Abstract
Podocyte depletion leads to glomerulosclerosis, but whether an impaired capacity of podocytes to respond to hypertrophic stress also causes glomerulosclerosis is unknown. We generated transgenic Fischer 344 rats that express a dominant negative AA-4E-BP1 transgene driven by the podocin promoter; a member of the mammalian target of rapamycin complex 1 (mTORC1) pathway, 4E-BP1 modulates cap-dependent translation, which is a key determinant of a cell’s hypertrophic response to nutrients and growth factors. AA-4E-BP1 rat podocytes expressed the transgene and had normal kidney histology and protein excretion at 100 g of body weight but developed ESRD by 12 months. Proteinuria and glomerulosclerosis were linearly related to both increasing body weight and transgene dose. Uni-nephrectomy reduced the body weight at which proteinuria first developed by 40%–50%. The initial histologic manifestation of disease was the appearance of bare areas of glomerular basement membrane from the pulling apart of podocyte foot processes, followed by adhesions to the Bowman capsule. Morphometric analysis confirmed the mismatch between glomerular tuft volume and total podocyte volume (number × size) per tuft in relation to weight gain and nephrectomy. Proteinuria and glomerulosclerosis did not develop if dietary calorie restriction prevented weight gain and glomerular enlargement. In summary, failure of podocytes to match glomerular tuft growth in response to growth signaling through the mTORC1 pathway can trigger proteinuria, glomerulosclerosis, and progression to ESRD. Reducing body weight and glomerular growth may be useful adjunctive therapies to slow or prevent progression to ESRD.
A direct causative relationship exists between degree of podocyte depletion and the development of proteinuria and glomerulosclerosis.1–5 Furthermore, once a critical degree of podocyte depletion has occurred, angiotensin II–dependent glomerular destabilization supervenes, such that glomeruli continue to lose podocytes in association with progressive glomerulosclerosis (FSGS) until glomeruli become globally depleted of podocytes at ESRD.6 These concepts account for how progression can be triggered and sustained after a critical degree of podocyte injury and loss. However, they do not account for why growth-associated processes, such as obesity, large body size, glomerulomegaly, and nephronopenia, can trigger FSGS or for why FSGS is particularly prevalent during phases of rapid body growth in childhood and adolescence. If podocyte depletion is a common mechanism underlying glomerulosclerosis, then growth itself may be able to trigger podocyte depletion, leading to progression in susceptible individuals.
One mechanism for how glomerular growth could result in relative podocyte depletion would be if podocytes have limited capacity to increase in number and size, while other glomerular cells do not, thereby resulting in a “mismatch” between glomerular tuft volume and total podocyte complement (number × average cell volume).7,8 To test this hypothesis directly, we developed a transgenic rat whose podocytes have limited capacity to respond to hypertrophic stress.
Cell growth is driven by growth factors and nutrition through the mammalian target of rapamycin complex 1 (mTORC1) pathway and downstream via both 4E-BP1 and S6 kinase-dependent mRNA translation.9,10 The mTORC1 pathway is essential for normal podocyte function.11,12 4E-BP1–regulated CAP-dependent translation can be retarded using a dominant negative AA-4E-BP1 transgene in which threonine residues (37 and 46) critical for 4E-BP1 phosphorylation and activation are mutated to alanine.13 AA-4E-BP1 transgene expression causes reduced cell size in culture.10 To impair podocyte response to hypertrophic stimuli, we therefore expressed the AA-4E-BP1 transgene specifically in podocytes of Fischer 344 rats under control of the podocin promoter. Body growth on an ad libitum diet and nephrectomy, known to increase glomerular capillary number and surface area,14,15 were used to stress wild-type and transgenic rats to bring out the underlying principles.
Results
Glomerular Volume Is Directly Related to Body Weight
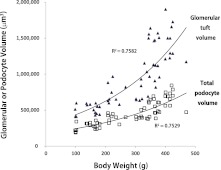
Glomerular volume increases in relation to body weight gain in wild type Fischer 344 intact rats (Figure 1). Podocyte volume also increases but does not increase in proportion to glomerular volume (Figure 1). If the “mismatch hypothesis” is correct, then impairing podocyte capacity to respond to hypertrophic stress should result in accelerated proteinuria and FSGS in relation to growth.
Figure 1.
Relationships between body weight and glomerular tuft volume (closed triangles) and body weight and total podocyte volume as measured by the GLEPP1-positive tuft volume (open squares). In wild-type Fischer 344 intact rats, glomerular tuft volume increased exponentially in relation to body weight gain in rats kept on an ad libitum diet. Podocyte volume also increased in relation to body weight gain, but not at the same rate as the glomerular tuft volume, representing an apparent mismatch developing between these two variables as body weight increases.
AA-4E-BP1 Is Expressed by Podocytes of Transgenic Fischer 344 Rats
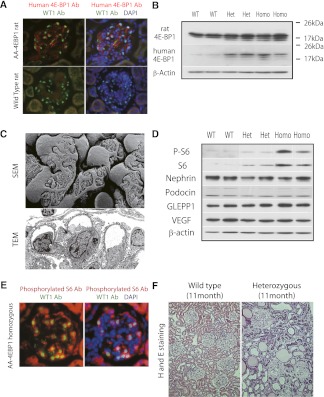
Transgenic AA-4E-BP1 rats, but not wild-type Fischer 344 rats, expressed human 4E-BP1 in their podocytes (Figure 2A). Western blot of isolated glomerular extracts demonstrated hypo-phosphorylated 4E-BP1 bands in both heterozygous and homozygous transgenic rats, along with expression of the human 4E-BP1 protein; these were not seen in wild-type rat glomerular extracts (Figure 2B). Thus, the human AA-4E-BP1 was expressed by transgenic rat podocytes.
Figure 2.
Characterization of AA-4E-BP1 transgenic rats. (A) Expression of human 4E-BP1 by immunofluorescence (red) in podocytes as identified by WT1-positive nuclear immunofluorescence (green) in homozygous transgenic rats (upper panels) and wild-type rats (lower panels). The right two panels show DAPI-stained nuclei for the same images. n=3. Ab, antibody. Original magnification, ×160 (B) Western blots of protein extracts from isolated glomeruli of wild-type (WT), heterozygous (Het), and homozygous (Homo) rats. Note that the heterozygous and homozygous glomeruli contain hypophosphorylated lower-molecular-weight bands not seen in wild-type glomeruli (upper blot), and antihuman 4E-BP1 antibodies recognize bands in the heterozygous and homozygous rat glomerular extracts not present in wild-type extracts (middle blot). β-actin is shown as a reference loading marker. n=2 per experiment, with two experiments performed. (C) SEM (top panel) and TEM (bottom panel) images of homozygous AA-4E-BP1 rats, illustrating normal podocyte structure at 100-g body weight. n=4 for SEM and n=2 for TEM. Original magnification ×2500. (D) Western blots of wild-type, heterozygous, and homozygous isolated glomerular extracts demonstrating increased S6 protein and phospho-S6 in heterozygous and homozygous rats compared with wild-type rats; there is no obvious difference in expression of podocyte products (nephrin, podocin, GLEPP1, and vascular endothelial growth factor [VEGF]). β-actin is shown as a reference loading marker. n=2 per experiment, with two experiments performed. (E) Glomerulus from a 100-g homozygous AA-4EBP1 rat developed for phospho-S6 immunofluorescence demonstrating predominant expression of phospho-S6 in podocytes as confirmed by WT1-positive (green) nuclei. The right panel shows DAPI-stained nuclei in the same image. n=3. Original magnification ×160. (F) Hematoxylin and eosin (H and E)–stained sections of rat renal cortex from an 11-month-old heterozygous AA-4EBP1 rat (left panel) versus the cortex from an 11-month-old wild-type Fischer 344 rat (right panel), demonstrating that ESRD had developed in the transgenic rat. n=7. Original magnification ×50.
