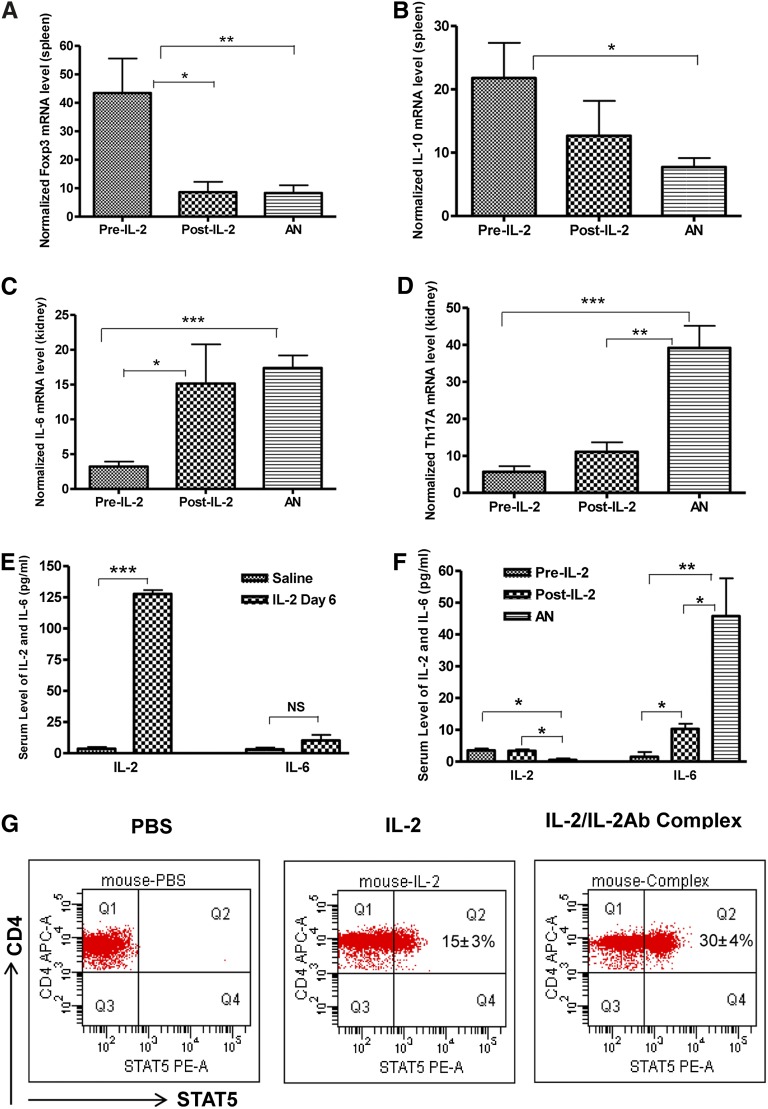
To determine the mechanisms by which the IL-2/IL-2Ab complex protects against injury, we examined cytokine levels in spleens and kidney at day 35 after adriamycin administration. We observed a significant reduction in IL-6 and IL-17A mRNA levels in the pre–IL-2 group compared with the AN and post–IL-2 groups (Figure 4, C and D). Foxp3 and IL-10, however, were higher in the pre–IL-2 group than in the AN and post–IL-2 groups (Figure 4, A and B). Thus, in addition to higher levels of Foxp3 consistent with Treg expansion, IL-2/IL-2Ab complex treatment led to reduction in inflammatory cytokines, including IL-6 and IL-17, and induction of protective anti-inflammatory cytokines, such as IL-10. This finding would be consistent with the IL-2/IL-2Ab group having direct expansion of Tregs (potentially the source of IL-10), which then limits ongoing inflammation, as reflected by improved histologic features and reduced IL-6. The reduced IL-17 may then result from a lack of the cytokines required for Th17 differentiation, such as IL-6.
Figure 4.
Real-time quantitative PCR of cytokine and Foxp3 mRNA levels from spleen and kidney. At the completion of the AN model, the pre–IL-2 group had significantly higher mRNA levels of Foxp3 (A) and IL-10 (B) in spleen and lower levels of IL-6 (C) and Th17A (D) than did the AN and post–IL-2 groups. Serum cytokine levels of IL-2 significantly increased at day 6 after IL-2 complex injection compared with saline-treated mice (E). However, at the completion of the AN, serum IL-2 returned to baseline. In contrast, IL-6 was elevated in the AN group but was significantly lower in both IL-2–treated groups (F). Murine splenocytes treated with IL-2 (100 ng/ml) and IL-2/IL-2Ab complex (IL-2, 100 ng/ml; anti–IL-2, 500 ng/ml of anti-IL-2; preincubated for 30 minutes at 37°C) in vitro showed a rapid increase in phosphorylation of STAT5. Phosphorylation was seen in 15%±3% of CD4+ T cells after 20 minutes of IL-2 stimulation; 30%±4% of CD4+ T cells showed phosphorylation of STAT5 after 20 minutes of IL-2/IL-2Ab complex treatment (G). Mean ± SD; *P<0.05, **P<0.01, ***P<0.001; n=5. PE-A, phycoerytherin.
