Abstract
Clinical trials of off-pump coronary artery bypass grafting (CABG) have largely excluded patients with CKD. Here, we sought to determine whether pump status affects outcomes in patients with CKD. Using a nonrandomized cohort of 742,909 non-emergent, isolated CABG cases, which included 158,561 off-pump cases, in the Society of Thoracic Surgery Database from 2004 through 2009, we evaluated the association between pump status (off-pump versus on-pump) and in-hospital death or incident renal replacement therapy (RRT) across strata of preoperative renal function. We used propensity methods to adjust patient- and center-level analyses for imbalances in baseline patient risk. Patients who received on-pump and off-pump CABG had similar mean age and distribution of preoperative estimated GFR (eGFR). In a propensity-weighted analysis, off-pump CABG was associated with a reduction in the composite in-hospital death or RRT, with patients having lower preoperative renal function exhibiting greater benefit, on average. The risk difference (on-pump minus off-pump) ranged from 0.05 (95% confidence interval, −0.06 to 0.16) per 100 patients for eGFR ≥90 ml/min per 1.73 m2 to 3.66 (95% confidence interval, 2.14–5.18) per 100 patients for eGFR 15–29 ml/min per 1.73 m2. Both component endpoints suggested the same trend. In summary, these data suggest that patients with CKD experience less death or incident RRT when treated with off-pump compared with on-pump CABG. The reduction in incident RRT, not death, drove this effect on the composite among patients with low eGFR. Prospective trials comparing these procedures in patients with impaired preoperative renal function are warranted.
AKI complicates 2.3% of isolated CABG cases, with an incidence as high as 14%–15% among patients with preoperative CKD.1 Although a progression to stage 3 AKI (e.g., such as renal replacement therapy [RRT]) is rare, it is associated with a 50%–70% in-hospital mortality and up to 50% of patients need chronic dialysis.2,3
Because prolonged cardiopulmonary bypass (CPB) time has been associated with postoperative AKI, some have advocated the use of off-pump coronary artery bypass (OPCAB) techniques for high-risk patients. Although randomized trials of OPCAB have not demonstrated benefits in a general population,4,5 patients with CKD have composed <10% of those studied, including <8% of those in the recent Veterans Affairs Randomized On/Off Bypass (ROOBY) trial.4
In this analysis, we examined a nationally representative patient cohort who underwent elective, isolated CABG at 1000 centers in the Society of Thoracic Surgeons Adult Cardiac Surgery Database (STS ACSD) to test the following hypotheses: (1) off-pump procedures are associated with a lower incidence of cardiac surgery–associated AKI compared with on-pump CABG, and (2) the degree of renal protection associated with off-pump CABG is directly related to the extent of preoperative renal dysfunction.
Results
Patient Characteristics
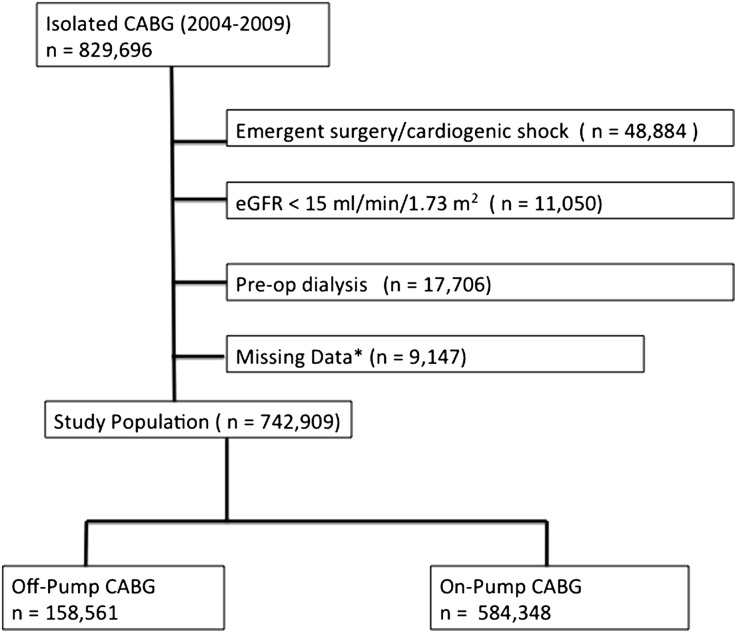
Of the 742,909 patients who underwent non-emergent, isolated CABG at 1000 STS ACSD centers, 158,561 (21.4%) included an intended off-pump approach (Figure 1). An unplanned cross-over from off-pump to on-pump procedures was observed in 2.9% of patients in the off-pump cohort, and the following reasons were cited for unintended cross-over: inadequate exposure or visualization (19.3%), bleeding (3.1%), inadequate size and/or diffuse distal vessel disease (7.1%), hemodynamic instability (60.2%), poor conduit quality and/or trauma (3.0%), and other reasons (7.3%). Conversion patients experienced a higher incidence of both in-hospital death (5.4%) and RRT (2.9%) than the remaining OPCAB cohort (Table 1).
Figure 1.
Patients included in analyses.
Table 1.