Heterozygous AA-4E-BP1 Rats at 100-g Body Weight Are Structurally and Functionally Normal, Whereas Homozygous Rats Have Smaller Podocytes and Glomerular Tufts
By 100-g body weight, transgenic rats were normal in appearance, had no increase in proteinuria (Figure 3A), were not hypertensive, had normal histologic features of the kidney by light microscopy (Figure 4A), and had no abnormality detected by scanning electron microscopy (SEM) and transmission electron microscopy (TEM) (Figure 2C). Morphometric examination at 100-g body weight showed no difference between wild-type and heterozygous rats, but homozygous rats had a smaller glomerular volume, a smaller total podocyte volume made up of smaller podocyte volume, and slightly reduced podocyte number (Table 1, left columns). Western blot showed increased expression of ribosomal protein S6 and S6 phosphorylation (activation) in both heterozygous and homozygous rat glomerular extracts (Figure 2D). Figure 2E shows that phosphorylated S6 in homozygous AA-4E-BP1 glomeruli was predominantly localized to podocytes. Thus, expression of the AA-4E-BP1 transgene designed to reduce traffic through the 4E-BP1 pathway resulted in a compensatory increase in activity in the S6 pathway. However, there was no obvious reduction in expressed levels of podocyte proteins in transgenic rats, including nephrin, podocin, glomerular epithelial protein 1 (GLEPP1), and vascular endothelial growth factor (Figure 2D). Therefore, transgene expression appeared to be well compensated in both heterozygous and homozygous rats at 100 g.
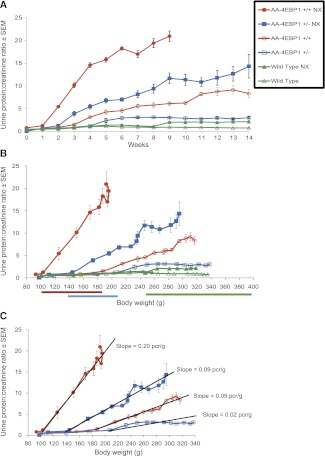
Figure 3.
Relationship between proteinuria, age, and weight. (A) Time course (starting at 100-g body weight) for development of proteinuria of wild-type, wild-type uni-nephrectomized (NX), heterozygous, heterozygous uni-nephrectomized, homozygous, and homozygous uni-nephrectomized rats. Note that the time course of proteinuria varied depending on transgene dose and whether the rats had been uni-nephrectomized. (B) Same data plotted against body weight on the x-axis, demonstrating that as weight increases a weight proteinuria threshold is crossed; beyond this weight threshold, the proteinuria increases linearly in relation to weight gain. Nephrectomy reduced this threshold by 40%–50% for each rat strain (wild-type, heterozygous, or homozygous). (C) For the uni-nephrectomized rat strains, the slope of proteinuria versus weight gain in homozygous rats is approximately twice that for heterozygous rats under both nephrectomy and intact conditions, thereby demonstrating that the gene dose plays a critical role in determining both the weight proteinuria threshold and the relationship between weight gain and proteinuria. Data are shown as the mean ± SEM.
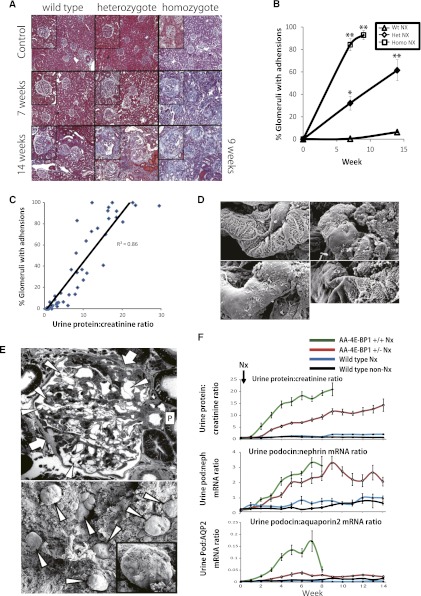
Figure 4.
Relationships between histologic features, proteinuria, and urine podocyte biomarkers in wild-type, heterozygous, and homozygous nephrectomized AA-4E-BP1 rats. Original magnification ×60. (A) Masson trichrome–stained histologic sections at 7 weeks and 14 weeks after nephrectomy (at 100-g body weight). Homozygous rats were euthanized at 9 weeks because they developed uremic symptoms. (B) Proportion of glomeruli that contained adhesions at 7 and 14 weeks (9 weeks for homozygous nephrectomized rats). Het, heterozygous; Homo, homozygous; Wt, wild-type. *P<0.05 and **P<0.01, as assessed by Kruskal-Wallis test and then Scheffe's test. (C) There was a direct relationship between the urine protein-to-creatinine ratio and the proportion of glomeruli that contained adhesions. (D) SEM images from heterozygous nephrectomized rats demonstrating that foot processes were mostly intact (upper left panel) but that glomeruli contained patches of bare GBM where the foot processes had apparently been pulled apart (three examples shown). Foot processes abutting these areas remained relatively intact. Original magnification ×4000. (E) Upper panel shows a TEM image demonstrating two adhesions (arrows) distant from the pole of the glomerulus (P), and podocytes adjacent to these areas contained granules and cysts (arrowheads). Most capillary loops were lined by intact foot processes, although occasional areas of effaced foot processes were present (not shown). Also shown are plump parietal epithelial cells adjacent to the adhesions. Original magnification ×260. Glomerular tufts could not be visualized by SEM because capsules remained attached to the tuft, presumably because of adhesions (lower panel). Original magnification ×70. (F) Time course of urine protein-to-creatinine ratio (upper panel), urine podocin-to-nephrin mRNA ratio as a measure of podocyte stress (middle panel), and urine podocin-to-aquaporin2 mRNA ratio per as a measure of the rate of podocyte loss into the urine. Note that there was no measurable increase in the rate of podocyte loss in wild-type rats, although podocyte stress was increased throughout the 14-week observation period. In contrast, podocyte stress was markedly increased in both heterozygous and homozygous rats, and the rate of podocyte loss was higher in homozygous than in heterozygous rats. As homozygous rats reached ESRD, glomeruli became depleted of podocytes, as reflected by a reduced rate of podocyte loss. Data are shown as the mean ± SEM.