Furthermore, serum protein levels of IL-2, IL-4, IL-6, IFN-γ, TNF, IL-17A, and IL-10 were assessed. There was a significant increase in serum IL-2 at day 6 after IL-2 complex injection compared with saline treatment (P<0.001; Figure 4E); however, in IL-2 complex–treated AN mice, IL-2 was at baseline at week 5 (P<0.05; Figure 4F). In contrast, serum IL-6 at week 5 was increased in the AN group but was significantly lower in the pre–IL-2 and post–IL-2 groups (P<0.01 and P<0.05, respectively; Figure 4F). Serum IL-4, IFN-γ, TNF, IL-17A, and IL-10 were undetectable in all three groups of mice. This finding was similar to the pattern seen with RNA expression but was less sensitive than the RNA measurements; this may reflect activity within the kidney that was not present systemically.The cytokines IL-2 and TGF-β appear to be crucial for the development, expansion, and function of Tregs. Both the TGF-β SMAD signaling pathway through SMAD2 and SMAD3 and the IL-2 signal of transducer and activator of transcription-5 (STAT-5) signaling pathway have been shown to be important for Foxp3 and Treg induction.10
IL-2 is known to activate the STAT pathway.16,17 Therefore, we evaluated the degree of STAT-5 activation by IL-2 or the IL-2/IL-2Ab complex through phosphorylation of STAT-5 in CD4 T cells. Murine splenocytes were treated with PBS, IL-2, or IL-2/IL-2Ab complex and analyzed for phosphorylation of STAT5 by flow cytometry. In vitro IL-2 and IL-2/IL-2Ab complex treatment produced a rapid increase in the phosphorylation of STAT-5. Of CD4+ T cells, 15%±3% showed phosphorylation after 20 minutes of IL-2 treatment, whereas 30%±4% showed phosphorylation of STAT-5 after 20 minutes with IL-2/IL-2Ab complex treatment (Figure 4G).
Tregs appear to be protective in AN, as shown by antibody removal using the anti-CD25 antibody PC-61 or by therapeutic injection of natural Tregs or Foxp3-transduced Tregs.4,5 In this model, although Treg expansion is only transient, the effect is maintained; this finding is in keeping with the results in EAE and islet transplantation.13 This finding also suggests that the expanded Tregs have a profound and long-lasting beneficial effect on inflammation in AN. The lesser effect found in the post-treatment group may be the result of expansion of CD25-expressing effector T cells in addition to Treg expansion, as previously described in the EAE model.13
The Treg-directed role of the IL-2/IL-2Ab complex may be functionally different from, and therapeutically superior to, the CD28-mediated expansion that was unsuccessful in human trials. Ongoing experiments in mouse models with a novel CD28 agonist suggest an increase in inflammatory activity with such compounds that necessitates steroid suppression; thus, their clinical use is highly unlikely because of associated inflammatory risks.17 The importance of this pathway clinically has been emphasized by the recent reports of low-dose IL-2 providing protection in human trials against hepatitis C injury and graft-versus-host disease. The mechanism of this was due to expansion of Treg populations, suggesting that IL-2 treatment may be a promising approach for autoimmune renal disease in which Tregs play a protective role.18–20
In conclusion, in vivo expansion of Tregs with IL-2/IL-2Ab complex leads to protection against chronic proteinuric renal injury and suggests that this may be a promising avenue for further therapeutic studies aimed at avoiding the general T cell activation found in human trials of Treg expansion with CD28 agonists.
Concise Methods
Mice
Male BALB/c mice were obtained from the Animal Resources Centre in Perth, Australia. All mice were maintained free of pathogens in the Westmead Hospital Animal House. Eight-week-old mice (weight, 20–22 g) were used in all groups. Experiments were carried out in accordance with protocols approved by the Animal Ethics Committee of Western Sydney Local Health District.
