Abstract
Eliminating autoantigen-specific B cells is an attractive alternative to global B-cell depletion for autoimmune disease treatment. To identify the potential for targeting a key autoimmune B-cell specificity in type 1 diabetes, insulin-binding B cells were tracked within a polyclonal repertoire using heavy chain B-cell receptor (BCR) transgenic (VH125Tg) mice. Insulin-specific B cells are rare in the periphery of nonautoimmune VH125Tg/C57BL/6 mice and WT/NOD autoimmune mice, whereas they clearly populate 1% of mature B-cell subsets in VH125Tg/NOD mice. Autoantigen upregulates CD86 in anti-insulin B cells, suggesting they are competent to interact with T cells. Endogenous insulin occupies anti-insulin BCR beginning with antigen commitment in bone marrow parenchyma, as identified by a second anti-insulin monoclonal antibody. Administration of this monoclonal antibody selectively eliminates insulin-reactive B cells in vivo and prevents disease in WT/NOD mice. Unexpectedly, developing B cells are less amenable to depletion, despite increased BCR sensitivity. These findings exemplify how a critical type 1 diabetes B-cell specificity escapes immune tolerance checkpoints. Disease liability is corrected by eliminating this B-cell specificity, providing proof of concept for a novel therapeutic approach for autoimmune disease.
Type 1 diabetes arises from immune-mediated destruction of insulin-producing β-cells in the pancreas. T cells directly mediate β-cell destruction; however, clinical trials have also uncovered an important role for B cells in type 1 diabetes, as global B-cell depletion preserves β-cell function in newly diagnosed type 1 diabetic patients (1) and preferentially impairs insulin autoantibody formation (2). Insulin autoantibody levels, but not GAD or IA-2 levels, correlate with disease progression in children, as does the age at which the first islet autoantibody is observed, suggesting that loss of tolerance for the insulin autoantigen may be of particular importance (3). Preclinical data for these studies came from the NOD mouse model of type 1 diabetes, which shares many human disease features. NOD mice in which insulin lacks a critical epitope for T-cell recognition are also protected from disease (4). Immunoglobulin (Ig)-transgenic NOD mice (VH281Tg/NOD) differing in two amino acids necessary for insulin binding fail to develop disease (5), whereas those harboring the anti-insulin specificity as all (125Tg/NOD) or part (VH125Tg/NOD) of the B-cell repertoire support disease (5,6), highlighting the critical importance of B cell–islet antigen specificity. B cell–specific expression of the correct major histocompatibility complex class II haplotype is also required for disease, demonstrating that B cells function pathogenically as antigen-presenting cells (APC) (7–9). Determining when and how B-cell tolerance for insulin fails could provide important clues toward specifically blocking their transition into dangerous APC and thus identify ways to restore immune tolerance to prevent type 1 diabetes pathogenesis.
Autoantigen encounter censors self-reactivity by functionally silencing B cells (anergy) or by removing them from the repertoire (receptor editing or deletion), broadly termed immune tolerance. Insulin-reactive B cells are censored in the bone marrow (BM) of healthy subjects (10), whereas they escape into the periphery in rheumatoid arthritis and systemic lupus erythematosus patients (11,12). A PTPN22 variant is linked with defective central tolerance (13), as well as type 1 diabetes development (14), predicting similar tolerance flaws in type 1 diabetic patients. To contribute to autoimmune disease, autoreactive B cells must compete with nonautoreactive B cells for survival factors and entry into follicular niches. These events are modeled in anti-insulin heavy chain transgenic mice (VH125Tg/NOD) that possess a polyclonal repertoire in which only 1 to 2% of mature B cells recognize insulin (5).
Anti-insulin monoclonal antibodies (mAb) specific for different epitopes allow detection of B cells for which surface B-cell receptors (BCR) are occupied by endogenous insulin (15). VH125Tg/NOD mice permit anti-insulin B-cell tracking as they navigate through immune tolerance hurdles for survival and thus identify how tolerance breaches of this specificity can be specifically corrected. Using this approach, we show that despite binding autoantigen, insulin-reactive B cells escape immune tolerance in type 1 diabetes-prone mice. Costimulatory molecule upregulation critical for T-cell cross-talk is intact in autoreactive B cells following insulin autoantigen exposure. Anti-insulin B cells are specifically eliminated by mAb therapy that targets BCR bound to insulin, whereas the broad repertoire is preserved. This therapy impairs disease progression in WT/NOD mice, in which the frequency of insulin-binding B cells is very low. When applied to VH125Tg/NOD mice, in which the anti-insulin B-cell population is increased, this approach unexpectedly reveals resistance of developing anti-insulin B cells to BCR-targeted elimination compared with mature B cells. These findings suggest a different approach to remove autoreactive B cells while avoiding the complications of global B-cell depletion. The data also indicate that differential sensitivity to BCR targeting may be present at each B-cell developmental stage, highlighting key considerations for the design of future therapeutics applying this tactic to the prevention of autoimmune disease.
RESEARCH DESIGN AND METHODS
Animals.
The anti-insulin VH125Tg [Cg-Tg(Igh-6/Igh-V125)2Jwt/JwtJ] and non–insulin-binding VH281Tg [Tg(Igh-6/Igh-V281)3Jwt/JwtJ] mice used in this study harbor a nontargeted heavy chain transgene on C57BL/6 or NOD backgrounds, as described previously (5,15). Sera from 125Tg/NOD mice [NOD.Cg-Tg(IGk-V125)1Jwt/JwtJ mice intercrossed with VH125Tg mice] served as positive controls in enzyme-linked immunosorbent assay (ELISA) to detect anti-insulin Ab (15). Age ranges are indicated in figure legends. All data are derived from lines backcrossed >20 generations to C57BL/6 or NOD that are hemizygous for all transgenes indicated. All mouse lines are available from The Jackson Laboratory. All mice were housed under sterile, specific pathogen-free housing conditions, and all studies were approved by the Institutional Animal Care and Use Committee of Vanderbilt University.
Cell isolation and culture.
BM was eluted from long bones, and spleens were macerated with Hanks’ balanced salt solution (HBSS; Invitrogen) plus 10% fetal bovine serum (FBS; HyClone). Red blood cells were lysed using Tris-NH4Cl. Freshly isolated pancreata were digested with 3 mL of 1 mg/mL collagenase P diluted in HBSS for 30 min, then tissue was disrupted using an 18-gauge needle. HBSS plus 10% FBS was immediately added to inhibit collagenase activity. Cells were directly analyzed by flow cytometry. Alternatively, 10 × 106 cells/mL splenocytes was cultured in complete culture medium (Dulbecco’s modified Eagle’s medium, 10% FBS, l-glutamine, HEPES, minimum essential medium sodium pyruvate, nonessential amino acids, gentamycin, 2 × 10−5 M β-mercaptoethanol [Invitrogen]) for 18 h in a 37°C CO2 incubator and stimulated with human insulin (Sigma-Aldrich) or anti-IgM (Jackson ImmunoResearch Laboratories).