Table 1.
Morphometric variables measured in wild-type, heterozygous, and homozygous AA-4E-BP1 Fischer 344 rats with and without prior nephrectomy
| Variable | Wild Type | Heterozygous | Homozygous | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wk 0, 100 g (n=5) | No NX | NX | Wk 0, 100 g (n=10) | No NX | NX | Wk 0, 100 g (n=5) | No NX | NX | ||||||||||||||
| Wk 14 (n=5) | Fold Change over Wk 0 | Wk 14 (n=5) | Fold Change over Wk 0 | Wk 14 (n=5) | Fold Change over Wk 0 | Wk 14 (n=10) | Fold Change over Wk 0 | Wk 14 (n=5) | Fold Change over Wk 0 | Wk 9 (n=5) | Fold Change over Wk 0 |
|||||||||||
| Glomerular tuft volume (μm3) | ||||||||||||||||||||||
| mean | 469,012 | 1,040,081 | 2.2 | 1,394,319 | 3.0 | 465,429 | 1,049,942 | 2.3 | 2,220,893 | 4.8 | 332,473 | 1,285,271 | 3.9 | 2,688,464 | 8.1 | |||||||
| SD | 84,100 | 54,891 | 189,865 | 92,952 | 212,906 | 729,859 | 54,000 | 295,202 | 303,846 | |||||||||||||
| P value within WT, heterozygous, or homozygous group | <0.01 | <0.01 | <0.01 | 0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |||||||||||||
| P value between WT, heterozygous, and homozygous groups | NS | NS | 0.03 | 0.01 | NS | NS | 0.02 | NS | <0.01 | |||||||||||||
| GLPP1-positive tuft area (%) | ||||||||||||||||||||||
| mean | 47 | 40 | 0.9 | 36 | 0.8 | 44 | 41 | 0.9 | 22 | 0.5 | 35 | 36 | 1.1 | 6 | 0.3 | |||||||
| SD | 6 | 4 | 4 | 6 | 6 | 18 | 2 | 6 | 3 | |||||||||||||
| P value within WT, heterozygous, or homozygous group | 0.02 | NS | <0.01 | NS | <0.01 | <0.01 | NS | <0.01 | <0.01 | |||||||||||||
| P value between WT, heterozygous, and homozygous groups | NS | NS | NS | <0.01 | NS | NS | <0.01 | NS | <0.01 | |||||||||||||
| GLPP1-positive volume (μm3) | ||||||||||||||||||||||
| mean | 221,747 | 415,217 | 1.9 | 495,051 | 2.2 | 203,676 | 426,971 | 2.1 | 399,118 | 2.0 | 117,259 | 468,379 | 3.9 | 158,365 | 1.4 | |||||||
| SD | 61,861 | 47,401 | 58,717 | 42,568 | 53,570 | 270,773 | 19,676 | 128.703 | 89,343 | |||||||||||||
| P value within WT, heterozygous, or homozygous group | <0.01 | <0.01 | <0.01 | 0.01 | 0.02 | 0.02 | <0.01 | <0.01 | NS | |||||||||||||
| P value between WT, heterozygous, and homozygous groups | NS | NS | NS | <0.01 | NS | NS | <0.01 | NS | <0.01 | |||||||||||||
| Podocytes per tuft (n) | ||||||||||||||||||||||
| mean | 120 | 130 | 1.1 | 122 | 1.0 | 118 | 125 | 1.1 | 93 | 0.8 | 103 | 137 | 1.3 | 53 | 0.5 | |||||||
| SD | 9 | 11 | 9 | 13 | 17 | 55 | 11 | 28 | 26 | |||||||||||||
| P value within WT, heterozygous, or homozygous group | NS | NS | NS | NS | NS | NS | <0.01 | <0.01 | <0.01 | |||||||||||||
| P value between WT, heterozygous, and homozygous groups | NS | NS | NS | 0.04 | NS | NS | 0.03 | NS | <0.01 | |||||||||||||
| GV/P (μm3) | ||||||||||||||||||||||
| mean | 3907 | 8070 | 2.2 | 11379 | 2.9 | 3939 | 8391 | 2.1 | 52,853 | 16.0 | 3,225 | 9,621 | 3.0 | 62,044 | 19.2 | |||||||
| SD | 663 | 1060 | 1215 | 555 | 1106 | 64,753 | 428 | 2,744 | 34,002 | |||||||||||||
| P value within WT, heterozygous, or homozygous group | <0.01 | <0.01 | <0.01 | <0.01 | 0.01 | 0.01 | NS | <0.01 | <0.01 | |||||||||||||
| P value between WT, heterozygous, and homozygous groups | NS | NS | NS | 0.01 | NS | NS | NS | NS | 0.01 | |||||||||||||
| Mean podocyte volume (μm3) | ||||||||||||||||||||||
| mean | 1833 | 3229 | 1.8 | 4059 | 2.2 | 1734 | 3440 | 2.0 | 4176 | 2.4 | 1133 | 3422 | 3.0 | 2945 | 2.6 | |||||||
| SD | 403 | 592 | 534 | 332 | 377 | 1552 | 116 | 660 | 1217 | |||||||||||||
| P value within WT, heterozygous, or homozygous group | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | NS | <0.01 | |||||||||||||
| P value between WT, heterozygous, and homozygous groups | NS | NS | NS | <0.01 | NS | NS | 0.01 | NS | NS | |||||||||||||
| GLEPP1-negative tuft volume (μm3) | ||||||||||||||||||||||
| mean | 247,264 | 624,863 | 2.3 | 899,269 | 3.6 | 261,754 | 622,971 | 2.4 | 1,821,775 | 7.0 | 215,214 | 816,892 | 3.8 | 2,530,099 | 12.2 | |||||||
| SD | 42,767 | 43,760 | 152,524 | 67,173 | 176,023 | 897,848 | 35,969 | 195,808 | 363,279 | |||||||||||||
| P value within WT, heterozygous, or homozygous group | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |||||||||||||
| P value between WT, heterozygous, and homozygous groups | NS | NS | 0.04 | NS | NS | NS | NS | 0.03 | <0.01 | |||||||||||||
Male rats at 100 g either nephrectomy or remained intact. The kidneys obtained by nephrectomy at 100 g were used as a baseline. After 14 weeks on an ad libitum diet, rats were euthanized (with the exception of the homozygous nephrectomy group, which reached ESRD and were euthanized at 9 weeks after nephrectomy). Morphometric variables were measured as shown in the table. The P values for each data set in the upper line compare values within each group. P values are shown in three columns. The left column compares 100-g rats to the intact rat 14 weeks later. The middle column compares the intact rat at 14 weeks to the nephrectomized rat at 9 weeks. The right column compares the nephrectomized rat at 9 weeks to the 100-g rat at 0 weeks. The P values shown for each data set in the lower line compare data for the same variables between wild-type, heterozygous, and homozygous groups. The P values in the wild-type column show values comparing wild-type to heterozygous rats. The data in the middle columns compare heterozygous rats to homozygous rats. The values in the homozygous columns compare homozygous rats to wild-type rats. NX, nephrectomy; WT, wild-type; GV/P, glomerular volume per podocyte.