Unadjusted associations between treatment received and outcome and between center preference and outcome
| Event | Treatment Received | Center Preference Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| On Pump | Off Pump | Risk Difference (%)a | On Pump | Off Pump | Risk Difference (%)b | |||||
| Patients and Events (n) | % | Patients and Events (n) | % | Patients and Events (n) | % | Patients and Events (n) | % | |||
| Overall cohort | 584,348 | 158,561 | 265,497 | 26,904 | ||||||
| death or RRT | 11,665 | 2.0 | 2853 | 1.8 | 0.20 | 5029 | 1.9 | 461 | 1.7 | 0.18 |
| death | 7849 | 1.3 | 1970 | 1.2 | 0.10 | 3366 | 1.3 | 325 | 1.2 | 0.06 |
| RRT | 5724 | 1.0 | 1351 | 0.9 | 0.13 | 2450 | 0.9 | 201 | 0.7 | 0.18 |
| eGFR ≥90 | 141,859 | 36,729 | 63,939 | 6060 | ||||||
| death or RRT | 1236 | 0.9 | 293 | 0.8 | 0.07 | 522 | 0.8 | 34 | 0.6 | 0.26 |
| death | 1006 | 0.7 | 244 | 0.7 | 0.04 | 422 | 0.7 | 28 | 0.5 | 0.20 |
| RRT | 408 | 0.3 | 80 | 0.2 | 0.07 | 165 | 0.3 | 10 | 0.2 | 0.09 |
| eGFR 60–89 | 301,603 | 80,968 | 137,085 | 14,027 | ||||||
| death or RRT | 4025 | 1.3 | 950 | 1.2 | 0.16 | 1672 | 1.2 | 170 | 1.2 | 0.01 |
| death | 3186 | 1.1 | 772 | 1.0 | 0.10 | 1341 | 1.0 | 145 | 1.0 | −0.06 |
| RRT | 1488 | 0.5 | 342 | 0.4 | 0.07 | 585 | 0.4 | 47 | 0.3 | 0.09 |
| eGFR 30–59 | 133,129 | 37,959 | 60,890 | 6396 | ||||||
| death or RRT | 5160 | 3.9 | 1250 | 3.3 | 0.58 | 2308 | 3.8 | 215 | 3.4 | 0.43 |
| death | 3253 | 2.4 | 853 | 2.2 | 0.20 | 1443 | 2.4 | 138 | 2.2 | 0.21 |
| RRT | 2794 | 2.1 | 622 | 1.6 | 0.46 | 1258 | 2.1 | 109 | 1.7 | 0.36 |
| eGFR 15–29 | 7757 | 2905 | 3583 | 421 | ||||||
| death or RRT | 1244 | 16.0 | 360 | 12.4 | 3.65 | 527 | 14.7 | 42 | 10.0 | 4.73 |
| death | 404 | 5.2 | 101 | 3.5 | 1.73 | 160 | 4.5 | 14 | 3.3 | 1.14 |
| RRT | 1034 | 13.3 | 307 | 10.6 | 2.76 | 442 | 12.3 | 35 | 8.3 | 4.02 |
Number of patients with the outcome per 100 patients treated on pump minus number of patients with the outcome per 100 patients treated off pump.
Number of patients with the outcome per 100 patients treated at centers with a preference for on-pump CABG (on-pump centers) minus the number of patients with the outcome per 100 patients treated at centers with a preference for off-pump CABG (off-pump centers).
Compared with on-pump coronary artery bypass (ONCAB) patients, OPCAB patients were slightly older and more often female, with a lower burden of both comorbidities and coronary artery disease (CAD) (Table 2), including a higher incidence of one-vessel (10.6% versus 2.4%; P<0.001) and two-vessel disease (25.0% versus 18.0%; P<0.001). CKD was present in a significant proportion of the overall cohort, with an estimated GFR (eGFR) of 30–59 in 22.8% and an eGFR of 15–29 in 1.3%; however, baseline renal function was similar across the two treatment groups. After propensity weighting, demographics and baseline comorbidities were well balanced across treatment groups in the overall cohort (Table 2), as well as among each of the four eGFR strata.
Table 2.
Characteristics of patients stratified by intended use of cardiopulmonary bypass pump
| Characteristic | Baseline | Propensity Weighted | |||
|---|---|---|---|---|---|
| On-Pump (n=584,348) | Off-Pump (n=158,561) | Standardized Difference (%)a | Standardized Difference (%)a | P Value | |
| Age (yr), median (IQR) | 65 (58, 73) | 66 (58, 74) | 6.5 | 0.1 | 0.83 |
| Female (%) | 26.2 | 29.4 | 7.1 | 0.01 | 0.99 |
| BSA, median (IQR) | 2.0 (1.8, 2.2) | 2.0 (1.8, 2.1) | 8.8 | 0.02 | 0.95 |
| Hypertension | 82.7 | 82.6 | 0.2 | 0.2 | 0.44 |
| Diabetes | 38.6 | 36.2 | 4.9 | 0.2 | 0.45 |
| Chronic lung disease | 21.7 | 21.9 | 0.5 | 0.09 | |
| eGFR | |||||
| ≥90 | 24.3 | 23.2 | 2.6 | 0.03 | 0.93 |
| 60–89 | 51.6 | 51.1 | 1.1 | 0.1 | 0.85 |
| 30–59 | 22.8 | 23.9 | 2.7 | 0.02 | 0.95 |
| 15–29 | 1.3 | 1.8 | 4.0 | 0.1 | 0.78 |
| Prior cardiovascular surgery | 4.9 | 3.9 | 3.9 | 0.4 | 0.23 |
| Prior MI | 43.9 | 41.6 | 0.6 | 0.1 | 0.80 |
| CHF | 12.8 | 13.1 | 0.7 | 0.1 | 0.62 |
| Ejection fraction, median (IQR) | 55 (45, 60) | 55 (45, 60) | 6.8 | 0.7 | 0.02 |
| Left main >50% | 30.8 | 28.3 | 5.4 | 0.1 | 0.81 |
| CAD, no. of vessels | |||||
| 1 | 2.4 | 10.6 | 33.6 | 0.01 | 0.97 |
| 2 | 18.0 | 25.0 | 17.1 | 0.2 | 0.38 |
| 3 | 79.4 | 64.0 | 34.6 | 0.2 | 0.42 |
| Procedure status | |||||
| elective | 47.9 | 51.5 | 7.2 | 0.4 | 0.24 |
| urgent | 52.1 | 48.6 | 7.2 | 0.4 | 0.24 |
IQR, interquartile range; BSA, body surface area; MI, myocardial infarction; CHF, congestive heart failure; CAD, coronary artery disease.