Flow cytometry.
Ab reagents reactive with B220 (6B2), IgMa (DS-1), IgMb (AF6-78), CD19 (1D3), CD21 (7G6), CD23 (B3B4), CD86 (GL1), 7-aminoactinomycin D, DAPI (BD Biosciences), or IgM (μ chain specific; Invitrogen) were used for flow cytometry. Biotinylated human insulin (16) was detected with fluorochrome-labeled streptavidin (BD Biosciences). To restrict analysis to antigen-specific cells: insulin-specific B cells = % insulin-binding B cells, no inhibition (Fig. 1A, top) − % insulin-binding B cells, 10× unlabeled insulin competitor (Fig. 1A, bottom); this was further confirmed by a linear relationship of insulin binding and IgMa. BCR occupancy with endogenous insulin or BCR preloaded with 50 ng/mL human insulin on ice was detected using a second anti-insulin antibody, mAb123, which was biotinylated. mAb123 binds a distinct insulin epitope from mAb125 (from which VH125Tg is derived) and detects anti-insulin 125Tg BCR occupancy with endogenous insulin (15), but not insulin bound to the hormone receptor (17). Flow cytometry analysis was performed using an LSRII (BD Biosciences) and FlowJo software (Tree Star).
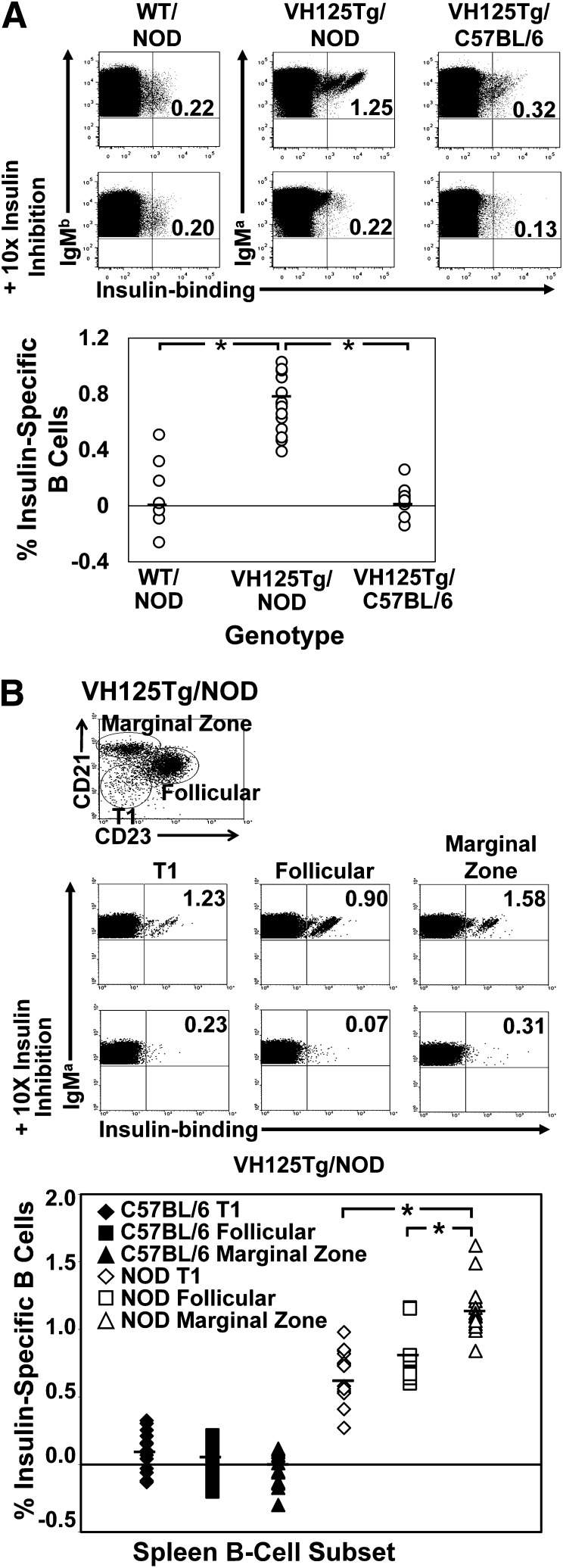
FIG. 1.
Anti-insulin B cells escape central tolerance to populate transitional, follicular, and marginal zone B-cell subsets in the spleen of VH125Tg/NOD mice. Freshly isolated spleen cells from NOD or C57BL/6 VH125Tg mice were analyzed using flow cytometry to detect insulin-binding B cells. Duplicate samples were incubated with 10-fold excess unlabeled insulin to show binding specificity. The percent age of insulin-specific B cells was calculated as in RESEARCH DESIGN AND METHODS. A: Top: Representative plots, gated on B220+ IgMa+ live lymphocytes. Bottom: Independent WT/NOD, VH125Tg/NOD, or VH125Tg/C57BL/6 mice are plotted; n ≥ 7 mice, n ≥ 4 experiments. B: Top and middle: Representative plots gated on B220+ IgMa+ live lymphocytes and further gated on CD21 and CD23 expression to identify insulin-specific B cells in T1 (CD21lo CD23lo), follicular (CD21lo CD23high), or marginal zone (CD21high CD23lo) B-cell compartments. Bottom: Individual mice are plotted; black symbols are C57BL/6, and white symbols are NOD. B-cell compartments indicated as follows: T1: diamonds (♦, ◇), follicular: squares (■, □), or marginal zone: triangles (▲, △). n ≥ 14 8- to 14-week-old mice; n ≥ 2 experiments. *P < 0.001 as calculated by a two-tailed t test.
Administration of mAb and disease study.
In short-term mAb administration experiments, 100 μg of anti-insulin mAb123 or IgG1 isotype control antibody (ATCC #HB-123 or #CRL-2395 hybridoma supernatant purified by the Vanderbilt Antibody and Protein Resource, respectively) in 100 μL 1× PBS (pH 7.4) and injected i.p. into VH125Tg/NOD mice weekly for 3 weeks. After 2–14 d, BM and spleens were harvested, and flow cytometry analysis was performed as above. For long-term administration of mAb in disease studies, 100 μg mAb123 was injected i.p. into WT/NOD or VH125Tg/NOD mice every other week starting at 3 weeks of age and continued throughout the disease study. Blood glucose was monitored weekly in untreated or mAb123-treated mice beginning at 10 weeks of age; diabetes was diagnosed following two consecutive >200 mg/dL blood glucose readings.
RESULTS
An increased frequency of anti-insulin B cells escapes into the mature polyclonal repertoire of VH125Tg/NOD mice.