However, by 1 year of age, most heterozygous and homozygous rats (male and female) had reached ESRD (Figure 2F). Therefore, podocyte-specific expression of the AA-4E-BP1 transgene under the direction of the podocin promoter that comes on late during the capillary-loop stage of glomerular development did not significantly alter podocyte structure or function in young rats, but conferred a markedly increased propensity to develop ESRD.
Body Weight Gain as a Driver of Increased Proteinuria and Glomerulosclerosis in Relation to the Transgene Dose, and Acceleration by Uni-nephrectomy
Figure 3A shows the time course (starting at 100-g body weight and continuing for 14 weeks) of proteinuria for wild-type, heterozygous, and homozygous Fischer 344 rats both in the intact state and after uni-nephrectomy performed at 100 g of body weight. Intact wild-type rats did not have significantly increased proteinuria by 14 weeks. In contrast, uni-nephrectomy of wild-type rats caused normal urine protein excretion for 8 weeks, followed by a small but significant increase in proteinuria at 9 weeks that persisted until week 14. Intact heterozygous rats had no increase in proteinuria until week 5, after which they developed proteinuria. Nephrectomy of heterozygous rats resulted in normal protein excretion for 2 weeks and then an increase in proteinuria by week 3 after nephrectomy. Intact homozygous rats had normal proteinuria for 3 weeks and then developed increased proteinuria by week 4. Nephrectomy of homozygous rats resulted in a normal level of proteinuria for 1 week and then sharply increasing proteinuria thereafter until these animals developed ESRD by 9 weeks. Thus, in each case (except the intact wild-type rats) during the 14-week period of observation, there was normal protein excretion for a period of time, after which some threshold was crossed and urine protein excretion then increased.
Figure 3B shows these same proteinuria data plotted against body weight. There is a weight threshold above which rats develop proteinuria (the “weight-proteinuria threshold”). This threshold varied depending on the dose of the transgene and on whether the rats had been nephrectomized. For example, we previously reported that wild-type intact Fischer 344 male rats developed proteinuria at about 400 g.8 Nephrectomized wild-type rats developed proteinuria at about 250-g body weight (a 40% lower weight-proteinuria threshold caused by nephrectomy). Similarly, nephrectomy reduced the weight-proteinuria threshold of heterozygous AA-4E-BP1 rats from about 210 to 140 g (a 35% reduction), and for AA-4E-BP homozygous rats from 190 to 100 g (a 47% reduction). Each rat strain therefore had its own weight-proteinuria threshold for the intact animal and an approximately 40%–50% lower weight-proteinuria threshold if one kidney had been removed.
Figure 3C emphasizes the linear relationship between weight gain and proteinuria, which is particularly well illustrated by comparing the nephrectomized homozygous and heterozygous transgenic rats. The slope for nephrectomized homozygous rats was twice that for heterozygous rats, thereby demonstrating a proportional gene dose effect not only on the weight-proteinuria threshold but also on the weight-proteinuria slope. These data demonstrate that genes expressed only by the podocyte can have a remarkable effect both in lowering the weight threshold at which proteinuria and FSGS develop as well as the slope of the relationship between weight gain and proteinuria. Furthermore, reduction in nephron mass caused by nephrectomy lowers this weight-proteinuria threshold by about 40%–50% and increases the weight-proteinuria slope two-fold in a predictable way that depends on the podocyte’s genetic profile.
Figure 4, A and B, shows Masson trichrome–stained histologic features at 14 weeks, together with quantitation of adhesions per glomerular tuft. Wild-type rats showed no detectable adhesions to the Bowman capsule by 7 weeks, but after nephrectomy 5% of glomerular tufts had adhesions by 14 weeks. Adhesions were present in increasing numbers in linear proportion to proteinuria in all groups (Figure 4C). Nephrectomized homozygous rats developed polyuria associated with the ruffled fur and reduced intake of food typical of ESRD; they were euthanized at 9 weeks, by which time almost every glomerulus had adhesions to the Bowman capsule. Heterozygous transgenic rats developed adhesions in 35% of glomeruli by 7 weeks and 60% of glomeruli by 14 weeks after uni-nephrectomy (Figure 4B).
Table 1 shows morphometric data documenting how glomerular tuft volume, the GLEPP1-positive (podocyte) area of the tuft, podocyte number, total podocyte volume, the glomerular volume per podocyte, and the tuft nonpodocyte volume change with normal growth in the intact rat on an ad libitum diet, and how this can be accelerated by nephrectomy. In wild-type intact rats, tuft volume increased 2.2-fold during the 14 weeks of growth, and nephrectomy caused a further increase in tuft volume to 3-fold above baseline. In rats whose podocytes carried the AA-4E-BP1 transgene, glomerular tuft volume increased to a greater extent than in wild-type rats, particularly after nephrectomy, to 4.8-fold and 8.1-fold in heterozygous and homozygous rats, respectively. The total podocyte volume (number × size) increased only 2.2-fold, 2.0-fold, and 1.4-fold in nephrectomized wild-type, heterozygous, and homozygous rats, respectively. Therefore, although the glomerular tuft volume that was not GLEPP1 positive (i.e., nonpodocyte tuft volume) increased 3.6-fold in wild-type nephrectomized rats (which was appropriate for the 3-fold increase in glomerular tuft volume), in heterozygous and homozygous transgenic rats the nonpodocyte glomerular volume increased 7-fold and 12.2-fold, respectively. Thus, the glomerular volume per podocyte increased 2.9-fold in the wild-type rats during the 14-week period but 16-fold in the heterozygous and 19.2-fold in the homozygous transgenic rats. Therefore, the total podocyte complement (number × volume) did not keep up with glomerular tuft enlargement, particularly after the added hypertrophic stimulus of nephrectomy. Collectively, these data strongly support the concept that in the presence of defective podocyte function, glomerular tufts have an enhanced capacity to enlarge in the presence of normal growth environment and after nephrectomy, whereas the podocyte number × size has a much more limited capacity for growth. This creates a “mismatch” between tuft volume and the podocyte complement, which was associated with development of proteinuria, adhesions to the Bowman capsule, and glomerulosclerosis.
