Abstract
Peritonitis is a major complication of peritoneal dialysis, but the relationship between peritonitis and mortality among these patients is not well understood. In this case-crossover study, we included the 1316 patients who received peritoneal dialysis in Australia and New Zealand from May 2004 through December 2009 and either died on peritoneal dialysis or within 30 days of transfer to hemodialysis. Each patient served as his or her own control. The mean age was 70 years, and the mean time receiving peritoneal dialysis was 3 years. In total, there were 1446 reported episodes of peritonitis with 27% of patients having ≥2 episodes. Compared with the rest of the year, there were significantly increased odds of peritonitis during the 120 days before death, although the magnitude of this association was much greater during the 30 days before death. Compared with a 30-day window 6 months before death, the odds for peritonitis was six-fold higher during the 30 days immediately before death (odds ratio, 6.2; 95% confidence interval, 4.4–8.7). In conclusion, peritonitis significantly associates with mortality in peritoneal dialysis patients. The increased odds extend up to 120 days after an episode of peritonitis but the magnitude is greater during the initial 30 days.
Annual mortality for peritoneal dialysis (PD) patients is between 10% and 20%.1 Infectious causes of death account for a variable proportion of deaths on PD (5.9%–33%), depending on the publication examined and the population studied.1–3 The single most common cause of these infections in PD patients is peritonitis, with rates varying between centers.2,4 However, it is difficult to ascertain the definition of infection-associated death, particularly that associated with peritonitis, because often it is a clinical diagnosis without any clearly defined criteria.
The evidence that peritonitis increases a patient’s risk of death is primarily descriptive in nature or extrapolated from peritonitis outcomes in nondialysis patients.5,6 There has been limited statistical analysis formally examining the relationship between peritonitis and death, primarily related to the brevity and intermittent nature of the at-risk period.4 The patient who has peritonitis and sepsis then dies, which clearly indicates that there is likely a causal relationship between the two. However, the inflammatory state that an infection creates within an individual may also predispose the patient to vascular events, especially in those with pre-existing disease, possibly leading to cardiovascular or cerebrovascular death.7–9 These events may even occur a period of time after an infection.10
Our aim was to explore the relationship between mortality and peritonitis in PD patients by determining whether peritonitis was more likely to occur in the time immediately before death than in periods distant to death. We utilize a case-crossover design to compare the likelihood of peritonitis at different times, in which individual patients serve as their own controls.11
Results
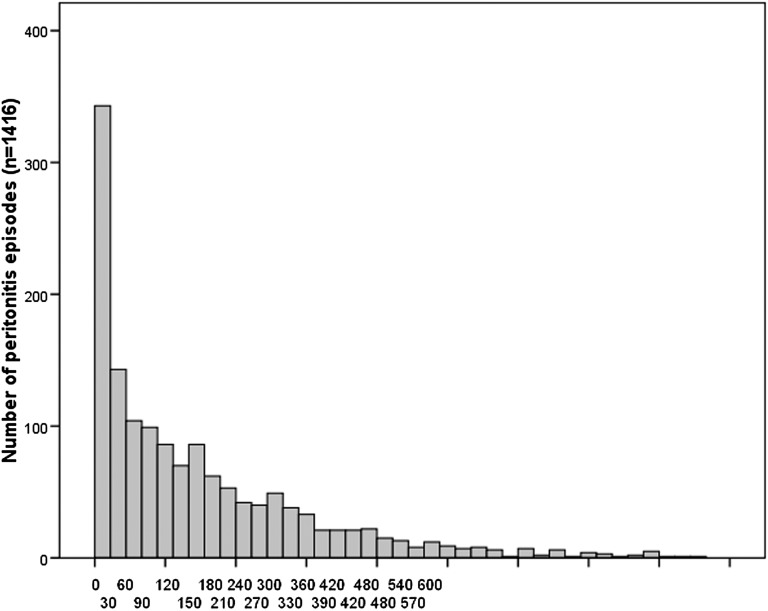
Our analyses included 1316 PD patients who died while receiving PD treatment (or within 30 days of transfer to hemodialysis), 44% of whom were female. Patients’ mean age at death was 70.4±11.7 years, with a mean time on PD of 2.9±2.0 years (Table 1). Patients were predominantly Caucasian (78%), with 10.7% Asians and 8.9% Aboriginal or Torres Strait Islanders. A total of 1446 peritonitis episodes were documented, with 56% of patients experiencing ≥1 episode and 27% experiencing ≥2. Mortality was attributed to peritonitis in 6% of deaths. The median (25th and 75th percentile) time between peritonitis episode and death was 247 days (64–552) (Figure 1).
Table 1.