Calculated as difference in group means divided by an estimate of the pooled SD; rounded to the first significant digit after the decimal point.
In-Hospital Outcomes
Death or Incident RRT
In the overall cohort, the incidence of in-hospital death or need for new RRT was 2.0%, with a slightly lower incidence observed in the off-pump versus on-pump cohorts (1.8% versus 2.0%; risk difference in on-pump minus off-pump, 0.20; 0.12–0.27) (Table 1).
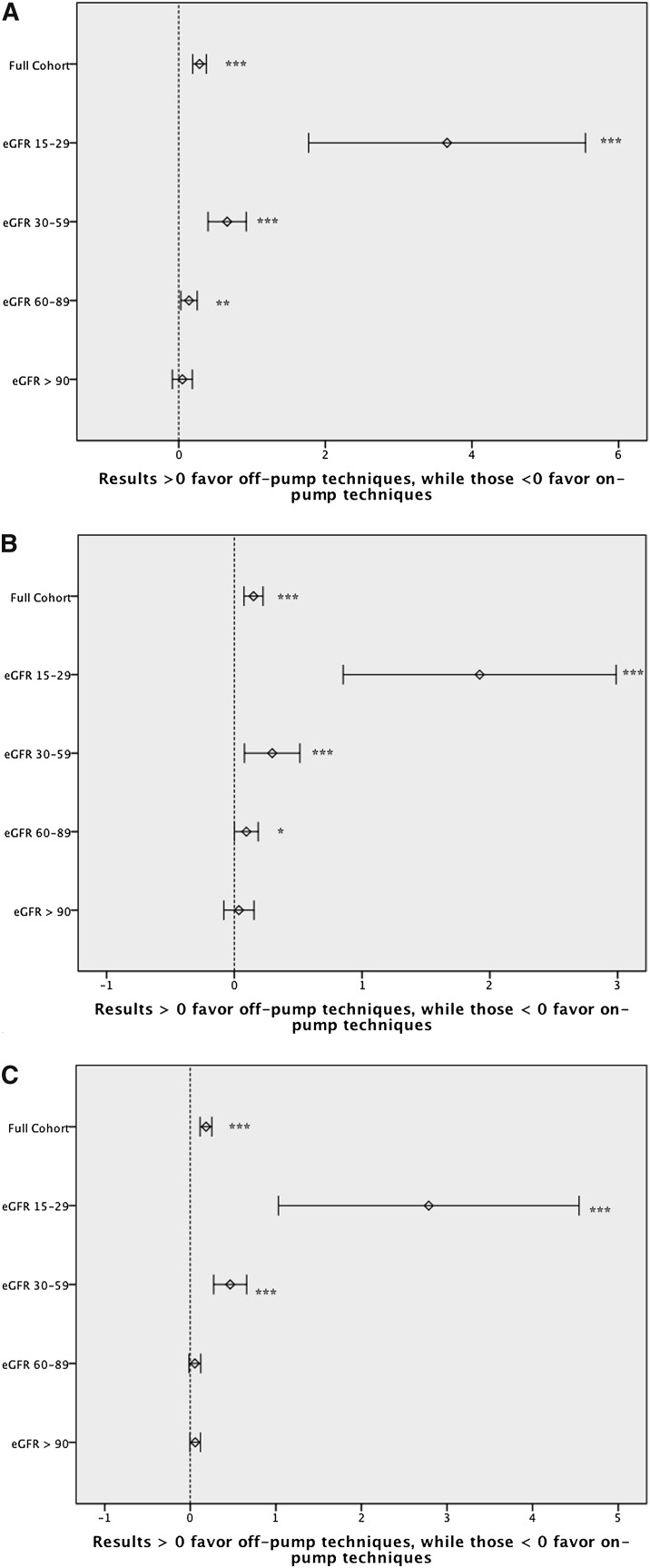
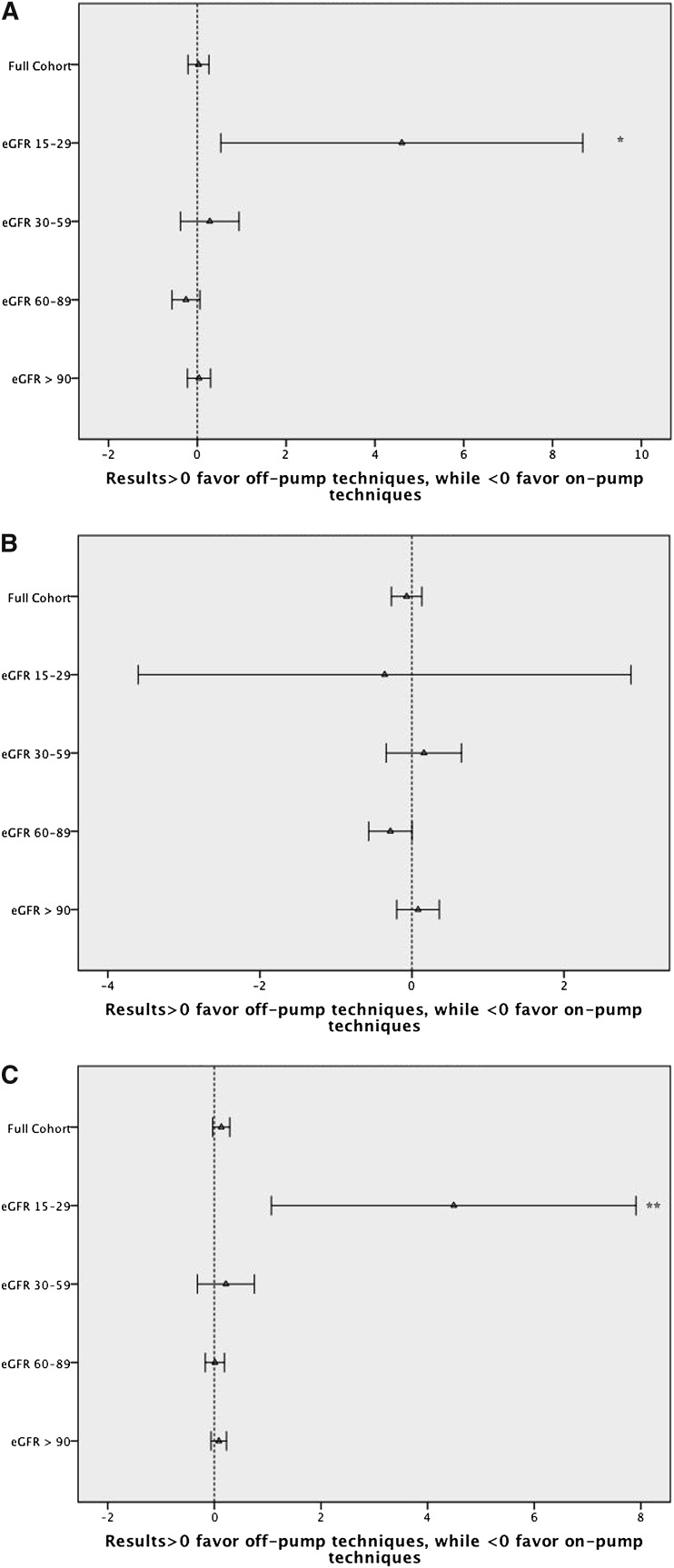
Patients with the poorest preoperative renal function had the highest incidence of in-hospital death or RRT (Table 1). The attributable benefit for in-hospital death or incident RRT associated with OPCAB (versus ONCAB) was progressively greater across strata of worsening baseline renal function. Using both propensity weighting and a center-level approach, this result was durable and remained statistically significant (Table 3 and Figures 2 and 3).
Table 3.
Conventional and instrumental variable estimates of the risk difference (on pump minus off pump) associated with on-pump versus off-pump CABG
| Event | Estimated Risk Difference per 100 Patients (95% CI) | |||
|---|---|---|---|---|
| Conventional Unadjusted | Conventional Propensity Weighteda | Unadjusted Center Preference Analysis | Adjusted Center Preference Analysis | |
| Overall cohort | ||||
| death or RRT | 0.20 (0.12, 0.27)b | 0.28 (0.21, 0.36)b | 0.18 (0.02, 0.34)c | 0.03 (−0.21, 0.26) |
| death | 0.10 (0.04, 0.16)d | 0.15 (0.09, 0.21)b | 0.06 (−0.08, 0.20) | −0.07 (−0.27, 0.13) |