SEM of glomeruli from both heterozygous and homozygous transgenic rats in this study showed areas of patchy absence of podocytes from the capillary loop surface (Figure 4D). At later time points, TEM showed that adhesions to the Bowman capsule had developed (Figure 4E, upper panel). By SEM, glomerular tuft could not be visualized because all capsules remained adherent to the tuft (Figure 4E, lower panel), presumably because of adhesions. Of note, podocyte foot processes were not diffusely effaced, although there were localized areas of foot process effacement (not shown) and cyst formation within podocytes (Figure 4E, upper panel). This result is compatible with the hypothesis that as glomerular tufts enlarge the capacity of transgenic podocytes to hypertrophy and cover the glomerular capillary surface area becomes locally inadequate, so that podocytes pull apart to reveal bare areas of glomerular basement membrane (GBM). These lesions are the likely source of proteinuria and give rise to the adhesions to the Bowman capsule seen by light microscopy and TEM, as described by Kriz and LeHir.16 This finding would also be consistent with the linear relationship between the proportion of glomerular tufts with adhesions and the degree of proteinuria (Figure 4C).
Urine podocyte biomarker analysis provides real-time information about podocyte stress and rate of loss.6,17 As shown in Figure 4F, the urine podocin-to-nephrin mRNA ratio (a measure of podocyte stress) was not statistically significantly upregulated after nephrectomy in wild-type rats but was significantly increased after nephrectomy in both heterozygous and homozygous rats. The urine podocin-to-aquaporin2 ratio (a measure of the rate of podocyte loss) was not detectably upregulated in nephrectomized wild-type rats but was increased by 3 weeks after nephrectomy to a greater extent in homozygous than heterozygous rats. We interpret this result to demonstrate that although podocyte number per tuft was not detectably reduced in glomeruli of heterozygous nephrectomized transgenic rats by 14 weeks (Table 1), the rate of podocyte loss was increased, reflecting increased podocyte turnover in these animals where podocyte replacement was able to approximately keep up with the rate of loss. In contrast, in homozygous nephrectomized transgenic rats the higher rate of podocyte loss could not be compensated for and was therefore associated with a significant reduction in the number of glomerular tuft podocytes by 14 weeks (Table 1).
Calorie Restriction Prevents Weight Gain, Glomerular Enlargement, Proteinuria, and FSGS
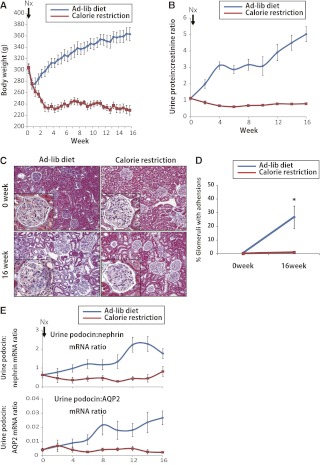
If glomerular enlargement is driving the development of FSGS, we would hypothesize that prevention of glomerular growth would prevent development of proteinuria and FSGS in AA-4E-BP1 transgenic rats. To test this hypothesis, we used 300-g male heterozygous AA-4E-BP1 rats (to avoid exposing growing rats to the 40% reduced calorie diet). As shown in Figure 5A, 40% calorie restriction resulted in 20% weight loss to a weight of about 240 g (the average weight of male rats in the wild).18 Rats fed ad libitum continued to gain weight. After nephrectomy, calorie-restricted rats did not develop proteinuria, adhesions to the Bowman capsule, or glomerulosclerosis (Figure 5, B–D). Morphometric analysis (Table 2) showed that in calorie-restricted rats, glomerular tuft volume did not increase, the podocyte complement as a proportion of the tuft volume did not change, and urine podocyte biomarkers did not show an increase in the rate of podocyte loss from glomeruli (Figure 5E). In contrast, ad libitum–fed transgenic rats showed increased volume of glomerular tufts, a decrease in the podocyte complement (number × volume) of glomerular tufts in proportion to glomerular volume, development of progressive proteinuria in relation to weight gain, increase in urine podocyte biomarkers, and FSGS. This result demonstrates that reduced nutritional signaling in the glomerulus has a powerful determinative effect on whether glomerular enlargement occurs together with its downstream consequences in terms of podocyte stress and loss, development of proteinuria, adhesions, and FSGS. This result also strongly supports the mismatch hypothesis.
Figure 5.
Effect of calorie restriction in preventing podocyte stress, podocyte loss, and adhesion formation in 300-g nephrectomized heterozygous AA-4EBP1 rats. (A) Forty percent calorie restriction resulted in loss of about 70 g in weight, whereas ad libitum–fed rats continued to gain weight over the 16-week period of observation. (B) Urine protein-to-creatinine ratio remained at baseline in calorie-restricted rats and increased in ad libitum–fed rats. (C) Representative Masson trichrome–stained sections illustrating the development of adhesions present in ad libitum–fed rats, which were not present in calorie-restricted rats. Original magnification ×110. (D) Quantitation of the adhesions. *P<0.05, as assessed by t test. (E) Both the urine podocin-to-nephrin mRNA ratio as a measure of podocyte stress and the urine podocin-to-aquaporin2 ratio as a measure of the rate of podocyte loss remained at baseline in calorie-restricted rats but increased in ad libitum–fed rats. Data are shown as the mean ± SEM.
Table 2.
Morphometric variables for ad libitum–fed and calorie-restricted heterozygous AA-4E-BP1 rats nephrectomized at 300-g body weight
| Variable | Ad Libitum Diet | Calorie-Restricted Diet | Fold Change: Ad Libitum versus Calorie-Restricted Diets | ||||
|---|---|---|---|---|---|---|---|
| Control (n=5) | 8 wk (n=5) | Fold Change versus Control | Control (n=5) | 8 wk (n=5) | Fold Change versus Control | ||
| Glomerular tuft volume (μm3) | |||||||
| mean | 988,394 | 1,537,509 | 1.6 | 988,509 | 829,346 | 0.8 | 1.9 |
| SD | 178,504 | 331,376 | 189,784 | 149,557 | |||
| P value within each group | <0.01 | NS | |||||
| P value between groups | NS | <0.01 | |||||
| GLPP1-positive tuft area (%) | |||||||
| mean | 57 | 43 | 0.8 | 55 | 53 | 1.0 | 0.8 |
| SD | 6 | 9 | 4 | 4 | |||
| P value within each group | <0.01 | NS | |||||
| P value between groups | NS | NS | |||||
| GLPP1-positive volume (μm3) | |||||||
| mean | 557,405 | 640,941 | 1.1 | 538,970 | 435,234 | 0.8 | 1.5 |
| SD | 101,091 | 41,783 | 104,964 | 75,625 | |||
| P value within each group | 0.03 | NS | |||||
| P value between groups | NS | <0.01 | |||||
| Podocytes per tuft (n) | |||||||
| mean | 116 | 132 | 1.1 | 106 | 128 | 1.2 | 1.0 |
| SD | 11 | 41 | 8 | 26 | |||
| P value within each group | NS | NS | |||||
| P value between groups | NS | NS | |||||
| GV/P (μm3) | |||||||
| mean | 8552 | 12453 | 1.5 | 9253 | 6518 | 0.7 | 1.9 |
| SD | 1676 | 3927 | 1302 | 696 | |||
| P value within each group | <0.01 | <0.01 | |||||
| P value between groups | NS | 0.01 | |||||
| Mean podocyte volume (μm3) | |||||||
| mean | 4826 | 5221 | 1.1 | 5032 | 3436 | 0.7 | 1.5 |
| SD | 970 | 1627 | 637 | 466 | |||
| P value within each group | NS | <0.01 | |||||
| P value between groups | NS | 0.05 | |||||
| GLEPP1-negative tuft volume (μm3) | |||||||
| mean | 430,989 | 896,567 | 2.1 | 449,538 | 394,112 | 0.9 | 2.3 |
| SD | 102,443 | 332,279 | 99,656 | 83,445 | |||
| P value within each group | <0.01 | NS | |||||
| P value between groups | NS | 0.01 | |||||
Fold differences are used to summarize the comparison between the ad libitum and calorie-restricted animals after 16 weeks of diet. Note that calorie restriction prevented glomerular enlargement, podocyte hypertrophy, and nonpodocyte glomerular volume expansion. GV/P, glomerular volume per podocyte.