Insulin autoantibodies are commonly detected in WT/NOD mice prior to type 1 diabetes onset (18). We therefore employed flow cytometry to identify insulin-binding B cells in the spleen. Live B220+IgMa+ lymphocytes and insulin-specific B cells were detected as in research design and methods. Insulin-specific B cells are difficult to reliably track in WT/NOD mice (Fig. 1A); therefore, to understand how B cells with this critical specificity are regulated during development, the VH125Tg BCR-transgenic model was employed, in which anti-insulin VH125 pairs with endogenous light chains in NOD mice to form a small population of B cells (1–2%) that recognize insulin (5). An increased frequency of anti-insulin B cells exists in the spleen of autoimmune VH125Tg/NOD mice compared with nonautoimmune VH125Tg/C57BL/6 mice (Fig. 1A).
The transitional 1 (T1) B-cell stage in the spleen is a major tolerance checkpoint that censors autoreactive specificities in the developing repertoire (10–12). Flow cytometry was therefore used to identify whether insulin-binding B cells may be culled at this checkpoint. T1 (CD21lowCD23low), follicular (CD21midCD23high), and marginal zone (CD21highCD23mid) B-cell subsets were identified within live B220+IgMa+ lymphocytes, and insulin-specific B cells were detected as in Fig. 1A in VH125Tg/C57BL/6 and VH125Tg/NOD mice. Insulin-specific B cells are present in the T1 (0.65 ± 0.19), as well as mature follicular (0.78 ± 0.17) and marginal zone (1.15 ± 0.20) B-cell compartments of VH125Tg/NOD mice (Fig. 1B). No significant decrease in the percentage of insulin-binding B cells is observed between the T1 to follicular or marginal zone B-cell compartments within VH125Tg/NOD mice; rather, a significantly increased frequency of insulin-binding B cells is found among marginal zone B cells (P < 0.0001), a site associated with autoreactive B-cell accumulation (19). In contrast, VH125Tg/C57BL/6 mice show a markedly reduced (or absent) percentage of insulin-binding B cells among the T1 (0.10 ± 0.14), follicular (0.04 ± 0.13), and marginal zone (−0.02 ± 0.12) spleen B-cell compartments. These data show that NOD anti-insulin B cells fail to be effectively censored at the T1 stage and compete effectively within a polyclonal repertoire for entry into mature B-cell subsets.
Anti-insulin B cells retain BCR-induced costimulatory potential critical for T-cell cross-talk but do not secrete detectable insulin autoantibody.
Anti-insulin B cells maintain functions necessary to promote β-cell destruction in VH125Tg/NOD mice (5). To identify whether insulin autoantigen specifically evokes costimulatory molecule upregulation in anti-insulin B cells, VH125Tg/NOD splenocytes were cultured in the presence or absence of insulin in vitro, and CD86 expression was measured using flow cytometry. The change in CD86 expression (mean fluorescence intensity [MFI]) was significantly increased approximately twofold (P < 0.01) in insulin-binding B cells exposed to insulin in vitro compared with those cultured in the absence of insulin. CD86 expression remained unchanged in non–insulin-binding B cells present in the same cultures, indicating CD86 upregulation was driven by specific insulin recognition through the BCR (Fig. 2A). To further investigate whether the potential to upregulate CD86 was different between anti-insulin and non–insulin-binding B cells, VH125Tg/NOD splenocytes were stimulated in vitro with anti-IgM to provide uniform BCR stimulation. Insulin-binding B cells and non–insulin-binding B cells showed comparable CD86 upregulation (Fig. 2B). CD86 expression was found to be significantly increased two- to threefold in insulin-binding B cells (P < 0.001), compared with non–insulin-binding B cells identified in freshly isolated pancreata (Fig. 2C). These data suggest that endogenous levels of autoantigen induce upregulation of CD86 in the disease setting. Similar outcomes were obtained in studies examining CD40 upregulation (not shown).
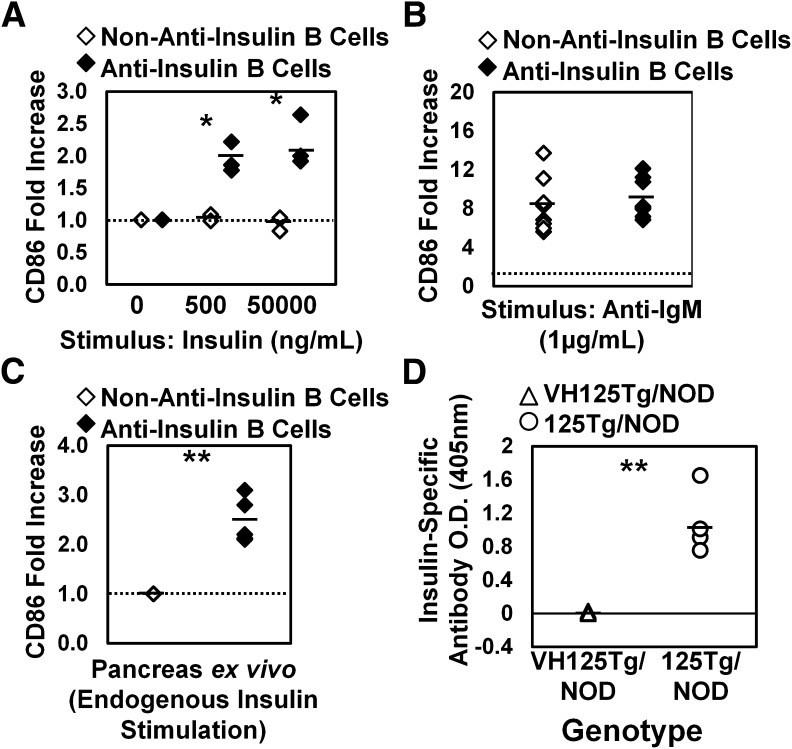
FIG. 2.
Insulin invokes CD86 upregulation in anti-insulin B cells, preparing them to interact with autoreactive T cells, whereas autoantibody production is silenced. Spleens were harvested from VH125Tg/NOD mice (10–13 weeks old), and cells were cultured for 18 h in complete media plus stimulus indicated. Flow cytometry was used to assess the MFI of CD86 on non–insulin-binding or insulin-binding immature B cells (live B220midIgM+ lymphocytes). A–C: Non–insulin-binding (◇) or insulin-binding (♦) B cells are indicated. A: Splenocytes were cultured with 0, 0.5, or 50 µg/mL human insulin, and the CD86 MFI of insulin-stimulated cells was divided by the CD86 MFI of unstimulated cells to calculate CD86 fold upregulation of non–insulin-binding or insulin-binding B cells. n = 3 mice; one experiment. B: VH125Tg/C57BL/6 or VH125Tg/NOD splenocytes were cultured with no stimulus or 1 μg/mL anti-IgM, and CD86 fold upregulation was determined. n = 8 mice; n = 2 experiments. C: Cells were freshly isolated from pancreata and stained on ice with 50 ng/mL human insulin (to ensure BCR occupancy), and insulin-binding B cells were identified using mAb123 staining. CD86 fold upregulation was identified among live B220+ IgMa+ lymphocytes by dividing the CD86 MFI of insulin-binding B cells by the CD86 MFI of non–insulin-binding B cells present in the same organ. n = 5 mice; n = 2 experiments. D: ELISA was used as described in RESEARCH DESIGN AND METHODS to detect anti-insulin IgMa antibodies in sera harvested from unimmunized mice of the indicated genotypes. n = 14 VH125Tg/NOD (△) or n = 4 125Tg/NOD (○) positive control 6- to 14-week-old mice. n = 3 experiments. *P < 0.01; **P < 0.001, as calculated by a two-tailed t test.