| RRT | 0.13 (0.08, 0.18)b | 0.18 (0.13, 0.24)b | 0.18 (0.07, 0.29)d | 0.13 (−0.03, 0.29) |
| eGFR ≥90 | ||||
| death or RRT | 0.07 (−0.03, 0.18) | 0.05 (-0.06, 0.16) | 0.26 (0.06, 0.46)d | 0.10 (−0.21, 0.41) |
| death | 0.05 (−0.05, 0.14) | 0.04 (-0.06, 0.13) | 0.20 (0.02, 0.38)c | 0.08 (−0.20, 0.36) |
| RRT | 0.07 (0.02, 0.13)c | 0.05 (-0.01, 0.11) | 0.09 (−0.02, 0.20) | 0.08 (−0.06, 0.23) |
| eGFR 60–89 | ||||
| death or RRT | 0.16 (0.08, 0.25)b | 0.14 (0.05, 0.23)d | 0.01 (−0.18, 0.20) | −0.26 (−0.57, 0.06) |
| death | 0.10 (0.03, 0.18)c | 0.09 (0.02, 0.17)c | −0.06 (−0.23, 0.12) | −0.28 (−0.57, 0.00) |
| RRT | 0.07 (0.02, 0.12)d | 0.05 (−0.00, 0.11) | 0.09 (−0.01, 0.19) | 0.01 (−0.17, 0.19) |
| eGFR 30–59 | ||||
| death or RRT | 0.58 (0.38, 0.79)b | 0.66 (0.45, 0.87)b | 0.43 (−0.04, 0.90) | 0.28 (−0.38, 0.94) |
| death | 0.20 (0.03, 0.37)c | 0.30 (0.12, 0.47)b | 0.21 (−0.16, 0.59) | 0.16 (−0.34, 0.65) |
| RRT | 0.46 (0.31, 0.61)b | 0.47 (0.31, 0.62)b | 0.36 (0.03, 0.70)c | 0.22 (−0.32, 0.75) |
| eGFR 15–29 | ||||
| death or RRT | 3.65 (2.20, 5.10)b | 3.66 (2.14, 5.18)b | 4.73 (1.64, 7.82)d | 4.60 (0.53, 8.68)c |
| death | 1.73 (0.90, 2.56)b | 1.92 (1.06, 2.78)b | 1.14 (−0.70, 2.98) | −0.36 (−3.60, 2.88) |
| RRT | 2.76 (1.41, 4.11)b | 2.79 (1.37, 4.20)b | 4.02 (1.17, 6.87)c | 4.49 (1.07, 7.91)d |
Inverse probability weighted estimates after balancing treatment groups using propensity score.
P value <0.001.
P value <0.05.
P value <0.01.
Figure 2.
Conventional estimates associated with on-pump versus off-pump CABG. (A) Inhospital mortality or need for RRT. (B) Inhospital mortality. (C) Need for RRT. *P<0.05. **P<0.01, ***P<0.001.
Figure 3.