Discussion
Increase in glomerular tuft volume is remarkably related to body weight gain. As previously demonstrated by Nyengaard and colleagues, this increase in glomerular tuft volume results from increased glomerular capillary number and surface area.14,15 At the same time, podocyte number per tuft did not increase in proportion to glomerular volume. This means that compensation for glomerular tuft enlargement must occur largely through podocyte hypertrophy, as previously noted for aging ad libitum–fed rats.8 This finding raises the possibility that failure of podocyte hypertrophy to match glomerular enlargement could potentially drive development of glomerulosclerosis. To test this concept, we engineered transgenic rats to selectively compromise the capacity of their podocytes to respond to hypertrophic stress. The resulting enhanced sensitivity of these transgenic rats to develop proteinuria and FSGS in response to both body growth (weight gain) and nephrectomy, and the prevention of these events by reducing nutritional signaling by calorie restriction, strongly supports the “mismatch” hypothesis.
The mechanism by which proteinuria occurs in the AA-4E-BP1 model appears to be by mechanical failure of the podocyte epithelial layer. The integrity of the glomerular filtration surface requires exact coverage of the GBM surface by podocyte foot processes. As tufts hypertrophy in response to growth, the filtration surface area increases; thus, in the setting of reduced capacity to hypertrophy, the podocyte interdigitating foot processes eventually pull apart at certain sites to expose bare areas of GBM. These bare areas of glomerular capillary surface facilitate protein leak and are where adhesions to the Bowman capsule then develop (as demonstrated by Masson trichrome staining at the light microscopic level and by TEM). These events lead to development of FSGS lesions and progression to glomerulosclerosis in the sequence described by Kriz and LeHir.16 The linear relationship between the adhesions and proteinuria is compatible with a direct causative relationship.
Morphometry data emphasize that glomerular tuft volume increases more in transgenic rats than in wild-type rats, and in homozygous transgenic rats versus heterozygous transgenic rats. Therefore, wild-type rats were better able to control glomerular tuft volume in the presence or absence of hypertrophic stress compared with transgenic rats, and heterozygous rats were better able to control glomerular tuft volume compared with homozygous rats. There was therefore a transgene dose-dependent effect. This must mean that one job of the normal podocyte is to control tuft volume, and that it requires active podocyte function by an unidentified mechanism to achieve this important regulatory process. Thus, the mismatch resulting from podocyte loss or dysfunction is a compounded result of both a reduction in effective podocyte volume (size × number) and an increase in glomerular tuft volume. The mechanism by which this loss of tuft volume control occurs is not yet identified but could provide an important target for intervention to prevent progression.
We demonstrate a remarkable direct linear relationship between body weight gain and development of proteinuria and glomerulosclerosis, and how this relationship is affected by podocyte-specific expression of gene variants, which compromise podocyte capacity to adapt to hypertrophic stress. This result is compatible with the general concept that every individual has a body weight above which proteinuria will develop (the weight-proteinuria threshold). The development of proteinuria and FSGS for any individual will therefore depend on several variables: (1) body weight and closely associated glomerular tuft volume (governed by both genetic and environmental factors); (2) nephron number (influenced by birth weight and maternal and genetic factors, as well as by loss of renal mass caused by nephrectomy [such as occurs in kidney donation, transplantation, and cancer surgery]); (3) the genetically endowed capacity of their podocytes to adapt to hypertrophic stress; and (4) accelerated loss of nephrons or podocytes caused by diverse disease mechanisms (both genetic and acquired). The well established relationships between nephron mass, body size, glomerular volume, and hypertension were recently reviewed by Luyckx and Brenner,19 although the key role of the podocyte in these relationships is not well recognized. Understanding the biology behind these relationships can provide improved opportunities for targeted therapeutic intervention to prevent progression.
The mechanism of podocyte depletion demonstrated by this model system is different from direct podocyte damage and death causing reduced podocyte number, leading to podocyte depletion and glomerulosclerosis.2,4,6 Growth-associated podocyte failure provides a mechanistic explanation for a separate group of diseases leading to FSGS or global glomerulosclerosis. These include (1) obesity and large body size, associated with proteinuria, glomerulomegaly FSGS, and progression to ESRD for all causes;20–23 (2) idiopathic FSGS and FSGS due to known genetic causes that are most prevalent during periods of rapid growth in childhood and adolescence; (3) nephronopenic and glomerulomegalic conditions (congenital and acquired);24 and (4) diabetic glomerulosclerosis, in which glomerulomegaly occurs in the setting of diabetes-associated renal hypertrophy, leading to reduced podocyte density and subsequent podocyte loss.25–29
The remarkable enhancement of FSGS caused by increased body growth in rats fed an ad libitum diet (and the even more remarkable total protection against FSGS caused by calorie restriction) could help explain the increased prevalence of FSGS reported in diverse ethnic populations30–39 in the setting of a worldwide increase in body mass index.40 The data are compatible with the concept that dietary modification and therapeutic modulation of growth/nutrition signaling pathways could be powerful adjunctive strategies to prevent progression of kidney diseases.