To identify whether anti-insulin B cells in VH125Tg/NOD mice are functionally silent for autoantibody production, sera were tested for the presence of IgMa insulin autoantibody using ELISA (see research design and methods). No insulin autoantibody was detected in the serum of VH125Tg/NOD mice compared with positive control 125Tg/NOD mice, which possess a small amount of insulin autoantibody (Fig. 2C). These data indicate that anti-insulin B cells that escape into the periphery of VH125Tg/NOD mice remain competent to upregulate costimulatory molecules in response to their cognate antigen. They do not produce detectable insulin autoantibody, suggesting that tolerance breakdown in VH125Tg/NOD mice includes the retention of T-cell stimulation capability necessary for APC function that is independent of autoantibody production.
Insulin autoantigen occupies the BCR of insulin-binding B cells in the spleen.
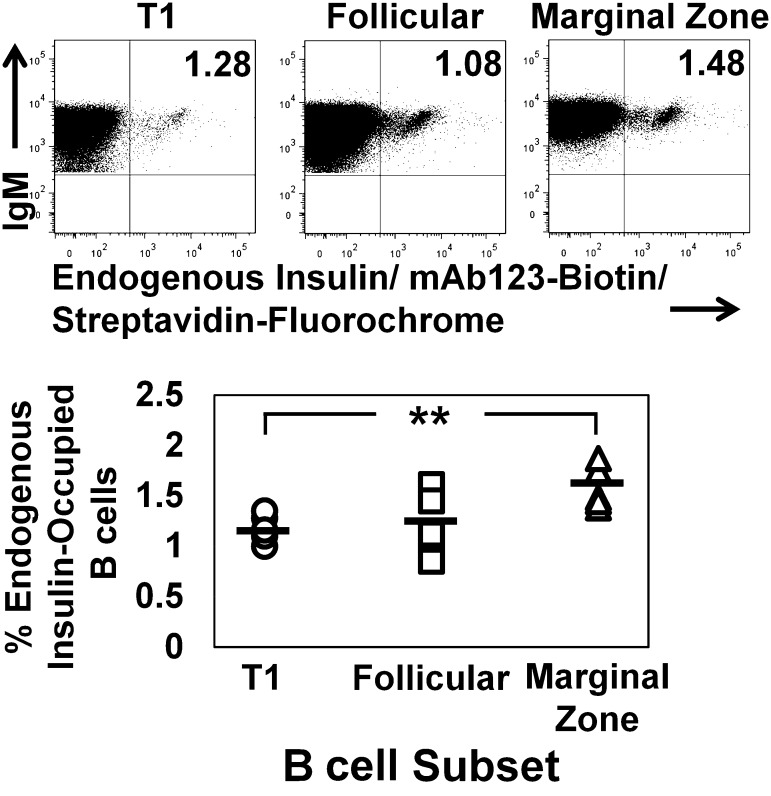
Flaws in tolerance revealed in VH125Tg/NOD mice suggest that BCR signaling following endogenous insulin encounter does not sufficiently evoke a mechanism to cull these cells (Fig. 1). To identify whether these cells are clonally ignorant, due to low concentrations of circulating insulin, mAb123-biotin was used to identify whether T1, follicular, or marginal zone BCR were occupied by insulin using flow cytometry. T1 (1.18 ± 0.14), follicular (1.22 ± 0.30), and marginal zone (1.58 ± 0.21) B-cell populations are clearly stained with mAb123-biotin (Fig. 3). The percentages detected fall within the range detected with biotinylated insulin (Fig. 1). These data suggest that BCR recognition of insulin is occurring, despite the low concentration of autoantigen physiologically present.
FIG. 3.
Endogenous insulin autoantigen occupies the BCR of developing and mature insulin-binding B cells in the spleen. Freshly isolated splenocytes were harvested from VH125Tg/NOD mice and stained with mAb123-biotin to recognize BCRs occupied with endogenous rodent insulin. Live B220+ IgM+ lymphocytes were further gated as in Fig. 1 to identify T1 (○), follicular (□), and marginal zone (△) B-cell subsets. Top: Representative plots are shown. Bottom: Summary graphs show n = 5 6- to 12-week-old mice; n = 2 experiments.
Autoantigen-targeted mAb engages the BCR to specifically eliminate autoreactive B cells while preserving the broad repertoire.
The BCR of anti-insulin B cells in VH125Tg/NOD are occupied by insulin. The macroself model demonstrates the feasibility of BCR-mediated deletion of B cells in the periphery upon strong antigen cross-linking of the BCR (20). We therefore hypothesized that mAb123 administered in vivo would bind specifically to insulin-occupied B cells and enhance BCR signaling to reinforce tolerance as a strategy to specifically remove anti-insulin B cells. VH125Tg/NOD mice were injected weekly for 3 weeks with either 100 μg of mAb123 or 100 μg IgG1 isotype control mAb. BM and spleens were harvested 2, 7, or 14 d following antibody treatment, and the percentage of insulin-binding B cells in the BM and spleen was assessed using flow cytometry (Fig. 1). mAb123 does not recognize insulin when it is bound to the insulin hormone receptor (17). The expected normoglycemia was observed, and no significant change in blood glucose levels was observed between the mAb123 or isotype control antibody-injected cohorts of mice (n = 11; P = 0.84) as calculated by a two-tailed t test.