Cohort demographic and clinical characteristics
| Characteristic | Value |
|---|---|
| Number of patients | 1316 |
| Female | 582 (44.2) |
| Age at start of PD (yr) | 67.5 (11.8)a |
| Age at death (yr) | 70.4 (11.7)a |
| Time on PD (yr) | 2.3 (1.4–3.9)b |
| State of residence | |
| New South Wales and Australian Capital Territory | 556 (42.2) |
| Victoria | 234 (17.8) |
| Western Australia | 125 (9.5) |
| Queensland | 271 (20.6) |
| Tasmania | 21 (1.6) |
| South Australia | 88 (6.7) |
| Northern Territory | 21 (1.6) |
| Region of residence | |
| major city | 720 (54.7) |
| regional | 345 (26.2) |
| remote | 69 (5.3) |
| unknown | 182 (13.8) |
| Ethnicity | |
| Caucasian | 1023 (77.8) |
| Asian | 141 (10.7) |
| Pacific | 34 (2.6) |
| Aboriginal and Torres Strait Islander | 117 (8.9) |
| Cause of death | |
| peritonitis | 78 (5.9) |
| other infection | 115 (8.7) |
| cardiac | 578 (43.9) |
| cerebrovascular | 107 (8.1) |
| peripheral vascular | 48 (3.6) |
| malignancy | 96 (7.3) |
| withdrawal | 174 (13.2) |
| other | 120 (9.1) |
| Number of peritonitis episodes | |
| 0 | 584 (44.4) |
| 1 | 380 (28.9) |
| 2 | 170 (12.9) |
| ≥3 | 182 (13.8) |
| Median days from peritonitis to death | 247 (64–552)b |
| Smoking status | |
| current | 145 (11.0) |
| former | 581 (44.1) |
| never | 590 (44.8) |
| Body mass index (kg/m2) | |
| <20 | 106 (8.1) |
| 20.0–24.9 | 456 (34.9) |
| 25.0–29.9 | 472 (36.1) |
| ≥30 | 273 (20.9) |
| Diabetes | 53 (4.0) |
| type I | 330 (25.1) |
| type II noninsulin requiring | 312 (23.7) |
| type II insulin requiring | |
| Other comorbidities | |
| chronic lung disease | 369 (28.0) |
| coronary artery disease | 1029 (78.2) |
| peripheral vascular disease | 710 (54.4) |
| cerebrovascular disease | 508 (38.6) |
| no comorbidity | 78 (5.9) |
| Membrane transport status | |
| low | 52 (4.0) |
| low average | 327 (24.8) |
| high average | 479 (36.4) |
| high | 191 (14.5) |
| unknown | 267 (20.3) |
| Kt/V (total) | 1.8 (0.6)a |
Data are presented as n (%) unless otherwise noted.
Mean (SD)
25th and 75th percentile.
Figure 1.
Days between peritonitis episode and death.
Of the 250 patients with an episode of peritonitis in the 30 days before death, 69 (27.6%) had peritonitis as the stated cause of death. For the remaining patients, the cause of death was categorized as cardiac (n=68, 27.2%), withdrawal (n=40, 16%), nonperitoneal infection (n=20, 8%), cerebrovascular (n=13, 5.2%), malignancy (n=7, 2.8%), peripheral vascular events (n=5, 2%), and other causes including bowel infarction, gastrointestinal hemorrhage, cachexia, and abdominal perforation (n=28, 11.2%).
Determination of the Duration of the Window Period
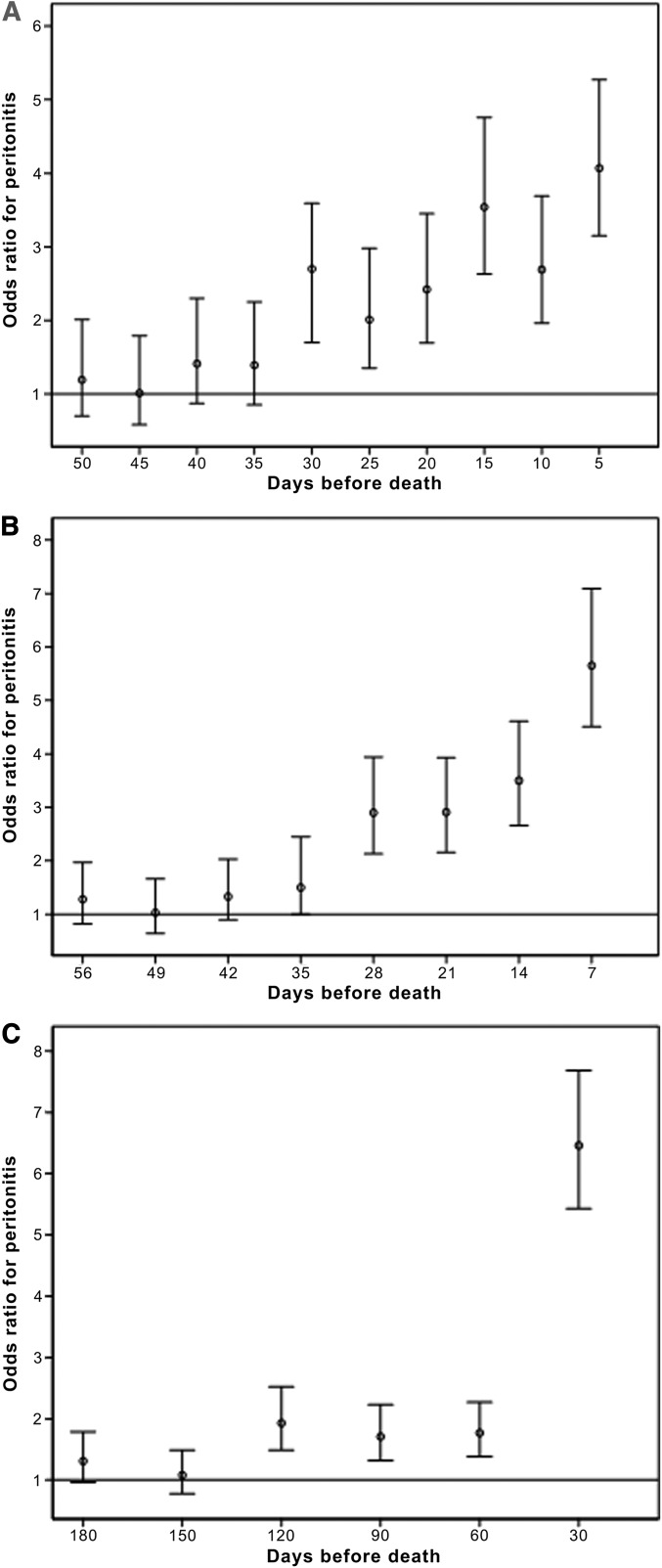