Instrumental variable estimates associated with on-pump versus off-pump CABG. (A) Inhospital mortality or need for RRT. (B) Inhospital mortality. (C) Need for RRT. *P<0.05. **P<0.01, ***P<0.001.
In-Hospital Mortality
In the overall cohort, the observed incidence of in-hospital mortality was slightly lower among patients treated with off-pump versus on-pump CABG (1.2% versus 1.3%; risk difference, 0.10; 0.04–0.16) (Table 1).
The unadjusted incidence of mortality in patients with a reduced eGFR (15–29 and 30–59) was lowest among the OPCAB (versus ONCAB) cohort (3.5% versus 5.2% and 2.2% versus 2.4%, respectively); however, the incidence was similar among patients with a preserved eGFR (60–89, 1.0% versus 1.1%; ≥90, 0.7% versus 0.7%). Although the associated survival advantage of OPCAB among low eGFR patients was maintained in the propensity analysis, no difference in survival was observed when the center-level analysis was performed (Table 3).
Incident RRT
In the overall cohort, the incidence of new RRT was 1.0%, with a slightly lower incidence observed among OPCAB (versus ONCAB) patients (0.9% versus 1.0%; risk difference, 0.13; 0.08–0.18) (Table 1).
Patients with the poorest preoperative renal function experienced the highest incidence of postoperative RRT (Table 1). The associated benefit of OPCAB (versus ONCAB) for incident RRT was greatest among patients with impaired preoperative renal function (Table 1). This pattern was statistically significant and maintained in both the propensity and center-level analyses (Table 3).
Among the patients who developed an incident need for RRT, those with the poorest preoperative renal function experienced better survival compared with those with more intact preoperative renal function (Table 4).
Table 4.
Inhospital mortality of patients requiring postoperative RRT stratified by preoperative eGFR
| Overall (n=7075) | GFR ≥90 (n=488) | GFR 60–89 (n=1830) | GFR 30–59 (n=3416) | GFR 15–29 (n=1341) | P Valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | ||
| Overall | 2376 | 33.58 | 209 | 42.83 | 813 | 44.43 | 1112 | 32.55 | 242 | 18.05 | <0.001 |
| ONCAB | 1908 | 33.33 | 178 | 43.63 | 649 | 43.62 | 887 | 31.75 | 194 | 18.76 | <0.001 |
| OPCAB | 468 | 34.64 | 31 | 38.75 | 164 | 47.95 | 225 | 36.17 | 48 | 15.64 | <0.001 |
P values determined by Mantel–Haenszel test of H0: Equal mortality rate across four eGFR groups.
Discussion
Randomized trials comparing on-pump versus off-pump CABG have not demonstrated a consistent benefit of off-pump techniques; however, these trials have included limited samples of patients with reduced renal function, a group in whom off-pump procedures may confer an acute benefit. In this large, contemporary cohort of isolated CABG patients, we have shown that OPCAB is associated with reduced in-hospital mortality or incident RRT among patients with CKD, a result driven by a reduction in the need for postoperative RRT. In this cohort, the benefit of OPCAB was inversely related to the baseline renal function (Table 3), and although patients with CKD experienced a stepwise benefit with a dose-response effect, those patients with normal renal function experienced no associated benefit (Figures 2 and 3). These data are not inconsistent with prior randomized trials and suggest a need for further evaluation in patients at high risk for postoperative AKI.
The link between CPB and AKI is plausible, especially among patients with limited preoperative renal reserve.6 Low mean arterial pressures (<60 mmHg) commonly maintained during CPB may be particularly deleterious in CKD patients with known impairments in renal autoregulation.7,8 In the setting of reduced perfusion, kidneys with impaired autoregulation are more vulnerable to ischemia, particularly in the low oxygen renal medulla.8 In addition, nonpulsatile renal perfusion,3,9 microemboli, and systemic inflammation associated with extracorporeal therapies3 may contribute to postoperative AKI. Despite this mechanistic association, prospective controlled trials of off-pump CABG have included few patients with CKD (Table 5).
Table 5.
Summary of previous studies
| Study | n | Baseline CKD, n (%) |
|---|---|---|
| Ascione et al. (1999)11 | 50 | 0 (0) |
| Nathoe et al. (2003) | 281 | 0 (0) |
| Tang et al. (2002) | 40 | 0 (0) |
| Legare et al. (2003) | 300 | 11 (3.7) |
| Puskas et al. (2003) | 197 | 4 (2) |
| Straka et al. (2004) | 388 | 3 (0.8) |
| Khan et al. (2004) | 104 | 0 (0) |
| Gerola et al. (2004) | 160 | 0 (0) |
| Wan et al. (2004) | 37 | 0 (0) |
| Staton et al. (2005) | 197 | 0 (0) |
| Jensen et al. (2006) | 120 | 0 (0) |
| Motallebzadeh et al. (2006) | 210 | 0 (0) |
| Sajja et al. (2007)17 | 116 | 116 (100) |
| Magee et al. (2008) | 3014 | 67 (2.2) |
| Paulitsch et al. (2009) | 92 | 0 (0) |
| Shroyer et al. (2009)4 | 2203 | 173 (7.9) |
| Hueb et al. (2010) | 308 | 0 (0) |
| Moller et al. (2010) | 339 | 13 (3.8) |
| Total | 8156 | 387 (4.7) |
A complete list of references for these studies is provided in Supplemental Material.
Prior studies examining the association between pump status and postoperative renal function have been inconclusive.10–16 However, these studies have been generally underpowered to examine the association in patients with CKD. The ROOBY trial, which remains the largest randomized comparison of off-pump versus on-pump CABG, did not show a benefit of OPCAB for postoperative renal function; however, <8% of the patients enrolled in this study had pre-existing CKD.4 In the one dedicated assessment of pump status among patients with preoperative CKD, Sajja et al. observed an association between OPCAB and improved renal outcomes.17 Congruent with both of these studies, we have demonstrated that OPCAB is only associated with renal protection in patients with impaired preoperative renal function. This finding may explain some of the heterogeneity of effects observed across prior studies.