The percentage of insulin-binding B cells was significantly reduced (2, 7, and 14 d, respectively) in immature B cells harvested from mice injected with mAb123 (0.33 ± 0.05, 0.45 ± 0.21, and 0.45 ± 0.20) compared with isotype control (0.72 ± 0.06, 0.93 ± 0.07, and 0.82 ± 0.07), as well as in T1 spleen B cells (mAb123: 0.11 ± 0.09, 0.42 ± 0.27, and 0.36 ± 0.17; isotype control: 0.56 ± 0.15, 1.05 ± 0.41, and 1.13 ± 0.34, respectively). However, complete depletion was not achieved in either of these developing compartments (Fig. 4A). The frequency of insulin-binding B cells was further reduced in follicular B cells isolated from mice treated with mAb123 (0.10 ± 0.05, 0.12 ± 0.05, and 0.12 ± 0.04) compared with isotype control antibody-treated mice (0.97 ± 0.17, 1.44 ± 0.11, and 1.27 ± 0.11), as well as marginal zone B cells (mAb123: 0.07 ± 0.01, 0.10 ± 0.11, and 0.09 ± 0.03; isotype control, 1.33 ± 0.19, 1.58 ± 0.42, and 2.46 ± 0.33, respectively; Fig. 4A). Elimination of anti-insulin B cells was also observed in the lymph nodes, blood, and peritoneal cavity (not shown). The percentages of total B cells present within B-cell developmental subsets were unchanged (not shown), suggesting that B cells were not depleted nonspecifically.
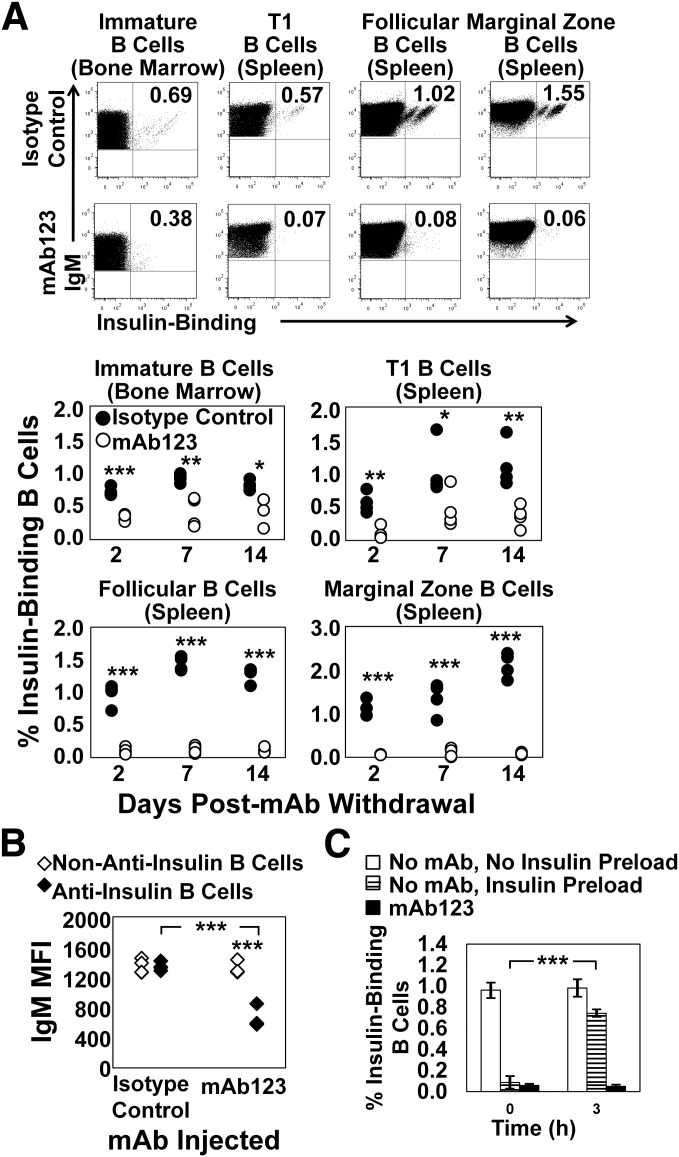
FIG. 4.
Autoantigen-targeted mAb specifically eliminates insulin-binding B cells while preserving the broad repertoire. A and B: VH125Tg/NOD mice were injected i.p. with 0.1 mg of mAb123 or 0.1 mg of a mouse IgG1 isotype control antibody once weekly for 3 weeks (four 7- to 12-week-old mice per group per time point). After 2, 7, or 14 days, BM and spleens were harvested. A: Insulin-binding B cells were identified in the BM—immature B cells (B220+IgMa+CD23− live lymphocytes)—or the spleen (B220+IgMa+ live lymphocyte gated): T1 B cells (CD21lo CD23lo), follicular B cells (CD21mid CD23hi), or marginal zone B cells (CD21hi CD23mid). Top: Representative plots from 2 days after final mAb injection. Bottom: Individual mice are plotted: isotype control-treated mice (●) and mAb123-treated mice (○). B-cell subset is indicated above chart. n = 4. Similar data were obtained in at least three experiments. B: The IgMa MFI is shown for non–insulin-binding (◇) or insulin-binding (♦) immature B cells from isotype control or mAb123-treated mice from A; organs harvested 2 days after final mAb injection. C: Spleens were harvested from VH125Tg/NOD mice that were untreated (white bars) or injected i.p. weekly for 3 weeks with 0.1 mg mAb123 (black bars). As a positive control, spleens were also harvested from untreated mice, and splenocytes were incubated with 50 µg/mL insulin to competitively inhibit binding by biotinylated insulin (striped bars). Splenocytes were plated in complete media and incubated for 0–3 h at 37°C. Cells were then immediately stained with reagents reactive with B220, IgMa, and 7-aminoactinomycin D, as well as biotinylated insulin. The percentage of live B220+IgMa+ lymphocytes detected by biotinylated insulin is plotted for each condition. n = 3 mice; data are representative of two experiments. *P < 0.05; **P < 0.01; ***P < 0.001 as calculated by a two-tailed t test.
Tolerance induction in developing B cells that encounter insulin has been shown to result in BCR downregulation (21). To test whether IgM levels were reduced in insulin-binding B cells that had not yet undergone depletion, IgMa MFI was compared among non–insulin-binding and insulin-binding B cells present in VH125Tg/NOD mice treated with isotype control or mAb123 antibodies. As shown in Fig. 4B, the IgMa MFI was significantly reduced in insulin-binding immature B cells harvested from mAb123-treated mice (650 ± 133) when compared with non–insulin-binding B cells from the same mice (1,312 ± 79, P < 0.001) or with insulin-binding B cells present in isotype control antibody-treated mice (1,340 ± 54; P < 0.001), but was unchanged in non–insulin-binding B cells harvested from mice treated with isotype control (1,342 ± 92) or mAb123 antibodies (1,312 ± 79; P = 0.64). mAb123 bound to insulin occupying the BCR does not block detection of IgMa (Fig. 3) and thus does not account for the reduced IgMa observed. These findings are consistent with the hypothesis that mAb123 induces BCR downregulation in immature B cells in an antigen-specific manner.