To ascertain the preferred duration of the window to be used in this analysis, 5- and 7-day windows were used at intervals before death and the occurrence of peritonitis during that window was compared with all of the preceding periods up to 12 months before death (Figure 2, A and B). The odds of peritonitis in any 5-day period >30 days before death were no different than any other early period in the year before death (Figure 2A). However, patients were >2.5 times more likely to have peritonitis in the window 30 days before death (95% confidence interval [95% CI], 1.7–3.6; P<0.001) than during any earlier period and the odds ratio (OR) increased to 4.1 times in the 5 days immediately before death (95% CI, 3.2–5.3; P<0.001). A similar pattern was observed using 7-day windows, with the risk of peritonitis significantly increased from 28 days to 7 days immediately before death (OR range, 2.9–5.7). Because there were statistically significant ORs for both 5- and 7-day windows until 30 days before death, 30 days was utilized as the duration of the windows to examine the association between mortality and peritonitis.
Figure 2.
Odds of peritonitis immediately before death compared with the preceding 12 months using different periods of time. (A) Odds of peritonitis in different 5-day periods before death compared with all other preceding 5-day periods in the year before death. (B) Odds of peritonitis in different 7-day periods before death compared with all other preceding 7-day periods in the year before death. (C) Odds of peritonitis in different 30-day periods before death compared with all other preceding 30-day periods in the year before death.
Potential At-Risk Period of the Association between Peritonitis and Mortality
Upon utilizing a 30-day window at different intervals before death and comparing the incidence of peritonitis with all other 30-day windows in the 12 months before death, there were significantly increased odds of peritonitis up to 120 days before death. However, the magnitude of this association was much greater in the first 30 days (OR, 6.5; 95% CI, 5.4–7.7; P<0.001) (Figure 2C).
Magnitude of the Odds of Peritonitis before Death
In the 30 days immediately before death, 250 patients (19.0%) experienced an episode of peritonitis, compared with 88 (6.7%) in the 30 days 6 months before death. The OR of peritonitis in the 30 days before death compared with a 30-day window 6 months before death was statistically significant at 6.2 (95% CI, 4.4–8.7) (Table 2). Similar statistically significant associations were seen when the comparator period was 3 and 9 months before death.
Table 2.
ORs of peritonitis in the 30 days before death compared with the 30-day period 3, 6, and 9 months before death
| Comparator Month | n | OR | 95% CI |
|---|---|---|---|
| All patients | |||
| 3 mo | 1316 | 4.07 | 3.04–5.45 |
| 6 mo | 1316 | 6.23 | 4.44–8.74 |
| 9 mo | 1221 | 6.97 | 4.84–10.04 |
| Patients with 1 episode | |||
| 3 mo | 380 | 8.36 | 4.80–14.55 |
| 6 mo | 380 | 5.09 | 3.25–7.95 |
| 9 mo | 359 | 11.10 | 5.81–21.20 |
Association of Peritonitis and Cause of Death–Specific Mortality
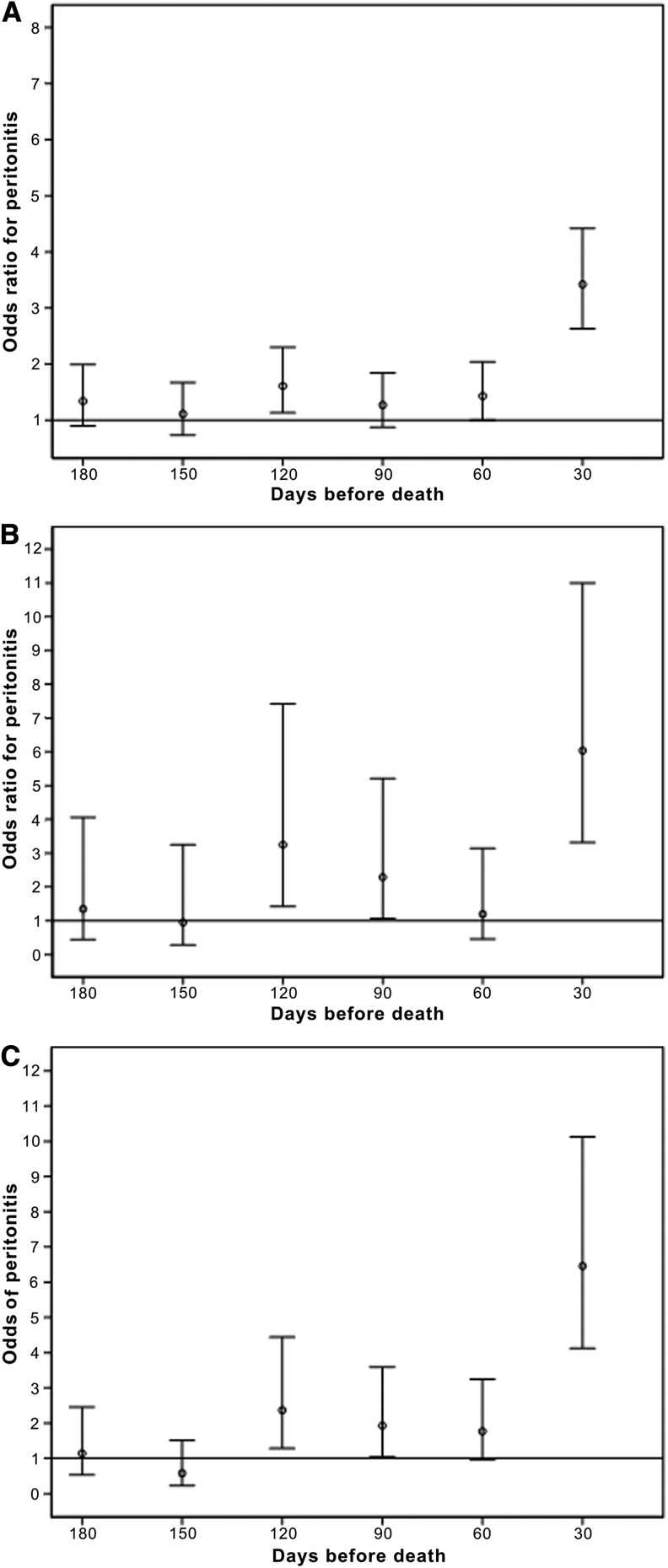
