Abstract
ddY mice spontaneously develop IgA nephropathy (IgAN) with a variable age of disease onset. Establishing a model with early-onset IgAN could aid the investigation of mechanisms that underlie the pathogenesis of this disease. On the basis of histologic grading in serial biopsies, we previously classified ddY mice into early-onset, late-onset, and quiescent groups. Here, we selectively mated mice with the early-onset phenotype for >20 generations and established “grouped ddY” mice that develop IgAN within 8 weeks of age. Similar to human IgAN, the prognosis was worse for male mice than females. These mice homogeneously retained genotypes of four marker loci previously associated with the early-onset phenotype, confirming a close association of these loci with early-onset IgAN in ddY mice. Grouped ddY mice comprised two sublines, however, which had distinct genotypes at a susceptibility locus for high serum IgA levels, which maps within the Ig heavy-chain gene complex. The subline bearing the Igh-2a IgA allotype had a more rapid course of fatal disease and lower oligosaccharide content, suggesting that aberrant IgA glycosylation may promote the progression of murine IgAN. Taken together, these data indicate that grouped ddY mice may be a useful model for the identification of susceptibility genes and the underlying molecular mechanisms involved in the pathogenesis of human IgAN.
IgA nephropathy (IgAN) is one of the most frequent forms of GN worldwide, accounting for 25%–50% of patients with primary GN. IgAN was initially considered to be a benign chronic nephropathy. Although it is dependent on renal biopsy policies in each country and timing of renal biopsy, accumulating evidence now suggests that 30%–40% of patients progress to ESRD within 20 years of clinical course.1–3 There are no effective treatment strategies, mostly because of the lack of a comprehensive understanding of IgAN pathogenesis. Although the only diagnostic criterion for IgAN is IgA deposition in the glomerular mesangium,4 clinical and histopathologic findings of IgAN patients are heterogeneous. Recently, many studies have convincingly demonstrated that general impairment of immune regulation in the mucosa–bone marrow axis plays an important role in IgAN pathogenesis.5–8 Fundamental pathogenic factors are present external to the kidney, as evidenced by the fact that about half of IgAN patients develop recurrent disease after renal transplantation.9
Animal models are useful tools for studying the dynamic and complex immune axis involved in the development of IgAN, although there are differences in IgA immune responses and the ability to induce GN in different species. The ddY mouse strain is a well known model of spontaneous IgAN, which develops GN with a striking deposition of IgA in the mesangium, as well as co-deposition of IgG, IgM, and C3.10 Nevertheless, a major disadvantage of the ddY mouse model is the high degree of variability in the age of onset and severity of the disease, because the strain has been maintained as an outbred stock.11–13 The high IgA (HIGA) mouse strain was established by interbreeding of ddY strains with high serum levels of IgA to assess the correlation of serum IgA levels with the development of IgAN.14 However, although HIGA mice have high IgA levels, serum IgA levels are not associated with the severity of glomerular injury and incidence of the disease.15
We recently reported that ddY mice could be classified into three groups: early-onset (approximately 20 weeks), late-onset (approximately 40 weeks), and quiescent groups, based on the serial histologic confirmation of glomerular lesions and IgA deposits.15 Genome-wide association analyses comparing early-onset and quiescent groups identified four marker loci (D1Mit216, D1Mit16, D9Mit252, and D10Mit86) linked with the early-onset phenotype.15 D1Mit16 is located close to the selectin gene: single-nucleotide polymorphisms of this gene are associated with human IgAN,16 and D10Mit86 lies within a region of synteny with human 6q22–23 containing IGAN1, which is implicated in familial IgAN.17 These results strongly suggest that IgAN in ddY mice and humans may be, at least partly, regulated by the same susceptibility genes. In addition, susceptibility to high serum IgA levels has been shown to be associated with D12Mit20,15 which is located within the Ig heavy-chain gene complex.
To overcome the problem of the genetic heterogeneity and the high degree of variability in the age of onset and severity of the disease in ddY mice, which hinders the progress toward understanding the pathogenesis of IgAN, we have established a novel strain of ddY mice, termed grouped ddY mice, by selective mating of early-onset ddY mice. We analyzed age of onset and disease phenotype in this IgAN-prone strain and confirmed the association between IgAN and the previously identified susceptibility loci. Our results demonstrate an early onset of IgAN with a 100% incidence in these mice, indicating that the newly established grouped ddY mice would be a suitable experimental model to elucidate the pathogenesis of IgAN.
Results
Establishment of Grouped ddY Mice with Early-Onset of Proteinuria and Progressive Renal Failure
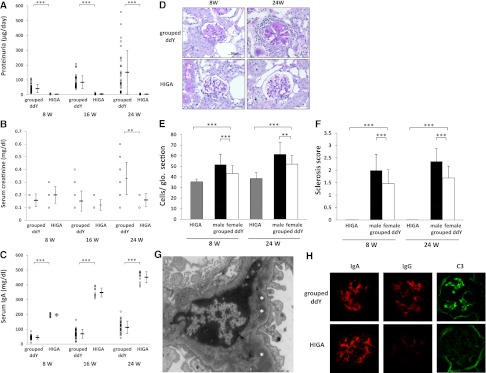
We previously classified ddY mice into early-onset, late-onset, and quiescent groups, based on histologic grading in serial biopsies.15 To obtain a mouse line consistently developing IgAN early in life, we intercrossed among an early-onset group of ddY mice, in which the development of IgAN was documented by the presence of proteinuria before mating and confirmed by mesangial IgA depositions and glomerular lesions through renal biopsies before 20 weeks of age. After selective intercrossing for >20 generations, we established a novel 100% early-onset grouped ddY mice model. All grouped ddY individuals developed proteinuria within 8 weeks of birth. The urinary protein level in female grouped ddY mice was markedly elevated compared with HIGA mice even at 8 weeks of age (Figure 1A) and the serum creatinine level was increased in association with renal failure at 24 weeks of age (Figure 1B). In HIGA mice, the serum IgA level was significantly higher than that in female grouped ddY mice at 8 weeks of age onward (Figure 1C); however, there was no evidence for the increase in levels of urinary protein and serum creatinine. Histopathologic analysis revealed that severity of glomerular injury was absolutely different in these two strains of female mice (Figure 1D). Female grouped ddY mice showed severe glomerular and tubulointerstitial lesions with mesangial proliferation, mesangial matrix expansion, and tubulointerstitial infiltrations. In contrast, HIGA mice showed faint pathologic alterations even at 24 weeks of age. Indeed, glomerular cell numbers and glomerular sclerosis scores at 8 and 24 weeks of age in female grouped ddY mice were significantly higher than those in HIGA mice (Figure 1, E and F). Electron microscopy showed electron-dense deposits mainly in the paramesangial area similar to those found in human IgAN (Figure 1G). Immunofluorescence staining showed glomerular deposits of IgA with IgG and C3 co-deposits in female grouped ddY mice, whereas only IgA deposits were found in HIGA mice (Figure 1H). The degree of glomerular IgA deposition was comparable in both strains of female mice.