Anti-insulin BCRs that are loaded with endogenous insulin are not 100% occupied, as they are further stained by fluorescently labeled insulin in vitro (not shown). To eliminate the possibility that mAb123 was capturing endogenous insulin and promoting 100% BCR occupancy, and thus blocking detection of anti-insulin B cells by biotinylated insulin, spleens from isotype control or mAb123-treated mice were placed at 37°C for 0–3 h to permit BCR turnover. As a positive control, spleens were harvested from untreated mice and incubated with excess human insulin to establish 100% BCR occupancy and were also placed at 37°C for 0–3 h. Whereas detection of insulin-binding B cells using biotinylated human insulin reappeared in the control spleens loaded with unlabeled insulin following incubation at 37°C, no insulin-binding B cells were observed in the spleens harvested from mAb123-treated mice, even after 3 h (Fig. 4C). As expected, insulin-binding B cells were present in isotype control Ab-treated mice at all time points (not shown). These data suggest that anti-insulin B cells are being specifically depleted from the repertoire, rather than being masked by mAb123 binding. Thus, mAb123 selectively enhances removal of anti-insulin B cells from the polyclonal repertoire. These data suggest that this strategy is highly effective at removing anti-insulin B cells from the mature repertoire, but less effective for developing B cells.
Autoantigen-targeted mAb therapy provides disease protection that is overcome by enhanced BM production of anti-insulin B cells.
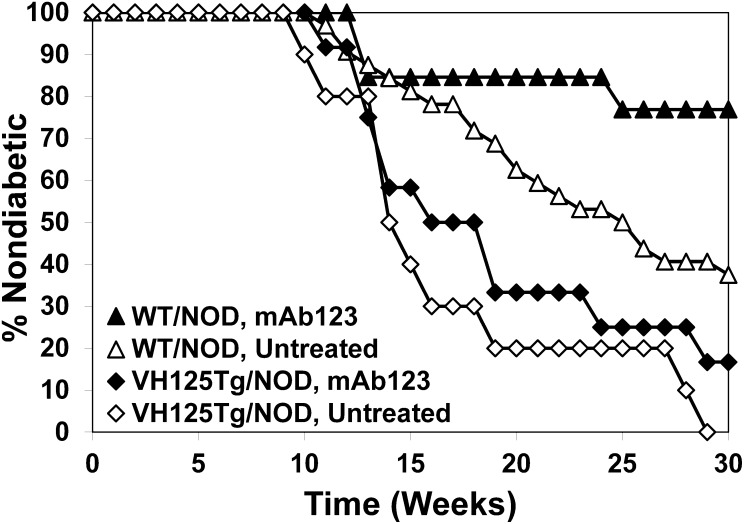
Anti-insulin B cells are critical for type 1 diabetes (5,8,22,23). This specificity is depleted from the repertoire in vivo by mAb123 treatment (Fig. 4); therefore, we tested whether this strategy could prevent disease in NOD mice. mAb123 was administered to VH125Tg/NOD or WT/NOD mice every other week beginning at 3 weeks of age, and diabetes outcomes were determined (see RESEARCH DESIGN AND METHODS). Significant disease protection is observed for mAb123-treated versus untreated WT/NOD mice (Fig. 5; P = 0.046). In contrast, disease protection is not observed in mAb123-treated VH125Tg/NOD mice (compared with untreated). These data suggest that mAb123 protects against disease in a polyclonal repertoire in which the frequency of anti-insulin B-cell formation in the developing repertoire is very low, but the same regimen is not sufficient to prevent disease if BM production of anti-insulin B cells is increased.
FIG. 5.
Selective depletion of anti-insulin B cells by mAb123 protects against disease in WT/NOD mice. WT/NOD or VH125Tg/NOD mice were injected with 0.1 mg of mAb123 once every other week beginning at 3 weeks of age. Diabetes outcome was monitored as in RESEARCH DESIGN AND METHODS. WT/NOD untreated mice (△; n = 32), WT/NOD mAb123-treated mice (▲; n = 13), VH125Tg/NOD untreated mice (◇; n = 10), and VH125TgNOD mAb123-treated mice (♦; n = 12) are shown in a Kaplan-Meier survival plot. WT/NOD mAb123 versus WT/NOD untreated: P < 0.05 as calculated by a log-rank test.
DISCUSSION
The findings presented in this study reveal how B-cell tolerance for insulin can be restored to eliminate this critical APC specificity to prevent type 1 diabetes. Tracking of islet antigen-specific B cells shows that multiple checkpoints fail to remove anti-insulin B cells and provides a realistic picture of how immune tolerance is lost in a multigenic autoimmune disease. Anti-insulin B cells escape into the peripheral repertoire, where they are functional to upregulate CD86 when stimulated with insulin (Figs. 1 and 2), highlighting their dangerous potential to activate autoreactive T cells. Developing and mature anti-insulin BCR are occupied by endogenous insulin, despite low levels of circulating autoantigen. Targeting autoantigen-occupied BCR specifically eliminates this pernicious specificity, but is unexpectedly less effective at targeting the developing repertoire (Fig. 4). This new approach protects against type 1 diabetes in WT/NOD mice; however, increased anti-insulin B-cell production in the BM of VH125Tg/NOD mice overwhelms this protective effect (Fig. 5). Together, these data suggest that BM production of anti-insulin B cells combines with tolerance flaws to create liabilities for the pathogenesis of type 1 diabetes, which can be overcome with effective specific targeting and elimination of autoantigen-specific B cells.
Breaches in tolerance represented by autoantibodies to insulin and other islet autoantigens are reliable predictors of disease progression (18,24,25). Maternal transmission of autoantibody promotes disease (26), and antibodies serve as cofactors in experimental type 1 diabetes when islet expression of the antigenic target is engineered (27). They are, however, neither necessary nor sufficient, as disease is observed in IgM isotype-restricted VH125Tg/NOD and 125Tg/NOD mice in the absence of anti-insulin autoantibody (5), and transfer of serum containing islet autoantibodies is insufficient to drive type 1 diabetes (7). These studies employ the VH125Tg/NOD model to probe how islet-reactive B cells escape tolerance to acquire APC function to drive type 1 diabetes, independent of peripheral tolerance blockade of plasma cell differentiation and autoantibody production.
CD21 and CD23 acquisition occurs normally for anergic anti-insulin B cells in a restricted repertoire (6), in contrast with other described states of anergy (28). These studies demonstrate that in a polyclonal repertoire, maturation and effective competition for follicular niches by anti-insulin B cells remain intact (Fig. 1). An increased proportion of insulin-binding B cells mature into marginal zone B cells (Fig. 1), which are superior to follicular B cells in activating naive CD4 T cells (29). Anti-insulin B cells retain the ability to upregulate CD86 following insulin engagement (Fig. 2), further highlighting the dangerous potential of these cells. The critical role of B cells as APC in driving type 1 diabetes has been well documented (7–9). These findings suggest that anergy is not as stringently enforced among anti-insulin B cells as for other autoantigens (6,30–32). Anti-insulin B cells are thus poised to activate anti-insulin T cells, a pathogenic function validated by the fact that they fully support the development of type 1 diabetes in NOD mice (5,6).