Concise Methods
All animal studies were approved by the University of Michigan Committee on Use and Care of Animals. Male Fischer 344 rats (Harlan, Indianapolis, IN) fed an ad libitum diet were used for these experiments because they do not spontaneously develop diabetes or hypertension. Calorie restriction was as previously described using the National Institute on Aging rat protocol and pellets purchased from this agency for this purpose as previously described.8 Urine was collected weekly using metabolic cages. Systolic BP; serum and urine creatinine, urine protein, and urine mRNA assays; and Western blots of glomerular extracts under reducing conditions were as previously described.2,4,6,8
AA-4E-BP1 Fischer 344 Rat Model
The AA-EFI4EBP1 cDNA, a double point mutant of EFI4EBP1 at phosphorylation sites A37 and A46, was isolated from a pACTAG-2-h4E-BP1 plasmid kindly provided by Dr. Anne-Claude Gingras.13 The AA-EFI4EBP1 cDNA was inserted at the 3′ position of the 2.5-kb human podocin (Nphs2) promoter at the NcoI site of plasmid p2.5P-nlacF, which we previously showed drives podocyte-specific LacZ expression in mice and human diphtheria toxin receptor expression in rats.4,41 The podocin promoter/AA-EFI4EBP1 Tg construct was isolated from plasmid (p2.5PnLacF_EFI4EBP1 Thr37Ala/Thr46Ala) by XbaI/HindIII restriction enzyme digestion and injected into the pronuclei of fertilized Fischer344 rat ova using standard techniques. Three Tg founders were obtained, only one of which (#110) produced Tg offspring. Transgenic rats were identified by PCR analysis of tail DNA using the primer pair: p2.5_EGFdetect.fwd (5′-ACCCGACGGTCTTTAGGG-3′); h4E-BP1_FixB.rev (5-ATCCCCCATGGATTCCTTG AGCACA AGTCTCT CTTAAATGTCC ATCTCAAA CTGTGACTC-3), which produced a 550-bp amplicon. Crossing of heterozygous AA-4E-BP1 transgenic rats resulted in the expected ratio of 1:2:1 wild-type:heterozygous:homozygous offspring ratios (data not shown).
Reagents and Antibodies
The following primary antibodies were used for immunofluorescence and Western blot. Nephrin and podocin (rabbit polyclonal) were supplied by Dr. Lawrence Holzman. GLEPP1 (also more recently designated in the tyrosine phosphatase literature as PTPro [protein tyrosine phosphatase ro]) mouse monoclonal antibody was raised against the recombinant rat GLEPP1 extracellular domain and used as described previously.2 Vascular endothelial growth factor (no. ab46154) and human 4E-BP1 (no. ab32130) antibodies were obtained from Abcam (Cambridge, MA). Phospho-S6 (no. 2215), S6 (no. 2217), and 4E-BP1 (no. 9644) antibodies were obtained from Cell Signaling Technology (Beverley, MA). β-actin antibody (no. A5441) was obtained from Sigma-Aldrich (St. Louis, MO).
Histomorphometry
Immunofluorescence and immunoperoxidase staining were performed as previously described.2,4,6,8 All histologic testing was performed on paraformaldehyde-lysine-periodate–perfused and fixed paraffin-embedded tissue sectioned on a Reichert-Jung 820-II microtome (Cambridge Instruments, Nussloch, Germany). For quantification of glomerular tuft adhesions, 3-μm sections were stained with Masson trichrome and read by a blinded observer. The proportion of glomeruli demonstrating adhesions in 100 consecutive glomeruli was measured. Glomerular volume was estimated by measuring the mean glomerular radius (r) using the Metamorph Imaging program, and then using the Weibel formula to calculate the mean maximal tuft radius (R) and hence the mean glomerular volume (4/3πR3) as previously described.42 For measurement of GLEPP1-positive area, 50 consecutive glomerular tuft areas were measured and the percentage of each glomerular tuft that stained positive using GLEPP1 immunoperoxidase was measured using the Metamorph Imaging program as previously described.6 The mean GLEPP1-positive (podocyte) volume per glomerulus was estimated by multiplying the mean glomerular volume by the mean percentage of GLEPP1-positive area. The number of podocytes per glomerular tuft was measured using WT1 immunofluorescence, as previously described.6 The mean individual podocyte volume was estimated by dividing the mean GLEPP1-positive tuft volume (estimated as outlined above) by the mean podocyte number per tuft. The nonpodocyte volume was estimated by subtracting the mean podocyte volume from the glomerular volume.
Transmission and SEM Analysis
The kidney samples were fixed with Sorensen phosphate buffer containing 4% paraformaldehyde and 2.5% glutaraldehyde. The processed samples were analyzed by Philips CM100 TEM and AMRAY 1910 field emission SEM.
Western Blot and Antibodies
Glomeruli were isolated as previously described.2,4 Purified glomeruli from one rat was lysed in lysis buffer (10 mM Tris-HCl [pH, 7.5], 100 mM NaCl, 1% NP-40, 50 mM NaF, 20 mM β-glycerophosphate, 2 mM EDTA, 1 mM dithiothreitol, 1 mM PMSF, 10 μg/ml leupeptin, and 10 μg/ml aprotinin) as a lysate sample. Lysates were then boiled in SDS sample buffer (20 mM Tris [pH, 6.8], 8% SDS, 0.05% bromophenol blue, 40% glycerol, 20% 2-mercaptoethanol) and subjected to SDS-PAGE and Western blotting according to standard techniques.
Urine mRNA Analysis
Urine was collected in metabolic cages and the overnight urine pellet was used for purification of RNA; production of cDNA; and quantitation of podocin, nephrin, and aquaporin2 cDNAs as previously described.6,17
Statistical Analyses
All results were presented as mean ± SEM except where otherwise noted. Differences among two groups was tested by a t test and, among more than two groups, by Kruskal-Wallis test. When the result of the Kruskal-Wallis test was significant, a Scheffe test was carried out for post hoc analysis. For data shown in the tables, an unpaired t test was used for most comparisons; for comparisons of data from the same animals, a paired t test was used.
Disclosures
None.
Acknowledgments
We are grateful to Bryan Wharram for his help in producing the AA-4E-BP1 transgenic rat.
This work was supported by the National Institutes of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (grants DK RO1 46073 and P30 DK081943).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Glomerular Homeostasis Requires a Match between Podocyte Mass and Metabolic Load,” on pages 1273–1275.