Figure 1.
Phenotypes of IgAN in grouped ddY and HIGA mice. Urinary protein (A), serum creatinine (B), and serum IgA (C) levels in female grouped ddY and HIGA mice at 8, 16, and 24 weeks of age. Means ± SDs are shown. All 35 grouped ddY female mice were proteinuric at 8 weeks of age. The levels of urinary protein and serum creatinine in grouped ddY female mice increased with age. On the other hand, the serum IgA level of HIGA mice were significantly higher than female ddY mice at any weeks of age, but none of 10 HIGA mice showed proteinuria. (D) Representative histologic appearance of glomerular lesion. Grouped ddY female mice showed mesangial proliferation and extracellular matrix expansion that were developed with age, whereas there was no clear glomerular damage in HIGA mice even at 24 weeks of age. Glomerular cell numbers (E) and mesangial sclerosis scores (F) in male and female grouped ddY mice and HIGA mice at 8 and 24 weeks of age (mean of 10 mice ± SD). These parameters of histologic changes in female grouped ddY mice were significantly higher than those in HIGA mice and changes in male grouped ddY mice were significantly higher than those in female grouped ddY mice. (G) Representative appearance of electron microscopy. Female grouped ddY mice at 8 weeks of age showed electron-dense deposits (*) in the paramesangial area. (H) Representative appearance of glomerular IgA, IgG, and C3 depositions in female grouped ddY and HIGA mice at 8 weeks of age. Although IgA deposition was identical in both strains of female mice, clear co-depositions of IgG and C3 was observed only in grouped ddY mice. **P<0.01, ***P<0.001. Original magnification, ×400. Original magnification, ×400 in D and H; ×3200 in G.
Inheritance of the Four IgAN Susceptibility Loci in the Grouped ddY Mice
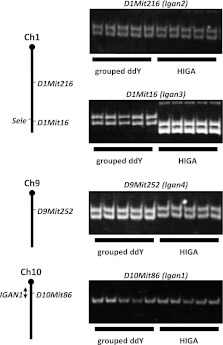
Our previous genome-wide association analyses identified four marker loci (D1Mit216, D1Mit16, D9Mit252, and D10Mit86) linked with the early onset of IgAN.15 To evaluate the effect of these loci on disease in grouped ddY mice, we genotyped for these four marker loci and obtained evidence that all of these marker loci were homogeneously retained in grouped ddY mice (Figure 2).Therefore, we designated the locus linked to D10Mit86 as Igan1 (IgA nephropathy 1), because it corresponds to the IGAN1 locus for human IgAN,17 and the three other loci, D1Mit216, D1Mit16, D9Mit252, as Igan2, Igan3, and Igan4, respectively (Table 1). In contrast, HIGA mice only share two of the alleles with grouped ddY mice: D10Mit86 (Igan1) and D1Mit216 (Igan2) (Table 1). Thus, the Igan3 and Igan4 loci, unique to grouped ddY mice, could play a pivotal role for early onset in grouped ddY mice, in combination with other genes.
Figure 2.
Genotypes of the four susceptibility loci linked to early onset of IgAN in grouped ddY and HIGA mice. All were homogeneous in each of the grouped ddY and HIGA mice. Genotypes of D1Mit16 (Igan3) and D9Mit252 (Igan4) in grouped ddY mice are different from those in HIGA mice.
Table 1.
IgAN susceptibility loci in the grouped ddY mice compared with those in HIGA mice
| Susceptibility Loci | Markers | Candidate Gene/Locus for Human IgAN | Igan Loci in HIGA Mice |
|---|---|---|---|
| Igan1 | D10Mit86 (Ch10, 24.3Mb)a | IGAN1 | + |
| Igan2 | D1Mit216 (Ch1, 79.8Mb) | + | |
| Igan3 | D1Mit16 (Ch1, 169.9Mb) | SELE | − |
| Igan4 | D9Mit252 (Ch9, 36.8Mb) | − |
Chromosomal locations of markers are indicated as Mb (megabases) from the centromere.
Development of More Severe IgAN in Grouped ddY Male Mice than in Female Counterparts
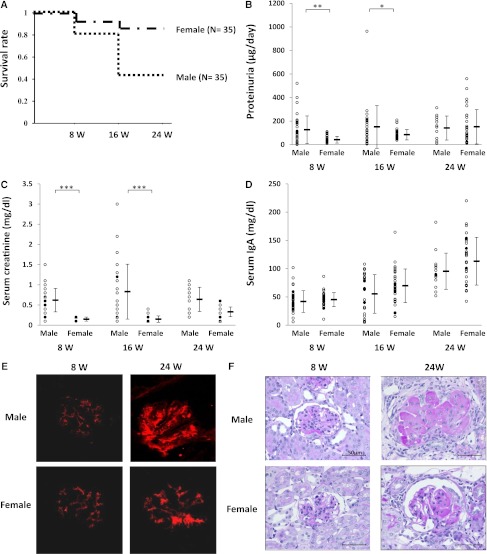
It has been reported that male sex is a risk factor for poor prognosis in patients with IgAN.18 Therefore, we compared the possible differences in the extent of disease severity between grouped ddY male and female mice. The results showed that the survival rate of grouped ddY male mice was lower at 24 weeks of age than that of female mice (40% versus 86%; P<0.001) (Figure 3A). In addition, male mice had significantly higher levels of urinary protein and serum creatinine than female mice at 8 and 16 weeks of age (Figure 3, B and C). In contrast, there were no sex differences in serum IgA levels (Figure 3D).