Significantly increased odds of peritonitis in the 30 days before death were detected in patients who died from cardiovascular, cerebrovascular, or peripheral vascular disease (OR, 3.4; 95% CI, 2.4–4.6) (Figure 3A). Similar findings were detected in patients who died from infectious causes and those who were coded as having withdrawn from dialysis (Figure 3, B and C).
Figure 3.
Odds of peritonitis in the 30-day period before death compared with all other preceding 30-day periods in the preceding year, from difference causes of death. (A) Odds of peritonitis in different 30-day periods before death from vascular disease compared with all other preceding 30-day periods in the year before death (n=733). (B) Odds of peritonitis in different 30-day periods before death from infection compared with all other preceding 30-day periods in the year before death (n=115). (C) Odds of peritonitis in different 30-day periods before death from withdrawal from dialysis compared with all other preceding 30-day periods in the year before death (n=174).
Sensitivity Analyses
Sensitivity analyses of the 30-day window demonstrated that there was no significant difference in the odds ratio of peritonitis at varying time periods before death more remote than the period immediately before death (Table 3). This was consistent when only PD patients with one episode of peritonitis were included.
Table 3.
Sensitivity analysis comparing the odds ratio of peritonitis in a 30-day window at two different periods of time before death
| Time Period | n | OR | 95% CI |
|---|---|---|---|
| All patients | |||
| 6 mo versus 9 mo | 1221 | 0.95 | 0.64–1.48 |
| 6 mo versus 12 mo | 1120 | 0.81 | 0.51–1.27 |
| Patients with 1 episode | |||
| 6 mo versus 9 mo | 359 | 1.10 | 0.47–2.59 |
| 6 mo versus 12 mo | 328 | 0.91 | 0.39–2.14 |
Demographic and Clinical Predictors of Peritonitis in the 30 Days before Death
Peritonitis in the 30 days before death was significantly associated with history of coronary artery disease, time spent on PD, and the number of peritonitis episodes they had previously. The odds ratio of peritonitis in the 30 days before death increased by 1.07 for each additional year spent on PD (95% CI, 1.00–1.15; P=0.049) and by 1.70 for each additional case of peritonitis (95% CI, 1.55–1.89; P<0.001). However, patients with a history of coronary artery disease were less likely to have peritonitis in the 30 days before death compared with those without this comorbidity (OR, 0.71; 95% CI, 0.51–0.99; P=0.048). (Table 4).
Table 4.
Results from logistic regression showing odds of peritonitis in the 30 days before death for selected patient demographic and clinical characteristics (n=1316)
| Characteristic | OR | 95% CI | P Value |
|---|---|---|---|
| History of coronary artery disease | 0.71 | 0.51–0.99 | 0.048 |
| Time on PD (yr)a | 1.07 | 1.00–1.15 | 0.049 |
| Number of peritonitis episodesa | 1.71 | 1.55–1.89 | <0.001 |
OR refers to each unit increase.