Induction of AN and IL-2/IL-2Ab Complex Administration
Male BALB/c mice were divided into four groups, each consisting of six mice: AN (adriamycin only), pre–IL-2 (adriamycin + IL-2/IL-2Ab complex administered before adriamycin), post–IL-2 (adriamycin + IL-2/IL-2Ab complex administered after adriamycin) and normal (control group, injected with saline). AN was induced by a single tail-vein injection of adriamycin (9.8 mg/kg, doxorubicin hydrochloride; Pharmacia & Upjohn, Perth, Australia). Pre–IL-2 mice were intraperitoneally injected with IL-2/IL-2Ab complex (1 μg/5 μg) daily for 3 days 1 week before adriamycin administration. Post–IL-2 mice were injected daily at days 5, 6, and 7 and days 12, 13, and 14 after adriamycin using the same doses as for pre–IL-2 mice.
Renal Function
Blood, spleen, and kidney samples were obtained from mice in each group on day 35. Renal function was assessed by 16-hour measurement of urine protein and serum creatinine using methods described elsewhere.4
RNA Isolation and Real-Time PCR and RT-PCR
RNA was isolated from mouse cells using TRIzol (Invitrogen, Life Technologies, Australia) according to the manufacturer’s instructions. The total amount of RNA was reverse-transcribed using Superscript II reverse transcriptase (Invitrogen, Life Technologies) and random primers (Promega, Madison, WI) in a final volume of 20 μl. PCR amplification was tested on each primer pair at different cycles to ensure amplification in the linear range.
Foxp3, IL-10, IL-6, and IL-17A mRNA levels were quantified by real-time PCR using TaqMan Gene Expression (Applied Biosystems) and Rotor-Gene software (Corbett Research). Glyceraldehyde 3-phosphate dehydrogenase was used as control. PCR condition and primers were as described elsewhere.4
Intracellular Staining for STAT5
Splenocytes were stained with anti-CD4 FITC and anti-CD25 allophycocyanin (BD Biosciences). The cells were treated with PBS, IL-2 (100 ng/ml), or IL-2/IL-2Ab complex (IL-2, 100 ng/ml; anti–IL-2, 0.5 μg/ml) for 20 minutes at 37°C.21 The cells were fixed with Lyse/Fix Buffer (BD Biosciences) for 10 minutes at 37°C, permeabilized by Phosflow Perm Buffer III (BD Biosciences) on ice for 30 minutes, and then stained with phycoerytherin mouse anti-phophoSTAT5 (BD Biosciences).
BD Cytometric Bead Array
The BD CBA Mouse Th1/Th2/Th17 Cytokine Kit was used to detect IL-2, IL-4, IL-6, IFN-γ, TNF, IL-17A, and IL-10 serum protein, following the manufacturer’s instructions (BD Bioscience). The data were analyzed by FCAP Array software (BD).
Flow Cytometric Analysis
Antibodies used for flow cytometry included FITC-conjugated antimouse CD25 and allophycocyanin-conjugated antimouse CD4 (BD Bioscience). Foxp3 intracellular staining was performed by using phycoerytherin anti–mouse/rat Foxp3 Staining Set (eBioscience, clone: FJK-16s), following the manufacturer’s instructions. All samples were analyzed on a LSRII-10 Color Flow Cytometer (Becton Dickinson, Mountain View, CA). BD FACSDiva software was used for acquisition and analysis (Becton Dickinson, Australia).
Histology and Morphometric Evaluation
The kidneys were removed on day 35. Kidneys were fixed in neutral buffered formalin at room temperature for 24 hours, embedded in paraffin stained with periodic acid-Schiff reagent, and assessed by light microscopy. Cortex of the kidney was snap frozen in liquid nitrogen for RNA and immunohistochemistry using Optimal Cutting Temperature media (Sakura Finetek Inc., Torrance, CA). The degree of renal injury was assessed by two blinded observers as described elsewhere.4
Immunohistochemistry
Immunohistochemical staining was performed for Foxp3+ cells (eBioscience, antimouse Foxp3, clone FJK-16s). Secondary antibody used was biotinylated rabbit antirat immunoglobulin (Dako Corporation, Carpinteria, CA), and 5-μm sections were cut. Endogenous peroxidase activity and endogenous avidin binding activity were blocked. Normal rat immunoglobulin was used for control. Sections were incubated with secondary antibodies and 3,3-diaminobenzidin (Dako). Slides were counterstained with hematoxylin (Sigma). For assessment of interstitial infiltration, positively stained cells located in interstitium only were counted from five random cortical fields (magnification ×400) in each section, and the numbers were averaged for each field. Glomerular macrophage infiltration was evaluated as the number of macrophages per 10 glomerular cross-sections.
Statistical Analyses
Statistical analyses were performed by one-way ANOVA. Results are expressed as the group mean ± SD. Two group differences were analyzed by t test. A P value (two-tailed) < 0.05 (GraphPad Prism software V4) was considered to represent a statistically significant difference.