Figure 3.
Poor prognosis in male grouped ddY mice. (A) Kaplan–Meier analysis of the survival rate between male and female grouped ddY mice (P<0.001). Levels of urinary protein (B), serum creatinine (C), and serum IgA (D) in male and female grouped ddY mice at 8, 16, and 24 weeks of age. Results of means ± SDs are shown. There was no sex difference associated with serum IgA level. However, the levels of urinary protein and serum creatinine in male mice were significantly higher than those in female mice at 8 and 16 weeks of age. (E) Representative appearance of glomerular IgA deposition in male and female grouped ddY mice at 8 and 24 weeks of age. All 20 grouped ddY mice showed mesangial IgA deposition at 8 weeks of age and the amounts of deposition increased with age. (F) Representative histologic appearance of glomerular lesion. All 20 grouped ddY mice displayed mesangioproliferative lesions at 8 weeks of age and these worsened with age. *P<0.05, **P<0.01, ***P<0.001. Original magnification, ×400 in E and F.
Immunohistochemical examination of kidneys at 8 and 24 weeks of age showed no differences in the extent of IgA deposition in glomeruli between males and females (Figure 3E). However, histologically, glomerular lesions characterized by mesangial cell proliferation and sclerotic changes of glomeruli were more severe in male mice than those in female mice (Figures 1, E and F, and 3F), consistent with the decreased survival rate in male mice.
Two Different Sublines of Grouped ddY Mice with Different IgA Allotype, Glycosylation, and Mortality Rate
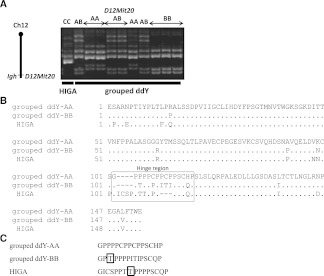
We previously showed the strong linkage of the D12Mit20 marker with serum levels of IgA in ddY mice.15 Notably, we observed the presence of three genotypes of D12Mit20 among the grouped ddY mice, designated types AA, BB, and AB (Figure 4A). On the other hand, HIGA mice carry a different genotype of D12Mit20, namely type CC.
Figure 4.
Heterogeneity of sequences of the hinge region in grouped ddY mice. (A) Genotype of D12Mit20, the susceptibility locus linked to high serum IgA levels. In grouped ddY mice, there were three genotypes: type AA, type BB, and type AB, whereas the genotype of HIGA mice is different (type CC). (B) Predicted amino acid sequences of the IgA constant region of grouped ddY and HIGA mice. Most differences were concentrated in the hinge region (boxed) among type AA and type BB grouped ddY mice and HIGA mice. (C) Comparison of the IgA hinge region amino acid sequences. Type BB grouped ddY mice and HIGA mice have the potential O-glycosylation acceptor site, the first threonine (T) residue adjacent prolines (P) (boxed).20
Because the D12Mit20 marker is located within the Ig heavy-chain gene complex, the observed D12Mit20 polymorphism could reflect the difference in the IgA allotype among the grouped ddY and HIGA mice. Indeed, the cDNA nucleotide sequence analysis revealed that the predicted amino-acid sequence of the IgA heavy-chain constant region, especially in the hinge region, differs among types AA and BB grouped ddY mice as well as HIGA mice (Figure 4B). Accordingly, the IgA allotypes of types AA and BB grouped ddY mice correspond to Igh-2b (C57BL/6 type) and Igh-2a (BALB/c type), respectively (Table 2), whereas the IgA allotype of HIGA mice is Igh-2c (DBA/2 type) as reported.19 More significantly, the Igh-2a hinge region of type BB grouped ddY and HIGA mice carry a potential O-glycosylation acceptor site, the first threonine residue adjacent to proline residue,20 which is not the case for IgA of type AA grouped ddY mice (Figure 4C). Notably, the presence of O-linked glycans in the hinge region of murine IgA bearing the Igh-2a allotype has recently been demonstrated.21
Table 2.
D12Mit20 polymorphism, IgA allotypes, and monosaccharide composition of IgA in grouped ddY mice
| D12Mit20 Polymorphisms | Type AA | Type BB |
|---|---|---|
| IgA allotype | Igh-2b | Igh-2a |
| sugar | ||
| mannose | 0.207 | 0.158 |
| GlcNAc | 0.106 | 0.092 |
| GalNAc | ND | ND |
| galactose | 0.197 | 0.153 |
| SA | 0.115 | 0.097 |
| IS | 1.000 | 1.000 |
Data are expressed as average from two experiments relative to standard sugars and normalized to internal standard, based on the area under the peak in the chromatograms. Ten micrograms of IgA was used per sample. GlcNAc, N-acetylglucosamine; GalNAc, N-acetylgalactosamine; IS, internal standard; ND, not detected; SA, sialic acid.
We further determined the monosaccharide composition of purified serum IgA from type AA and type BB grouped ddY mice. Gas-liquid chromatography analyses revealed that IgA from type BB had lower content of glycans compared with that from type AA mice (Table 2). However, none of the two IgA preparations contained N-acetylgalactosamine, a sugar residue characteristic of O-glycans that is attached to serine or threonine residues of glycoproteins.
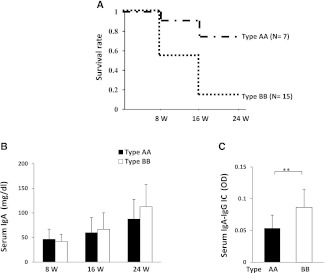
Intriguingly, whereas our previous studies showed no linkage between the D12Mit20 marker and the early onset of disease in ddY mice,15 type BB male mice had an early mortality rate compared with type AA male mice (13% versus 71% at 24 weeks of age; P<0.001) (Figure 5A). This is consistent with the notion that type BB genotype contributes to the acceleration of disease severity in combination with the other four IgAN susceptibility loci reserved in grouped ddY mice. Thus, we termed this locus contributing to the accelerated development of IgAN as Igan5, the possible candidate is Igh-2b allotype. The difference in Igh-2 allotype in types AA and BB male mice did not affect the serum level of IgA at any age (Figure 5B), but the serum level of IgA-IgG immune complexes (ICs) in type BB grouped ddY male mice was significantly higher than those in type AA grouped ddY male mice at 8 weeks of age (Figure 5C).