Renalism is a term coined by Chertow et al.18 in 2004 and refers to the notion that high-risk patients with kidney disease tend to “receive more conservative therapy for cardiovascular diseases, even though the relative benefits of therapy tend to be greater” (p 2462). Although CKD has a stronger association with cardiovascular events than any of the classic Framingham risk factors (i.e., family history, hypercholesterolemia, smoking, hypertension, and diabetes mellitus),19,20 patients with CKD have been systematically excluded from large cardiovascular clinical trials.21 In some cases, this trend has been a function of concern for increased toxicity of cardiovascular medications in patients with impaired metabolite clearance; in others, it has been a function of perceived therapeutic futility. Certainly, caution is always appropriate when considering the inclusion of patients with high-risk features in clinical trials; however, our findings reaffirm that a systematic exclusion of these patients is ill advised when a plausible mechanistic link suggests additive therapeutic benefit in one or more of the high-risk subgroups.
Among patients requiring incident RRT, a higher associated incidence of in-hospital mortality was observed among those with more intact preoperative renal function compared with those with a lower baseline eGFR. These results are provocative and are consistent with a prior analysis demonstrating worse outcomes among critically ill patients who develop incident AKI requiring RRT compared with similarly matched critically ill patients with baseline ESRD who required RRT.22 The mechanism underlying this observation is unclear; further speculation is beyond the scope of this report. Future studies should be conducted to further elaborate on this observation and test potential mechanistic pathways.
This analysis has many strengths, including robust CKD subgroups and an intention-to-treat design, but it also has several limitations. Most importantly, this study is retrospective in design. Without randomized treatment allocation, imbalances in unmeasured patient characteristics may have biased our results. In fact, the center preference analysis suggests that a portion of the OPCAB mortality benefit observed in the propensity-weighted analysis may have been the result of residual bias. However, confirmation of the benefit of OPCAB on the postoperative incidence of RRT with both propensity score and center-level analyses adds validity to these results. Second, although the observed rate of off-pump to on-pump cross-over in this cohort (2.9%) was within the bounds of previously reported studies,23 it is substantially lower than the 12% cross-over rate reported by the ROOBY investigators.4 The bias that may have resulted from inclusion of these additional cross-over cases in the on-pump cohort was directly addressed by our center preference analysis. In addition, extra precautions were taken to ensure the results were not biased by center effects associated with the treatment strategy preference. Finally, OPCAB has a significant learning curve, and the nuances of individual center and surgical team effects are not easily captured in this type of analysis. However, on average, OPCAB was associated with a reduction in the need for postoperative RRT in this cohort. To test our hypothesis that OPCAB compared with ONCAB improves renal outcomes and survival in CKD patients, we believe that an appropriately powered prospective study should be conducted. Based on our analysis, the number of patients needed for such a study would range from 1300 to 7000 patients depending on the incidence of RRT/death rate, effect size, and power (Supplemental Appendix 1).
In conclusion, OPCAB is associated with a lower incidence of postoperative RRT compared with ONCAB in patients with poor preoperative renal function. Future efforts should focus on an assessment of risks and benefits in this high-risk subgroup.
Concise Methods
Data Sources and Patient Population
Since 1989, the STS ACSD has collected perioperative data on cardiac operations at hospitals across North America as a part of a continuous quality improvement effort. The ACSD ensures high-quality data through an independent auditing process, which has verified a 96% correlation between ACSD data and that obtained through chart review.24
For this analysis, we examined patients undergoing non-emergent, isolated CABG from January 1, 2004, through December 31, 2009. We excluded patients with cardiogenic shock and those with a preoperative eGFR <15 ml/min per 1.73 m2 or those who were receiving dialysis preoperatively. Patients were also excluded when missing data for CPB utilization, preoperative serum creatinine, postoperative dialysis, postoperative renal failure, or in-hospital mortality.
Unadjusted and adjusted comparisons of OPCAB versus ONCAB were estimated in the overall cohort and within each of the following four eGFR subgroups: (1) eGFR 15–29 ml/min per 1.73 m2, (2) eGFR 30–59 ml/min per 1.73 m2, (3) eGFR 60–89 ml/min per 1.73 m2, and (4) eGFR ≥90 ml/min per 1.73 m2. The Duke University School of Medicine Institutional Review Board granted a waiver of informed consent and authorization for this study.
Data Definitions
The STS ACSD collects information on in-patient mortality and morbidity, including the need for dialysis and postoperative renal failure. Detailed definitions of risk factors and complications are provided on the STS web site (www.sts.org). Preoperative eGFR is based on the last serum creatinine closest to the date and time of the CABG procedure, as collected within the STS ACSD. OPCAB was defined as an intent to operate off pump, including patients undergoing CABG with no CPB, or unplanned use of CPB as collected in the data collection form. Likewise, the ONCAB group was defined as patients with planned CPB utilization. eGFR was calculated using the Modified Diet in Renal Disease equation, and was based on available preoperative data.25
Study End Points
Because of the nontrivial competing risk of in-hospital death in this population, the primary outcome for this study was a composite of in-hospital death and incident in-hospital RRT. Secondary outcomes included the two component endpoints of in-hospital mortality and RRT.
Statistical Analyses
We estimated the effect of CPB on in-hospital death and incident RRT using a conventional patient-level propensity analysis and a center-level analysis in which the exposure variable was center preference for on-pump versus off-pump procedures.