Global B-cell depletion therapy (rituximab) shows promise for the treatment of type 1 diabetes (33), highlighting a pivotal role for B cells in disease. Unwanted side effects resulting from immune suppression (34) could be avoided through targeted silencing or elimination of autoreactive B cells. Developing B cells in the BM and T1 stage in the spleen undergo cell death following BCR stimulation (35). Given the enhanced BCR sensitivity of immature B cells (36–39), developing B cells were expected to be the most sensitive B-cell subset to this type of approach. Surprisingly, the opposite was observed, whereby mature anti-insulin B cells were more readily culled, and the immature BM and T1 spleen B cells were somewhat more resistant to elimination (Fig. 4A). Insulin-binding B cells are continuously generated in the BM, making the immature and T1 B-cell compartments more dynamic than the longer-lived mature B-cell compartment. We propose that the feasibility of therapeutic depletion using this strategy is enhanced among B cells that are further from the source of the leak in the BM. The surviving insulin-binding immature B cells show markedly decreased surface BCR expression (Fig. 4B), consistent with tolerance induction for insulin (21). We propose that anti-insulin B cells may be programmed for receptor editing or deletion when mAb123 intensifies insulin-occupied BCR stimulation in vivo. Antibody-dependent cell-mediated cytotoxicity impacts clearance of B cells in anti-CD20 treated mice (40); thus, it is likely also a factor in this treatment scenario. Future studies utilizing mAb123 F(ab’)2 fragments will be necessary to uncover the role that Fc receptor recognition is playing in anti-insulin B-cell depletion and whether BCR engagement alone can invoke depletion.
These results suggest that reinforcing B-cell tolerance for insulin is sufficient to protect against disease (Fig. 5). The frequency of insulin-binding B cells in WT/NOD mice is very low, whereas this population is clearly increased in VH125Tg/NOD mice (Fig. 1). Insulin-binding B cells were detected in many of the mAb123-treated VH125Tg/NOD mice following disease development and study removal (not shown). We hypothesize that a crucial threshold exists, beyond which the effect of this mAb123 dosage is overwhelmed by continuous BM production. Exquisite depletion efficiency is thus a key requirement for successful autoreactive B cell–directed mAb candidates. It is also possible that VH125 skews the repertoire toward other islet specificities not targeted by mAb123.
These data add to the growing body of evidence to suggest that insulin is a critical autoantigen in type 1 diabetes (4,5) and thus a clear target for immune tolerance restoration. The human B-cell repertoire for insulin may be restricted (41), emphasizing the potential feasibility of this approach in either prevention of disease or protection against future attack of β-cells in islet grafts. These findings provide proof of concept for how anti-insulin B cells might be therapeutically removed to prevent autoimmune disease. This concept could be broadly applied to additional autoimmune diseases, provided the targeted autoantigen is present on the surface of the B cells to be depleted or that highly selective anti-idiotype reagents could be generated. A panel of mAb specific for multiple autoantigens may show the best potential for therapeutic intervention by removing the complete array of autoreactive B cells from the repertoire. The success of intravenous γ globulin in treating many autoimmune disorders, although variable, may use the same fundamental principal, as heterogeneous preparations contain multiple specificities.
On the basis of our findings, we propose the following model: tolerance failure seeds anti-insulin B cells into the mature repertoire, where they subsequently escape peripheral tolerance and upregulate CD86 following insulin stimulation to provide critical APC functions for autoaggressive T cells. This breach in tolerance is only partial, as anti-insulin B cells remain impaired for antibody production. This outcome can be circumvented by autoantigen-specific targeting to selectively deplete self-reactive B cells to prevent disease. These studies identify multiple checkpoints at which tolerance fails to circumvent autoreactivity in type 1 diabetes-prone mice and provide proof of concept for the development of a new strategic approach for the elimination of escaped autoreactive B cells.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants 5T32-HL-069765, 5T32-AR-059039, R01-AI-051448, and R01-DK-08246, as well as Juvenile Diabetes Research Foundation Grant 1-2008-108.
No potential conflicts of interest relevant to this article were reported.
R.A.H. designed and performed the experiments and wrote the manuscript. P.L.K. and J.W.T. provided experimental design and discussion input, as well as critical manuscript review. P.L.K. also assisted in the disease study experiment. J.W.T. is the guarantor of this work, and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this work were presented as a short talk in Workshop 2A at the Keystone Symposia B Cells: New Insights into Normal versus Dysregulated Function, Whistler, British Columbia, Canada, 12–17 April 2011.
The authors thank Amita Rachakonda, Brett Case, and Allison Sullivan for technical support (Vanderbilt University), as well as the Vanderbilt Medical Center Flow Cytometry Shared Resource (supported by the Vanderbilt Ingram Cancer Center [P30 CA68485] and the Vanderbilt Digestive Disease Research Center [DK058404]), the Vanderbilt Antibody and Protein Resource, the Vanderbilt Hormone Assay & Analytical Services Core, and the Vanderbilt Diabetes Research and Training Center. The authors also thank Eugene Oltz (Washington University, St. Louis, MO), Daniel Moore, Mark Boothby, Kristen Hoek, and Patrick Collins (Vanderbilt University) for critical manuscript review.