Figure 5.
Different prognosis between two sublines in grouped ddY mice. (A) Kaplan–Meier analysis of the survival rate between types AA and BB grouped ddY male mice (P<0.001). (B) Serum levels of IgA in type AA and type BB grouped ddY mice at 8, 16, and 24 weeks of age. Results of means ± SDs are shown. There were no differences in serum levels of IgA between type AA and type BB grouped ddY mice at any week of age. (C) Serum levels of IgA-IgG ICs in type AA and type BB grouped ddY mice at 8 weeks of age. Results of means ± SDs are shown. Serum levels of IgA-IgG ICs were significantly higher in type BB mice than those in type AA mice. **P<0.01.
Discussion
Elucidation of the pathogenesis of IgAN using appropriate animal models is urgently required in order to develop effective treatments for IgAN. There have been many attempts at establishing animal models of IgAN by genetic manipulation of individual candidate genes; however, a majority of them were only successful in causing mesangial IgA deposition.22 In this study, we have shown that grouped ddY mice, which were established by selective mating of ddY mice with the 100% early-onset phenotype, spontaneously developed mesangial co-deposition of IgA, IgG, and C3, severe proteinuria, mesangioproliferation, and expansion of extracellular matrix by 8 weeks of age, followed by renal failure within a short period. These features and the clinical course closely resemble severe IgAN in human patients.
In humans, the importance of disease susceptibility genes has been indicated by studies of familial IgAN, differences in disease incidence rates among different ethnicities, and sporadic patients with familial history of asymptomatic hematuria.23 Although linkage analysis of familial IgAN revealed close association with the five loci,17,24,25 precise causative genes have not been identified. Moreover, many candidate genes have been associated with IgAN, such as those for human leukocyte antigen,26 MHC,27 uteroglobin,28–30 Ig mu-binding protein 2,31 polymeric Ig receptor,32 selectin,16 and angiotensin-converting enzyme33,34; however, none of these studies have been sufficiently replicated or confirmed functionally. It is noteworthy that the combined effect of multiple susceptibility genes may contribute to the development of IgAN. However, it is practically difficult to analyze such genetic interactions in humans. On the other hand, genetic factors are likely to play a critical role in the onset and progression of disease in grouped ddY mice as well, because they inherited all four susceptibility loci linked with the early-onset phenotype of IgAN. Therefore, our grouped ddY mice model can be useful to analyze such genetic interactions.
Grouped ddY mice develop mesangial IgA deposits with co-deposits of IgG and C3 and severe glomerular lesions as early as 8 weeks of age, which is before any significant elevation in serum IgA levels. In contrast, even though HIGA mice showed a marked increase of serum IgA and significant mesangial IgA deposits, these mice did not fully develop mesangial co-deposits of IgG or C3 and GN even by 24 weeks of age. It was reported that in HIGA mice, pathologic alterations in the glomeruli become evident from 40 weeks of age.35 These data suggest that the induction and progression of IgAN are not simply determined by serum levels of IgA, but rather by the physicochemical properties or specificities of pathogenic IgA or a component of immune complexes, such as IgG autoantibodies specific for aberrantly glycosylated IgA,36,37 which may subsequently result in activation of complement.38 It is possible that the delayed onset with a lower incidence of IgAN in HIGA mice, despite high serum levels of IgA, could be due to the lack of two IgAN susceptibility loci, Igan3 and Igan4, mapped to D1Mit16 and D9Mit252, which may contribute to the generation of nephritogenic IgA and IgA-IgG ICs. The elucidation of candidate genes for all four susceptibility loci and roles of these genes for disease phenotypes is of paramount importance for shedding light on the genetic mechanisms that control the progression of IgAN. Further studies are underway in our laboratories by establishing congenic mouse lines carrying each of these loci separately or a combination of these loci.
The poor prognosis of male grouped ddY mice is consistent with the observations in the Japanese cohort showing that male sex is a risk factor for poor prognosis in human IgAN.18 Sex differences in human IgAN have been considered to be influenced by stress, because many IgAN patients are affected at the meridian of social life. Recently, it has been proposed that IgAN may be one of the autoimmune diseases in which aberrantly glycosylated IgA1 is the autoantigen36,39,40 and there are many reports stating that sex differences associated with the incidence or progression of autoimmune diseases may involve sex chromosomes or sex hormones.41 Thus, the observed sex effect in our grouped ddY model is likely to be dependent on biologic factors, such as sex hormones or susceptibility loci on sex chromosomes. Our grouped ddY mice will be important tools to investigate the effect of sex on the progression of human IgAN.
The nephritogenic roles of aberrantly glycosylated IgA have long been discussed in the pathogenesis of IgAN. In human primary and/or secondary IgAN, abnormal glycosylation of N-linked as well as O-linked glycans in IgA1,42 heterogeneity of structure and composition of O-glycans in the hinge region of IgA1,40 and involvement of IgG autoantibodies recognizing the aberrantly glycosylated IgA136,37 have been reported. In addition, it has also been reported that aberrant glycosylation of the N-linked glycan in mice lacking β1,4-galactosyltransferase-I induces murine IgAN.43 Furthermore, recent studies raised a possibility that degree of O-glycosylation in the hinge region as well as structure of N-glycans in the CH1 domain of murine IgA may determine the nephritogenicity.21 Collectively, these findings suggest that structural abnormalities of carbohydrates of serum IgA, presumably in association with the development of glycan-specific IgG autoantibodies, are involved in the development of human and murine IgAN, whether the carbohydrates are O-glycans or N-glycans. However, the clinical relevance of this to IgAN pathogenesis remains largely unknown because of the lack of a reliable mouse model of IgAN.