Propensity Score Analysis (Patient-Level)
The on-pump and off-pump groups were compared using risk difference (in percentages), that is, the estimated probability difference (on-pump group minus the off-pump group) between two groups. Propensity scores were estimated by fitting a nonparsimonious logistic model within the overall cohort (c statistic 0.63) and each of the four eGFR strata (eGFR 15–29: c statistic, 0.65; eGFR 30–59: c statistic, 0.63; eGFR 60–89: c statistic, 0.63; eGFR ≥90: c statistic, 0.64). The five-number summaries (minimum, 20th percentile, median, 80th percentile, maximum) describing propensity score distributions for on-pump and off-pump treatment groups were as follows: eGFR 15–29 (0.06, 0.18, 0.24, 0.33, and 0.88 versus 0.09, 0.21, 0.29, 0.41, and 0.81), eGFR 30–59 (0.05, 0.15, 0.20, 0.26, and 0.80 versus 0.08, 0.17, 0.23, 0.32, and 0.78), eGFR 60–89 (0.07, 0.15, 0.20, 0.24, and 0.76 versus 0.08, 0.16, 0.21, 0.30, and 0.80), and eGFR ≥90 (0.07, 0.14, 0.18, 0.23, and 0.74 versus 0.07, 0.16, 0.21, 0.30, and 0.82) (Supplemental Appendix 2). The high degree of overlap across treatment groups suggests that the use of propensity score methods was appropriate in this cohort. In this case, the propensity score represented the estimated probability of receiving OPCAB (versus ONCAB) as a function of the patient’s baseline demographics and comorbidities as well as preoperative cardiac medications (70 observed covariates; Supplemental Appendix 3). Adjusted risk differences were estimated using linear models, including a single covariate for treatment and weighting each observation by the inverse of the estimated propensity score.26 When estimating 95% confidence intervals, robust sandwich variance estimates were used to account for the patients’ dependence with sites. The balance of baseline characteristics achieved across the two treatment groups after propensity weighting was assessed through visual inspection of propensity distributions and through an evaluation of the standardized difference (defined as difference in group means divided by an estimate of the pooled SD). There was a high degree of overlap of the distribution of propensity scores for each of the treatment groups in the overall cohort and in each of the eGFR strata. Observed differences in covariates across the treatment groups were small, and in all cases were <5% of the estimated SD, indicating a good balance of baseline risk factors.27 To further limit bias from treatment selection, patients were excluded if their propensity score was outside the area of treatment group overlap, as previously described.28
Center Preference Analysis (Center-Level)
Although the STS ACSD collects data on conversion from an intended off-pump to on-pump approach, conversion was indicated in only 2.9% of off-pump cases (versus 12.4% in the recently published ROOBY trial4), raising the concern that a substantial proportion of off-pump to on-pump conversions were recorded as on-pump procedures in the database. A strong association between unplanned conversion and poor operative outcomes was previously demonstrated,29 which would bias our analysis results against the on-pump technique. A center preference analysis was performed to address both the potential bias introduced by cross-over procedures and the likely unmeasured confounding associated with the treatment selection. In this analysis, we used center preference for on-pump versus off-pump procedures as the exposure of interest. To the extent that outcomes across these centers varied only as the effect of preference for on-pump versus off-pump procedures, this analysis would produce an unbiased estimate of the effect of pump status on outcomes. An institutional preference for off-pump CABG was defined as the use of off-pump techniques in >90% of CABG cases, to allow for a 10% expected rate of off-pump to on-pump conversion.4 Likewise, an institutional preference for on-pump CABG was defined as the use of on-pump techniques in >95% of CABG cases, to allow for a 5% incidence of factors such as a heavily calcified aorta that may compel an unplanned off-pump approach. Regardless of the actual CPB usage, all of the cases from the 51 centers preferring an off-pump strategy were compared with cases from the 347 centers preferring an on-pump strategy. The comparison of observed patient characteristics at on-pump versus off-pump centers revealed minor differences, including a slightly lower proportion of three-vessel disease at off-pump centers (Supplemental Appendix 4). A comparison of outcomes of a common and relevant on-pump procedure (aortic valve replacement plus CABG) across on-pump versus off-pump centers revealed no significant difference in outcomes across these two types of centers (Supplemental Appendix 5), suggesting that the centers preferring on-pump versus off-pump techniques are not systematically different in overall surgical skill and quality of care delivered. Despite these results suggesting general similarities in patient characteristics and center-level outcomes, we used propensity score methods to risk adjust this analysis for both patient-level covariates (n=70) and the estimated center-specific random effects, in which the center-specific random effects were estimated using a hierarchical model for in-hospital motility within the aortic valve replacement plus CABG procedures. The propensity score was the probability of a patient being in an on-pump versus off-pump center. Both unadjusted and risk-adjusted estimates were calculated for each of the primary and secondary endpoints.
Descriptive statistics are based on nonmissing values and presented as median and interquartile range (25th to 75th percentile) for continuous variables, or frequency and percentage for categorical variables. The Wilcoxon rank sum test compared the distribution of continuous variables, whereas the Mantel–Haenszel test was used for categorical variable comparisons. Missing data were rare (<0.5% for all variables). Missing values of body surface area and body mass index were imputed to sex-specific median values. Missing values of ejection fraction were imputed to sex-specific median values for patients with known congestive heart failure, were otherwise imputed to 50%. Missing values of the remaining risk factors were defaulted to their most common value. SAS statistical software (version 9.1; SAS Institute, Cary, NC) was used for all calculations.
Disclosures
None.
Acknowledgments
The authors thank Richard Amdur for reviewing this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012020122/-/DCSupplemental.