REFERENCES
- 1.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Type 1 Diabetes TrialNet Anti-CD20 Study Group . Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med 2009;361:2143–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu L, Herold K, Krause-Steinrauf H, et al. Type 1 Diabetes TrialNet Anti-CD20 Study Group . Rituximab selectively suppresses specific islet antibodies. Diabetes 2011;60:2560–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steck AK, Johnson K, Barriga KJ, et al. Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA-2 autoantibodies, predict age of diagnosis of type 1 diabetes: diabetes autoimmunity study in the young. Diabetes Care 2011;34:1397–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakayama M, Abiru N, Moriyama H, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 2005;435:220–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hulbert C, Riseili B, Rojas M, Thomas JW. B cell specificity contributes to the outcome of diabetes in nonobese diabetic mice. J Immunol 2001;167:5535–5538 [DOI] [PubMed] [Google Scholar]
- 6.Acevedo-Suárez CA, Hulbert C, Woodward EJ, Thomas JW. Uncoupling of anergy from developmental arrest in anti-insulin B cells supports the development of autoimmune diabetes. J Immunol 2005;174:827–833 [DOI] [PubMed] [Google Scholar]
- 7.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol 1998;161:3912–3918 [PubMed] [Google Scholar]
- 8.Silveira PA, Johnson E, Chapman HD, Bui T, Tisch RM, Serreze DV. The preferential ability of B lymphocytes to act as diabetogenic APC in NOD mice depends on expression of self-antigen-specific immunoglobulin receptors. Eur J Immunol 2002;32:3657–3666 [DOI] [PubMed] [Google Scholar]
- 9.Noorchashm H, Lieu YK, Noorchashm N, et al. I-Ag7-mediated antigen presentation by B lymphocytes is critical in overcoming a checkpoint in T cell tolerance to islet beta cells of nonobese diabetic mice. J Immunol 1999;163:743–750 [PubMed] [Google Scholar]
- 10.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science 2003;301:1374–1377 [DOI] [PubMed] [Google Scholar]
- 11.Yurasov S, Wardemann H, Hammersen J, et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med 2005;201:703–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samuels J, Ng YS, Coupillaud C, Paget D, Meffre E. Impaired early B cell tolerance in patients with rheumatoid arthritis. J Exp Med 2005;201:1659–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menard L, Saadoun D, Isnardi I, et al. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J Clin Invest 2011;121:3635–3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bottini N, Musumeci L, Alonso A, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet 2004;36:337–338 [DOI] [PubMed] [Google Scholar]
- 15.Rojas M, Hulbert C, Thomas JW. Anergy and not clonal ignorance determines the fate of B cells that recognize a physiological autoantigen. J Immunol 2001;166:3194–3200 [DOI] [PubMed] [Google Scholar]
- 16.Henry RA, Kendall PL, Woodward EJ, Hulbert C, Thomas JW. Vkappa polymorphisms in NOD mice are spread throughout the entire immunoglobulin kappa locus and are shared by other autoimmune strains. Immunogenetics 2010;62:507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor SI, Schroer JA, Marcus-Samuels B, McElduff A, Bender TP. Binding of insulin to its receptor impairs recognition by monoclonal anti-insulin antibodies. Diabetes 1984;33:778–784 [DOI] [PubMed] [Google Scholar]
- 18.Yu L, Robles DT, Abiru N, et al. Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci USA 2000;97:1701–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Martin F, Forbush KA, Perlmutter RM, Kearney JF. Evidence for selection of a population of multi-reactive B cells into the splenic marginal zone. Int Immunol 1997;9:27–41 [DOI] [PubMed] [Google Scholar]
- 20.Aït-Azzouzene D, Verkoczy L, Duong B, Skog P, Gavin AL, Nemazee D. Split tolerance in peripheral B cell subsets in mice expressing a low level of Igkappa-reactive ligand. J Immunol 2006;176:939–948 [DOI] [PubMed] [Google Scholar]
- 21.Henry RA, Acevedo-Suárez CA, Thomas JW. Functional silencing is initiated and maintained in immature anti-insulin B cells. J Immunol 2009;182:3432–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serreze DV, Chapman HD, Varnum DS, et al. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD.Ig mu null mice. J Exp Med 1996;184:2049–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akashi T, Nagafuchi S, Anzai K, et al. Direct evidence for the contribution of B cells to the progression of insulitis and the development of diabetes in non-obese diabetic mice. Int Immunol 1997;9:1159–1164 [DOI] [PubMed] [Google Scholar]
- 24.Palmer JP, Asplin CM, Clemons P, et al. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science 1983;222:1337–1339 [DOI] [PubMed] [Google Scholar]
- 25.Baekkeskov S, Nielsen JH, Marner B, Bilde T, Ludvigsson J, Lernmark A. Autoantibodies in newly diagnosed diabetic children immunoprecipitate human pancreatic islet cell proteins. Nature 1982;298:167–169 [DOI] [PubMed] [Google Scholar]
- 26.Greeley SA, Katsumata M, Yu L, et al. Elimination of maternally transmitted autoantibodies prevents diabetes in nonobese diabetic mice. Nat Med 2002;8:399–402 [DOI] [PubMed] [Google Scholar]
- 27.Silva DG, Daley SR, Hogan J, et al. Anti-islet autoantibodies trigger autoimmune diabetes in the presence of an increased frequency of islet-reactive CD4 T cells. Diabetes 2011;60:2102–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature 1991;353:765–769 [DOI] [PubMed] [Google Scholar]
- 29.Attanavanich K, Kearney JF. Marginal zone, but not follicular B cells, are potent activators of naive CD4 T cells. J Immunol 2004;172:803–811 [DOI] [PubMed] [Google Scholar]
- 30.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature 1994;371:389–395 [DOI] [PubMed] [Google Scholar]
- 31.Mandik-Nayak L, Bui A, Noorchashm H, Eaton A, Erikson J. Regulation of anti-double-stranded DNA B cells in nonautoimmune mice: localization to the T-B interface of the splenic follicle. J Exp Med 1997;186:1257–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mason DY, Jones M, Goodnow CC. Development and follicular localization of tolerant B lymphocytes in lysozyme/anti-lysozyme IgM/IgD transgenic mice. Int Immunol 1992;4:163–175 [DOI] [PubMed] [Google Scholar]
- 33.Townsend MJ, Monroe JG, Chan AC. B-cell targeted therapies in human autoimmune diseases: an updated perspective. Immunol Rev 2010;237:264–283 [DOI] [PubMed] [Google Scholar]
- 34.Gea-Banacloche JC. Rituximab-associated infections. Semin Hematol 2010;47:187–198 [DOI] [PubMed] [Google Scholar]
- 35.Norvell A, Mandik L, Monroe JG. Engagement of the antigen-receptor on immature murine B lymphocytes results in death by apoptosis. J Immunol 1995;154:4404–4413 [PubMed] [Google Scholar]
- 36.Benschop RJ, Melamed D, Nemazee D, Cambier JC. Distinct signal thresholds for the unique antigen receptor-linked gene expression programs in mature and immature B cells. J Exp Med 1999;190:749–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benschop RJ, Brandl E, Chan AC, Cambier JC. Unique signaling properties of B cell antigen receptor in mature and immature B cells: implications for tolerance and activation. J Immunol 2001;167:4172–4179 [DOI] [PubMed] [Google Scholar]
- 38.Metcalf ES, Klinman NR. In vitro tolerance induction of bone marrow cells: a marker for B cell maturation. J Immunol 1977;118:2111–2116 [PubMed] [Google Scholar]
- 39.Raff MC, Owen JJ, Cooper MD, Lawton AR, 3rd, Megson M, Gathings WE. Differences in susceptibility of mature and immature mouse B lymphocytes to anti-immunoglobulin-induced immunoglobulin suppression in vitro. Possible implications for B-cell tolerance to self. J Exp Med 1975;142:1052–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uchida J, Hamaguchi Y, Oliver JA, et al. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med 2004;199:1659–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castaño L, Ziegler AG, Ziegler R, Shoelson S, Eisenbarth GS. Characterization of insulin autoantibodies in relatives of patients with type I diabetes. Diabetes 1993;42:1202–1209 [DOI] [PubMed] [Google Scholar]