Discussion
Patients on PD have an annual mortality rate of 10%–20%.1 In Australia and New Zealand, 15% of PD patients who died were coded as having done so due to infections, with approximately 6% due to peritonitis.1 In fact, 19% of PD patients died with peritonitis occurring in the preceding 30 days. We have demonstrated that in those patients that died on PD, there was an approximately six-fold increase in the odds of peritonitis in the 30 days before death compared with the 30-day period 6 months before death.
There is currently no standard definition of peritonitis-associated mortality. Not surprisingly, there is appreciable variation in the reported prevalence of peritonitis-associated mortality in the literature, ranging from 5.9% up to 33% of deaths.2,3,12 Although a proportion of this variability reflects differences in case mix and racial origins, coding differences are also likely to contribute.
In many cases, the diagnosis of peritonitis-associated death is made by the treating physician and is somewhat subjective. Some physicians may only diagnose peritonitis-associated mortality in cases in which the patient presented with systemic manifestations of sepsis and immediately died, whereas others include all deaths after a period of time after an episode of peritonitis. For example, a previous series of publications from the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry defined peritonitis-associated mortality as “death directly attributable to peritonitis in the clinical opinion of the treating nephrologist”.13–21 In contrast, Perez-Fontan et al. defined peritonitis-associated mortality as death “a) during the course of a clinically active peritonitis, or b) during the week after complete clinical, bacteriologic, and cytological remission of an episode of peritonitis, or c) in the case of a refractory peritonitis demanding catheter removal, before hospital discharge for reinitiation of regular dialysis therapy (PD or HD)”.22 Alternatively, Szeto et al. defined peritonitis-associated mortality as “death from any cause during antibiotic treatment (generally 2–3 weeks, depending on the organism) or death during temporary hemodialysis (generally 4 weeks after catheter removal).”23
It is difficult to define the length of the at-risk period after peritonitis. It may be that the risk of mortality is elevated only during the couple of weeks of active inflammation or it may extend past this point. These variable and subjective approaches to diagnosing peritonitis-associated death make it exceedingly difficult to compare, contrast, and explore observed differences in peritonitis-associated death rates between different centers, regions, and countries. Our results would make a case for diagnosing peritonitis-associated death as any death within 30 days of an episode of peritonitis. Our finding of significantly greater odds of peritonitis in the period just before death was consistent using different periods of time distant from death. The sensitivity analysis confirmed that this finding was not related to increased peritonitis risk with time on dialysis.
Most studies that have examined factors that may influence patient survival in PD populations have failed to include peritonitis as an independent variable.2,24,25 In a single-center retrospective study of 423 PD patients, Sipahioglu et al. demonstrated that for every increase in peritonitis rate by one episode per 12 patient months, there was an associated 1.87-fold increased relative risk of mortality.4 This was performed by entering the peritonitis rates into a Cox proportional hazards model, which may not be appropriate because these models are not well suited to intermittent exposures with time-varying effects.26 This approach would also have difficulties in selecting an appropriate control group (i.e., patients who did not die on PD). These patients are likely to be fundamentally different from those who die on PD and this approach may introduce control-selection biases.
We chose to use a case-crossover design. This method is used to determine the relationship between an intermittent exposure and an outcome.27,28 A case-crossover study differs from a case-control study in that all participants are cases (i.e., all have experienced the outcome) and all have had the opportunity to be exposed and unexposed at different study periods because the risk posed by the exposure is transient. Rather than having case and control patients, the case-crossover design uses case and control periods of time to determine whether the exposure occurs more often in the case period, immediately before the event, than in the control period, more distant to the outcome.27 Therefore, the case-crossover design assesses the timing of the exposure relative to the outcome, using participants as their own controls.
The advantage of the case-crossover design is that it examines the association between time-varying exposures on an outcome while controlling for constant patient-level confounders and avoiding control-selection bias.11 The main limitations of case-crossover studies are that they do not account for within-person (time-varying) confounders or account for the role of chronic exposures.27 The design we used ensured that we controlled for stable patient factors such as age, ethnicity, and baseline comorbidity; however, the design did not account for patient factors that may have changed rapidly, such as nutritional status, anemia, or volume status.
We selected this design given that PD patients may be repeatedly exposed and unexposed to peritonitis during their treatment and that the risk of mortality posed by peritonitis is unknown; however, it would be reasonable to assume that if an increased risk exists it is likely to be time limited. The case-crossover design allowed us to determine whether peritonitis was more likely to occur in the period immediately before death for PD patients than during earlier periods. Vlak et al. used this methodology to identify eight potential trigger factors immediately before the rupture of intracranial aneurysms in 250 patients.29 Similarly, mobile phones were demonstrated to be associated with motor vehicle accidents using this technique.30
The finding in this investigation of an increased risk of death for up to 120 days after an episode of peritonitis may be potentially explained by a persistent systemic inflammatory state, which may predispose patients to cardiovascular events.7–9,31 This inflammatory state may lead to cardiovascular or cerebrovascular events that are remote to the time of the peritonitis.7–9 Our finding that there is a significant association between peritonitis and death from vascular disease supports this hypothesis. There are other possible mechanisms explaining why peritonitis may be associated with increased mortality, including through its effects on increased frailty, requirement for medications, possible hospitalization, and adverse effects on nutritional status.32 Further exploration is required into our documented association between peritonitis and death reported as “withdrawal.” This may be mislabeling by the ANZDATA Registry and a better understanding of the reasons for withdrawal may provide additional insight into this association.