It has long been believed that O-glycans are absent in the hinge region of murine IgA. However, a recent study has demonstrated the presence of O-linked glycans in the hinge region of an IgA rheumatoid factor bearing the Igh-2a allotype and its potential to induce IgAN-like glomerular lesions was associated with increased levels of O-glycosylation.21 Notably, we observed the poor prognosis in a subgroup (type BB) of grouped ddY mice that carry the Igh-2a IgA allotype, compared with the second group (type AA) of grouped ddY mice that bear a different IgA allotype (Igh-2b). Thus, differences in the IgA allotype may be partly responsible for the poor prognosis of type BB grouped ddY mice. Although the analysis of sugar composition of serum IgA of both lines of ddY mice failed to show the presence of O-glycan–specific N-acetylgalactosamine, it should be emphasized that the occupancy of O-glycosylation site is usually only partial.21,44,45 Indeed, O-glycans were detected only in one of the four murine IgA monoclonal proteins bearing of the Igh-2a allotype analyzed in the past,21,46,47 and in even the one that carries O-glycan, its occupancy is partial.21 Thus, one cannot exclude the possibility that only a minor fraction of IgA is O-glycosylated and exhibits a high pathogenic potency in type BB mice. Whatever the presence of O-glycans in IgA of type BB grouped ddY mice may be, we noted that sugar contents in serum IgA of type BB grouped ddY mice are lower than those of type AA mice. In this regard, it is worth noting that the structure of N-linked glycans present in the CH1 domain was markedly different between nephritogenic and non-nephritogenic IgA: bi- and triantennary complex-type in the former and the hybrid-type with features of both high mannose- and complex-type oligosaccharides in the latter.21 This could raise another possibility that a change of IgA in conformation or biochemical property as a result of modification of carbohydrate structures could promote formation of nephritogenic ICs. Indeed, we observed that serum levels of IgA-IgG ICs of type BB grouped ddY mice were significantly higher than those of type AA grouped ddY mice. Thus, differences in the IgA allotype or in monosaccharide composition of IgA may be partly responsible for the poor prognosis of type BB grouped ddY mice. Clearly, further studies are required to determine whether a reduced content of oligosaccharides may promote the development of IgG autoantibodies against sugar moiety exposed on IgA in type BB mice, as in the case of human IgAN.36 For example, an inbred model from each subline of grouped ddY mice by sib-mating and generation of stable B cell clones from the inbred models may provide further clues for the role of glycosylation.
In conclusion, we have established a novel mouse model of IgAN with a 100% incidence of severe disease at a young age. Analysis of this disease model will provide useful insights into the pathogenesis of human IgAN, because it should help the eventual identification of susceptibility genes and elucidation of underlying molecular mechanisms and also define the role of IgA polymorphisms in context of IgA glycosylation and the effect of sex difference on the progression of IgAN.
Concise Methods
Mice and Breeding Environment
Grouped ddY mice, which were established by selective mating of early-onset ddY mice15 for >20 generations, were maintained at the animal facility of Juntendo University with regular chow (Oriental Yeast, Tokyo, Japan) and water ad libitum in a specific-pathogen-free room. The natural history of 70 grouped ddY mice (35 males, 35 females) was documented by obtaining serum and urinary samples, and genotyping. Histologic examination was performed on each of the 20 grouped ddY mice (10 males, 10 females) at 8 and 24 weeks of age. Ten female HIGA mice (it is commercially unable to obtain male HIGA mice), which were inbred mice established by selective mating among high serum IgA ddY mice,14 were also maintained under similar conditions and used as controls. The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Juntendo University Faculty of Medicine.
The grouped ddY mice are now maintained by brother-sister mating to establish the inbred strain of mice and these mice will be freely available through Dr. Yusuke Suzuki (Division of Nephrology, Department of Internal Medicine, Juntendo University Faculty of Medicine; yusuke@juntendo.ac.jp) to appropriately qualified researchers.
Serum and Urinary Analyses
Blood samples were obtained from the buccal vein at 8, 16, and 24 weeks of age. Serum IgA were measured using a sandwich ELISA kit (Bethyl Laboratories). Serum IgA-IgG ICs were detected by sandwich ELISA, using the modified method based on our previous report.37 Purified rat anti-mouse IgG antisera (BD Biosciences, Pharmingen, San Diego, CA) and horseradish peroxidase-conjugated goat anti-mouse IgA (Zymed Laboratories, San Francisco, CA) were used. Data were expressed as OD. Serum creatinine levels were measured using an autoanalyzer (Fuji Dry-Chem 5500; Fujifilm, Tokyo, Japan). Urinary samples obtained at 8, 16, and 24 weeks of age were pooled. Urinary albumin was measured by immunoassay (DCA 2000 system; Siemens Healthcare Diagnostics, Tokyo, Japan).
Histologic Analyses
For light microscopy, renal specimens were fixed in 15% formaldehyde and embedded in paraffin, and 3-μm sections were collected. Tissue sections were stained with hematoxylin and eosin and periodic acid–Schiff stains. The nuclear number per glomerular cross-section was determined in a blind manner with 20 glomeruli per one mouse. For the quantitative analysis of glomerular sclerosis, 20 glomeruli/cross-section were observed and following scores were assigned, depending on the modified method based on the previous report by Raij et al.48 as follows: 0 points, no glomerular sclerosis; 1 point, mild glomerular sclerosis (approximately 25%); 2 points, moderate glomerular sclerosis (approximately <50%); and 3 points, severe glomerular sclerosis (approximately >50%). Sclerosis scores were calculated as follows: [∑(each score × number of glomeruli)]/20.