References
- 1.Shahian DM, O’Brien SM, Filardo G, Ferraris VA, Haan CK, Rich JB, Normand SL, DeLong ER, Shewan CM, Dokholyan RS, Peterson ED, Edwards FH, Anderson RP, Society of Thoracic Surgeons Quality Measurement Task Force : The Society of Thoracic Surgeons 2008 cardiac surgery risk models: Part 1—coronary artery bypass grafting surgery. Ann Thorac Surg 88[Suppl]: S2–S22, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M: Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: A prospective cohort study. J Am Soc Nephrol 15: 1597–1605, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Rosner MH, Okusa MD: Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 1: 19–32, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Shroyer AL, Grover FL, Hattler B, Collins JF, McDonald GO, Kozora E, Lucke JC, Baltz JH, Novitzky D, Veterans Affairs Randomized On/Off Bypass (ROOBY) Study Group : On-pump versus off-pump coronary-artery bypass surgery. N Engl J Med 361: 1827–1837, 2009. 19890125 [Google Scholar]
- 5.Takagi H, Matsui M, Umemoto T: Off-pump coronary artery bypass may increase late mortality: A meta-analysis of randomized trials. Ann Thorac Surg 89: 1881–1888, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Barai S, Gambhir S, Prasad N, Sharma RK, Ora M: Functional renal reserve capacity in different stages of chronic kidney disease. Nephrology (Carlton) 15: 350–353, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Abuelo JG: Normotensive ischemic acute renal failure. N Engl J Med 357: 797–805, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Loutzenhiser R, Griffin K, Williamson G, Bidani A: Renal autoregulation: New perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol 290: R1153–R1167, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haines N, Wang S, Undar A, Alkan T, Akcevin A: Clinical outcomes of pulsatile and non-pulsatile mode of perfusion. J Extra Corpor Technol 41: 26–29, 2009 [PMC free article] [PubMed] [Google Scholar]
- 10.Boulton BJ, Kilgo P, Guyton RA, Puskas JD, Lattouf OM, Chen EP, Cooper WA, Vega JD, Halkos ME, Thourani VH: Impact of preoperative renal dysfunction in patients undergoing off-pump versus on-pump coronary artery bypass. Ann Thorac Surg 92: 595–601, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Sellke FW, DiMaio JM, Caplan LR, Ferguson TB, Gardner TJ, Hiratzka LF, Isselbacher EM, Lytle BW, Mack MJ, Murkin JM, Robbins RC, American Heart Association : Comparing on-pump and off-pump coronary artery bypass grafting: Numerous studies but few conclusions: A scientific statement from the American Heart Association council on cardiovascular surgery and anesthesia in collaboration with the interdisciplinary working group on quality of care and outcomes research. Circulation 111: 2858–2864, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Ascione R, Lloyd CT, Underwood MJ, Gomes WJ, Angelini GD: On-pump versus off-pump coronary revascularization: Evaluation of renal function. Ann Thorac Surg 68: 493–498, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Schwann NM, Horrow JC, Strong MD 3rd, Chamchad D, Guerraty A, Wechsler AS: Does off-pump coronary artery bypass reduce the incidence of clinically evident renal dysfunction after multivessel myocardial revascularization? Anesth Analg 99: 959–964, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Chukwuemeka A, Weisel A, Maganti M, Nette AF, Wijeysundera DN, Beattie WS, Borger MA: Renal dysfunction in high-risk patients after on-pump and off-pump coronary artery bypass surgery: A propensity score analysis. Ann Thorac Surg 80: 2148–2153, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Weerasinghe A, Athanasiou T, Al-Ruzzeh S, Casula R, Tekkis PP, Amrani M, Punjabi P, Taylor K, Stanbridge R, Glenville B: Functional renal outcome in on-pump and off-pump coronary revascularization: A propensity-based analysis. Ann Thorac Surg 79: 1577–1583, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Hix JK, Thakar CV, Katz EM, Yared JP, Sabik J, Paganini EP: Effect of off-pump coronary artery bypass graft surgery on postoperative acute kidney injury and mortality. Crit Care Med 34: 2979–2983, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Sajja LR, Mannam G, Chakravarthi RM, Sompalli S, Naidu SK, Somaraju B, Penumatsa RR: Coronary artery bypass grafting with or without cardiopulmonary bypass in patients with preoperative non-dialysis dependent renal insufficiency: A randomized study. J Thorac Cardiovasc Surg 133: 378–388, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Chertow GM, Normand SL, McNeil BJ: “Renalism”: Inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. J Am Soc Nephrol 15: 2462–2468, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Debella YT, Giduma HD, Light RP, Agarwal R: Chronic kidney disease as a coronary disease equivalent—a comparison with diabetes over a decade. Clin J Am Soc Nephrol 6: 1385–1392, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Coca SG, Krumholz HM, Garg AX, Parikh CR: Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA 296: 1377–1384, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Clermont G, Acker CG, Angus DC, Sirio CA, Pinsky MR, Johnson JP: Renal failure in the ICU: Comparison of the impact of acute renal failure and end-stage renal disease on ICU outcomes. Kidney Int 62: 986–996, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Patel NC, Patel NU, Loulmet DF, McCabe JC, Subramanian VA: Emergency conversion to cardiopulmonary bypass during attempted off-pump revascularization results in increased morbidity and mortality. J Thorac Cardiovasc Surg 128: 655–661, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Welke KF, Ferguson TB, Jr, Coombs LP, Dokholyan RS, Murray CJ, Schrader MA, Peterson ED: Validity of the Society of Thoracic Surgeons National Adult Cardiac Surgery Database. Ann Thorac Surg 77: 1137–1139, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ: Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care 45[Supl 2]: S103–S107, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Austin PC: A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med 27: 2037–2049, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Glynn RJ, Schneeweiss S, Stürmer T: Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol 98: 253–259, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novitzky D, Baltz JH, Hattler B, Collins JF, Kozora E, Shroyer AL, Grover FL: Outcomes after conversion in the Veterans Affairs randomized on versus off bypass trial. Ann Thorac Surg 92: 2147–2154, 2011 [DOI] [PubMed] [Google Scholar]