The strengths of this study include its large sample size and inclusiveness. We included all patients in Australia during the study period who died while on PD or within 30 days of transferring to hemodialysis, which greatly enhanced the external validity of our findings. In addition, the study methodology reduces the potential for confounding and accounts for the nature of the exposure. These strengths should be balanced against the study’s limitations, the principal ones being that those patients sick enough to die may also be more likely to develop peritonitis and that we could not adjust for rapidly changing patient factors. Our study does not prove a causal link between peritonitis and mortality. Similarly to other registries, ANZDATA is a voluntary registry and there is no external audit of data accuracy, including the diagnosis of peritonitis and cause of death. Consequently, the possibility of coding/classification bias cannot be excluded. It is also possible that physicians report peritonitis events more often when they occur in close proximity to death. The study design also will not correct for any potential time-varying confounders. Because the data for this study are obtained from a single region and were limited to those patients that had been on PD for a minimum of 7 months, the results may not necessarily be generalizable to other PD populations.
In conclusion, we have established for the first time that there is a significant association between mortality and peritonitis. We recommend that a new definition ofperitonitis-associated mortality be made that includes any death within 30 days after an episode of peritonitis to reduce the chances of mislabeling.
Concise Methods
All patients in the ANZDATA Registry who died on PD or within 30 days of transferring from PD to hemodialysis, between May 1, 2004 and December 31, 2009, were included in this study. Patients also had to be on PD for a minimum of 7 months to allow a control period of time, distant to the time of death, with which to compare the peritonitis rates. Ethics approval was received from the University of Western Australia.
The start date for peritonitis was defined as the date of diagnosis, based on clinical features of peritonitis (abdominal pain or cloudy dialysate) and dialysate leukocytosis (white blood cell count >100/µl with >50% neutrophils).33 Variables collected from the ANZDATA Registry included dates of all diagnoses of peritonitis, patient demographic characteristics (e.g., age at time of death, sex, race, geographic remoteness at commencement of dialysis), and clinical characteristics (e.g., body mass index, cause of primary renal disease, comorbidities at the start of dialysis, smoking status, Kt/V, membrane transport characteristics, and cause of death). These data are collected by nursing and medical staff in each renal unit in Australia and New Zealand and are submitted to the ANZDATA Registry annually.
Statistical Analyses
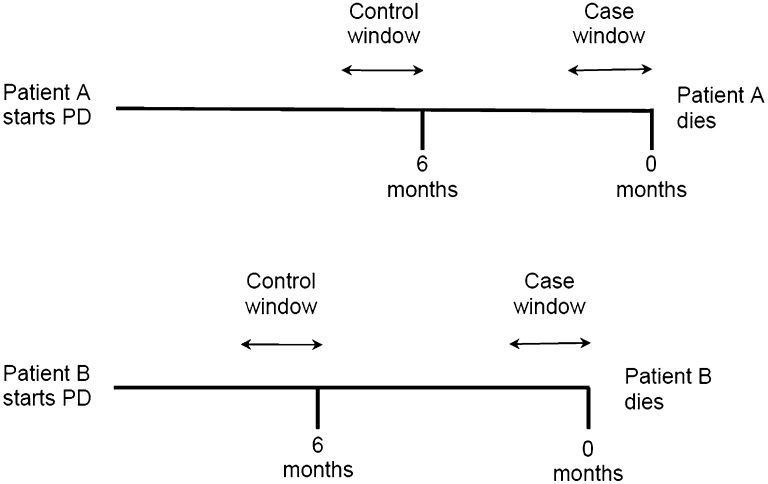
We used a time-stratified case-crossover design whereby each patient acted as his or her own control (Figure 4).11,34 Two sampling periods were determined: (1) the case window, immediately before the patient’s death, and (2) a control window, of equal duration, distant from the time of death. We varied the duration of these windows and the distance from the time of death in our analyses. Conditional logistic regression was used to compare the odds of peritonitis in the case window compared with the control window for the same patient. This method eliminated the influence of stable patient-level confounders such as sex, race, and comorbidities. This method was used to assess the association between peritonitis and all-cause mortality, death due to vascular disease (defined as death due to cardiovascular, cerebrovascular, or peripheral vascular disease), death due to infection, and death due to withdrawal of dialysis. Cause of death was defined by the treating physician.
Figure 4.
Case-crossover method in which individual patients serve as their own controls. The odds of peritonitis occurring within the case window (period immediately before death), which can be of varying lengths, is compared with a control window (period remote from death, as shown 6 months prior in this figure) of equal duration.
Peritonitis may be more likely to occur the longer a patient is on dialysis; thus, a sensitivity analysis was performed examining the relative risk of peritonitis in two 30-day windows that are both distant from the time of death, including 6, 9, and 12 months. Another sensitivity analysis was performed examining patients with one episode separately from patients with multiple episodes of peritonitis.