Immunofluorescence Analyses
Kidneys were obtained after perfusion with normal saline. Renal specimens were mounted in OCT compound (Sakura Finetek, Tokyo, Japan) and stored at −80°C. Specimens embedded in OCT compound were cut into 3-μm sections and fixed with acetone at −20°C for 4 minutes. IgA, IgG, and C3 were stained by the immunofluorescence method. Briefly, sections were washed with PBS, blocked with a blocking agent (DS Pharma Biomedical, Osaka, Japan) for 30 minutes at room temperature, and then incubated with goat anti-murine IgA and IgG (Bethyl Laboratories, Montgomery, TX) and rat anti-C3 (Hycult Biotechnology B.V., Uden, Netherlands) as the primary antibody for 60 minutes at room temperature. After three washes with PBS, slides were incubated with secondary antibodies compatible with primary antibodies to some degree for 30 minutes at room temperature, washed, and mounted with a mounting medium (Dako, Tokyo, Japan). Samples were analyzed and imaged using confocal laser microscopy (Olympus Corporation, Tokyo, Japan).
Electron Microscopy Analyses
For electron microscopy, the specimens were fixed in phosphate-buffered glutaraldehyde for 2 hours, postfixed in 2% osmium tetroxide for 2 hours, and then embedded in Epon resin after dehydration. Ultrathin sections were sliced at 70 nm, stained with 4% uranyl acetate and lead citrate, and then examined under an electron microscope (Hitachi 7100; Hitachi, Tokyo, Japan).
Genotyping of Susceptibility Loci
Genomic DNA was obtained from mouse tails using the QIAamp DNA Mini Kit (Qiagen, Valencia, CA).
Primer information for susceptibility loci of age of onset (D1Mit216, D1Mit16, D9Mit252, and D10Mit86)15 and high serum IgA levels (D12Mit20)15 are available from the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/). A reaction volume of 25 µl containing 50 ng of genomic DNA, 5× reaction buffer, 2.5 mM dNTP mix, 4 µl Taq polymerase in 0.125 µl (Promega, Fitchburg, WI), and 100 nM of each primer was prepared for PCR in 96-well plates. After 5 minutes of incubation at 95°C, the reaction mixtures were amplified for 45 cycles of 30 seconds at 95°C, 40 seconds at 55°C, and 1 minute at 72°C, followed by 7 minutes at 72°C. Detection of PCR products was performed by PAGE on a 15% gel.
RT-PCR and cDNA Sequencing
RNA from spleen cells was purified with TRIzol reagent (Invitrogen AG, Basel, Switzerland). For nucleotide sequencing of the IgA constant regions, cDNA was amplified with Pfu DNA polymerase (Stratagene Cloning Systems, La Jolla, CA) using the following pairs of primers: VH1BACK forward primer (5′-AGGTSMARCTGCAGSAGTCWGG-3′) and 3′-UT Cα reverse primer (5′-GAAGTGCAGGGATACTTTGG-3′).49 The nucleotide sequence corresponding to the IgA constant region was determined by the dideoxynucleotide chain termination method.
Monosaccharide Compositional Analyses of IgA by Gas-Liquid Chromatography
IgA was purified by affinity chromatography using anti-mouse IgA antibodies immobilized on CNBr-Sepharose from sera of type AA and type BB grouped ddY mice collected at 8 weeks of age. Purity of the preparations was assessed by SDS-PAGE. TCA-precipitated 10 μg protein for each IgA sample was analyzed by gas-liquid chromatography with sorbitol as the internal standard. The analyses were performed with a gas chromatograph (model 5890; Hewlett-Packard, Sacramento, CA) equipped with a 25-m fused silica (0.22-mm inner diameter) OV-1701 WCOT column (Chrompack, Bridgewater, NJ) and electron capture detector. Standard sugars were used for quantification. The results were expressed relative to the internal standard and specific sugar standard, using the area under the peak in chromatograms.50,51
Statistical Analyses
Comparison of groups was performed using univariate ANOVA. A P value of <0.05 was considered significant. All statistical analyses were performed using the Windows version of StatView 5.0 software (Abacus Concepts, Berkeley, CA).
Disclosures
None.
Acknowledgments
We thank Ms. Terumi Shibata for her excellent technical assistance. We also thank Ms. Takako Ikegami and Ms. Tomomi Ikeda (Division of Molecular and Biochemical Research, Juntendo University Graduate School of Medicine) for their excellent technical assistance.
This study was supported in part by a Grant-in-Aid for Progressive Renal Diseases Research, Research on Intractable Disease, from the Ministry of Health, Labour, and Welfare of Japan and by a grant from the Swiss National Foundation for Scientific Research. J.N. was supported in part by grants from the National Institutes of Health (DK083663, DK078244, DK082753, DK075868, and GM098539).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Appel GB, Waldman M: The IgA nephropathy treatment dilemma. Kidney Int 69: 1939–1944, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Barratt J, Feehally J: IgA nephropathy. J Am Soc Nephrol 16: 2088–2097, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Julian BA, Novak J: IgA nephropathy: An update. Curr Opin Nephrol Hypertens 13: 171–179, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Berger J, Hinglais N: [Intercapillary deposits of IgA-IgG]. J Urol Nephrol (Paris) 74: 694–695, 1968 [PubMed] [Google Scholar]
- 5.van den Wall Bake AW, Beyer WE, Evers-Schouten JH, Hermans J, Daha MR, Masurel N, van Es LA: Humoral immune response to influenza vaccination in patients with primary immunoglobulin A nephropathy. An analysis of isotype distribution and size of the influenza-specific antibodies. J Clin Invest 84: 1070–1075, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Wall Bake AW, Daha MR, Evers-Schouten JH, van Es LA: Serum IgA and the production of IgA by peripheral blood and bone marrow lymphocytes in patients with primary IgA nephropathy: Evidence for the bone marrow as the source of mesangial IgA. Am J Kidney Dis 12: 410–414, 1988 [DOI] [PubMed] [Google Scholar]
- 7.van den Wall Bake AW, Daha MR, van Es LA: Immunopathogenetic aspects of IgA nephropathy. Nephrologie 10: 141–145, 1989 [PubMed] [Google Scholar]