Disclosures
None.
Acknowledgments
We thank the staff of the Westmead Hospital Animal House Facility for care of the animals. We thank Professor Abbas for his comments.
This work was supported by research grants from the National Health and Medical Research Council of Australia (NHMRC Grants 571343 and 632519).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Hori S, Nomura T, Sakaguchi S: Control of regulatory T cell development by the transcription factor Foxp3. Science 299: 1057–1061, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T: Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev 212: 8–27, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S: Regulatory T cells in the past and for the future. Eur J Immunol 38: 901–937, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Wang YM, Zhang GY, Wang Y, Hu M, Wu H, Watson D, Hori S, Alexander IE, Harris DC, Alexander SI: Foxp3-transduced polyclonal regulatory T cells protect against chronic renal injury from adriamycin. J Am Soc Nephrol 17: 697–706, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Mahajan D, Wang Y, Qin X, Wang Y, Zheng G, Wang YM, Alexander SI, Harris DC: CD4+CD25+ regulatory T cells protect against injury in an innate murine model of chronic kidney disease. J Am Soc Nephrol 17: 2731–2741, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Kim HP, Kelly J, Leonard WJ: The basis for IL-2-induced IL-2 receptor alpha chain gene regulation: Importance of two widely separated IL-2 response elements. Immunity 15: 159–172, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Beyersdorf N, Ding X, Blank G, Dennehy KM, Kerkau T, Hünig T: Protection from graft-versus-host disease with a novel B7 binding site-specific mouse anti-mouse CD28 monoclonal antibody. Blood 112: 4328–4336, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Varker KA, Kondadasula SV, Go MR, Lesinski GB, Ghosh-Berkebile R, Lehman A, Monk JP, Olencki T, Kendra K, Carson WE, 3rd: Multiparametric flow cytometric analysis of signal transducer and activator of transcription 5 phosphorylation in immune cell subsets in vitro and following interleukin-2 immunotherapy. Clin Cancer Res 12: 5850–5858, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Takabatake Y, Li XK, Mizui M, Miyasato K, Matsui I, Kawada N, Imai E, Hünig T, Takahara S, Wada T, Furuichi K, Rakugi H, Isaka Y: A superagonistic monoclonal antibody for CD28 ameliorates crescentic glomerulonephritis in wistar-kyoto rats. Mol Med 17: 686–696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohkura N, Hamaguchi M, Sakaguchi S: FOXP3+ regulatory T cells: Control of FOXP3 expression by pharmacological agents. Trends Pharmacol Sci 32: 158–166, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Nelson BH: IL-2, regulatory T cells, and tolerance. J Immunol 172: 3983–3988, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Tomala J, Chmelova H, Mrkvan T, Rihova B, Kovar M: In vivo expansion of activated naive CD8+ T cells and NK cells driven by complexes of IL-2 and anti-IL-2 monoclonal antibody as novel approach of cancer immunotherapy. J Immunol 183: 4904–4912, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J: In vivo expansion of T reg cells with IL-2-mAb complexes: Induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med 206: 751–760, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barron L, Dooms H, Hoyer KK, Kuswanto W, Hofmann J, O’Gorman WE, Abbas AK: Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J Immunol 185: 6426–6430, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson MS, Pesce JT, Ramalingam TR, Thompson RW, Cheever A, Wynn TA: Suppression of murine allergic airway disease by IL-2:anti-IL-2 monoclonal antibody-induced regulatory T cells. J Immunol 181: 6942–6954, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phelan JD, Orekov T, Finkelman FD: Cutting edge: mechanism of enhancement of in vivo cytokine effects by anti-cytokine monoclonal antibodies. J Immunol 180: 44–48, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Gogishvili T, Langenhorst D, Lühder F, Elias F, Elflein K, Dennehy KM, Gold R, Hünig T: Rapid regulatory T-cell response prevents cytokine storm in CD28 superagonist treated mice. PLoS ONE 4: e4643, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V, Sene D, Cacoub P, Klatzmann D: Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med 365: 2067–2077, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP, 3rd, Armand P, Cutler C, Ho VT, Treister NS, Bienfang DC, Prasad S, Tzachanis D, Joyce RM, Avigan DE, Antin JH, Ritz J, Soiffer RJ: Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med 365: 2055–2066, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bluestone JA: The yin and yang of interleukin-2-mediated immunotherapy. N Engl J Med 365: 2129–2131, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Boyman O, Surh CD, Sprent J: Potential use of IL-2/anti-IL-2 antibody immune complexes for the treatment of cancer and autoimmune disease. Expert Opin Biol Ther 6: 1323–1331, 2006 [DOI] [PubMed] [Google Scholar]