Logistic regression was used to determine the patient demographic and clinical characteristics predictive of peritonitis in the 30 days before death. Univariate models were initially used to examine the relationship between each variable and peritonitis within 30 days of death. The categorical variables examined were sex, age group (<60, 60–74, and ≥75 years), state of residence, Aboriginal or Torres Strait Islander status, body mass index category (underweight, <20; healthy, 20–24.9; overweight, 25–29.9; and obese, ≥30), smoking status (current, former, or never), membrane transport status category (low, <0.50; low average, 0.50–0.64; high average, 0.65–0.80; and high, ≥0.81), and comorbid diabetes, chronic lung disease, coronary heart disease, peripheral vascular disease, and cerebrovascular disease (yes/no). The continuous variables examined were time on PD (years) and number of peritonitis episodes. The linearity assumption was tested by examining the interaction between these variables and their log transformations, and was not violated in either case.35 Those variables significantly predicting peritonitis within 30 days of death (P<0.05) were included in a multivariate logistic regression model (coronary heart disease, time on PD, and number of peritonitis episodes).
Conditional logistic regression analyses were conducted using SAS software (version 9.2). All other statistical procedures were conducted with IBM SPSS Statistics software (version 19).
Disclosures
N.B. previously received research funds from Roche; travel grants from Roche, Amgen, and Jansen-Cilag; and speaking honoraria from Roche. W.L. is on the advisory board for Novartis, Genzyme, Bristol Myer Squibb, and Pfizer; he has also received research grants from Novartis, Genzyme, and Pfizer, as well as speaking honoraria from Novartis and Genzyme. S.P.M. has received speaking honoraria from Amgen Australia, Fresenius Australia, and Solvay Pharmaceuticals as well as travel grants from Amgen Australia, Genzyme Australia, and Jansen-Cilag. K.M.B. is a consultant for Baxter Healthcare Pty Ltd and has served on their clinical advisory board and received speaking honoraria. F.G.B. is a consultant for Baxter and Fresenius and has received travel grants from Amgen and Roche. D.W.J. is a consultant for Baxter Healthcare Pty Ltd and Fresenius Medical Care and previously received research funds from Baxter; he has also received speakers’ honoraria and research grants from Fresenius Medical Care and Baxter.
Acknowledgments
The authors gratefully acknowledge the substantial contributions of the entire Australia and New Zealand nephrology community (physicians, surgeons, database managers, nurses, renal operators, and patients) in providing information for and maintaining the ANZDATA Registry database.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Excell L, Livingston B, McDonald SP: ANZDATA Registry Report 2010, Adelaide, South Australia, Australia and New Zealand Dialysis and Transplant Registry, 2010 [Google Scholar]
- 2.Fang W, Qian J, Lin A, Rowaie F, Ni Z, Yao Q, Bargman JM, Oreopoulos DG: Comparison of peritoneal dialysis practice patterns and outcomes between a Canadian and a Chinese centre. Nephrol Dial Transplant 23: 4021–4028, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Hiramatsu M, Japanese Society for Elderly Patients on Peritoneal Dialysis : How to improve survival in geriatric peritoneal dialysis patients. Perit Dial Int 27[Suppl 2]: S185–S189, 2007 [PubMed] [Google Scholar]
- 4.Sipahioglu MH, Aybal A, Unal A, Tokgoz B, Oymak O, Utas C: Patient and technique survival and factors affecting mortality on peritoneal dialysis in Turkey: 12 years’ experience in a single center. Perit Dial Int 28: 238–245, 2008 [PubMed] [Google Scholar]
- 5.Abraham G, Kumar V, Nayak KS, Ravichandran R, Srinivasan G, Krishnamurthy M, Prasath AK, Kumar S, Thiagarajan T, Mathew M, Lesley N: Predictors of long-term survival on peritoneal dialysis in South India: A multicenter study. Perit Dial Int 30: 29–34, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Malik AA, Wani KA, Dar LA, Wani MA, Wani RA, Parray FQ: Mannheim Peritonitis Index and APACHE II—prediction of outcome in patients with peritonitis. Ulus Travma Acil Cerrahi Derg 16: 27–32, 2010 [PubMed] [Google Scholar]
- 7.Rost NS, Wolf PA, Kase CS, Kelly-Hayes M, Silbershatz H, Massaro JM, D’Agostino RB, Franzblau C, Wilson PW: Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack: The Framingham study. Stroke 32: 2575–2579, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Tuomainen AM, Hyvärinen K, Ehlers PI, Mervaala E, Leinonen M, Saikku P, Kovanen PT, Jauhiainen M, Pussinen PJ: The effect of proatherogenic microbes on macrophage cholesterol homeostasis in apoE-deficient mice. Microb Pathog 51: 217–224, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Hansson GK, Libby P, Schönbeck U, Yan ZQ: Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res 91: 281–291, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Lam MF, Leung JC, Lam CW, Tse KC, Lo WK, Lui SL, Chan TM, Tam S, Lai KN: Procalcitonin fails to differentiate inflammatory status or predict long-term outcomes in peritoneal dialysis-associated peritonitis. Perit Dial Int 28: 377–384, 2008 [PubMed] [Google Scholar]
- 11.Maclure M: The case-crossover design: A method for studying transient effects on the risk of acute events. Am J Epidemiol 133: 144–153, 1991 [DOI] [PubMed] [Google Scholar]