- 8.van Es LA, van den Wall Bake AW, Stad RK, van den Dobbelsteen ME, Bogers MJ, Daha MR: Enigmas in the pathogenesis of IgA nephropathy. Contrib Nephrol 111: 169–175, discussion 175–176, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Floege J: Recurrent IgA nephropathy after renal transplantation. Semin Nephrol 24: 287–291, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Imai H, Nakamoto Y, Asakura K, Miki K, Yasuda T, Miura AB: Spontaneous glomerular IgA deposition in ddY mice: An animal model of IgA nephritis. Kidney Int 27: 756–761, 1985 [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi E, Doi T, Shimada T, Muso E, Maruyama N, Yoshida H: Retroviral gp70 antigen in spontaneous mesangial glomerulonephritis of ddY mice. Kidney Int 35: 638–646, 1989 [DOI] [PubMed] [Google Scholar]
- 12.Shimizu M, Tomino Y, Abe M, Shirai T, Koide H: Retroviral envelope glycoprotein (gp 70) is not a prerequisite for pathogenesis of primary immunoglobulin A nephropathy in ddY mice. Nephron 62: 328–331, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Tomino Y, Shimizu M, Koide H, Abe M, Shirai T: Effect of monoclonal antibody CD4 on glomerulonephritis of ddY mice, a spontaneous animal model of IgA nephropathy. Am J Kidney Dis 21: 427–432, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Muso E, Yoshida H, Takeuchi E, Yashiro M, Matsushima H, Oyama A, Suyama K, Kawamura T, Kamata T, Miyawaki S, Izui S, Sasayama S: Enhanced production of glomerular extracellular matrix in a new mouse strain of high serum IgA ddY mice. Kidney Int 50: 1946–1957, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Suzuki H, Suzuki Y, Yamanaka T, Hirose S, Nishimura H, Toei J, Horikoshi S, Tomino Y: Genome-wide scan in a novel IgA nephropathy model identifies a susceptibility locus on murine chromosome 10, in a region syntenic to human IGAN1 on chromosome 6q22-23. J Am Soc Nephrol 16: 1289–1299, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Takei T, Iida A, Nitta K, Tanaka T, Ohnishi Y, Yamada R, Maeda S, Tsunoda T, Takeoka S, Ito K, Honda K, Uchida K, Tsuchiya K, Suzuki Y, Fujioka T, Ujiie T, Nagane Y, Miyano S, Narita I, Gejyo F, Nihei H, Nakamura Y: Association between single-nucleotide polymorphisms in selectin genes and immunoglobulin A nephropathy. Am J Hum Genet 70: 781–786, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gharavi AG, Yan Y, Scolari F, Schena FP, Frasca GM, Ghiggeri GM, Cooper K, Amoroso A, Viola BF, Battini G, Caridi G, Canova C, Farhi A, Subramanian V, Nelson-Williams C, Woodford S, Julian BA, Wyatt RJ, Lifton RP: IgA nephropathy, the most common cause of glomerulonephritis, is linked to 6q22-23. Nat Genet 26: 354–357, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Goto M, Wakai K, Kawamura T, Ando M, Endoh M, Tomino Y: A scoring system to predict renal outcome in IgA nephropathy: A nationwide 10-year prospective cohort study. Nephrol Dial Transplant 24: 3068–3074, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oida E, Nogaki F, Kobayashi I, Kamata T, Ono T, Miyawaki S, Serikawa T, Yoshida H, Kita T, Muso E: Quantitative trait loci (QTL) analysis reveals a close linkage between the hinge region and trimeric IgA dominancy in a high IgA strain (HIGA) of ddY mice. Eur J Immunol 34: 2200–2208, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Phillips-Quagliata JM: Mouse IgA allotypes have major differences in their hinge regions. Immunogenetics 53: 1033–1038, 200221, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Otani M, Nakata J, Kihara M, Leroy V, Moll S, Wada Y, Izui S: O-glycosylated IgA rheumatoid factor induces IgA deposits and glomerulonephritis. J Am Soc Nephrol 23: 438–446, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki Y, Tomino Y: Potential immunopathogenic role of the mucosa-bone marrow axis in IgA nephropathy: Insights from animal models. Semin Nephrol 28: 66–77, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Kiryluk K, Julian BA, Wyatt RJ, Scolari F, Zhang H, Novak J, Gharavi AG: Genetic studies of IgA nephropathy: past, present, and future. Pediatr Nephrol 25: 2257–2268, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bisceglia L, Cerullo G, Forabosco P, Torres DD, Scolari F, Di Perna M, Foramitti M, Amoroso A, Bertok S, Floege J, Mertens PR, Zerres K, Alexopoulos E, Kirmizis D, Ermelinda M, Zelante L, Schena FP; European IgAN Consortium: Genetic heterogeneity in Italian families with IgA nephropathy: suggestive linkage for two novel IgA nephropathy loci. Am J Hum Genet 79: 1130–1134, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paterson AD, Liu XQ, Wang K, Magistroni R, Song X, Kappel J, Klassen J, Cattran D, St George-Hyslop P, Pei Y: Genome-wide linkage scan of a large family with IgA nephropathy localizes a novel susceptibility locus to chromosome 2q36. J Am Soc Nephrol 18: 2408–2415, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Fennessy M, Hitman GA, Moore RH, Metcalfe K, Medcraft J, Sinico RA, Mustonen JT, D’Amico G: HLA-DQ gene polymorphism in primary IgA nephropathy in three European populations. Kidney Int 49: 477–480, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Akiyama F, Tanaka T, Yamada R, Ohnishi Y, Tsunoda T, Maeda S, Takei T, Obara W, Ito K, Honda K, Uchida K, Tsuchiya K, Nitta K, Yumura W, Nihei H, Ujiie T, Nagane Y, Miyano S, Suzuki Y, Fujioka T, Narita I, Gejyo F, Nakamura Y: Single-nucleotide polymorphisms in the class II region of the major histocompatibility complex in Japanese patients with immunoglobulin A nephropathy. J Hum Genet 47: 532–538, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Kim YS, Kang D, Kwon DY, Park WY, Kim H, Lee DS, Lim CS, Han JS, Kim S, Lee JS: Uteroglobin gene polymorphisms affect the progression of immunoglobulin A nephropathy by modulating the level of uteroglobin expression. Pharmacogenetics 11: 