References
- 1.Kriz W, Gretz N, Lemley KV: Progression of glomerular diseases: Is the podocyte the culprit? Kidney Int 54: 687–697, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, Kershaw D, Wiggins R: Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int 60: 957–968, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Kriz W: Podocyte is the major culprit accounting for the progression of chronic renal disease. Microsc Res Tech 57: 189–195, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Fukuda A, Wickman LT, Venkatareddy MP, Sato Y, Chowdhury MA, Wang SQ, Shedden KA, Dysko RC, Wiggins JE, Wiggins RC: Angiotensin II-dependent persistent podocyte loss from destabilized glomeruli causes progression of end stage kidney disease. Kidney Int 81: 40–55, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagata M, Kriz W: Glomerular damage after uninephrectomy in young rats. II. Mechanical stress on podocytes as a pathway to sclerosis. Kidney Int 42: 148–160, 1992 [DOI] [PubMed] [Google Scholar]
- 8.Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, Kuick RD, Wiggins RC: Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: Prevention by calorie restriction. J Am Soc Nephrol 16: 2953–2966, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Inoki K, Kim J, Guan KL: AMPK and mTOR in cellular energy homeostasis and drug targets. Annu Rev Pharmacol Toxicol 52: 381–400, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Fingar DC, Salama S, Tsou C, Harlow E, Blenis J: Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev 16: 1472–1487, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, Blattner SM, Ikenoue T, Rüegg MA, Hall MN, Kwiatkowski DJ, Rastaldi MP, Huber TB, Kretzler M, Holzman LB, Wiggins RC, Guan K-L: mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest 121: 2181–2196, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gödel M, Hartleben B, Herbach N, Liu S, Zschiedrich S, Lu S, Debreczeni-Mór A, Lindenmeyer MT, Rastaldi MP, Hartleben G, Wiech T, Fornoni A, Nelson RG, Kretzler M, Wanke R, Pavenstädt H, Kerjaschki D, Cohen CD, Hall MN, Rüegg MA, Inoki K, Walz G, Huber TB: Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest 121: 2197–2209, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N: Regulation of 4E-BP1 phosphorylation: A novel two-step mechanism. Genes Dev 13: 1422–1437, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nyengaard JR: Number and dimensions of rat glomerular capillaries in normal development and after nephrectomy. Kidney Int 43: 1049–1057, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Marcussen N, Nyengaard JR, Christensen S: Compensatory growth of glomeruli is accomplished by an increased number of glomerular capillaries. Lab Invest 70: 868–874, 1994 [PubMed] [Google Scholar]
- 16.Kriz W, LeHir M: Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 67: 404–419, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Sato Y, Wharram BL, Lee SK, Wickman L, Goyal M, Venkatareddy M, Chang JW, Wiggins JE, Lienczewski C, Kretzler M, Wiggins RC: Urine podocyte mRNAs mark progression of renal disease. J Am Soc Nephrol 20: 1041–1052, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis DE: The weight of wild brown rats at sexual maturity. Anat Rec 99: 575, 1947 [PubMed] [Google Scholar]
- 19.Luyckx VA, Brenner BM: The clinical importance of nephron mass. J Am Soc Nephrol 21: 898–910, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Afshinnia F, Wilt TJ, Duval S, Esmaeili A, Ibrahim HN: Weight loss and proteinuria: Systematic review of clinical trials and comparative cohorts. Nephrol Dial Transplant 25: 1173–1183, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Ritz E, Koleganova N, Piecha G: Is there an obesity-metabolic syndrome related glomerulopathy? Curr Opin Nephrol Hypertens 20: 44–49, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Eknoyan G: Obesity and chronic kidney disease. Nefrologia 31: 397–403, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Burton JO, Gray LJ, Webb DR, Davies MJ, Khunti K, Crasto W, Carr SJ, Brunskill NJ: Association of anthropometric obesity measures with chronic kidney disease risk in a non-diabetic patient population. Nephrol Dial Transplant 27: 1860–1866, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Bhathena DB: Glomerular basement membrane length to podocyte ratio in human nephronopenia: Implications for focal segmental glomerulosclerosis. Am J Kidney Dis 41: 1179–1188, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW: Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer TW, Bennett PH, Nelson RG: Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia 42: 1341–1344, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Steffes MW, Schmidt D, McCrery R, Basgen JM, International Diabetic Nephropathy Study Group : Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int 59: 2104–2113, 2001 [DOI] [PubMed] [Google Scholar]
- 28.White KE, Bilous RW, Marshall SM, El Nahas M, Remuzzi G, Piras G, De Cosmo S, Viberti G: European Study for the Prevention of Renal Disease in Type I diabetes (ESPRIT): Podocyte number in normotensive type I diabetic patients with albuminuria. Diabetes 51: 3083–3089, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Dalla Vestra M, Masiero A, Roiter AM, Saller A, Crepaldi G, Fioretto P: Is podocyte injury relevant in diabetic nephropathy? Studies in patients with type 2 diabetes. Diabetes 52: 1031–1035, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Haas M, Spargo BH, Coventry S: Increasing incidence of focal-segmental glomerulosclerosis among adult nephropathies: A 20-year renal biopsy study. Am J Kidney Dis 26: 740–750, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Bonilla-Felix M, Parra C, Dajani T, Ferris M, Swinford RD, Portman RJ, Verani R: Changing patterns in the histopathology of idiopathic nephrotic syndrome in children. Kidney Int 55: 1885–1890, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Gulati S, Sharma AP, Sharma RK, Gupta A: Changing trends of histopathology in childhood nephrotic syndrome. Am J Kidney Dis 34: 646–650, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Braden GL, Mulhern JG, O’Shea MH, Nash SV, Ucci AA, Jr, Germain MJ: Changing incidence of glomerular diseases in adults. Am J Kidney Dis 35: 878–883, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Filler G, Young E, Geier P, Carpenter B, Drukker A, Feber J: Is there really an increase in non-minimal change nephrotic syndrome in children? Am J Kidney Dis 42: 1107–1113, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Dragovic D, Rosenstock JL, Wahl SJ, Panagopoulos G, DeVita MV, Michelis MF: Increasing incidence of focal segmental glomerulosclerosis and an examination of demographic patterns. Clin Nephrol 63: 1–7, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Swaminathan S, Leung N, Lager DJ, Melton LJ, 3rd, Bergstralh EJ, Rohlinger A, Fervenza FC: Changing incidence of glomerular disease in Olmsted County, Minnesota: A 30-year renal biopsy study. Clin J Am Soc Nephrol 1: 483–487, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Borges FF, Shiraichi L, da Silva MP, Nishimoto EI, Nogueira PC: Is focal segmental glomerulosclerosis increasing in patients with nephrotic syndrome? Pediatr Nephrol 22: 1309–1313, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Zhou FD, Zhao MH, Zou WZ, Liu G, Wang H: The changing spectrum of primary glomerular diseases within 15 years: a survey of 3331 patients in a single Chinese centre. Nephrol Dial Transplant 24: 870–876, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Das U, Dakshinamurty KV, Prayaga A: Pattern of biopsy-proven renal disease in a single center of south India: 19 years experience. Indian J Nephrol 21: 250–257, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M, Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index) : National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 377: 557–567, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Two gene fragments that direct podocyte-specific expression in transgenic mice. J Am Soc Nephrol 13: 1561–1567, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Sanden SK, Wiggins JE, Goyal M, Riggs LK, Wiggins RC: Evaluation of a thick and thin section method for estimation of podocyte number, glomerular volume, and glomerular volume per podocyte in rat kidney with Wilms’ tumor-1 protein used as a podocyte nuclear marker. J Am Soc Nephrol 14: 2484–2493, 2003 [DOI] [PubMed] [Google Scholar]