- 12.Lam MF, Tang C, Wong AK, Tong KL, Yu AW, Li CS, Cheung KO, Lai KN: ASPD: A prospective study of adequacy in Asian patients on long term, small volume, continuous ambulatory peritoneal dialysis. Perit Dial Int 26: 466–474, 2006 [PubMed] [Google Scholar]
- 13.Barraclough K, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, Bannister KM, Johnson DW: Polymicrobial peritonitis in peritoneal dialysis patients in Australia: Predictors, treatment, and outcomes. Am J Kidney Dis 55: 121–131, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Burke M, Hawley CM, Badve SV, McDonald SP, Brown FG, Boudville N, Wiggins KJ, Bannister KM, Johnson DW: Relapsing and recurrent peritoneal dialysis-associated peritonitis: A multicenter registry study. Am J Kidney Dis 58: 429–436, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Edey M, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, Bannister KM, Johnson DW: Enterococcal peritonitis in Australian peritoneal dialysis patients: Predictors, treatment and outcomes in 116 cases. Nephrol Dial Transplant 25: 1272–1278, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Fahim M, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, Bannister KM, Johnson DW: Culture-negative peritonitis in peritoneal dialysis patients in Australia: Predictors, treatment, and outcomes in 435 cases. Am J Kidney Dis 55: 690–697, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Fahim M, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, Bannister KM, Johnson DW: Coagulase-negative staphylococcal peritonitis in Australian peritoneal dialysis patients: Predictors, treatment and outcomes in 936 cases. Nephrol Dial Transplant 25: 3386–3392, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Govindarajulu S, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, Bannister KM, Johnson DW: Staphylococcus aureus peritonitis in Australian peritoneal dialysis patients: Predictors, treatment, and outcomes in 503 cases. Perit Dial Int 30: 311–319, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Miles R, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, Bannister KM, Johnson DW: Predictors and outcomes of fungal peritonitis in peritoneal dialysis patients. Kidney Int 76: 622–628, 2009 [DOI] [PubMed] [Google Scholar]
- 20.O’Shea S, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, Bannister KM, Johnson DW: Streptococcal peritonitis in Australian peritoneal dialysis patients: Predictors, treatment and outcomes in 287 cases. BMC Nephrol 10–19, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siva B, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, Bannister KM, Johnson DW: Pseudomonas peritonitis in Australia: Predictors, treatment, and outcomes in 191 cases. Clin J Am Soc Nephrol 4: 957–964, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pérez Fontan M, Rodríguez-Carmona A, García-Naveiro R, Rosales M, Villaverde P, Valdés F: Peritonitis-related mortality in patients undergoing chronic peritoneal dialysis. Perit Dial Int 25: 274–284, 2005 [PubMed] [Google Scholar]
- 23.Szeto CC, Kwan BC, Chow KM, Law MC, Pang WF, Leung CB, Li PK: Repeat peritonitis in peritoneal dialysis: Retrospective review of 181 consecutive cases. Clin J Am Soc Nephrol 6: 827–833, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jager KJ, Merkus MP, Dekker FW, Boeschoten EW, Tijssen JG, Stevens P, Bos WJ, Krediet RT, NECOSAD Study Group : Mortality and technique failure in patients starting chronic peritoneal dialysis: Results of The Netherlands Cooperative Study on the Adequacy of Dialysis. Kidney Int 55: 1476–1485, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, Mujais S, Mexican Nephrology Collaborative Study Group : Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol 13: 1307–1320, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Cortese G, Scheike TH, Martinussen T: Flexible survival regression modelling. Stat Methods Med Res 19: 5–28, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Maclure M, Mittleman MA: Should we use a case-crossover design? Annu Rev Public Health 21: 193–221, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Mittleman MA, Maclure M, Robins JM: Control sampling strategies for case-crossover studies: An assessment of relative efficiency. Am J Epidemiol 142: 91–98, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Vlak MH, Rinkel GJ, Greebe P, van der Bom JG, Algra A: Trigger factors and their attributable risk for rupture of intracranial aneurysms: a case-crossover study. Stroke 42: 1878–1882, 2011 [DOI] [PubMed] [Google Scholar]
- 30.McEvoy SP, Stevenson MR, McCartt AT, Woodward M, Haworth C, Palamara P, Cercarelli R: Role of mobile phones in motor vehicle crashes resulting in hospital attendance: A case-crossover study. BMJ 331: 428, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fine A: Relevance of C-reactive protein levels in peritoneal dialysis patients. Kidney Int 61: 615–620, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Johansen KL, Chertow GM, Jin C, Kutner NG: Significance of frailty among dialysis patients. J Am Soc Nephrol 18: 2960–2967, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, Johnson DW, Kuijper EJ, Lye WC, Salzer W, Schaefer F, Struijk DG, International Society for Peritoneal Dialysis : Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 30: 393–423, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Lu Y, Symons JM, Geyh AS, Zeger SL: An approach to checking case-crossover analyses based on equivalence with time-series methods. Epidemiology 19: 169–175, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Hosmer DW, Leleshow S: Applied Logistic Regression, 2nd Ed., Hoboken, NJ, John Wiley and Sons, 2000 [Google Scholar]