299–305, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Narita I, Saito N, Goto S, Jin S, Omori K, Sakatsume M, Gejyo F: Role of uteroglobin G38A polymorphism in the progression of IgA nephropathy in Japanese patients. Kidney Int 61: 1853–1858, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Menegatti E, Nardacchione A, Alpa M, Agnes C, Rossi D, Chiara M, Modena V, Sena LM, Roccatello D: Polymorphism of the uteroglobin gene in systemic lupus erythematosus and IgA nephropathy. Lab Invest 82: 543–546, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Ohtsubo S, Iida A, Nitta K, Tanaka T, Yamada R, Ohnishi Y, Maeda S, Tsunoda T, Takei T, Obara W, Akiyama F, Ito K, Honda K, Uchida K, Tsuchiya K, Yumura W, Ujiie T, Nagane Y, Miyano S, Suzuki Y, Narita I, Gejyo F, Fujioka T, Nihei H, Nakamura Y: Association of a single-nucleotide polymorphism in the immunoglobulin mu-binding protein 2 gene with immunoglobulin A nephropathy. J Hum Genet 50: 30–35, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Obara W, Iida A, Suzuki Y, Tanaka T, Akiyama F, Maeda S, Ohnishi Y, Yamada R, Tsunoda T, Takei T, Ito K, Honda K, Uchida K, Tsuchiya K, Yumura W, Ujiie T, Nagane Y, Nitta K, Miyano S, Narita I, Gejyo F, Nihei H, Fujioka T, Nakamura Y: Association of single-nucleotide polymorphisms in the polymeric immunoglobulin receptor gene with immunoglobulin A nephropathy (IgAN) in Japanese patients. J Hum Genet 48: 293–299, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Schena FP, D’Altri C, Cerullo G, Manno C, Gesualdo L: ACE gene polymorphism and IgA nephropathy: An ethnically homogeneous study and a meta-analysis. Kidney Int 60: 732–740, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Narita I, Goto S, Saito N, Song J, Ajiro J, Sato F, Saga D, Kondo D, Akazawa K, Sakatsume M, Gejyo F: Interaction between ACE and ADD1 gene polymorphisms in the progression of IgA nephropathy in Japanese patients. Hypertension 42: 304–309, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Yoshimura H, Ito M, Kuwahara Y, Ishii A, Tsuritani K, Nakamura A, Hirasawa Y, Nagamatsu T: Downregulated expression in high IgA (HIGA) mice and the renal protective role of meprinbeta. Life Sci 82: 899–908, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, Lee JY, Robinson J, Tomana M, Tomino Y, Mestecky J, Novak J: Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest 119: 1668–1677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki H, Suzuki Y, Aizawa M, Yamanaka T, Kihara M, Pang H, Horikoshi S, Tomino Y: Th1 polarization in murine IgA nephropathy directed by bone marrow-derived cells. Kidney Int 72: 319–327, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Gadjeva MG, Rouseva MM, Zlatarova AS, Reid KB, Kishore U, Kojouharova MS: Interaction of human C1q with IgG and IgM: Revisited. Biochemistry 47: 13093–13102, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Mestecky J, Tomana M, Moldoveanu Z, Julian BA, Suzuki H, Matousovic K, Renfrow MB, Novak L, Wyatt RJ, Novak J: Role of aberrant glycosylation of IgA1 molecules in the pathogenesis of IgA nephropathy. Kidney Blood Press Res 31: 29–37, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novak J, Julian BA, Tomana M, Mestecky J: IgA glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Semin Nephrol 28: 78–87, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zandman-Goddard G, Peeva E, Shoenfeld Y: Gender and autoimmunity. Autoimmun Rev 6: 366–372, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Novak J, Julian BA: Sugars and alcohol: IgA-associated renal diseases in alcoholic cirrhosis. Kidney Int 80: 1252–1254, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Nishie T, Miyaishi O, Azuma H, Kameyama A, Naruse C, Hashimoto N, Yokoyama H, Narimatsu H, Wada T, Asano M: Development of immunoglobulin A nephropathy- like disease in beta-1,4-galactosyltransferase-I-deficient mice. Am J Pathol 170: 447–456, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mattu TS, Pleass RJ, Willis AC, Kilian M, Wormald MR, Lellouch AC, Rudd PM, Woof JM, Dwek RA: The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fc alpha receptor interactions. J Biol Chem 273: 2260–2272, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Wada Y, Tajiri M, Ohshima S: Quantitation of saccharide compositions of O-glycans by mass spectrometry of glycopeptides and its application to rheumatoid arthritis. J Proteome Res 9: 1367–1373, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Young NM, Jackson GE, Brisson JR: The glycopeptides of the mouse immunoglobulin A T15. Mol Immunol 27: 1083–1090, 1990 [DOI] [PubMed] [Google Scholar]
- 47.Lipniunas P, Grönberg G, Krotkiewski H, Angel AS, Nilsson B: Investigation of the structural heterogeneity in the carbohydrate portion of a mouse monoclonal immunoglobulin A antibody. Arch Biochem Biophys 300: 335–345, 1993 [DOI] [PubMed] [Google Scholar]
- 48.Raij L, Azar S, Keane W: Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int 26: 137–143, 1984 [DOI] [PubMed] [Google Scholar]
- 49.Orlandi R, Güssow DH, Jones PT, Winter G: Cloning immunoglobulin variable domains for expression by the polymerase chain reaction. Proc Natl Acad Sci U S A 86: 3833–3837, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Renfrow MB, Cooper HJ, Tomana M, Kulhavy R, Hiki Y, Toma K, Emmett MR, Mestecky J, Marshall AG, Novak J: Determination of aberrant O-glycosylation in the IgA1 hinge region by electron capture dissociation fourier transform-ion cyclotron resonance mass spectrometry. J Biol Chem 280: 19136–19145, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Raska M, Takahashi K, Czernekova L, Zachova K, Hall S, Moldoveanu Z, Elliott MC, Wilson L, Brown R, Jancova D, Barnes S, Vrbkova J, Tomana M, Smith PD, Mestecky J, Renfrow MB, Novak J: Glycosylation patterns of HIV-1 gp120 depend on the type of expressing cells and affect antibody recognition. J Biol Chem 285: 20860–20869, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]